Abstract
Recent evidence suggests that subsets of lung fibroblasts differentially contribute to fibrogenic progression. We have previously shown that a subset of rat lung fibroblasts with fibrogenic characteristics [Thy-1 (−) fibroblasts] responds to stimuli (bleomycin, interleukin-4, etc) with increased latent transforming growth factor (TGF)-β activation, whereas non-fibrogenic Thy-1-expressing [Thy-1 (+)] fibroblasts do not. Activation of latent TGF-β1 by interstitial lung fibroblasts is critical for fibrogenic responses. To better understand the susceptibility of fibrogenic fibroblasts to the stimulation of TGF-β activation, we examined the role of latent TGF-β-binding proteins (LTBPs), key regulators of TGF-β bioavailability and activation, in TGF-β1 activation by these fibroblasts. Treatment of fibroblasts with bleomycin up-regulated LTBP-4 mRNA, protein, and soluble LTBP-4-bound large latent TGF-β1 complexes in Thy-1 (−) fibroblasts to significantly higher levels than in Thy-1 (+) fibroblasts. Bleomycin-induced TGF-β1 activation required LTBP-4, since lung fibroblasts deficient in LTBP-4 did not activate TGF-β1. Expression of LTBP-4 restored TGF-β1 activation in response to bleomycin, but expression either of LTBP-4 lacking the TGF-β-binding site or only the TGF-β-binding domain did not. Bleomycin treatment of mice increased LTBP-4 expression in the lung. Thy-1 knockout mice had increased levels of both LTBP-4 expression and TGF-β activation, as well as enhanced Smad3 phosphorylation compared with wild-type mice. Together, these data identify a critical role for LTBP-4 in the regulation of latent TGF-β1 activation in bleomycin-induced lung fibrosis.
Excessive transforming growth factor (TGF)-β activity is central to the development of pulmonary fibrosis.1,2,3 TGF-β is initially synthesized as an inactive latent molecule (termed the small latent TGF-β complex or SLC), which consists of an N-terminal pro-domain, the latency associated peptide (LAP), and a C-terminal mature TGF-β domain (active TGF-β).4,5 During posttranslational processing, the LAP and mature TGF-β domains are cleaved by the endopeptidase furin, but the two peptides remain non-covalently associated.6,7 The association of the mature domain with LAP prevents mature TGF-β from binding to its cell surface receptors.5,8 Activation of latent TGF-β, which involves either the release of the mature domain from the latent complex or the exposure of mature TGF-β as a consequence of alterations in the structure or folding of the latent complex, is required for TGF-β signaling.
Expression of latent TGF-β1 protein is insufficient to stimulate pulmonary fibrosis. Rats treated with recombinant adenovirus expressing constitutively active TGF-β1 developed lung fibrosis, but not rats treated with adenovirus expressing latent TGF-β1.9 Previously we showed that a subset of lung fibroblasts, which are localized to fibroblastic foci in the lungs of patients with idiopathic pulmonary fibrosis,10 have an increased capacity to activate latent TGF-β in response to fibrogenic stimuli as compared with a non-fibrogenic fibroblast subset, which predominates in the normal lung interstitium.11 The fibrogenic subset is characterized by a lack of expression of Thy-1 (CD90), a glycosyl phosphatidylinositol-anchored glycoprotein. These Thy-1 (−) lung fibroblasts exhibit increased motility, contractility, and expression of myofibroblastic characteristics as compared with Thy-1 (+) cells.12,13,14,15,16
TGF-β bioavailability is regulated by complex factors, including agents that control activation, synthesis and processing, and deposition in the extracellular matrix (ECM).17 Latent TGF-β binding proteins (LTBP-1, -2, -3, and -4) are a family of fibrillin-like molecules that contain multiple unique 8-cysteine repeats and multiple EGF-like domains. LTBP-1, -3, and -4 are covalently linked to the small latent TGF-β complex through a pair of cysteine residues in the N-terminal region of LAP and the third 8-cysteine repeat in LTBP to form the large latent TGF-β complex (LLC).18,19,20,21 LTBP-2 does not bind to the SLC.22 In addition, a number of splice variants have been identified for each of the LTBPs.23 However, specific functions for individual LTBP splice variants are not known.
Besides acting as matrix components, LTBPs have important functions in the regulation of TGF-β bioavailability and activity. LTBPs facilitate latent TGF-β secretion, mediate latent TGF-β targeting to the ECM for storage, and regulate latent TGF-β activation.19,24,25,26 Anti-LTBP-1 antibodies block latent TGF-β activation in both in vitro co-cultures of endothelial cells and smooth muscle cells and in ex vivo collagen gel cultures of embryonic heart.27,28 Integrin αvβ6-mediated epithelial TGF-β activation requires LTBP-1 interaction with fibronectin in the extracellular matrix.29 A recent study shows that LTBP-1 is one of the essential components of the ECM-integrin-cytoskeleton machinery involved in myofibroblast contraction-induced latent TGF-β1 activation.30 Hypomorphic LTBP-4 mice develop severe pulmonary emphysema, cardiomyopathy and colorectal cancer, conditions associated with deficient TGF-β activation.31 Lung fibroblasts isolated from these mice exhibit decreased active TGF-β, but increased total TGF-β, as compared with wild-type lung fibroblasts,32 suggesting that decreased LTBP-4 impairs TGF-β activation. Despite prior evidence that lack of LTBP-4 expression is associated with reduced TGF-β signaling in the lung, there have been no studies that demonstrate that LTBP-4 is increased in the fibrotic lung or studies that establish a critical role for LTBP-4 in the process of lung fibrogenesis.
In this study, we provide evidence from both in vitro and in vivo systems establishing the importance of LTBP-4 expression and its binding to the small latent TGF-β1 complex for activation of latent TGF-β in a model of lung fibrosis. Studies using a fibrogenic subset [Thy-1 (−)] of lung fibroblasts showed that the ability of these cells to activate latent TGF-β in response to bleomycin is dependent on LTBP-4 expression and its association with the small latent complex. In vivo studies in a bleomycin-induced mouse model of lung fibrosis show that bleomycin up-regulates LTBP-4 expression in the lung, both in the interstitium and in inflammatory cells. Furthermore, Thy-1 knockout mice with exacerbated lung fibrosis have increased LTBP-4 expression as compared with wild-type mice. The enhanced LTBP-4 expression in Thy-1 knockout mice is associated with increased latent TGF-β activation as well as Smad3-dependent TGF-β signal transduction. Together, these studies suggest that LTBP-4 is a key factor regulating latent TGF-β1 bioavailability and activation in lung fibrogenesis.
Materials and Methods
Antibodies, Plasmids, and Reagents
A rabbit polyclonal antibody (#33-4) against a peptide in the fourth 8-cys repeat of LTBP-4,33 and H-293, a rabbit polyclonal antibody against the N-terminus of LTBP-4 (Santa Cruz Biotechnology, Santa Cruz, CA), were used for Western blotting and immunohistochemical staining, respectively, for LTBP-4 analyses. Ab39, a rabbit antiserum against LTBP-1, was a gift from Dr. Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden).24 Anti-LTBP-3 antibody (anti-L3C) has been described previously.34 No cross-reactivity was found among antibodies specific for LTBP-1, LTBP-3, and LTBP-4. Goat anti-LAP(β1) antibody and neutralizing antibodies against TGF-β1, TGF-β2, TGF-β3, and TGF-β1, 2, and 3 were purchased from R&D Systems (Minneapolis, MN). Anti-Flag and anti-α-smooth muscle actin antibodies were purchased from Sigma (St. Louis, MO). Anti-phospho-Smad3 antibody (Ser423/425) was purchased from Cell Signaling (Beverly, MA). Anti-hemagglutinin (HA) antibody and anti-β tubulin antibody were purchased from Santa Cruz Biotechnology.
Both the full length human LTBP-4 cDNA and the mutated splice variant LTBP-4 cDNA lacking the third 8-cysteine repeat (LTBP-4Δ8-cys3rd) were cloned into the expression vector pEFIRES-P.35,36 The cDNA encoding the third 8-Cys repeat of LTBP-4 (L4ECR3E) was cloned into the pcDNA3HA expression vector and expressed as a HA-tagged fusion protein.37 A pEF6/V5 vector expressing Flag-tagged small latent TGF-β1 complex (pFlag-SLC) was constructed as described previously.38
Recombinant human TGF-β1 was from R&D Systems. LTBP-4 specific siRNAs (ID 00063593 and ID 0063594) and puromycin were from Sigma. Bleomycin sulfate was from Calbiochem (San Diego, CA). G418 was from ICN Biomedicals (Aurora, Ohio). Zeocin was from Invitrogen (Carlsbad, CA). Luciferase 1000 Assay System was from Promega (Madison, WI).
Cell Culture, Transfection, and Treatment
Rat lung fibroblasts stably expressing murine Thy-1.2 cDNA (Thy-1 positive) and empty vector-transfected control cell line (Thy-1 negative) were generated as described previously.11,12 Cells were maintained in F12K media (Cellgro Herndon, VA) containing 10% PBS and 200 μg/ml Zeocin. Hypomorphic LTBP-4 mice were developed previously.31 Lung fibroblasts isolated from hypomorphic LTBP-4 mice were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.31 Transfection of plasmids into lung fibroblasts was performed with a Nucleofector device (Amaxa, Inc., Cologne Germany) following the manufacturer’s instructions. All transfections were performed with the combination of cell line Nucleofector Kit R and program O-017. Cell lines stably expressing LTBP-4 were selected with puromycin (2 to 5 μg/ml). For bleomycin and TGF-β neutralizing antibody treatment, cells were grown to 70% to 80% confluence and rendered quiescent in F12K or DMEM with 0.1% fetal bovine serum for 48 hours and in serum-free media for 2 hours. After removal of quiescing media, fresh serum-free media was added in the presence of 0.5 μg/ml of bleomycin, anti TGF-β1, -2, -3 (1 μg/ml), anti TGF-β1 (0.2 μg/ml), anti TGF-β2 (0.1 μg/ml) and anti TGF-β3 (2 μg/ml) neutralizing antibodies and incubated for 24 to 72 hours.
Preparation of Conditioned Media and Plasmin-Digested Extracellular Matrix
The culture supernatants (30 ml) from 150 mm plates were harvested and centrifuged at 1500 rpm for 10 minutes at 4°C to remove cell debris. Cell-free conditioned media (CM) was concentrated to 300 μl (100×) with Amicon Ultra-15 10,000 MWCO Centrifugal Filter Devices (Millipore, Bedford, MA). Protein concentration was determined using the Bio-Rad (Richmond, CA) protein assay. The concentrated CM was aliquoted in siliconized tubes and stored at −80°C until used. For preparation of the ECM, cell plates were washed twice with PBS and covered with 1 ml of ice-cold lysis buffer (50 mmol/L Tris-HCl at pH 7.2, 150 mmol/L NaCl, 1% NP-40, 0.5% sodium deoxycholate). The plates were scraped with rubber policemen. Deoxycholate-insoluble pellets were collected by centrifugation at 14,000 rpm for 10 minutes. The pellets were washed twice with 1 ml PBS and digested with plasmin (0.2 CU/ml) in 150 μl of matrix digestion buffer (PBS containing 1 mmol/L Ca2+, 1 mmol/L Mg2+, and 0.1% n-octyl-d-β-glycopyranoside) at 37°C for 1 hour. Proteins released by plasmin digestion were obtained by centrifugation and collection of the supernatant. These preparations contain proteins derived from the highly cross-linked, SDS-insoluble extracellular matrix.19
Northern Blotting and Reverse Transcription-PCR
Total cellular RNA was isolated with TRIzol reagent (Invitrogen). Fifteen μg of total RNA was denatured and fractionated in 1.2% formaldehyde agarose gels and transferred to Hybond N+ membrane for 16 hours. Verification of equal loading was achieved by comparison of 28S rRNA intensities after staining by ethidium bromide. After alkali fixation for 5 minutes, the blots were hybridized with a human LTBP−4 probe36 labeled with [α-32P]-dCTP (Amersham). Hybridization was performed at 42°C for 16 hours in the ULTRAhyb Hybridization Buffer (Ambion). After washing, membranes were exposed to X-ray film (Kodak) for varying lengths of time. For reverse transcription (RT)-PCR, the first strand cDNA was synthesized by using Cells-to-cDNA II kit (Ambion). A pair of human LTBP-4-specific primers (forward: 5′-GCTGTTCGCTGCCCATTCTG-3′, reverse: 5′-AGGGTACGAGGCTGGCTGAGT-3′) were used to identify transcriptional expression, specifically of human LTBP-4 in rat lung fibroblasts. These primers do not amplify rat LTBP-4 due to the mismatches between the rat and human sequences at the primer 3′ terminals. PCR was performed in a Mastercycler Personal (Eppendorf) with a standard protocol.
Western Blotting
Equal amounts of protein were loaded onto SDS-polyacrylamide gels under non-reducing conditions. After electrophoresis, the proteins were electrophoretically transferred from the gels to nitrocellulose at 100 V for 3 hours at 4°C. Membranes were blocked in casein solution (1% casein, 25 mmol/L Na2HPO4, pH 7.1) for 1 hour at room temperature. Membranes were incubated with primary antibodies diluted in TBS-T and casein solution (1:1): #33-4 at 1:300,33 H-293 at 1 μg/ml, Ab39 at 1:1000,24 anti-L3C at 1:5000,34 anti LAP(β1) at 0.02 μg/ml, and anti-β-tubulin at 0.1 μg/ml for 18 hours at 4°C. After extensive washing, membranes were incubated with appropriate peroxidase-conjugated secondary antibodies (0.1 μg/ml) diluted in TBS-T for 1 hour at room temperature. Immunodetection was performed by chemiluminescence.
Immunoprecipitation
The binding of LTBP-4 to the SLC in the conditioned media of Thy-1 (−) rat lung fibroblasts was determined by immunoprecipitation with anti-LAP(β1) (300 μg/ml) antibody followed Western blotting analysis with anti-LTBP-4 antibody (1 μg/ml). Immunoprecipitation was performed with ProFound Co-Immunoprecipitation Kit (Pierce, Rockford, IL) according to the manufacturer’s instructions.
To detect interactions between the third 8-cysteine repeat of LTBP-4 and the SLC, the ECR3E-HA plasmids37 (2 μg) were co-transfected with pFlag-SLC (2 μg) into hypomorphic LTBP-4 lung fibroblasts with a Nucleofector device (Amaxa, Inc., Cologne, Germany). Transfected cells were cultured in DMEM media supplemented with 10% fetal bovine serum for overnight. Cells were then washed twice with serum-free DMEM and were further cultured in serum-free DMEM for 48 hours. Conditioned media was collected and subjected to immunoprecipitation with Anti-HA Immunoprecipitation Kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions.
siRNA Knockdown of Rat LTBP-4
Two duplex oligo siRNAs specific to rat LTBP-4 (Sigma, St. Louis, MO) and Silencer Negative Control siRNA (Ambion, Austin, TX) were transfected into Thy-1 (−) rat lung fibroblasts at a final concentration of 50 nmol/L for each with a Nucleofector device as described above. 24 hours after transfection, cells were switched to serum free media and cultured further for 48 hours. Knockdown effect was determined by the levels of LTBP-4 protein in the conditioned media with immunoblotting analysis. To treat the transfected cells with bleomycin, transfected cells were rendered quiescent in serum free media for 24 hours. Bleomycin was added at a final concentration of 0.5 μg/ml and incubated with cells for 24 hours.
Bioassay of TGF-β Activity
TGF-β activity was determined by the plasminogen activator inhibitor−1 promoter luciferase reporter assay.39 Mink lung epithelial cells stably expressing the firefly luciferase reporter gene under the control of the TGF-β-response element of plasminogen activator inhibitor−1 promoter were plated in 24-well plates at a density of 2.5 × 105 cells/well with DMEM containing 10% fetal bovine serum, 2 mmol/L l-glutamine, 1% penicillin-streptomycin, and 200 μg/ml G418. Cells were allowed to attach for 4 hours and washed with serum-free media. For measurement of active TGF-β from conditioned media or bronchoalveolar lavage (BAL) fluid, 0.5 ml fresh conditioned media or BAL fluid were directly added to each well. For assay of total TGF-β, 0.05 ml conditioned media or BAL fluid were first heat-activated for 3 minutes at 100°C, mixed with 0.45 ml serum-free media, and a final volume of 0.5 ml medium was incubated with reporter cells for 18 hours at 37°C. Mink lung epithelial cell lysates from each well were prepared using reporter lysis buffer (Promega, Madison, WI). Luciferase activity was measured as relative light units, using an Orion Microplate Luminometer from Berthold (Pforzheim, Germany), and converted to TGF-β activity (picomoles) using a standard curve generated by human recombinant TGF-β1.
In Vivo Mouse Experiments: Bleomycin Administration, Bronchoalveolar Lavage, and Lung Tissue Fixation
Bleomycin was administered to 8 to 10-week-old female Thy-1 knockout (C57BL/6) mice and wild-type mice as described previously.40 Briefly, bleomycin (100 mg/kg body weight) or an equal volume of normal saline was placed into an osmotic minipump (Model 2001; Alza Corp., Palo Alto, CA) and implanted subcutaneously into the back, slightly posterior to the scapulae. The pump delivered bleomycin at a constant rate of 1 μl/hr for 7 days and induced lung fibrosis. Mice were sacrificed at day 8 and day 14. Mouse lungs were lavaged with serum-free DMEM while under anesthesia just before sacrifice. The BAL fluid was centrifuged at 400 × g for 5 minutes at 4°C. The supernatants were used for Western blotting analysis and TGF-β bioactivity assay. The lungs were inflated and fixed in 10% formalin, and embedded in paraffin for immunohistochemical analysis.
Immunohistochemistry and Morphometric Analysis
Lung tissue blocks were cut in 5 μm sections. Antigen unmasking was performed by immersing slides in 10 mmol/L sodium citrate buffer (pH 6.0) and heating at 100°C for 10 minutes. Sections were blocked with 5% normal goat serum and stained with the following antibodies: rabbit anti-LTBP-4 (H-293) at 4 μg/ml, rabbit anti-human phospho-Smad3 at 1 μg/ml and with equal concentrations of the corresponding non-immune IgG controls. Endogenous peroxidase activity was quenched with 3% H2O2. Staining was developed with biotinylated secondary antibodies, streptavidin-peroxidase and 3-amino-9-ethylcarbazole chromogen (Vector Laboratories, Burlingame, CA). Images were obtained with a Spot Insight CCD camera and MetaMorph software version 6.2 r4 (Universal Imaging Corp., Downington, PA). Digital image quantification for LTBP-4 and phospho-Smad3 staining was performed as described previously.10 Background in sections treated with non-immune serum was used to define threshold. Positive pixels were expressed as a percentage of total tissue area, averaged over six random fields.
Densitometry and Statistical Analysis
Images were scanned and bands were quantified by Scanalytic’s One-Dscan version 1.31. Fold increases in LTBP-4 mRNAs were normalized to 28S ribosomal RNAs. Fold increases or decreases in LTBP-4 protein were normalized to LTBP-3 protein. Statistical differences among treatment conditions were determined using one way analysis of variance (Newman-Keuls method for multiple comparisons). The analysis was performed with SigmaStat 3.0 software (SPSS Inc. Chicago, IL). Values of P < 0.01 or P < 0.05 were considered significant.
Results
Bleomycin-Induced Latent TGF-β Activation by Thy-1 (−) Lung Fibroblasts is Specific to Latent TGF-β1
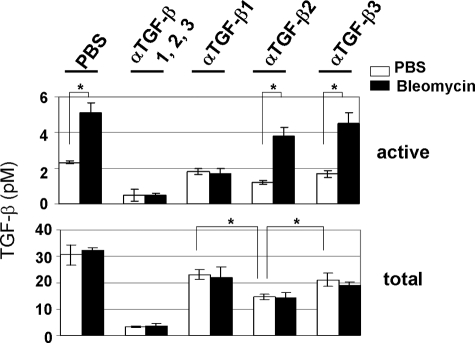
Previously, we showed that Thy-1 (−) rat lung fibroblasts respond to bleomycin with increased TGF-β activation, whereas Thy-1 (+) rat lung fibroblasts do not.11 In this study, we determined which specific TGF-β isoform was activated by Thy-1 (−) rat lung fibroblasts in response to bleomycin. Quiescent Thy-1 (−) rat lung fibroblasts were treated with bleomycin in the presence or absence of pan-specific or isoform-specific anti-TGF-β neutralizing antibodies for 24 hours. CM was collected and assayed for both active and total TGF-β. Results showed that bleomycin treatment increased active TGF-β whereas total TGF-β was not changed, indicating that bleomycin increases TGF-β activation. Addition of anti-TGF-β1 or pan-specific anti-TGF-β1, 2, 3 neutralizing antibodies blocked bleomycin-induced increases in active TGF-β, whereas anti-TGF-β2 or anti-TGF-β3 neutralizing antibodies did not reduce TGF-β activity (Figure 1). These results suggest that the TGF-β1 isoform is the primary isoform activated by Thy-1 (−) lung fibroblasts.
Figure 1.
Bleomycin-induced latent TGF-β activation by Thy-1 (−) rat lung fibroblasts is latent TGF-β1 specific. Quiescent Thy-1 (−) rat lung fibroblasts were cultured in serum-free media in the presence (solid bars) or absence (open bars) of bleomycin for 24 hours. Some cells were treated with neutralizing antibodies specific to TGF-β1, 2, 3, TGF-β1, TGF-β2, or TGF-β3. CM were collected and assayed for both active and total TGF-β. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01.
Bleomycin Up-Regulates LTBP-4 Expression by Thy-1 (−) Rat Lung Fibroblasts, Resulting in an Increase in Large Latent TGF-β1 Complex in the Conditioned Media
Since LTBPs are key factors regulating both TGF-β bioavailability and activation, we investigated whether LTBPs are involved in bleomycin-induced TGF-β1 activation by Thy-1 (−) rat lung fibroblasts. Thy-1 (−) and Thy-1 (+) rat lung fibroblasts were made quiescent and stimulated with 0.5 μg/ml bleomycin for 72 hours. Immunoblot analyses were performed to determine LTBP-1, LTBP-3, LTBP-4, and LAP•TGF-β1 levels in the CM and the ECM at baseline and in response to bleomycin. Under basal conditions, Thy-1 (−) lung fibroblasts expressed higher levels of LTBP-4 protein in both the CM and the ECM than Thy-1 (+) lung fibroblasts. Bleomycin treatment increased LTBP-4 protein levels in the CM as well as in the ECM by Thy-1 (−) lung fibroblasts. In contrast, bleomycin induction of LTBP-4 protein was not evident in Thy-1 (+) lung fibroblasts. In parallel with LTBP-4, Thy-1 (−) fibroblasts expressed higher levels of TGF-β1 LLC in the CM than did Thy-1 (+) lung fibroblasts under basal conditions. Bleomycin treatment further increased levels of soluble TGF-β1 LLC protein by Thy-1 (−) lung fibroblasts (Figure 2A, left panel, boxed).
Figure 2.
Bleomycin induces LTBP-4 expression by Thy-1 (−) rat lung fibroblasts, resulting in an increase in soluble LTBP-4-bound large latent TGF-β1. A: Quiescent Thy-1 (+) and Thy-1 (−) rat lung fibroblasts were stimulated with PBS or bleomycin for 72 hours. Cell-free conditioned media (CM) was collected and concentrated 100-fold. Seventy μg of concentrated CM was separated by SDS-PAGE under non-reducing conditions. Loading volumes of solubilized ECM proteins were corrected to load equal amounts of total cell lysate protein. B: Concentrated CM with equal amounts of protein were immunoprecipitated with 300 μg anti-LAP(β1) antibody. Proteins were separated by SDS-PAGE under reducing conditions and LTBP-4 detected by immunoblotting with anti-LTBP-4 antibody H-293. C: Quiescent cells were treated with bleomycin for the time period indicated. Levels of LTBP−4 mRNA were determined by Northern hybridization with a LTBP−4-specific probe. Relative mRNA levels were determined by scanning densitometry of the blots and normalized to 28S rRNA levels. The level of LTBP−4 mRNA from Thy-1 (+) fibroblasts in the absence of bleomycin treatment was set at 1.
In contrast, bleomycin increased LTBP-1 and TGF-β1 LLC levels in the ECM of Thy-1 (+) lung fibroblasts (Figure 2A, right panel, boxed). Expression of LTBP-1 in the CM and expression of LTBP-3 in the CM and the ECM were equivalent between Thy-1 (−) and Thy-1 (+) lung fibroblasts under both basal and bleomycin-stimulated conditions (Figure 2A).
Gel electrophoresis under non-reducing conditions showed that LTBP-4 co-migrated with TGF-β1 LLC in the CM, suggesting that soluble TGF-β1 LLC might be LTBP-4-bound (Figure 2A). To confirm this, we immunoprecipitated the CM from Thy-1 (−) and Thy-1 (+) lung fibroblasts with anti-LAP(β1) antibody. The immunoprecipitated proteins were subjected to immunoblot analysis with anti-LTBP-4 antibody. Results showed that anti-LAP(β1) antibody pulled down a LTBP-4 fragment from the CM of Thy-1 (−) lung fibroblasts. Bleomycin treatment increased the amount of this fragment by Thy-1 (−) lung fibroblasts (Figure 2B). The molecular mass of immunoprecipitated LTBP-4 is about 70 kDa, which is consistent with the mass of the TGF-β1 LLC (∼180 kDa: ∼70 kDa LTBP-4 fragment plus ∼100 kDa LAP•TGF-β1) observed in the CM of Thy-1 (−) lung fibroblasts (Figure 2A). In contrast, anti-LAP(β1) antibody failed to pull down LTBP-4 from the CM of Thy-1 (+) lung fibroblasts. These data suggest that LTBP-4 binds to the small latent TGF-β1 complex and regulates secretion of the large latent TGF-β1 complex by fibrogenic lung fibroblasts in response to bleomycin.
To determine whether bleomycin up-regulates LTBP-4 gene expression, Thy-1 (−) and Thy-1 (+) rat lung fibroblasts were made quiescent and stimulated with 0.5 μg/ml bleomycin for 24 hours. Northern blotting analyses showed that Thy-1 (−) lung fibroblasts had higher levels of LTBP-4 message than Thy-1 (+) lung fibroblasts at baseline. Bleomycin treatment resulted in approximately a 25-fold increase in LTBP-4 mRNA levels in the Thy-1 (−) fibroblasts, whereas Thy-1 (+) fibroblasts exhibited only a seven-fold increase in LTBP-4 mRNA, which is nearly equivalent to mRNA levels of basal Thy-1 (−) cells (Figure 2C). These data suggest that bleomycin up-regulates LTBP-4 gene expression by fibrogenic Thy-1 (−) lung fibroblasts, but to a highly attenuated extent in the non-fibrogenic Thy-1 (+) fibroblasts.
Knockdown of LTBP-4 Abrogates Bleomycin-Induced TGF-β1 Activation by Thy-1 (−) Rat Lung Fibroblasts
Since bleomycin up-regulates LTBP-4 expression by Thy-1 (−) lung fibroblasts and previous studies showed that lung fibroblasts isolated from hypomorphic LTBP-4 mice had decreased TGF-β activation,32 we investigated whether LTBP-4 is involved in bleomycin-induced TGF-β1 activation. We used a siRNA-based approach to knockdown LTBP-4 expression in Thy-1 (−) rat lung fibroblasts. LTBP-4 siRNA treatment decreased LTBP-4 expression at baseline by 82% and inhibited bleomycin-induced increase in LTBP-4. Treatment with irrelevant negative control siRNA did not affect LTBP-4 expression. The knockdown effect was LTBP-4-specific, since LTBP-4 siRNA did not influence levels of LTBP-1, LTBP-3, or β-tubulin expression. Knockdown of LTBP-4 resulted in decreased secretion of large latent TGF-β1 complex at baseline and in response to bleomycin (Figure 3A). Assay of TGF-β activity showed that control siRNA-transfected Thy-1 (−) rat lung fibroblasts responded to bleomycin treatment with increased active TGF-β. Total TGF-β was not changed. When LTBP-4 siRNA-transfected Thy-1 (−) rat lung fibroblasts were treated with bleomycin, neither active TGF-β nor total TGF-β was increased (Figure 3B). These data suggest that knockdown of LTBP-4 abrogates the ability of Thy-1 (−) rat lung fibroblasts to activate TGF-β1 in response to bleomycin.
Figure 3.
LTBP-4 knockdown abrogates bleomycin-induced TGF-β1 activation by Thy-1 (−) rat lung fibroblasts. A: A final concentration of 50 nmol/L LTBP-4 siRNAs or negative control siRNA was transfected into Thy-1 (−) rat lung fibroblasts with a Nucleofector device. Protein levels of LTBP-4, LAP•TGF-β1, LTBP-1, and LTBP-3 were detected by immunoblot analyses. To determine equal loading, LTBP-4 blots were stripped and re-blotted for β-tubulin. Relative LTBP-4 levels were determined by scanning densitometry of the blots and normalized to β-tubulin levels. The level of LTBP-4 protein at baseline was set at 1. B: siRNA-transfected and control siRNA-transfected Thy-1 (−) rat lung fibroblasts were made quiescent and cultured in serum-free media in the presence (solid bars) or absence (open bars) of bleomycin for 24 hours. CM was collected and assayed for active and total TGF-β. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01.
Thy-1 (−) Lung Fibroblasts Isolated from Hypomorphic LTBP-4 Mice Lose the Ability to Activate TGF-β1 in Response to Bleomycin; Transfection of Human LTBP-4 into Hypomorphic Lung Fibroblasts Restores Bleomycin-Induced TGF-β1 Activation
To further determine whether LTBP-4 is required for bleomycin-induced TGF-β1 activation, we used lung fibroblasts isolated from hypomorphic LTBP-4 mice and wild-type mice and tested their abilities to activate latent TGF-β1 in response to bleomycin. A previous study showed that lung fibroblasts isolated from hypomorphic LTBP-4 mice do not express detectable levels of either LTBP-4 mRNA or protein.32 Fluorescence-activated cell sorting analysis showed that 80% of lung fibroblasts isolated from hypomorphic LTBP-4 mice and 89% lung fibroblasts isolated from wild-type mice are Thy-1 negative (Figure 4A). Bleomycin treatment induced an increase in active TGF-β by wild-type lung fibroblasts, whereas total TGF-β was not changed, indicating that latent TGF-β was activated by bleomycin. However, when hypomorphic LTBP-4 lung fibroblasts were treated with bleomycin, neither active nor total TGF-β was changed (Figure 4B). These findings confirm that LTBP-4 deficiency abrogates the ability of Thy-1 (−) lung fibroblasts to activate latent TGF-β1 in response to bleomycin. Consistent with the finding from rat lung fibroblasts, anti-TGF-β1 neutralizing antibody, but not anti-TGF-β2 or anti-TGF-β3 neutralizing antibodies, blocked bleomycin-induced TGF-β activation, suggesting that bleomycin preferentially activates the TGF-β1 isoform in mouse lung fibroblasts (Figure 4B).
Figure 4.
Thy-1 (−) lung fibroblasts isolated from LTBP-4 hypomorphic mice lose the ability to activate TGF-β1 in response to bleomycin. Transfection of full length human LTBP−4 cDNA into hypomorphic cells restores bleomycin-induced TGF-β1 activation. A: Lung fibroblasts isolated from hypomorphic LTBP-4 mice or wild-type mice were stained with either non-immune IgG-FITC or anti-Thy-1.1, conjugated with FITC. Cell surface expression of Thy-1 was determined by flow cytometry. B: Quiescent cells were cultured in serum-free media in the presence (solid bars) or absence (open bars) of bleomycin for 24 hours. Some wild-type lung fibroblasts were treated with pan-specific or isoform-specific TGF-β neutralizing antibodies. CM was collected and assayed for active and total TGF-β. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01. C: Hypomorphic lung fibroblasts were transfected with human LTBP-4-expressing plasmid (hL4) or control vector (ctrl vector). Expression of hL4 by established stable cell lines was analyzed by RT-PCR. Transfected cells were made quiescent and stimulated with bleomycin for 24 hours. Some LTBP4-expressing hypomorphic lung fibroblasts were treated with anti-TGF-β neutralizing antibodies. CM was collected and assayed for both active and total TGF-β. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01.
To determine whether exogenous expression of functional LTBP-4 restores bleomycin-induced TGF-β1 activation by hypomorphic LTBP-4 lung fibroblasts, pEFIRESLTBP-4, a construct expressing the full length human LTBP-4, was transfected into hypomorphic cells and a stable cell line expressing LTBP-4 was established. Meanwhile, cells transfected with the empty vector were established as a control (Figure 4C). On treatment with bleomycin, hypomorphic lung fibroblasts expressing LTBP-4 responded with an increase in active TGF-β. Total TGF-β was not changed. As expected, neither active nor total TGF-β was changed in empty vector-transfected control cells. Furthermore, addition of anti-TGF-β1 neutralizing antibody, but not anti-TGF-β2- or anti-TGF-β3-neutralizing antibodies, blocked bleomycin-induced TGF-β activation by LTBP-4-expressing hypomorphic fibroblasts (Figure 4C). Together, these data suggest that restoration of LTBP-4 expression is sufficient to rescue the ability of hypomorphic LTBP-4 mouse lung fibroblasts to activate TGF-β1 in response to bleomycin.
LTBP-4-Regulated Latent TGF-β1 Activation Requires its Binding to the Small Latent TGF-β1 Complex
LTBP-4 binds to the small latent TGF-β1 complex through its third 8-cysteine repeat.22 To determine whether LTBP-4 binding to the small latent complex is important for LTBP-4-regulated TGF-β1 activation, we transfected a human LTBP-4 splice variant in which the third 8-Cys repeat has been deleted (LTBP-4Δ8-cys3rd) into hypomorphic LTBP-4 lung fibroblasts and established a cell line stably expressing LTBP-4Δ8-cys3rd. Expression of this non-TGF-β binding LTBP-4 variant failed to rescue bleomycin-induced latent TGF-β1 activation by hypomorphic lung fibroblasts (Figure 5A). In contrast, hypomorphic lung fibroblasts expressing full length LTBP-4 were able to activate latent TGF-β. These results suggest that LTBP-4-regulated latent TGF-β1 activation requires LTBP-4 binding to the small latent complex.
Figure 5.
LTBP-4-regulated TGF-β1 activation requires the third 8-cys repeat of LTBP-4 and unidentified additional LTBP-4 domains. A: Hypomorphic LTBP-4 lung fibroblasts were transfected with a construct expressing a human LTBP-4 splice variant lacking the third 8-cys repeat (hL4Δ8-cys3rd) or a control vector (Ctrl vector). Expression of hL4Δ8-cys3rd by the established stable cell line was determined by RT-PCR. Transfected cells were made quiescent and cultured in serum-free media in the presence (solid bars) or absence (open bars) of bleomycin for 24 hours. CM was collected and assayed for both active and total TGF-β. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01. B: Hypomorphic lung fibroblasts were transfected with a plasmid expressing HA-tagged third 8-cys repeat of human LTBP-4 (L4ECR3E). Expression of L4ECR3E in the CM was determined by immunoblot analysis with anti-HA antibody under reducing conditions. Transfected cells were made quiescent and cultured in serum-free media in the presence (solid bars) or absence (open bars) of bleomycin for 24 hours. CM was collected and assayed for both active and total TGF-β. Hypomorphic lung fibroblasts expressing full length L4 (hL4) were used as a control for bleomycin-induced TGF-β activation. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01. To determine the binding of L4ECR3E to the SLC to form a complex. Hypomorphic lung fibroblasts were cotransfected with HA-tagged L4ECR3E and Flag-tagged SLC. CM were collected and subjected to immunoprecipitation with anti-HA antibody (αHA). Immunoblotting analysis was performed with either anti-HA antibody or anti-Flag antibody (αFlag) under non-reducing conditions. Arrowheads indicate L4ECR3E monomer, L4ECR3E dimer, and L4ECR3E-SLC complex.
To determine whether binding of the third 8-cysteine repeat domain to the small latent complex is sufficient for TGF-β1 activation, LTBP-4 hypomorphic lung fibroblasts were transfected with a construct expressing the third 8-cysteine repeat of LTBP-4 as an HA tagged fusion protein (L4ECR3E).37 Expression of the L4ECR3E-SLC binding domain alone was not sufficient for TGF-β activation (Figure 5B). The ability of L4ECR3E to bind LAP(β1) was confirmed by immunoprecipitation and immunoblot analyses of conditioned media from LTBP-4 hypomorphic lung fibroblasts transfected with co-transfection of L4ECR3E and a construct (pFlag-SLC) expressing Flag-tagged small latent TGF-β1 (Figure 5B). Together, these data suggest that the third 8-cysteine repeat of LTBP-4 alone is not sufficient for bleomycin-induced TGF-β1 activation and that activation requires additional LTBP-4 domains.
Overexpression of Non-TGF-β1-Binding LTBP-4 Abrogates Bleomycin-Induced TGF-β1 Activation by Thy-1 (−) Lung Fibroblasts
Normal human lung fibroblasts express both full length LTBP-4 and the non-TGF-β1-binding LTBP-4Δ8-cys3rd splice variant.36 The biological function of the non-TGF-β1-binding LTBP-4 splice variant is not clear. In this study, we investigated whether overexpression of non-TGF-β1-binding LTBP-4 affected the ability of Thy-1 (−) lung fibroblasts to activate latent TGF-β1. Thy-1 (−) rat lung fibroblasts were transfected with the LTBP-4Δ8-cys3rd construct or the corresponding empty vector. Stable cell lines were established by puromycin selection. Expression of the LTBP-4Δ8-cys3rd splice variant was identified by RT-PCR with a pair of PCR primers specific to the human LTBP−4 sequence (Figure 6A). Established cell lines were treated with bleomycin for 24 hours. Assay for active and total TGF-β in the CM showed that overexpression of LTBP-4Δ8-cys3rd by Thy-1 (−) rat lung fibroblasts completely abrogated the ability of cells to activate latent TGF-β1 in response to bleomycin. In addition, overexpression of LTBP-4Δ8-cys3rd also decreased active TGF-β under basal conditions. However, total TGF-β was not changed (Figure 6B). These results suggest that increased non-TGF-β-binding LTBP-4 expression abrogates bleomycin-induced TGF-β1 activation by Thy-1 (−) rat lung fibroblasts.
Figure 6.
Overexpression of non-TGF-β-binding LTBP-4 abrogates bleomycin-induced TGF-β1 activation by Thy-1 (−) rat lung fibroblasts. A: Thy-1 (−) rat lung fibroblasts were transfected with hL4Δ8-cys3rd plasmid or control vector (Ctrl vector). Expression of hL4Δ8-cys3rd by established stable cell lines were determined by RT-PCR with a pair of primers specific to human LTBP−4. B: Transfected cells were made quiescent and cultured in serum-free media in the presence (solid bars) or absence (open bars) of bleomycin for 24 hours. CM was collected and assayed for both active and total TGF-β. Results are the means of three separate experiments ± SD, each performed in triplicate. *P < 0.01.
Bleomycin Treatment Increases LTBP-4 Expression in Mouse Lung; Thy-1 Knockout Mice Have Higher Levels of LTBP-4 Expression and TGF-β Activation than Do Wild-Type Mice
Previous studies showed that Thy-1 knockout (Thy-1−/−) mice treated with bleomycin had increased TGF-β1 activation and developed more severe lung fibrosis as compared with wild-type mice.10 In this study, we investigated whether LTBP-4 expression correlates with TGF-β1 activation in vivo in a bleomycin-induced mouse model of lung fibrosis. Bleomycin or saline was administered to Thy-1 knockout mice and to wild-type mice through subcutaneous osmotic minipumps.40,41 Bronchoalveolar fluid and lung tissues were collected at day 8 and day 14 when fibroblast activation in bleomycin-induced lung fibrosis model occurs.42 Consistent with the previous observation of increased TGF-β in the lungs of bleomycin-stimulated Thy-1 knockout mice, we also observed increased TGF-β signaling in lungs from bleomycin-treated Thy-1 knockout mice. Immunohistochemical staining showed that both knockout and wild-type mice have increased nuclear accumulation of phosphorylated Smad3 in the lungs with bleomycin treatment. Compared with bleomycin-treated wild-type mice, bleomycin-treated Thy-1 knockout mice have a significantly increased number of phosphorylated Smad3 positive nuclei (Figure 7A). Assay of active and total TGF-β in BAL fluid showed that bleomycin treatment increased levels of active and total TGF-β in both wild-type and Thy-1 knockout mice. Compared with wild-type mice, Thy-1 knockout mice had higher levels of active TGF-β at baseline and in response to bleomycin (Figure 7B). The percent active TGF-β in BAL fluid from wild-type mice was 5% at baseline and 26% in response to bleomycin. In contrast, the percent active TGF-β in BAL fluid from Thy-1 knockout mice was 24% at baseline and 66% in response to bleomycin. Smad3 phosphorylation and TGF-β activation were also examined in mice at day 14 after treatment with saline or bleomycin. Results similar to day 8 were obtained, although both the number of phosphorylated Smad3 positive nuclei and TGF-β activation slightly decreased by day 14 as compared with day 8 (data not shown).
Figure 7.
LTBP-4 is up-regulated in bleomycin-induced mouse model of lung fibrosis. Thy-1 knockout mice have higher levels of LTBP-4 expression and TGF-β activation than wild-type mice. A: Paraffin embedded lung sections from saline- or bleomycin-treated wild-type mice and Thy-1−/− mice were stained for phosphorylated Smad3. Arrows indicate representative phospho-Smad3 staining in nuclei of positively staining cells. Phospho-Smad3 positive nuclei were counted from immunohistochemical staining images (original magnification ×100) with MetaMorph software. The numbers were averaged from 15 to 20 areas. *P < 0.01. B: Lungs from bleomycin-treated (solid bars) and saline-treated mice (open bars) were lavaged with serum-free media. BAL fluid was assayed for both active and total TGF-β. *P < 0.01 and #P < 0.05. C: Equal volumes of BAL fluid were subjected to SDS-PAGE under non-reducing conditions. Levels of LTBP-4 protein were determined by immunoblot analysis with anti-LTBP-4 antibody (rabbit IgG). The membrane was stripped and re-blotted with anti-LAP(β1) (goat IgG). Immunoblot of LTBP-1 was used as a loading control. Relative LTBP-4, LAP(β1), and LTBP-1 levels were determined by scanning densitometry of the blots. The level of LTBP-4, LAP(β1), or LTBP-1 from bleomycin-treated wild-type mice was set at 1. *P < 0.01. D: Paraffin embedded lung sections from saline- or bleomycin-treated wild-type mice and Thy-1−/− mice were stained for LTBP-4 (original magnification ×20, ×40). Arrowheads indicate representative LTBP-4 staining in lung cells and inflammatory cells (alveolar macrophages). LTBP-4 staining (original magnification ×20) was quantified using morphometric analysis as described in Materials and Methods. *P < 0.01. All data shown in this figure were from mice treated with saline or bleomycin at day 8.
To determine whether LTBP-4 is differentially regulated during lung fibrosis in Thy-1 knockout and wild-type mice, LTBP-4 levels in BAL fluid and in lung tissues were evaluated. Immunoblot analyses showed that bleomycin treatment increases levels of LTBP-4, the large latent TGF-β1 complex and LTBP-1 in the BAL fluid in both Thy-1 knockout and wild-type mice. Bleomycin-treated knockout mice (day 8) have ∼ threefold higher levels of LTBP-4 and TGF-β1 LLC, but not LTBP-1, as compared with bleomycin-treated wild-type mice (Figure 7C). Consistent with the data from cultured lung fibroblasts (Figure 2A), LTBP-4 co-migrated with LAP(β1) in BAL fluid and the molecular mass of TGF-β1 LLC was ∼180 kDa. These data suggest that bleomycin increases LTBP-4 expression in mouse lungs, which is associated with an increase in TGF-β1 LLC expression. Immunohistochemical analyses showed that both saline-treated wild-type and Thy-1 knockout mice have weak LTBP-4 staining in the alveolar walls and the vasculature. Bleomycin treatment increased LTBP-4 staining in both wild-type and knockout mice: the area staining for LTBP-4 in knockout mice was more than twice that of wild-type mice. Significant increases in LTBP-4 expression were found in both interstitial and inflammatory cells (Figure 7D). Sections stained with non-immune rabbit IgG instead of primary antibody were negative (data not shown). LTBP-4 expression at day 14 was comparable to expression at day 8 (data not shown). Together, these data suggest that bleomycin treatment increases LTBP-4 expression in mouse lung, which is associated with increased latent TGF-β activation. Compared with wild-type mice, Thy-1 knockout mice have higher levels of LTBP-4 expression and TGF-β activation in the lung in response to bleomycin.
Discussion
The major findings in this study are that bleomycin treatment up-regulates LTBP-4 gene expression both in vitro in cultured lung fibroblasts and in vivo in the bleomycin-induced mouse model of lung fibrosis. The increased LTBP-4 expression promotes accumulation of soluble LTBP-4-bound large latent TGF-β1 complex by Thy-1 (−) fibrogenic lung fibroblasts, resulting in increased TGF-β1 activation. Furthermore, LTBP-4 regulated TGF-β1 bioavailability and activation requires the binding of LTBP-4 to the small latent TGF-β1 complex. These are the first studies to demonstrate the importance of LTBP-4 in the progression of lung fibrosis by interstitial lung fibroblasts.
The regulation of latent TGF-β bioavailability and its activation are complex processes, which are known to be influenced by the various LTBP isoforms. There are numerous lines of evidence suggesting that factors that increase latent TGF-β bioavailability through release from extracellular matrix storage pools facilitate increased TGF-β activity. Recent studies show that BMP1 releases large latent TGF-β from the ECM, presumably by cleavage of LTBP-1, facilitating subsequent TGF-β activation by Matrix Metalloproteinases (MMPs).43 Similarly, Membrane Type 1-Matrix Metalloproteinase (MT1-MMP) cleaves ECM-bound LTBP-1 to release the large latent TGF-β1 complex in PMA-treated endothelial cells.44 A C-terminal fibrillin-1 fragment encoded by exons 44 to 49 disrupts binding of LTBP-1-bound LLC to microfibrils, resulting in the release of TGF-β1 and increased Smad2 signaling.45 These studies suggest that solubilization of LLC from the ECM or prevention of LLC targeting to the ECM contributes to latent TGF-β activation. Our current studies support this idea, since the fibrogenic Thy-1 (−) lung fibroblasts increased soluble LTBP-4-bound large latent TGF-β1 complex and TGF-β1 activity in response to the fibrogenic stimulus, bleomycin. In contrast, the non-fibrogenic Thy-1 (+) fibroblasts had no detectable soluble LTBP-4-bound large latent TGF-β1 complex. Furthermore, disruption of LTBP-4 expression or expression of LTBP-4 lacking the latent TGF-β binding domain abrogates the stimulation of increased TGF-β activity by bleomycin.
Although solubilization of the LTBP-4 bound latent complex appears critical for latent TGF-β activation by Thy-1 (−) lung fibroblasts, it is not yet known whether LTBP-4 might directly participate in activation of the latent complex or alternately, facilitate its solubility and presentation to known activators. There is evidence that ECM-bound LTBPs may actively participate in TGF-β activation. In support of this, integrin αvβ6-regulated TGF-β activation has been shown to require ECM-bound LTBP-1 and matrix fibronectin.29,46,47 A recent study suggests that LTBP-1, which is also a component of the ECM-integrin-cytoskeleton mechanotransduction pathway, is essential for lung myofibroblast contraction-induced TGF-β1 activation.30 In our current studies, Thy-1 (+) lung fibroblasts respond to bleomycin with increased matrix deposition of LTBP-1 and TGF-β1 LLC, but not with increased TGF-β1 activation. A recent study showed that Thy-1 (+) lung fibroblasts have decreased collagen gel contractility as compared with Thy-1 (−) lung fibroblasts.14 Furthermore, Thy-1 interacts with cell surface integrins through a RGD-like motif.48,49,50 It is possible that the reduced contractile activity and possible interference of integrin-dependent mechanotransduction signaling pathways due to Thy-1-integrin interactions might account for the non-responsiveness of Thy-1 (+) lung fibroblasts to latent TGF-β1 activation, despite the presence of LTBP-1 bound latent complex in the extracellular matrix.
Previous studies show that fibroblasts isolated from fibronectin-null mice lack LTBP-1 in the ECM and that disruption of fibronectin matrix assembly by a 70 kDa N-terminal fibronectin fragment remarkably reduces LTBP-1 matrix deposition,51 suggesting that LTBP-1 incorporation into the ECM requires a fibronectin matrix scaffold. Unpublished observations show that Thy-1 (+) lung fibroblasts form a well-organized fibronectin matrix, whereas the fibronectin matrix formed by Thy-1 (−) lung fibroblasts is diffuse and poorly-organized (Anne Woods and James Hagood, unpublished data), indicating that Thy-1 (−) lung fibroblasts are deficient in fibronectin matrix assembly. The abnormal fibronectin matrix scaffold might explain the inability of Thy-1 (−) lung fibroblasts to respond to bleomycin with increased LTBP-1 and LLC matrix deposition. Furthermore, deposition of LTBP-1 into early ECM precedes deposition of either LTBP-4 or LTBP-3, and LTBP-4 is only deposited into a mature ECM.52 Thus, the increase in soluble LTBP-4 in stimulated Thy-1 (−) fibroblasts might also be a consequence of deficient ECM assembly.
LTBP-4-regulated TGF-β1 activation requires the binding of LTBP-4 to the small latent TGF-β1 complex. However, the isolated SLC binding motif (the third 8-cysteine domain of LTBP-4) is not sufficient to support TGF-β1 activation, suggesting that additional LTBP-4 domains of LTBP-4 are needed for correct presentation of the LLC for activation. Interestingly, overexpression of LTBP-4 lacking the TGF-β binding domain blocks the ability of Thy-1 (−) lung fibroblasts to activate latent TGF-β1. Although the mechanism whereby this non-TGF-β binding LTBP-4 variant blocks latent TGF-β activation is currently not clear, it is possible that the non-TGF-β binding regions of LTBP-4 competitively inhibit interactions necessary for LTBP-4 mediated support of TGF-β1 activation. In support of this idea, it was recently shown that LTBPs can bind to molecules such as fibronectin and heparan sulfate proteoglycans.53,54
In this study, we consistently observed a ∼180-kDa large latent TGF-β1 complex in both cultured rat lung fibroblasts and mouse BAL fluid that co-migrates with LTBP-4 in immunoblots run under non-reducing conditions. Immunoprecipitation confirmed that the LTBP bound to the small latent TGF-β1 complex is LTBP-4. The molecular mass of TGF-β1-binding LTBP-4 is about 70 kDa, suggesting it is not the full-length LTBP-4 molecule, which would be expected to migrate at ∼300 kDa. The smaller form of the LTBP-4 is not the result of expression of a truncated LTBP-4 transcript since the LTBP-4 transcript is the full predicted size (5.3 kb). These findings suggest that the soluble LTBP-4 bound to the latent TGF-β1 expressed by Thy-1 (−) lung fibroblasts might be a cleaved form of LTBP-4, possibly cleaved from the ECM. Our previous studies showed that bleomycin-induced TGF-β activation requires both TSP1 and MMP activity.11 Current studies are aimed at determining whether MMPs or other proteases, such as BMP1 and plasmin, are involved in LTBP-4 cleavage and release of the soluble TGF-β complex.
These in vitro findings are supported by demonstration of increased LTBP-4-associated latent complex in the BAL fluid and tissues of bleomycin-treated mice, an established model of lung fibrosis. Furthermore, Thy-1 (−) mice with exacerbated lung fibrosis have increased LTBP-4 expression and Smad3 signaling in tissues as compared with wild-type mice. These in vivo findings are consistent with the in vitro studies and support an important role of LTBP-4 in promoting latent TGF-β bioavailability and activation in lung fibrosis. Interestingly, robust LTBP-4 expression is seen in both interstitial fibroblasts and in inflammatory cells in mice treated with bleomycin. Expression of LTBP-4 by inflammatory cells has not been reported previously. Inflammatory cells, in particular alveolar macrophages, are a major cellular source of latent TGF-β1 in the lung. TGF-β activation by alveolar macrophages involves interaction of the latent complex with TSP1 bound to its receptor, CD36, and a subsequent cleavage of the TGF-β•LAP by plasmin.55 It will be interesting to determine whether LTBP-4 is similarly involved in regulation of TGF-β1 activation by alveolar macrophages.
The mechanisms by which Thy-1 expressing fibroblasts fail to up-regulate LTBP-4 expression in response to injury are currently not known. A previous microarray analysis has shown that Thy-1 (−) lung fibroblasts and Thy-1 (+) lung fibroblasts express distinct gene profiles (James Hagood et al, unpublished data), suggesting that cell surface Thy-1 regulates gene expression by lung fibroblasts. It is speculated that LTBP-4 expression might be inhibited by Thy-1-dependent cell signaling.56 Alternatively, there might be differential epigenetic regulation of LTBP-4 expression, such as DNA methylation of the promoter region, between Thy-1 (−) and Thy-1 (+) lung fibroblasts. The LTBP-4 promoter is poorly characterized and ongoing studies are aimed at determining whether Thy-1 signaling is involved in regulation of LTBP-4 expression.
In previous studies, immunohistochemical analyses showed that TGF-β1, -β2 and -β3 are ubiquitously expressed in normal lungs from both mice and rats.57,58 Consistent with these findings, we showed that total TGF-β in the conditioned media from rat and mouse lung fibroblasts contains all three TGF-β isoforms. However, our data show that bleomycin-induced TGF-β activation is specific to latent TGF-β1. These observations are consistent with data showing that LTBP-4 interacts with TGF-β1, but not TGF-β2 and -β322 and that TGF-β1 is the primary TGF-β isoform involved in fibrotic lung progression.58,59
In summary, these studies demonstrate that LTBP-4 is more highly expressed in the fibrotic lung and importantly, they establish a critical role for LTBP-4 in regulating the fibrogenic potential of lung fibroblast subsets through regulating latent TGF-β1 bioavailability and activation. These studies suggest that interventions targeting LTBP-4 action might be attractive, novel therapeutic approaches for the treatment of fibrotic lung diseases.
Acknowledgments
We thank Dr. Carl-Henrik Heldin for providing anti-LTBP-1 antiserum (Ab39) and Mariya Sweetwyne for her technical assistance.
Footnotes
Address reprint requests to Joanne E. Murphy-Ullrich, Ph.D., Department of Pathology, Volker Hall 668, 1530 3rd Ave S, Birmingham, AL 35294-0019. E-mail: murphy@uab.edu.
Supported by NIH grants DK60658 and HL086706 to J.E.M.-U.; American Heart Association Scientist Development Grant 0835432N to Y.Z.; NIH grant HL082818, American Lung Association Career Investigator Award and funding from the UAB Department of Pediatrics to J.S.H.; NIH Research Facilities Improvement Program Grant C06 RR 15490; NIH grants CA034282 and AR49698 to D.B.R., and Awards from the Academy of Finland to K.K.
References
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Gauldie J, Sime PJ, Xing Z, Marr B, Tremblay GM. Transforming growth factor-beta gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Curr Top Pathol. 1999;93:35–45. doi: 10.1007/978-3-642-58456-5_5. [DOI] [PubMed] [Google Scholar]
- Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- Lawrence DA, Pircher R, Kryceve-Martinerie C, Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984;121:184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Gentry LE, Lioubin MN, Purchio AF, Marquardt H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol. 1988;8:4162–4168. doi: 10.1128/mcb.8.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha X, Brunner AM, Purchio AF, Gentry LE. Transforming growth factor beta 1: importance of glycosylation and acidic proteases for processing and secretion. Mol Endocrinol. 1989;3:1090–1098. doi: 10.1210/mend-3-7-1090. [DOI] [PubMed] [Google Scholar]
- Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- Gentry LE, Nash BW. The pro domain of pre-pro-transforming growth factor beta 1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–6857. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, Pardo A, Selman M. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167:365–379. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol. 2004;165:659–669. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res. 2004;295:488–496. doi: 10.1016/j.yexcr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Rege TA, Pallero MA, Gomez C, Grenett HE, Murphy-Ullrich JE, Hagood JS. Thy-1, via its GPI anchor, modulates Src family kinase and focal adhesion kinase phosphorylation and subcellular localization, and fibroblast migration, in response to thrombospondin-1/hep I. Exp Cell Res. 2006;312:3752–3767. doi: 10.1016/j.yexcr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1 (−) lung fibroblasts. Am J Respir Cell Mol Biol. 2007;36:226–235. doi: 10.1165/rcmb.2006-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagood JS, Miller PJ, Lasky JA, Tousson A, Guo B, Fuller GM, McIntosh JC. Differential expression of platelet-derived growth factor-alpha receptor by Thy-1 (−) and Thy-1 (+) lung fibroblasts. Am J Physiol. 1999;277:L218–L224. doi: 10.1152/ajplung.1999.277.1.L218. [DOI] [PubMed] [Google Scholar]
- Hagood JS, Lasky JA, Nesbitt JE, Segarini P. Differential expression, surface binding, and response to connective tissue growth factor in lung fibroblast subpopulations. Chest. 2001;120:64S–66S. doi: 10.1378/chest.120.1_suppl.s64. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleizes PE, Beavis RC, Mazzieri R, Shen B, Rifkin DB. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-beta binding protein-1 that mediates bonding to the latent transforming growth factor-beta1. J Biol Chem. 1996;271:29891–29896. doi: 10.1074/jbc.271.47.29891. [DOI] [PubMed] [Google Scholar]
- Saharinen J, Taipale J, Keski-Oja J. Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996;15:245–253. [PMC free article] [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins. LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Saharinen J, Hedman K, Keski-Oja J. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–889. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth factor-beta binding protein in storage of latent TGF-beta in the extracellular matrix and as a structural matrix protein. J Cell Biol. 1995;131:539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin CH, Rifkin DB. Role of the latent TGF-beta binding protein in the activation of latent TGF-beta by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120:995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Miyazono K, Kato M, Takase M, Yamagishi T, Nakamura H. Extracellular fibrillar structure of latent TGF beta binding protein-1: role in TGF beta-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. J Cell Biol. 1997;136:193–204. doi: 10.1083/jcb.136.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koli K, Wempe F, Sterner-Kock A, Kantola A, Komor M, Hofmann WK, von Melchner H, Keski-Oja J. Disruption of LTBP-4 function reduces TGF-beta activation and enhances BMP-4 signaling in the lung. J Cell Biol. 2004;167:123–133. doi: 10.1083/jcb.200403067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen J, Taipale J, Monni O, Keski-Oja J. Identification and characterization of a new latent transforming growth factor-beta-binding protein LTBP-4. J Biol Chem. 1998;273:18459–18469. doi: 10.1074/jbc.273.29.18459. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dabovic B, Annes JP, Rifkin DB. Latent TGF-beta binding protein-3 (LTBP-3) requires binding to TGF-beta for secretion. FEBS Lett. 2002;517:277–280. doi: 10.1016/s0014-5793(02)02648-0. [DOI] [PubMed] [Google Scholar]
- Hobbs S, Jitrapakdee S, Wallace JC. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem Biophys Res Commun. 1998;252:368–372. doi: 10.1006/bbrc.1998.9646. [DOI] [PubMed] [Google Scholar]
- Koli K, Saharinen J, Karkkainen M, Keski-Oja J. Novel non-TGF-beta-binding splice variant of LTBP-4 in human cells and tissues provides means to decrease TGF-beta deposition. J Cell Sci. 2001;114:2869–2878. doi: 10.1242/jcs.114.15.2869. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ali T, Todorovic V, O'Leary JM, Kristina Downing A, Rifkin DB. Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes. J Mol Biol. 2005;345:175–186. doi: 10.1016/j.jmb.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem. 2004;279:38032–38039. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Harrison JH, Jr, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther. 1987;243:1185–1194. [PubMed] [Google Scholar]
- Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama H, Yoshimoto D, Okamoto Y, Kikkawa H, Asano S, Kinoshita M. Receptor-activated Smad localisation in bleomycin-induced pulmonary fibrosis. J Clin Pathol. 2007;60:283–289. doi: 10.1136/jcp.2006.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti O, Vehvilainen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res. 2008;314:2501–2514. doi: 10.1016/j.yexcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM. Fibrillin-1 regulates the bioavailability of TGFbeta1. J Cell Biol. 2007;176:355–367. doi: 10.1083/jcb.200608167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fassler R, Sakai LY, Rifkin DB. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- Leyton L, Schneider P, Labra CV, Ruegg C, Hetz CA, Quest AF, Bron C. Thy-1 binds to integrin beta(3) on astrocytes and triggers formation of focal contact sites. Curr Biol. 2001;11:1028–1038. doi: 10.1016/s0960-9822(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Saalbach A, Wetzel A, Haustein UF, Sticherling M, Simon JC, Anderegg U. Interaction of human Thy-1 (CD 90) with the integrin alphavbeta3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene. 2005;24:4710–4720. doi: 10.1038/sj.onc.1208559. [DOI] [PubMed] [Google Scholar]
- Wetzel A, Chavakis T, Preissner KT, Sticherling M, Haustein UF, Anderegg U, Saalbach A. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Immunol. 2004;172:3850–3859. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Koli K, Hyytiainen M, Ryynanen MJ, Keski-Oja J. Sequential deposition of latent TGF-beta binding proteins (LTBPs) during formation of the extracellular matrix in human lung fibroblasts. Exp Cell Res. 2005;310:370–382. doi: 10.1016/j.yexcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sivakumar P, Barley C, Peters DM, Gomes RR, Farach-Carson MC, Dallas SL. Potential role for heparan sulfate proteoglycans in regulation of TGF-beta by modulating assembly of latent TGF-beta binding protein-1 (LTBP1), J Biol Chem. 2007;282:26418–26430. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Yehualaeshet T, O'Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006;1763:991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana A, Saxena B, Noble NA, Gold LI, Marshall BC. Increased expression of transforming growth factor beta isoforms (beta 1, beta 2, beta 3) in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1995;13:34–44. doi: 10.1165/ajrcmb.13.1.7541221. [DOI] [PubMed] [Google Scholar]
- Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997;150:981–991. [PMC free article] [PubMed] [Google Scholar]
- Khalil N, O'Connor RN, Flanders KC, Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996;14:131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]