Abstract
Acute kidney injury evokes renal tubular cholesterol synthesis. However, the factors during acute kidney injury that regulate HMG CoA reductase (HMGCR) activity, the rate-limiting step in cholesterol synthesis, have not been defined. To investigate these factors, mice were subjected to 30 minutes of either unilateral renal ischemia or sham surgery. After 3 days, bilateral nephrectomy was performed and cortical tissue extracts were prepared. The recruitment of RNA polymerase II (Pol II), transcription factors (SREBP-1, SREBP-2, NF-κB, c-Fos, and c-Jun), and heat shock proteins (HSP-70 and heme oxygenase-1) to the HMGCR promoter and transcription region (start/end exons) were assessed by Matrix ChIP assay. HMGCR mRNA, protein, and cholesterol levels were determined. Finally, histone modifications at HMGCR were assessed. Ischemia/reperfusion (I/R) induced marked cholesterol loading, which corresponded with elevated Pol II recruitment to HMGCR and increased expression levels of both HMGCR protein and mRNA. I/R also induced the binding of multiple transcription factors (SREBP-1, SREBP-2, c-Fos, c-Jun, NF-κB) and heat shock proteins to the HMGCR promoter and transcription regions. Significant histone modifications (increased H3K4m3, H3K19Ac, and H2A.Z variant) at these loci were also observed but were not identified at either the 5′ and 3′ HMGCR flanking regions (±5000 bps) or at negative control genes (β-actin and β-globin). In conclusion, I/R activates the HMGCR gene via multiple stress-activated transcriptional and epigenetic pathways, contributing to renal cholesterol loading.
Diverse forms of tissue injury evoke cellular responses that confer protection against subsequent ischemic or toxic attack. This adaptation has been denoted by heterogeneous terms that include: acquired cytoresistance, the stunning phenomenon, the heat shock response, and ischemic preconditioning. It has been recognized for a century that the kidney can undergo this same injury adaptation.1 This is based on observations that exposing the kidney to one nephrotoxin elicits protection against the same, or a different (cross resistance), nephrotoxic agent.1 In 1984, this laboratory demonstrated that this phenomenon is not restricted to nephrotoxic injury: when mild ischemic renal injury was induced in rats, dramatic protection against subsequent, and more severe, renal ischemia resulted.2 Indeed, this was probably the first demonstration of the so-called ischemic preconditioning phenomenon, subsequently confirmed in many extra-renal tissues (eg, heart, liver, intestine, brain).3,4,5,6,7 It should be recognized that ischemic preconditioning is not a specific postischemic state. As noted above, this same preconditioning develops after toxic renal damage. Furthermore, nonischemic/nontoxic insults can also induce the renal cytoresistant state. Examples include heat shock,8,9 oxidant stress,10,11,12 endotoxemia,13,14,15 partial ablation of renal mass,16 urinary tract obstruction,17,18 and acute glomerulonephritis.19 Therefore, the phenomenon of ischemic preconditioning is best considered to be one of any number of stressors that can elicit subsequent protection against acute renal failure.
There are undoubtedly multiple pathways by which injured renal tubular cells acquire resistance to further damage. The one that has been most widely recognized is the induction of cytoprotective stress proteins, such as the heat shock protein-70 (HSP-70), HSP-32 (ie, heme oxygenase-1; HO-1), and ferritin.8,9,20,21 Alterations in lipid homeostasis may also be involved. For example, arachidonic acid release from phospholipids,22,23,24 as well as sphingomyelin-generated sphingolipid products,25,26,27 can evoke a cytoprotective response. Probably the most consistent, and stable, renal injury-induced lipid alteration is renal tubular cholesterol accumulation.15,28,29,30,31,32,33,34 For example, in each of the above-mentioned models of renal cytoresistance (IR; myoglobinuric acute renal failure, glomerulonephritis, nephrotoxins, heat shock, ureteral obstruction, endotoxemia), an ∼25 to 50% sustained increase in renal tubular cholesterol concentrations result.28,29,30,31,32,33,34 The precise mechanism(s) by which excess cholesterol exerts its protective action remains to be defined. However, the available data suggest that cholesterol increases plasma membrane and mitochondrial membrane rigidity, and this serves to maintain mitochondrial energetics and plasma membrane integrity during superimposed ischemic or toxic attack.28,30,32 Of note, after injury cholesterol accumulation and cytoresistance are not renal-specific phenomena. For example, we have observed that when acute myelogenous leukemia cells are exposed to cancer chemotherapeutic agents, cholesterol accumulates, conferring resistance to further chemotherapy.35,36,37 Therefore, the mechanisms that are responsible for injury-induced cholesterol accumulation likely have broad-based biological relevance.
Evidence gathered to date indicates that increased tubular cell cholesterol synthesis can contribute to the cholesterol loading state after injury. This assertion is based on observations that statin-mediated inhibition of HMG CoA reductase, the rate-limiting enzyme in cholesterol synthesis, abrogates cholesterol accumulation after injury.31,35,36,37,38,39 However, whether increased cholesterol synthesis reflects increased HMGCR gene transcription, and which transcription factors might stimulate this response, have not been assessed. Hence, the present study was undertaken to explore the following issues: i) Does renal ischemic preconditioning activate the HMGCR gene (as assessed by RNA polymerase II recruitment to its transcription sites)? ii) If so, which transcription factor(s) might be involved? iii) Do epigenetic modifications exist at the HMGCR gene, potentially facilitating an increased transcriptional state? Investigations into each of these issues form the basis of this study.
Materials and Methods
Ischemia-Reperfusion Injury
Male CD 1 mice (30 to 35 g; Charles River Laboratories, Wilmington, MA), maintained under routine vivarium conditions and subjected to Institutional Animal Care and Use Committee approved protocols were used for all experiments. They were subjected to 30 minutes of left renal pedicle occlusion performed through an abdominal incision under pentobarbital anesthesia (40 to 50 mg/kg; IP) and followed by two-layer abdominal wall suturing. Approximately 72 hours after surgery, they were re-anesthetized, and bilateral nephrectomy was performed.
Analyses
The renal cortices were dissected (4°C) and subjected to either lipid, protein, or RNA extraction or chromatin cross linking (formalin).40 Lipid extracts were analyzed for free and esterified cholesterol levels by gas chromatography (expressed as nmol/μmol phospholipid phosphate).29,40 Protein samples were probed for HMGCR protein by Western blotting/chemiluminescence.31 HMGCR mRNA was quantified by competitive polymerase chain reaction (PCR) (expressed as a ratio to simultaneously measured GAPDH product).34 The validity of using the contralateral kidneys as controls was ascertained by identifying a lack of cholesterol/cholesterol ester accumulation in them, compared with kidneys obtained from six mice undergoing sham unilateral ischemia surgery.
Microplate-Based Matrix Chromatin Immunoprecipitation (ChIP)
Supplies and Equipment
ChIP assays were done using the Matrix ChIP platform in 96-well polystyrene high-binding capacity microplates (no. 9018; Corning, Corning, NY).41 The following reagents and equipment were used: protein A (no. P7837; Sigma, St. Louis, MO); proteinase K (no. 25530hyphen]015; Invitrogen, Carlsbad, CA); formaldehyde (no. 2106-02; J.T. Baker, Phillipsburg, NJ); bovine serum albumin (no. A9647, Sigma); phenylmethyl sulfonyl fluoride (no. P-7626, Sigma); leupeptin (no. L-2884, Sigma); SYBR Green PCR master mix (2×SensiMix, no. QT6T3; Quantace, Norwood, MA); salmon sperm DNA (no. D1626, Sigma); Misonix Sonicator 3000 with micro tip (no. S3000; Misonix, Farmingdale, NY); ultrasonic bath (no. B3510-MT CPN-952-316; Branson, Danbury, CT); heat blocks (analog heat block, no. 13259032: VWR Scientific, West Chester, PA; Isotemp 125: Fisher Scientific, Pittsburgh, PA); quantitative PCR (ABI 7900HT system; ABI Biotechnology, Foster City, CA); and MixMate (Eppendorf, Westbury, NY).
Buffers
The following buffers were used: phosphate-buffered saline (PBS): 137 mmol/L NaCl, 10 mmol/L Na phosphate, 2.7 mmol/L KCl, pH 7.4; TE buffer: 10 mmol/L Tris, 1 mmol/L ethylenediaminetetraacetic acid, pH 7.0; immunoprecipitation (IP) buffer: 150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.5, 5 mmol/L ethylenediaminetetraacetic acid, NP-40 (0.5% v/v), Triton X-100 (1.0% v/v); blocking buffer: 5% bovine serum albumin, 100 μg/ml sheared salmon sperm DNA in IP buffer; elution buffer: 25 mmol/L Tris base, 1 mmol/L ethylenediaminetetraacetic acid, pH 9.8, 200 μg/ml proteinase K (20 mg/ml stock, stored at −20°C).
Sonication of Renal Cortex Chromatin
Approximately 25 mg of minced renal cortex was fixed with formaldehyde (final concentration 1.42% in PBS for 15 minutes; 22°C) and then quenched with 125 mmol/L glycine (5 minutes, 22°C). The cross-linked tissues were then extensively washed with PBS (4°C). To shear the chromatin, the washed cross-linked tissue pellets were resuspended in 1 ml of IP buffer (containing the following inhibitors: 0.5 mmol/L dithiothreitol, 10 μg/ml leupeptin, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 30 mmol/L p-nitrophenyl phosphate, 10 mmol/L NaF, 0.1 mmol/L Na3VO4, 0.1 mmol/L Na2MoO4, and 10 mmol/L β-glycerophosphate) and sheared using six rounds of sonication (power 5, 15 seconds, on ice). The suspension was cleared by centrifugation at 12,000 × g (10 minutes at 4°C), and the supernatant, representing sheared chromatin, was aliquoted and stored at −80°C.
Immunoprecipitation and DNA Isolation
Ninety-six-well plates were washed once with 200 μl of PBS per well and were incubated overnight with 0.2 μg of protein A in 100 μl of PBS per well. After washing (200 μl of PBS per well), well walls were blocked with 200 μl of blocking buffer (15 to 60 minutes, 22°C). The wells were cleared and the used antibodies (Table 1) were added with 100 μl of blocking buffer per well (60 minutes, 22°C). Chromatin samples (5.0-μl chromatin preparations/100 μl of blocking buffer) were added (100 μl/well) and plates were floated in an ultrasonic water bath (60 minutes, 4°C) to accelerate protein-antibody binding.41 The wells were washed three times with 200 μl of IP buffer and 1 times with 200 μl of TE buffer. Wells were incubated with 100 μl of elution buffer (15 minutes at 55°C, followed by 15 minutes at 95°C). Total DNA (input) was isolated using the same plate and concurrently with immunoprecipitated DNA by suspending 5.0 μl of chromatin in 100 μl of elution buffer (15 minutes at 55°C, followed by 15 minutes at 95°C). DNA samples were stored (−20°C) in the same Matrix ChIP plates for repeated use.
Table 1.
List of Antibodies Used in Matrix ChIP Assay
| Antibody | Type | Source | Catalog | Amount/ChIP |
|---|---|---|---|---|
| Pol II CTD (4h8) | Monoclonal | Gene Tex | GTX25408 | 0.25 μg |
| p65/Rel NF-κB | Rabbit anti-serum | See Reference 70 | na | 0.5 μl |
| c-Jun | Rabbit polyclonal | Santa Cruz | sc-044 | 0.5 μg |
| c-Fos | Rabbit polyclonal | Santa Cruz | sc-652 | 0.5 μg |
| SREBP-1 | Monoclonal | PharMingen | 67351A | 1.0 μg |
| SREBP-2 | Monoclonal | BD Pharmingen | 557037 | 1.0 mg |
| HO-1 | Rabbit polyclonal | Oncogene Research | PC340 | 1.0 μg |
| HSP-72/73 | Monoclonal | Oncogene Research | HSP01 | 1.0 μg |
| H2A.Z | Rabbit polyclonal | Abcam | ab4174 | 0.5 μg |
| H3K4m3 | Rabbit polyclonal | Abcam | Ab8580 | 0.5 μg |
| H3K9Ac | Rabbit polyclonal | Cell Signaling | No. 9671 | 0.5 μg |
These antibodies were used in the ChIP assay, as described in the text.
Real-Time PCR (qPCR)
ChIP DNA samples were assayed by qPCR. The reaction mixture contained 2.5 μl of 2× SYBR Green PCR master mix (SensiMix, Quantace), 2.3 μl of DNA template, and 0.2 μl of primers (10 μmol/L) in 5-μl final volume in a 384-well optical reaction plate (Applied Biosystems, Foster City, CA). Amplification (three step, 40 cycles), data acquisition, and analyses were done using the 7900HT real-time PCR system and SDS Enterprise Database (Applied Biosystems). All PCR reactions were run in triplicate. At least four samples were run for each determination. The used qPCR primers are presented in Table 2. PCR calibration curves were generated for each primer pair from a dilution series of total mouse sheared genomic DNA. The PCR primer efficiency curve was fit to cycle threshold (Ct) versus loge (genomic DNA dilutions) using an r-squared best fit. DNA concentration values for each ChIP and input DNA samples were calculated from their respective average Ct values. Final results were expressed as percent input DNA.41
Table 2.
List of Primers Used for qPCR Analyses in ChIP Analyses
| Hmgcr (ID15357) | Right | 5′-CAGAACCAGAAGAGGCCTTG-3′ |
| −5′ (−5000 bp to TSS) | Left | 5′-GACCTCTGGCGGAGACATAC-3′ |
| Hmgcr | Right | 5′-GGAAGGACTGCGCTTACG-3′ |
| Promoter (−100 bp to TSS) | Left | 5′-GTTGTTAGGGAGACCGTTCG-3′ |
| Hmgcr | Right | 5′-AGGATCCAAGGACTGTGAGG-3′ |
| Exon1 | Left | 5′-AGGGCACTCATAATTCCAGC-3′ |
| Hmgcr | Right | 5′-TGTTCAAGGAGCATGCAAAG-3′ |
| Exon19 | Left | 5′-CTTACCTGTTGTGAACCATGTG-3′ |
| Hmgcr | Right | 5′-CAGCTCTCCTATCCAATGCC-3′ |
| +5′ flanking (+5000 bp to end of gene) | Left | 5′-TTGAAGGACATGCTGCTCAC-3′ |
| β-Actin (ID 11461) | Right | 5′-AGGAAGGAAGGCTGGAAGAG-3′ |
| Exon 1 | Left | 5′-GCTGAGAGGGAAATTGTTCG-3′ |
| β-Globin (ID 2440) | Right | 5′-ACCCATGATAGCAGAGGCAG-3′ |
| Int 1-Exon 2 | Left | 5′-GGTGCTTGGAGACAGAGGTC-3′ |
Primers used in qPCR analyses after chromatin immunoprecipitation.
Calculations and Statistics
All values are presented as means ± 1 SEM. Paired Student’s t-test was used to compare results obtained from left versus right kidney samples. Comparisons between different mice were performed by unpaired Student’s t-test. An n of four to eight tissue samples were used for all comparisons. Significance was judged by a P value of <0.05.
Results
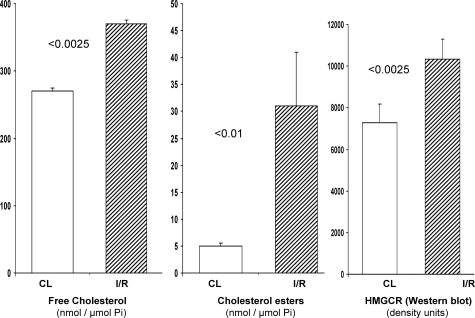
We previously demonstrated that by 18 to 24 hours after ischemia-reperfusion (I/R) injury, an ∼25 to 50% increase in renal cortical cholesterol levels result.28 The present study demonstrates the durability of this response: at 3 days after unilateral renal ischemia, a time that corresponds to persistent renal tubular histological injury (Figure 1), cholesterol levels were elevated by ∼35%, compared to the values observed in contralateral (nonischemic) control kidneys (Figure 2, left). Of note, the contralateral controls retained normal cholesterol levels, compared to sham-operated animals (data not shown). This indicates that the postischemic kidney elevations reflected the impact of I/R, rather than a nonspecific adaptation to surgical stress. The free cholesterol levels were accompanied by an approximate sixfold increase in cholesterol ester content (Figure 2, middle). Because acyl transferase-mediated esterification represents a shunting of excess free cholesterol into the cholesterol ester storage pool,40 cholesterol ester serves as a biomarker for degrees of cell cholesterol/sterol excess.29,42
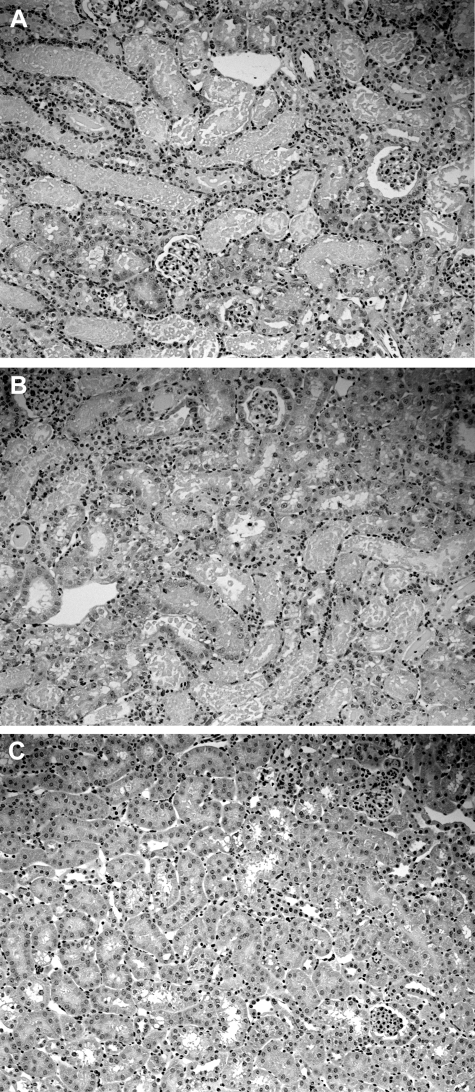
Figure 1.
Renal histological injury, as assessed at 3 days after unilateral ischemic injury. As a frame of reference for interpreting the biochemical data, 4-μm kidney sections were cut from 10% formalin-fixed tissues and stained with H&E. Extensive proximal tubular necrosis and cast formation was observed in the renal cortex (A) and in the outer medullary stripe (B). C: The contralateral kidney manifested normal histology. Thus, extensive renal injury was present in the kidney samples that were used to study the HMG CoA reductase pathway.
Figure 2.
Free cholesterol (left), cholesterol esters (middle), and HMG CoA reductase (HMGCR) protein levels (right, by Western blot) were assessed 3 days after unilateral I/R and in contralateral (CL) control kidneys. An ∼35% increase in free cholesterol levels was observed. This was accompanied by a sixfold increase in cholesterol esters, a cholesterol storage form. Increases in HMGCR protein were also observed, the magnitude of which paralleled the extent of the free cholesterol accumulation (each ∼35%).
HMGCR-mediated conversion of HMG CoA to mevalonate is the rate limiting step in cholesterol synthesis.43,45 Under physiological conditions, increased cellular cholesterol suppresses HMGCR expression via inhibition of the sterol regulatory element binding protein (SREBP) transcription pathway.43,44,45,46,47 Furthermore, cholesterol loading accelerates HMGCR protein catabolism.48,49 Therefore, if I/R were to induce cholesterol loading via a nonsynthetic pathway (eg, by low-density lipoprotein receptor-mediated uptake of plasma cholesterol), decreased HMGCR protein levels should result (decreased transcription/increased proteolysis). Conversely, an increase in HMGCR protein would be consistent with increased HMGCR transcription, translation, and hence cholesterol synthesis. To help differentiate between these two possibilities, HMGCR protein levels were assessed by Western blotting. As shown in Figure 2, right, at 72 hours after ischemia, an ∼35% increase in HMGCR protein levels was observed, paralleling the total cholesterol gain. Thus, these findings are consistent with an activated cholesterol synthesis pathway.
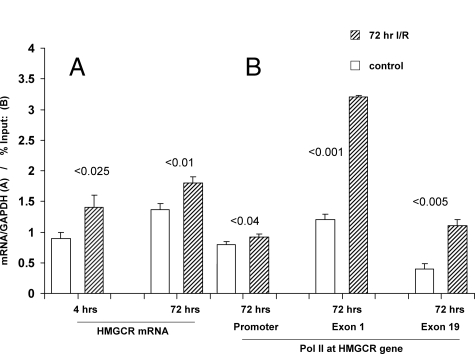
To gain additional support for the concept that I/R activates the HMGCR gene, HMGCR mRNA levels were assessed. As shown in Figure 3A, at both 4 and 72 hours after ischemia, HMGCR mRNA levels were elevated by ∼30 to 35%, quantitatively paralleling the HMGCR protein and cholesterol increases. The 4-hour mRNA elevations are noteworthy in that they preceded any increase in renal cholesterol content (data not shown), suggesting mechanistic relevance. HMGCR mRNA levels were still increased at the 72-hour time point, a time of marked cholesterol excess. Because suppressed HMGCR mRNA would be expected with cholesterol overload, a failure of feedback inhibition of mevalonate/cholesterol signaling pathway(s) clearly existed.43,44,45,46
Figure 3.
A: HMGCR mRNA levels in control and post-I/R cortical kidney samples obtained at 4 or 72 hours after surgery were quantified by PCR. In both instances, an ∼30% increase in mRNA levels was observed (expressed as a ratio to GAPDH). B: RNA polymerase II (Pol II) densities were assessed at the HMGCR promoter, and at the start and end exons of the HMGCR gene (exons 1 and 19). I/R caused a small, albeit statistically significant, Pol II increase at the HMGCR promoter. Conversely, I/R induced an approximately threefold increase in Pol II at both exons 1 and 19. As expected, the absolute Pol II amounts in both the control and post-I/R kidneys were greater at the start versus the end exon.
It could be argued that the HMGCR mRNA increases reflected increased mRNA stability, rather than increased gene transcription. Therefore, to lend additional support for the latter hypothesis, degrees of RNA polymerase II (Pol II) recruitment along the HMGCR gene were assessed. In this regard, Pol II is the critical enzyme that drives mRNA synthesis from the DNA template. Hence, the relative degree of Pol II recruitment at a target gene serves as a semiquantitative index of transcription rates.50,51,52,53 As shown in Figure 3B, I/R induced approximately threefold Pol II increases at both the start and end exons of the HMGCR gene. A smaller, although significant, increase was also observed at the HMGCR promoter. In sum, then, the evidence presented above indicates that I/R activated the entire cholesterol synthetic pathway (from increased Pol II binding to HMGCR → increased HMGCR mRNA → increased HMGCR protein → increased cholesterol → increased cholesterol esters).
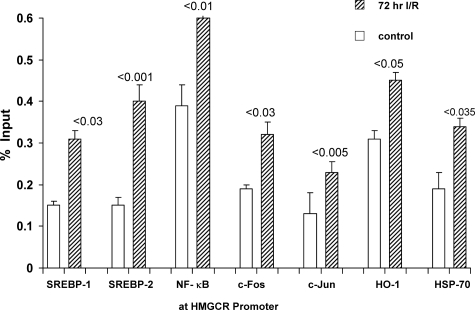
The maintenance of cellular cholesterol homeostasis is generally believed to be under the transcriptional control of SREBP-2, and to a lesser extent, SREBP-1.43,44,45 Under conditions of normal cholesterol supply, the SREBPs bind cholesterol and remain inactive, being anchored within the endoplasmic reticulum.43,44,45,46,47 Under conditions of cholesterol depletion, the SREBPs are mobilized from the endoplasmic reticulum, and they are escorted to the Golgi apparatus by SCAP (SREBP-cleavage-activating protein). Two proteolytic steps then ensue (via S1P and S2P), generating active transcription factors. These gain nuclear access via importin β, and they then bind to multiple genes that regulate lipid synthesis (including HMGCR). Under conditions of cholesterol excess, these signaling pathways should be suppressed. Therefore, to assess whether SREBP signaling is activated by I/R, SREBP-1 and SREBP-2 densities at the HMGCR promoter were determined. As shown in Figure 4, approximately threefold elevations of both SREBPs were observed at the promoter region. This indicates both a failure of physiological suppression and that the SREBPs likely participate in mediating the cholesterol loading state. The reason for the inappropriate SREBP nuclear translocation in the setting of cholesterol excess remains unknown. However, one possibility is that I/R activates intracellular/endoplasmic reticulum proteases, causing nonphysiological SREBP-endoplasmic reticulum release.
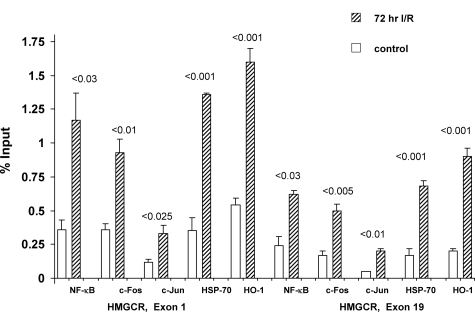
Figure 4.
The HMGCR promoter region was probed for: i) sterol-responsive element binding proteins (SREBP 1, 2); ii) general transcription factors (NF-κB, c-FOS, c-Jun); and iii) two heat shock proteins (HSP-70 and HSP-32, ie, HO-1). I/R caused twofold to threefold increases in the densities of both SREBP 1 and 2 at the HMGCR promoter. Significantly greater amounts of NF-κB, c-Fos, c-Jun, HSP-70, and HO-1 were also observed in the I/R versus control kidneys. Thus, multiple factors appear to activate HMGCR transcription as part of the stress response.
Although I/R increased SREBP nuclear binding, it remains possible that this is not the only transcription factor that might participate in activating the HMGCR gene. Hence, we addressed the possibility that other stress-activated early response genes54 might also participate. Toward this end, degrees of nuclear factor (NF)-κB (p65RelA), c-Fos, and c-Jun densities at the HMGCR promoter were assessed. In each instance, I/R caused dramatic increases (Figure 4). It has recently been suggested that heat shock proteins (HSPs) are also capable of translocation to the nucleus, potentially impacting gene transcription rates.55,56,57,58,59 Hence, we assessed whether I/R facilitates heat shock protein—nuclear access with subsequent binding to HMGCR. As shown in Figure 4, right, this was indeed the case: I/R-injured kidneys had 50 to 100% greater HSP-70 and HO-1 densities at the HMGCR promoter, compared to control kidney samples. Thus, it appears that classic cholesterol transcription factors (SREBPS), inducible transcription factors (c-Fos c-Jun, NF-κB), and HSPs may each participate in I/R-induced HMGCR activation.
Recently, there have been suggestions that transcription factors can bind along the length of an activated gene, rather than simply being confined to the promoter region. For example, Martone and colleagues60 documented NF-κB along the length of chromosome 22. This suggests that in addition to initiation of transcription, the classical transcription factor(s) may also participate in elongation and pre-mRNA processing. To test whether the above noted changes in NF-κB, c-Fos, c-Jun, HO-1, and HSP-70 might extend beyond the promoter, each was probed at both start and end exons of the HMGCR gene. As shown in Figure 5, the postischemic kidneys did, indeed, demonstrate increased amounts of each of these five proteins at these loci. Thus, these findings seemingly provide a new paradigm for stress-mediated cholesterol accumulation: the stress response, in this case induced by I/R, leads to binding of not only SREBPs, but also of inducible transcription factors, as well as HSPs, to the HMGCR promoter and transcribed regions.
Figure 5.
Localization of general transcription factors (NF-κB, c-Fos, c-Jun), HSP-70, and HO-1, at the start and end HMGCR exons. I/R induced significant increases of each protein at both HMGCR exons 1 and 19, compared to control kidneys. The absolute levels were consistently greater at the start versus the end exon (as would be expected with gene transcription).
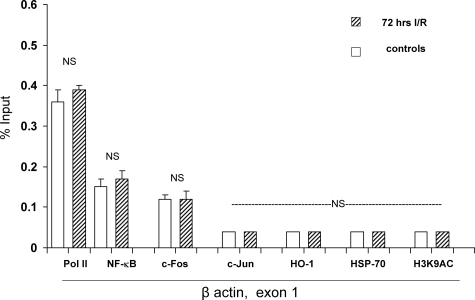
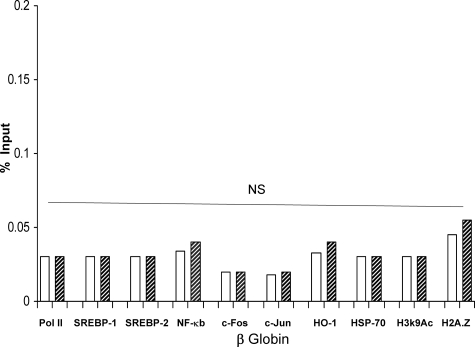
Given the dramatic results that are described above, we carefully considered the possibility that they might have represented nonspecific transcription factor/protein binding to chromatin, induced by the used tissue preparation technique. To exclude this possibility, extensive controls were undertaken. First, as shown in Figure 6, control and I/R kidney samples had virtually identical amounts of Pol II, NF-κB, c-Fos, c-Jun, HO-1, and HSP-70 at exon 1 of the β-actin gene (selected to serve as a negative control). Second, as shown in Figure 7 (and its legend), I/R did not increase Pol II, SREBP-1, SREBP-2, NF-κB, c-Fos, c-Jun, HO-1, or HSP-70 binding at the silent β-globin gene. Third, Pol II, NF-κB, c-Fos, c-Jun, HSP-70, and HO-1 densities were measured at the 5′ and 3′ flanking regions of HMGCR (−5 kb relative to the transcription start; +5 kb relative to the end of the gene). Again, no differences were seen between control and I/R kidney samples (Table 3); and fourth, it is notable that in each instance in which Pol II/transcription factor/or HSP binding was observed at the HMGCR gene, greater amounts were seen at exon 1, compared to exon 19. This is typical of Pol II recruitment, with the highest levels being observed at the transcription start sites, with progressive decreases along the gene.61 Had the binding results simply been an artifact, rather than a physiological process, one would have expected approximately equal binding at the start and end of HMGCR, as well as binding at its flanking regions, β-actin, and the β-globin genes. The relative importance of each of these proteins to gene activation, and ultimately mRNA synthesis (eg, elongation along the gene), remain to be defined.
Figure 6.
Assessments performed at a control gene (β-actin, exon 1). I/R did not significantly alter Pol II levels at β-actin, exon 1, serving as a negative control for the data shown at the right of Figure 2. Furthermore, I/R did not impact NF-κB, c-Fos, or c-Jun binding to β-actin, (negative control for the data in Figure 4). Finally, no difference in the extent of trimethylation of histone 3 lysine 4 (H3K4m3) was observed, indicating the relative specificity for data presented in Figure 8. [Note: H3K9AC and H2A.Z variants at β-actin were not assessed.]
Figure 7.
Assessments performed at the β-globin gene. As additional controls for the experiments depicted in Figures 1 to 5 and Figure 7, the SREBPs, general transcription factors, heat shock proteins, and histone modifications/variants were assessed at the normally silent β-globin gene (assessed between exon 1 and intron 1). In no instance was a statistically significant difference observed between control and the I/R kidney samples. Not shown (because of a different y axis scale), the amount of H3K4m3 was also not increased by I/R (2.2% versus 2.5% input for control and I/R samples; NS).
Table 3.
Pol II, Transcription Factors, Heat Shock Proteins, and Histone Modifications/Variants in HMGCR Flanking Regions (±5 kb)
| HMGCR flanking regions (±5 kb) | Contralateral kidney | 72 hours I/R |
|---|---|---|
| Pol II | ||
| −5 kb | 0.29 ± 0.02 | 0.24 ± 0.03 (NS) |
| +5 kb | 0.26 ± 0.06 | 0.31 ± 0.05 (NS) |
| SREBP-1 | ||
| −5 kb | 0.067 ± 0.01 | 0.064 ± 0.006 (NS) |
| +5 kb | 0.06 ± 0.01 | 0.052 ± 0.006 (NS) |
| SREBP-2 | ||
| −5 kb | 0.05 ± 0.005 | 0.049 ± 0.01 (NS) |
| +5 kb | 0.052 ± 0.007 | 0.055 ± 0.02 (NS) |
| NF-κB | ||
| −5 kb | 0.26 ± 0.05 | 0.26 ± 0.05 (NS) |
| +5 kb | 0.20 ± 0.01 | 0.18 ± 0.01 (NS) |
| c-Fos | ||
| −5 kb | 0.12 ± 0.01 | 0.14 ± 0.01 (NS) |
| +5 kb | 0.11 ± 0.01 | 0.14 ± 0.01 (NS) |
| c-Jun | ||
| −5 kb | 0.04 ± 0.005 | 0.04 ± 0.005 (NS) |
| +5 kb | 0.03 ± 0.005 | 0.03 ± 0.005 (NS) |
| HSP-70 | ||
| −5 kb | 0.08 ± 0.01 | 0.09 ± 0.01 (NS) |
| +5 kb | 0.03 ± 0.005 | 0.03 ± 0.005 (NS) |
| HO-1 | ||
| −5 kb | 0.06 ± 0.005 | 0.05 ± 0.005 (NS) |
| +5 kb | 0.03 ± 0.05 | 0.03 ± 0.005 (NS) |
| H2A.Z | ||
| −5 kb | 0.14 ± 0.01 | 0.12 ± 0.01 (NS) |
| +5 kb | 0.10 ± 0.01 | 0.11 ± 0.01 (NS) |
| H3K4m3 | ||
| −5 kb | 6.5 ± 0.4 | 6.5 ± 0.3 (NS) |
| +5 kb | 6.0 ± 0.02 | 6.3 ± 0.03 (NS) |
| H3K9Ac | ||
| −5 kb | 0.08 ± 0.01 | 0.09 ± 0.01 (NS) |
| +5 kb | 0.07 ± 0.01 | 0.08 ± 0.01 (NS) |
Values are percent of DNA input. No significant differences were observed between control (contralateral) and postischemic kidney samples.
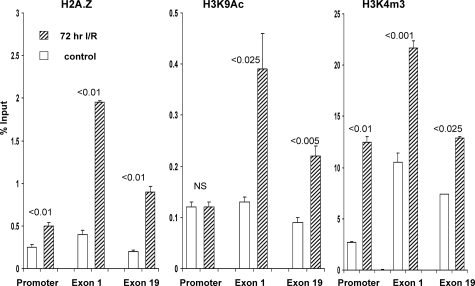
The final goal of this study was to ascertain whether the I/R-induced changes in transcription factor binding and Pol II expression at HMGCR were accompanied by epigenetic modifications at this gene. Two changes that are thought to enhance Pol II recruitment to target genes by opening chromatin structure are trimethylation of histone 3-lysine 4 (H3K4m3) and acetylation of H3K9 (H3K9Ac).62,63,64,65,66 As shown in Figure 8, I/R injury dramatically increased the levels of each, as noted by their densities at the promoter as well as at exons 1 and 19. Another epigenetic change that may enhance, or reflect, gene activity is replacement of canonical H2A with histone 2A.Z variant.67,68 I/R did, indeed, increase the deposition of H2A.Z at the promoter and transcribed regions (Figure 8). As negative controls, H3K4m3, H3K9Ac, and H2A.Z were probed at HMGCR flanking regions and at the β-actin or β-globin genes. In no instance did I/R increase any of these three histone marks (H3K4m3, H3K9Ac, H2A.Z) at these regions (Figures 6 and 7; Table 3). This strongly implies that these histone modifications at HMGCR loci were not simply nonspecific markers of cell stress.
Figure 8.
Histone variant H2A.Z levels and H3 histone modifications at the HMGCR promoter, and along the gene. I/R caused a twofold to fourfold increase in the amounts of H2A.Z variant and of H3K4m3 at all assessed sites. H3 lysine 19 acetylation was also increased at exons 1 and 19 (but not at the promoter).
Discussion
It has recently been suggested, based on data obtained in yeast, that HMGCR can function as a stress responsive element and that the resultant cholesterol increases can confer a survival advantage.69 The results of the present study demonstrate quite clearly that this same response can be observed in mammals (or, at least, in the mouse). As discussed above, in response to a classic form of renal stress, ie, I/R, a 35% increase in free cholesterol concentrations resulted, culminating in a sixfold increase in the cholesterol ester storage pool. That injury-induced cholesterol increases can confer a survival advantage on mammalian cells has previously been demonstrated in a variety of studies from this laboratory that used freshly isolated mouse proximal tubules, cultured human proximal tubular (HK-2) cells, and acute myelogenous leukemia cells.15,17,28,29,30,35,36,37 It needs to be underscored that these injury-induced cytoprotective cholesterol increases cannot be totally attributed to HMGCR-mediated cholesterol synthesis. Clearly, increased low-density lipoprotein receptor-mediated cholesterol uptake, as well as decreased cholesterol efflux (reverse cholesterol transport) may also be involved.14,39 However, to separate out each of these different components in vivo is extremely difficult because any single experimentally induced change in one particular cholesterol accumulation pathway (eg, statin-mediated HMGCR inhibition) can cause compensatory alteration(s) in the others (eg, a secondary increase in low-density lipoprotein receptor-mediated uptake). Hence, these types of determinations are most easily resolved in cell culture experiments in which individual pathways can be controlled.39
The purpose of this study was to focus on the molecular determinant(s) of just one critical cholesterol homeostatic pathway: the HMGCR gene. The stimulus for this investigation was the present observation that I/R caused increases in both HMGCR protein and its cognate mRNA. Hence, we sought to gain further support for increased HMGCR activity by attempting to document increased Pol II recruitment to multiple loci within the transcribed and promoter regions. Because this was observed, as discussed at length in the Results section, we sought to identify the transcription factors that might be involved. The results obtained indicated that classic sterol regulatory transcription factors (SREBP-1, SREBP-2), stress-activated transcription factors (NF-κB, c-Fos, C-Jun), and heat shock proteins (HSP-70, HO-1) may all play a role. That non-SREBP gene-activating elements may impact HMGCR regulation during cell stress represents, to the best of our knowledge, a novel insight.
Finally, we sought to test the hypothesis that I/R-induced HMGCR activation might either arise from, or conceivably induce, histone modifications at its gene loci. The results obtained support this possibility given that three potential gene activating epigenetic modifications at the HMGCR promoter and along the gene (increased H3K4m3, H3K9Ac, and H2A.Z) were observed. It remains to be proven that these modifications are mechanistically involved in activating the HMGCR gene, and hence, culminate in increased Pol II recruitment. An answer to this question remains a goal for future studies. These will undoubtedly require techniques to alter specific histone modifications at specific locations within the genome. Until such methodologies are available, the physiological relevance of the dramatic H3K4m3, H3K9AC, and H2A.Z changes noted in this study must be considered speculative as of this time.
Footnotes
Address reprint requests to Richard A. Zager, M.D., Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N, Room D2-190, Seattle, WA 98109. E-mail: dzager@fhcrc.org.
Supported by the National Institutes of Health (research grants DK-R37-38431 and DK-68520 to R.A.Z.; and DK-R37-45978 and GM45134 to K.B.).
References
- Honda N, Hishida A, Ikuma K, Yonemura K. Acquired resistance to acute renal failure. Kidney Int. 1987;31:1233–1238. doi: 10.1038/ki.1987.136. [DOI] [PubMed] [Google Scholar]
- Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- Reimer KA, Murry CE, Jennings RB. Cardiac adaptation to ischemia. Ischemic preconditioning increases myocardial tolerance to subsequent ischemic episodes. Circulation. 1990;82:2266–2268. doi: 10.1161/01.cir.82.6.2266. [DOI] [PubMed] [Google Scholar]
- Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2:313–318. doi: 10.1007/s11481-007-9089-8. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- Ambros JT, Herrero-Fresneda I, Borau OG, Boira JM. Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transpl Int. 2007;20:219–229. doi: 10.1111/j.1432-2277.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- Jo SK, Ko GJ, Boo CS, Cho WY, Kim HK. Heat preconditioning attenuates renal injury in ischemic ARF in rats: role of heat-shock protein 70 on NF-kappa B-mediated inflammation and on tubular cell injury. J Am Soc Nephrol. 2006;17:3082–3092. doi: 10.1681/ASN.2005101077. [DOI] [PubMed] [Google Scholar]
- Riordan M, Sreedharan R, Kashgarian M, Siegel NJ. Modulation of renal cell injury by heat shock proteins: lessons learned from the immature kidney. Nat Clin Pract Nephrol. 2006;2:149–156. doi: 10.1038/ncpneph0117. [DOI] [PubMed] [Google Scholar]
- Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- Platt JL, Nath KA. Heme oxygenase: protective gene or Trojan horse. Nat Med. 1998;4:1364–1365. doi: 10.1038/3947. [DOI] [PubMed] [Google Scholar]
- Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Alam J, Croatt AJ, Vercellotti GM, Nath KA. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab Invest. 1995;72:474–483. [PubMed] [Google Scholar]
- Zager RA, Johnson ACM, Hanson SY. Sepsis syndrome stimulates proximal tubule cholesterol synthesis and suppresses the SR-B1 cholesterol transporter. Kidney Int. 2003;63:123–133. doi: 10.1046/j.1523-1755.2003.00735.x. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Lund S. ‘Endotoxin tolerance’: TNF-alpha hyper-reactivity and tubular cytoresistance in a renal cholesterol loading state. Kidney Int. 2007;71:496–503. doi: 10.1038/sj.ki.5002092. [DOI] [PubMed] [Google Scholar]
- Zager RA, Baltes LA. Progressive renal insufficiency induces increasing protection against ischemic acute renal failure. J Lab Clin Med. 1984;103:511–523. [PubMed] [Google Scholar]
- Zager RA. Obstruction of proximal tubules initiates cytoresistance against hypoxic damage. Kidney Int. 1995;47:628–637. doi: 10.1038/ki.1995.80. [DOI] [PubMed] [Google Scholar]
- Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277:2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Shanley TP, Croatt A, Alam J, Johnson KJ, Nath KA. Glomerular inflammation induces resistance to tubular injury in the rat. A novel form of acquired, heme oxygenase-dependent resistance to renal injury. J Clin Invest. 1996;98:2139–2145. doi: 10.1172/JCI119020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla J, Nath KA, Balla G, Juckett MB, Jacob HS, Vercellotti GM. Endothelial cell heme oxygenase and ferritin induction in rat lung by hemoglobin in vivo. Am J Physiol. 1995;268:L321–L327. doi: 10.1152/ajplung.1995.268.2.L321. [DOI] [PubMed] [Google Scholar]
- Berenshtein E, Vaisman B, Goldberg-Langerman C, Kitrossky N, Konijn AM, Chevion M. Roles of ferritin and iron in ischemic preconditioning of the heart. Mol Cell Biochem. 2002;234:283–292. [PubMed] [Google Scholar]
- Zager RA, Schimpf BA, Gmur DJ, Burke TJ. Phospholipase A2 can protect renal tubules from oxygen deprivation injury. Proc Natl Acad Sci USA. 1993;90:8297–8301. doi: 10.1073/pnas.90.17.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhunaizi AM, Yaqoob MM, Edelstein CL, Gengaro PE, Burke TJ, Nemenoff RA, Schrier RW. Arachidonic acid protects against hypoxic injury in rat proximal tubules. Kidney Int. 1996;49:620–625. doi: 10.1038/ki.1996.89. [DOI] [PubMed] [Google Scholar]
- Zager RA, Conrad DS, Burkhart K. Phospholipase A2: a potentially important determinant of adenosine triphosphate levels during hypoxic-reoxygenation tubular injury. J Am Soc Nephrol. 1996;7:2327–2339. doi: 10.1681/ASN.V7112327. [DOI] [PubMed] [Google Scholar]
- Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–140. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- Iwata M, Herrington J, Zager RA. Sphingosine: a mediator of acute renal tubular injury and subsequent cytoresistance. Proc Natl Acad Sci USA. 1995;92:8970–8974. doi: 10.1073/pnas.92.19.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Webb I, Marber MS. Sphingosine kinase isoforms and cardiac protection. Cardiovasc Res. 2007;76:3–4. doi: 10.1016/j.cardiores.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Zager RA, Burkhart KM, Johnson ACM, Sacks BM. Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance.”. Kidney Int. 1999;56:1788–1797. doi: 10.1046/j.1523-1755.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- Zager RA, Kalhorn TF. Changes in free and esterified cholesterol; hallmarks of acute renal tubular injury and acquired cytoresistance. Am J Pathol. 2000;157:1007–1016. doi: 10.1016/S0002-9440(10)64613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA. Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int. 2000;58:193–205. doi: 10.1046/j.1523-1755.2000.00154.x. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson A. Renal cortical cholesterol accumulation is an integral component of the systemic stress response. Kidney Int. 2001;60:2299–2310. doi: 10.1046/j.1523-1755.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- Zager RA. P glycoprotein-mediated cholesterol cycling determines proximal tubular cell viability. Kidney Int. 2001;60:944–956. doi: 10.1046/j.1523-1755.2001.060003944.x. [DOI] [PubMed] [Google Scholar]
- Zager RA, Andoh T, Bennett WM. Renal cholesterol accumulation: a durable response after acute and subacute renal insults. Am J Pathol. 2001;159:743–752. doi: 10.1016/S0002-9440(10)61745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA, Shah VO, Shah HV, Zager PG, Johnson ACM, Hanson S. The mevalonate pathway during acute tubular injury. Am J Pathol. 2002;161:681–692. doi: 10.1016/S0002-9440(10)64224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker DE, Mayer SJ, Li HY, Willman CL, Appelbaum FR, Zager RA. Cholesterol synthesis and import contribute to protective cholesterol increments in acute myeloid leukemia cells. Blood. 2004;104:1816–1824. doi: 10.1182/blood-2004-01-0395. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Appelbaum FR, Willman CL, Zager RA, Banker DE. Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or over expression. Leuk Res. 2003;27:133–145. doi: 10.1016/s0145-2126(02)00085-1. [DOI] [PubMed] [Google Scholar]
- Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101:3628–3634. doi: 10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol. 2008;294:F187–F197. doi: 10.1152/ajprenal.00434.2007. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Hanson SY. Proximal tubular cholesterol loading after mitochondrial, but not glycolytic, blockade. Am J Physiol. 2003;285:F1092–F1099. doi: 10.1152/ajprenal.00187.2003. [DOI] [PubMed] [Google Scholar]
- Naito M, Bomsztyk K, Zager RA. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J Am Soc Nephrol. 2008;19:1321–1330. doi: 10.1681/ASN.2007121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagin S, Nelson JD, Castner DG, Denisenko O, Bomsztyk K. Microplate-based chromatin immunoprecipitation method, Matrix ChIP: a platform to study signaling of complex genomic events. Nucleic Acids Res. 2008;36:e17. doi: 10.1093/nar/gkn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DA. Regulation of the cholesterol ester cycle of cultured Leydig tumor cells. Eur J Biochem. 1987;164:351–356. doi: 10.1111/j.1432-1033.1987.tb11065.x. [DOI] [PubMed] [Google Scholar]
- Espenshade PJ. SREBPs: sterol-regulation transcription factors. J Cell Sci. 2006;119:973–976. doi: 10.1242/jcs.02866. [DOI] [PubMed] [Google Scholar]
- Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet. 2007;41:401–427. doi: 10.1146/annurev.genet.41.110306.130315. [DOI] [PubMed] [Google Scholar]
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol. 2007;19:215–222. doi: 10.1016/j.ceb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86:839–848. doi: 10.1016/j.biochi.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Chun KT, Bar-Nun S, Simioni RD. The regulated degradation of 3-hydroxy-3methyglutaryl-CoA reductase requires a short lived protein and occurs in the endoplasmic reticulum. J Biol Chem. 1990;265:22004–22010. [PubMed] [Google Scholar]
- Cronin SR, Khoury A, Ferry DK, Hampton RY. Regulation of HMG-CoA reductase degradation requires the p-type ATPase Cod1p/Spf1p. J Cell Biol. 2000;148:915–924. doi: 10.1083/jcb.148.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD. The molecular basis of eukaryotic transcription. Proc Natl Acad Sci USA. 2007;104:12955–12961. doi: 10.1073/pnas.0704138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- Marx J. Transcription enzyme structure solved. Science. 2001;292:411–414. doi: 10.1126/science.292.5516.411b. [DOI] [PubMed] [Google Scholar]
- Sng JCG, Taniura H, Yoneda Y. A tale of early response genes. Biol Pharm Bull. 2004;27:606–612. doi: 10.1248/bpb.27.606. [DOI] [PubMed] [Google Scholar]
- Zeise E, Kuhl N, Kunz J, Rensing L. Nuclear translocation of stress protein HSC70 during S phase in rat C6 glioma cells. Cell Stress Chaperones. 1998;3:94–99. doi: 10.1379/1466-1268(1998)003<0094:ntosph>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgitani E, Kobayashi K, Takeshita K, Imanishi J. Biphasic translocation of a 70 kDa heat shock protein in human cytomegalovirus-infected cells. J Virol. 1999;80:63–68. doi: 10.1099/0022-1317-80-1-63. [DOI] [PubMed] [Google Scholar]
- Longshaw VM, Chapple JP, Balda MS, Cheetham ME, Blatch GL. Nuclear translocation of the Hsp70/ Hsp90 organizing protein mST11 is regulated by cell cycle kinases. J Cell Sci. 2004;117:701–710. doi: 10.1242/jcs.00905. [DOI] [PubMed] [Google Scholar]
- Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Sacca P, Meiss R, Casas G, Mazza O, Calvo JC, Navone N, Vazquez E. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br J Cancer. 2007;97:1683–1689. doi: 10.1038/sj.bjc.6604081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone R, Euskirchen G, Bertone P, Hartman S, Royce TE, Luscombe NM, Rinn JL, Nelson FK, Miller P, Gerstein M, Weissman S, Snyder M. Distribution of NF-kappaB-binding sites across human chromosome 22. Proc Natl Acad Sci USA. 2003;100:12247–12252. doi: 10.1073/pnas.2135255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–296. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;9:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bantignies F, Cavalli G. Cellular memory and dynamic regulation of polycomb group proteins. Curr Opinion Cell Biol. 2006;18:275–283. doi: 10.1016/j.ceb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Chen L, Firozi P, Barton M, Templeton NS. Widespread, exceptionally high levels of histone H3 lysine 4 trimethylation largely mediate “privileged” gene expression. Gene Express. 2007;13:271–282. doi: 10.3727/000000006780666966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Ramaswamy A, Ioshikhes I. Global dynamics of newly constructed oligonucleosomes of conventional and variant H2A.Z histone. BMC Struct Biol. 2007;7:76. doi: 10.1186/1472-6807-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J, Thakar A. H2A.Z: view from the top. Structure. 2008;16:166–179. doi: 10.1016/j.str.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Robichon C, Dugail I. De novo cholesterol synthesis at the crossroads of adaptive response to extracellular stress through SREBP. Biochimie. 2007;89:260–264. doi: 10.1016/j.biochi.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Gordon KL, Couser WG, Bomsztyk K. IL-1b increases laminin B2 chain mRNA levels and activates NF-kB in rat glomerular epithelial cells. Am J Physiol. 1995;268:F273–F278. doi: 10.1152/ajprenal.1995.268.2.F273. [DOI] [PubMed] [Google Scholar]