Abstract
Recurrent Crohn’s disease originates with small erosions in the follicle-associated epithelium overlying the Peyer’s patches. Animal studies have illustrated mucosal immune regulation by dendritic cells located in the subepithelial dome. The aim of this study was to characterize the dendritic cells at this specific site in patients with Crohn’s disease. Ileal tissues were obtained after surgery performed on Crohn’s patients; ileal samples from noninflammatory bowel disease and ulcerative colitis served as standard and inflammatory controls, respectively. Flow cytometry of isolated intestinal mononuclear cells showed a larger subset of dendritic cells in Crohn’s samples compared with controls. This finding was corroborated by confocal microscopy, showing enhanced infiltrates of cells positive for the dendritic cell markers, DC-SIGN+ and CD83+, in the subepithelial dome. Moreover, the CD83+ cells in Crohn’s tissues showed reduced expression of the lymph node migratory receptor, CCR7, possibly contributing to the high numbers of dendritic cells. After exposure to nonpathogenic Escherichia coli in Ussing chambers, dendritic cells in the subepithelial dome of Crohn’s disease demonstrated increased co-localization with translocated bacteria. Immunohistochemical results revealed that DC-SIGN+ cells in Crohn’s tissues were found to express toll-like receptor 4 and produce tumor necrosis factor-α. In conclusion, nonmigrating dendritic cells that accumulate in the subepithelial dome and internalize nonpathogenic bacteria may be important for the onset and perpetuation of mucosal inflammation in Crohn’s disease.
Crohn’s disease (CD) is a multifactorial condition characterized by inappropriate and exaggerated mucosal immune responses.1,2,3,4,5 Evidence suggests that the disease originates from abnormal interplay between the intestinal microflora and the mucosal immune system in genetically susceptible individuals.6,7,8 This is likely to occur at the follicle-associated epithelium (FAE), which lines key mucosal inductive sites of the intestine.9,10,11
Unlike regular villous epithelium, the FAE is more exposed to luminal contents12 while simultaneously having closer contact with the mucosal immune system.13,14,15 M-cells that are part of the FAE are specialized to sample and transport luminal contents to underlying immune cells.16 However, the lack of an accepted human histochemical M-cell marker hampers studies in identifying M-cells in tissues.17 We have previously shown that FAE of human ileum is more effective at delivering antigens and bacteria to the subepithelial dome (SED) compared with villous epithelium.18 This controlled uptake of luminal contents along with the presence of the mucosal immune system is believed to be crucial for the induction of protective mucosal immunity. In CD, protective immunity is, however, disrupted and small erosions develop at the FAE, resulting in the initiation of recurrent ileal inflammation.19,20,21,22 Recently, we found increased transepithelial uptake of nonpathogenic bacteria in the FAE of ileal CD.23 Little is know, however, about the fate of bacteria after crossing the epithelial layer in intestinal inflammation.
Dendritic cells (DCs) are one of the cells that orchestrate the mucosal immune system. Serving as sentinels, resident and recruited mucosal DCs either play an important immunoregulatory or priming role. In mice, DCs expressing the chemokine receptor CCR6 migrate toward the ligand CCL20, which is predominantly expressed by FAE.24 Once in the SED, DCs, which expressing the chemokine receptor CCR7, internalize translocated commensal bacteria, mature, and migrate to the mesenteric lymph nodes.25 Under physiologically normal conditions, it is believed that DCs loaded with commensal bacteria do not penetrate beyond the mesenteric lymph nodes. Hence, immune induction is confined to the mucosa rather than leading to systemic activation.26 Indeed, although Peyer’s patches were susceptible to bacterial penetration,25 DCs isolated from these lymphoid follicles produced higher amounts of anti-inflammatory cytokine interleukin-10 than the ones isolated from other organs.27 This suggests that DCs in Peyer’s patches normally induce peripheral tolerance toward the intestinal microflora. Despite this information regarding intestinal DCs in rodents, limited information exists about human mucosal DCs in inflammatory bowel diseases (IBD).
Recent studies in human intestine have demonstrated that adult and pediatric patients with IBD have an imbalance in the numbers of DCs in the colonic mucosa.28,29,30,31 Using a unique antibody, M-DC8, it was shown that DCs are present in the SED of individuals with CD.32 Moreover, other studies have shown that intestinal epithelial cells in IBD tissues have increased expression of CCL20.33,34 CCL20 is a known chemoattractant for immature DCs.35,36 This abnormal expression of CCL20 could explain, in part, the imbalance of mucosal DCs observed in CD. However, no information is available regarding a potential association between CCL20, DCs, and bacterial uptake in Peyer’s patches of patients with CD.
The aim of this study was to characterize and investigate the functional properties of DCs found in the SED in ileal Peyer’s patches from individuals with CD. We found an abnormal accumulation of DCs that had greater propensity to internalize live bacteria. We also discuss the potential implication of these results in the immunopathogenesis of CD.
Materials and Methods
Patients and Tissue Specimens
Distal ileal tissues, adjacent to the ileocecal valve or from the neoterminal ileum, were freshly obtained from 29 patients with CD who underwent bowel resection at the University Hospital of Linköping. The mean age of the group was 41 years (range, 22 to 70 years) with 14 females and 15 males. Fourteen patients underwent re-resections and fifteen cases were primary resections. Nine of the patients were on mesalasine, four were given steroids, and four patients received azathioprine, whereas the rest had no anti-inflammatory medication. According to disease activity, assessed by Crohn’s disease activity index (CDAI), 10 patients were in the inactive stage (CDAI < 150), whereas 19 patients had active disease. Standard histological examination showed signs of low to mild inflammation in all patients. Control samples were obtained from macro- and microscopically normal ileal specimens of 18 patients with no IBD. There were 12 females and 6 males, average age 55 years (range, 29 to 91 years), who underwent surgery for colon cancer. The ileal specimens had no signs of generalized disease and none had received preoperative chemo- and radiotherapy. In addition, 15 patients with ulcerative colitis (UC), 9 females and 6 males, mean age of 42 years (range, 33 to 76 years), were used as inflammatory controls. Ileal samples were obtained during colectomy (n = 9) for chronic continuous disease or dysplasia or at routine follow-up colonoscopy (n = 6). All 15 patients were on maintenance treatment with mesalasine, 1 patient also received steroids, and 3 were on azathioprine. Histological assessment of the colonic biopsies showed chronic inflammation (lymphocyte infiltration) in all cases, whereas the ileal samples showed normal to low-grade inflammation, except for one patient with moderate backwash ileitis. Because of the range of experimental approaches, it was not technically feasible to conduct all of the different experiments on each of the patients’ tissues. Thus the number in each analysis is lower than the total number of patients. The study was approved by the Regional Human Ethics Committee in Linköping, Sweden, and all participants in the study had given their informed consent.
Antibodies
The antibodies and their working concentrations are specified in Table 1. We used DC-specific ICAM-3 grabbing nonintegrin (DC-SIGN), a 44-kDa type II lectin membrane protein, as a specific DC marker that is highly expressed by immature DCs.37,38 DC-SIGN is involved in antigen recognition and uptake and is primarily expressed in the intestine by myeloid DCs.30,39 However a small subset of macrophages in other organs have been shown to express DC-SIGN.38,40 To identify mature DCs, we stained for CD83, which plays a role in antigen presentation and CD4+ T-cell development.41 These markers have been previously used to stain for DCs in colonic tissue sections from patients with CD and non-IBD.30,31
Table 1.
The Antibodies and Working Concentrations Used for Immunofluorescence Staining
| Antibody | Dilution | Manufacturer |
|---|---|---|
| Goat CCL20 | 1:100 | R&D Systems, Oxon, UK |
| Goat CCR6 | 1:250 | Abcam Ltd., Cambridgeshire, UK |
| Goat CCR7 | 1:250 | Abcam Ltd. |
| Mouse CD83 | 1:40 | Santa Cruz Biotechnology Inc., Heidelberg, Germany |
| Mouse DC-SIGN | 1:40 | R&D Systems |
| Goat DC-SIGN | 1:30 | Santa Cruz Biotechnology Inc. |
| Goat TLR4 | 1:100 | Santa Cruz Biotechnology Inc. |
| Mouse TNF-α | 1:200 | R&D Systems |
| Goat neutrophil elastase | 1:100 | Santa Cruz Biotechnology Inc. |
| Fluorescein-conjugated donkey anti-goat antibody | 1:500 | Abcam Ltd. |
| Alexa Fluor 633-conjugated goat anti-mouse | 1:500 | Molecular Probes, Leiden, The Netherlands |
| Phalloidin-conjugated Alexa Fluor 581 | 1:500 | Molecular Probes |
| Normal mouse IgG1 | 1:40 | DAKO, Glostrup, Denmark |
| Normal goat IgG1 | 1:30 | DAKO |
Isolation of Intestinal Mononuclear Cells and Flow Cytometry Analysis
Intestinal mononuclear cells were isolated from five non-IBD control patients, six patients with CD, and six patients with UC. The muscles and myenteric plexus were stripped-off the ileal surgical tissue. The mucosa was cut into small pieces (∼0.5 to 1 cm in diameter) and digested with collagenase D (Roche Diagnostics, Mannheim, Germany) (1 mg/ml collagenase D and 20 mmol/L HEPES; Sigma-Aldrich, Steinheim, Germany), 10% fetal calf serum, 200 mmol/L l-glutamine, 2% penicillin-streptomycin in RPMI-1640 (all from Invitrogen, Paisley, Scotland), pH 7.4, for 2 hours at 37°C under constant agitation. The supernatant was collected and filtered through a cell strainer (pore size, 70 μm). The cells were then spun down, the media decanted, and the pellet was resuspended with RPMI 1640 containing 10% fetal calf serum. For in vitro isolation of mononuclear cells, Ficoll-Paque Plus (Amersham Bioscience, Uppsala, Sweden) was used according to the manufacturer’s instructions. Briefly, the suspended cells were gently placed over Ficoll-Paque Plus in centrifuge tubes and spun at 400 × g, for 25 minutes at 21°C without breaks. The interface between the Ficoll-Paque Plus and sample layer was collected and washed once. The cells were spun down, the buffer was decanted, and the pellet was resuspended in phosphate-buffered saline (PBS) buffer containing 1 mmol/L ethylenediaminetetraacetic acid and 0.02% sodium azide.
Flow cytometry data were acquired using a FACS (fluorescence-activated cell sorter) Calibur flow cytometer (Becton Dickinson, San Jose, CA). All of the antibodies used for the FACS analysis were purchased from BD Biosciences (Temese, Belgium). Using Lineage cocktail 1, DCs were identified as an HLA-DR+ Lineage (Lin) 1− (CD3−, CD14−, CD16−, CD19−, CD34−, CD56−), and within this gate the CD83− CD11c+ or CD83+ CD11+ populations were assessed. Analysis was performed using Becton Dickinson FACSDiva Software (version 5.0.1). The data are presented as the percentage population.
Identification and Immunofluorescence Staining of Ileal Peyer’s Patches
Peyer’s patches were identified as previously done in our laboratory.18 Briefly, once the muscularis layers were stripped-off, the remaining tissue was then placed on a dish, trans-illuminated from below, and the Peyer’s patches identified under a dissection microscope. Regions containing the Peyer’s patches were either mounted on modified Ussing chambers (Harvard Apparatus Inc., Holliston, MA) or frozen for immunofluorescence staining.
During the entire staining protocol, the cryosectioned specimens were placed at room temperature in a dark humidified box. Sections were initially pre-incubated with PBS containing 5% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich) (BSA/PBS) for 30 minutes, followed by incubation with mouse anti-DC-SIGN or CD83 antibodies (Table 1) for 60 minutes. The slides were washed three times with PBS, blocked with of 5% BSA for 30 minutes, and subsequently incubated with goat anti-CCR6, -CCR7, or -CCL20 antibodies for 60 minutes. Sections were washed and blocked again with 5% BSA in PBS for 30 minutes. For neutrophil staining, sections were incubated with goat anti-neutrophil elastase (Table 1) and either mouse anti-DC-SIGN or CD83 antibodies for 60 minutes. Secondary fluorescein-conjugated donkey anti-goat antibody was added for 60 minutes followed by Alexa Fluor 633-conjugated goat anti-mouse antibody for 60 minutes. Then sections were incubated for 10 minutes with Alexa Fluor 581-conjugated phalloidin, followed by washing five times with PBS. A drop of mounting media (Dako Cytomation, Carpinteria, CA) was added and stored at 4°C until visualization. Control staining was performed by either omitting the primary antibodies or using their isotype-matched control antibodies at similar dilution (Table 1). Tissue sections were examined under a Nikon Eclipse E600W laser-scanning confocal microscope (Nikon, Melville, NY) and image analysis performed using Nikon EZ-C1 software (Nikon, Lijnden The Netherlands).
Internalization of Live Escherichia coli HB101
To study internalization of live bacteria into DCs, Peyer’s patches isolated from five non-IBD controls and four CD and three UC patients were mounted on modified Ussing chambers42 following the procedure previously described.18 Briefly, Peyer’s patches were placed in the chamber system ensuring that the patches covered the entire exposed tissue area of 4.9 mm2, with the mucosal and serosal compartments filled with 1.5 ml of 10 mmol/L mannitol or 10 mmol/L glucose in Krebs buffer, respectively. The tissue segments were kept at 37°C and continuously oxygenated at 5% CO2/95% O2. Subsequently, previously transformed E. coli HB101 with green fluorescent protein (GFP)18 was added to the mucosal compartment at 1 × 108 CFU/ml. After 15 or 30 minutes of incubation, the tissue samples were washed with Krebs buffer, dismounted, placed in OCT, and frozen to −80°C. These tissue samples were cryosectioned and processed for immunofluorescence.
Quantification of DCs
Histological quantification was done by counting the number of positively stained cells in SED of scanned confocal images. Using the Nikon EZ-C1 software, a box with a defined area that covered the SED (not including the germinal center) was drawn on each image and the numbers of positively double-stained DCs within this area were counted. The sections were randomly selected and an average of 10 sections from each patient was examined. Data are presented as the number of DCs per mm2.
Immunohistochemistry Double Staining for Cytokines and DCs
Immunohistochemical staining was performed on sections obtained from paraffin-embedded blocks of 12 non-IBD controls and 17 CD, following previously described protocols.30 Briefly, endogenous peroxidase activity of the sections was blocked with 10% H2O2 and subsequently treated with 0.05% trypsin solution in 0.05% of CaCl2 (pH 7.8) at 37°C for 30 minutes to unmask antigenicity. After a wash step with PBS, sections were blocked with 10% normal human serum protein for 30 minutes. Incubations were performed overnight at 4°C with mouse anti-DC-SIGN and goat anti-TLR4 or goat anti-DC-SIGN and mouse anti-tumor necrosis factor (TNF)-α (Table 1). The slides were washed in PBS and incubated with biotinylated rabbit anti-goat (1:200; DAKO, Glostrup, Denmark) for 90 minutes. Washed slides were incubated with streptavidin-AP (1:100, DAKO) for 30 minutes, and then with the Fast Red substrate system (DAKO). A red color precipitate was obtained. After an extensive wash, the slides were incubated with biotinylated goat anti-mouse (1:25, DAKO) for 90 minutes. Subsequent to the washing, the slides were incubated with streptavidin-peroxidase for 30 minutes. The color reaction was performed using the DAB substrate kit (Pierce Biotechnology Inc., Rockford, IL) following the manufacturer’s instructions, yielding a brown precipitate. Slides were washed with PBS and counterstained with Mayer hematoxylin. Negative control staining was performed using isotype-matched antibodies (Table 1).
Statistical Analysis
Data were analyzed using the nonparametric Mann-Whitney U-test for comparisons between two groups, and with Kruskal-Wallis one-way analysis of variance for three groups. The data are presented with median values and the 25th and 75th interquartile range. A difference of P < 0.05 was considered significant.
Results
Increased HLA-DR+Lin1− and CD83+CD11c+ DCs in CD Ileum
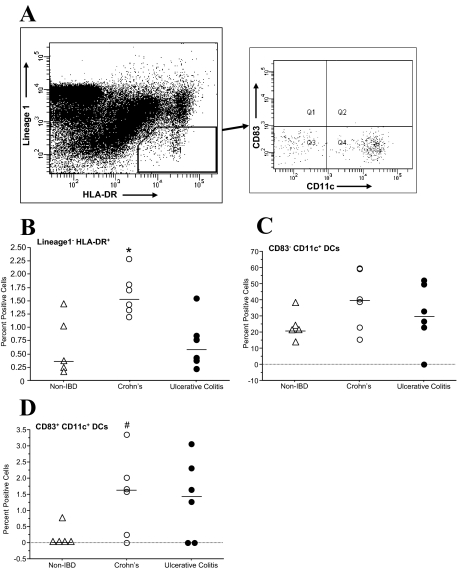
DCs isolated from whole ileal intestinal wall were identified as HLA-DR+ Lineage1− (Figure 1A for gate selection). A significant percentage of cells isolated from CD tissue were HLA-DR+ Lineage1− compared with the control and UC groups [CD, 1.6% (1.3 to 1.8%); non-IBD controls, 0.3% (0.2 to 1.1%); UC, 0.6% (0.4 to 0.8%; P < 0.05, comparison done using Kruskal-Wallis one-way analysis of variance, Figure 1B]. Of the HLA-DR+ Lineage1− cells, no difference in the proportion of CD83−CD11c+ DCs was found between the groups [non-IBD controls, 20.9% (19.0 to 27.1%); CD, 39.5% (22.9 to 59.0%); UC, 29.8% (23.0 to 49.5%); Figure 1C]. However, the percentage of CD83+ CD11c+ DCs was higher in CD when compared with non-IBD controls [1.6% (0.3 to 2.0%), 0.0% (0.0 to 0.2%), respectively; P = 0.04, using Mann-Whitney U-test], but not with UC [1.4% (0.0 to 2.3%); Figure 1D]. The isolation of the mononuclear cells does not give insight into which ileal compartment the DCs are located. Therefore, we conducted immunofluorescence staining focusing on the Peyer’s patches because we found very few DCs present in the lamina propria of regular villous epithelium.
Figure 1.
Identification and characterization of DCs from isolated mononuclear cells. Mononuclear cells were isolated from five non-IBD control patients, six CD patients, and six UC patients and were analyzed using multicolor flow cytometry. A: DCs were identified as HLA-DR+ and Lineage 1− (CD3−, CD14−, CD16−, CD19−, CD20−, and CD56−). Within this gate, CD83−CD11c+ and CD83+CD11c+ subsets were quantified. B: The percentage of HLA-DR+ Lineage1− cells was significantly greater in CD versus the non-IBD control and UC groups (*P < 0.05). C: No difference in the proportion of immature myeloid (CD83−CD11c+) DCs was found between the groups. D: The percentage of mature myeloid (CD83+CD11c+) DCs found in the HLA-DR+ Lineage1− was higher in CD when compared with non-IBD controls (#P < 0.05) but not to UC.
DCs Infiltrate the SED in CD
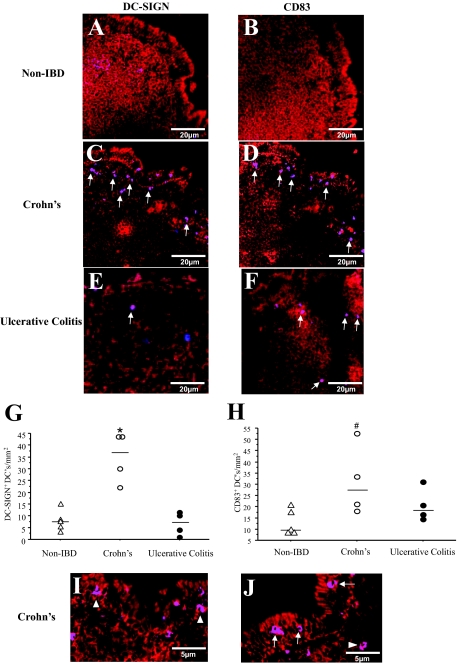
To characterize DCs, ileal Peyer’s patches from non-IBD controls, CD, and UC were stained with DC-SIGN and CD83 (Figure 2). DCs were primarily found in the SED, whereas very few were present in the germinal centers of Peyer’s patches. Compared with controls (Figure 2, A and B), there were more DC-SIGN+ and CD83+ cells in the SED of patients with CD (Figure 2, C and D, respectively). Meanwhile, UC tissues showed very scant staining of both DC-SIGN+ and CD83+ DCs in ileal Peyer’s patches (Figure 2, E and F). Quantification of the DC-SIGN+ and CD83+ DCs revealed significant number of DCs present in CD when compared with the non-IBD controls (Figure 2, G and H). The number of CD83+ DCs correlated significantly to disease assessed by CDAI (ρ = 0.71, P = 0.045), whereas DC-SIGN+ cell numbers did not correlated with CDAI (ρ = 0.36, P = 0.31).
Figure 2.
Expression of DC-SIGN and CD83+ DCs in tissue sections. Surgical sections from five non-IBD control, four CD, and four UC patients were stained with antibodies against DC-SIGN (blue) and CD83 (blue) and tissue morphology was visualized by staining F-actin (red). Few to no DC-SIGN+ DCs (A, purple) and CD83+ DCs (B, purple) were found in the non-IBD control sections. In CD tissue sections, DC-SIGN+ (C) and CD83+ (D) DCs were mainly found in the SED (arrows). In UC, fewer DC-SIGN+ DC (E) and CD83+ DCs (F) were observed. Quantification revealed significantly greater numbers of DC-SIGN+ DCs (G) and CD83+ DCs (H) present in CD compared with non-IBD controls. Magnification of CD tissues’ Peyer’s patches illustrated DCs to be in close association with (arrowheads, I, J) and between the FAE cells (arrows, J). I: DC-SIGN+ DCs. J: CD83+ DCs. Purple = DC-SIGN or CD83 (blue) co-localized with F-actin (red). A range of 7 to 10 randomly selected sections were examined from each patient tissue. *P = 0.01, #P = 0.05.
Magnification of the Peyer’s patches in the CD tissues illustrated DCs to be in close proximity to the FAE (Figure 2I, arrowheads). In addition, DCs were also found between the epithelial cells of the FAE (Figure 2J, arrows). Conversely in control tissues, DCs were rarely found to be associated with the epithelial cells of the FAE. In none of the tissue slides from controls, nor in UC, were there any intraepithelial DCs in the FAE. Staining of lamina propria in villous epithelium of CD sections illustrated very scant distribution of DC-SIGN+ DCs. This was similar to our previous observations.43 These DCs were mainly found in the crypt areas of villous epithelium (Supplementary Figure S1, see http://ajp.amjpathol.org).
Enhanced Internalization of E. coli HB101 by DCs in the SED in CD Tissues
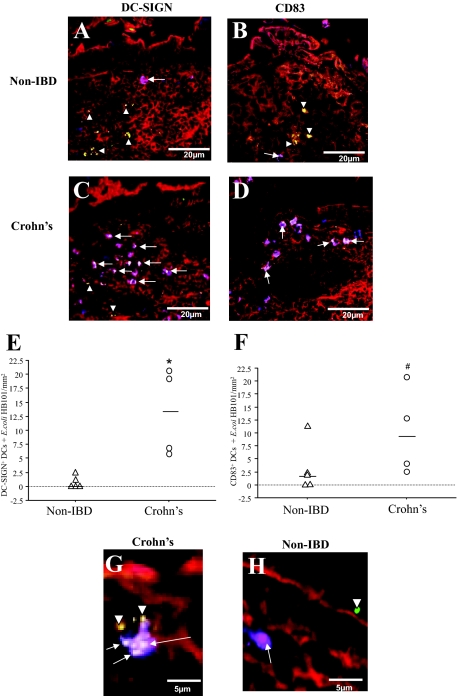
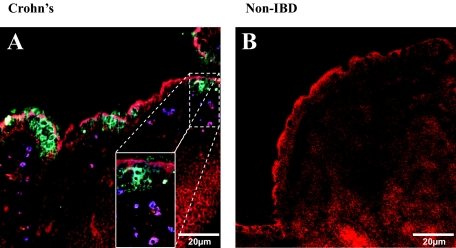
In the noninflammatory control tissues (non-IBD), few live GFP E. coli were found to co-localize with DC-SIGN+ DCs after Ussing chamber experiments (Figure 3A, arrows). In contrast, CD tissues exposed to live E. coli HB101 in Ussing chambers revealed frequent co-localization (Figure 3C, arrows). In the UC tissues, no co-localization between GFP E. coli and DC-SIGN+ DCs was found in stained sections (from three patients). Future experiments on UC tissues were excluded from the study because of the very limited number of DCs with internalized E. coli. Quantification of the number of DC-SIGN+ DCs that had taken up fluorescent E. coli was higher in tissue sections from patients with CD than in controls [13 (6 to 23) versus 0 (0 to 1) cells per mm2, respectively (P = 0.01), Figure 3E]. Furthermore, the DC-SIGN+ DCs in CD tissues were more prone to internalize E. coli, as the percentage of DC-SIGN+ DCs that co-localized with E. coli was 44 ± 5% versus 24 ± 5% in non-IBD controls (P < 0.05). Magnification of the SED illustrated that in CD, DC-SIGN+ DCs were able to sample more than one bacterium (Figure 3G), which was not the case for DCs in the non-IBD control tissue sections (Figure 3H).
Figure 3.
Enhanced internalization of fluorescent E. coli HB101 by DCs in CD. Sections of distal ileum containing Peyer’s patches from five non-IBD control, and four CD patients were mounted in Ussing chambers. Live GFP E. coli HB101 was added to the mucosal compartment and after 30 minutes of exposure, the tissues were processed for confocal microscopy. Staining of non-IBD control sections illustrated that there were few co-localizations of DC-SIGN+ DCs (A; purple,§ arrows) and CD83+ DCs (B) with E. coli. In CD tissue sections, a higher frequency of both DC-SIGN+ (C) and CD83+ (D) DCs were found to internalize bacteria (white, arrows) in the SED. E. coli was also found either in the extracellular matrix between cells or in cells other than DCs (green, arrowheads). E: Quantification of the co-localized DC-SIGN+ DCs and E. coli HB101 showed significant increase in CD (n = 4) versus non-IBD tissue sections (n = 5). F: Quantification showed that CD83+ DCs co-localization with E. coli were more frequent in CD (n = 4) than in non-IBD (n = 4) tissues. Magnification of the Peyer’s patches showed that in CD (G), the DCs were not only closely associated with the FAE, but were also able to internalize more than one bacterium, in contrast to observations in non-IBD controls (H). From each patient tissue, 10 to 12 sections were examined. Red = F-actin; green = fluorescently-labeled E. coli HB101; §purple = DC-SIGN or CD83 (blue) co-localized with F-actin (red); white = co-localization of DCs with E. coli. *P = 0.01, #P = 0.05.
DCs that have internalized bacteria are expected to mature and migrate out of the SED and into the interfollicular region (IFR) and lymph nodes. However our immunofluorescence staining of CD tissue revealed a large number of CD83+ DCs with internalized E. coli were still localized within the SED (Figure 3D), compared with the DCs in non-IBD controls (Figure 3B). There was an increase in the number of CD83+ DCs with E. coli HB101 in CD versus controls [8 (3 to 17) versus 2 (0 to 5) cells per mm2, respectively (P = 0.05), Figure 3F]. Meanwhile the percentage of CD83+ DCs that co-localized with bacteria was 35 ± 5% in CD and 27 ± 6% in controls (P = 0.1).
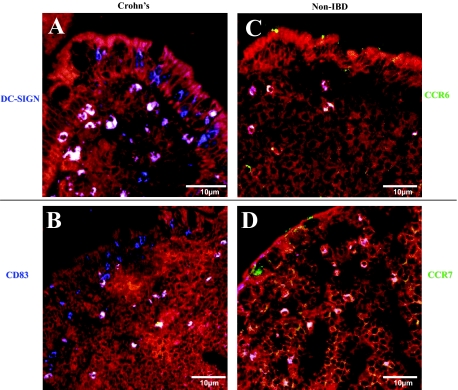
Fewer CD83+ DCs in the SED of CD Express CCR7
Finding high numbers of mature CD83+ DCs in the SED of CD tissue sections led us to examine the expression of their migratory receptors, CCR6 and CCR7. In CD, we found DC-SIGN+ CCR6+ DCs (white) and also DC-SIGN+ CCR6− DCs (blue) (Figure 4A). Meanwhile, staining for CD83 and CCR7 illustrated that there were lower amounts of CD83+ CCR7+ DCs located in the SED in CD tissue sections (Figure 4B) than in controls (Figure 4, C and D). The percentage of CD83+ DCs that expressed either CCR6+ or CCR7+ in CD tissue sections and controls are given in Table 2. We found that in CD, only 28% of the mature DCs were set to migrate out of the SED (CD83+ CCR7+) whereas 92% of the CD83+ DCs in the non-IBD controls expressed the CCR7 emigrating receptor (P < 0.01). Past studies have shown neutrophils to express CD83 and CCR6.44,45 In this study, we found no co-localization of CD83+ cells with neutrophil elastase (Supplementary Figure S2, see http://ajp.amjpathol.org).
Figure 4.
Tissue sections stained for migratory receptors CCR6 and CCR7. Surgical specimens from CD and non-IBD patients were double-stained for DC-SIGN (blue) and CCR6 (green, top), or CD83 (blue) and CCR7 (green, bottom). Co-localization of the maturity markers (DC-SIGN or CD83) with migratory receptors (CCR6 or CCR7) results in a white color. In CD, there were DC-SIGN+ DCs that were CCR6+ (white) or CCR6− (blue) (A), whereas there were mainly DC-SIGN+ CCR6+ DCs (white) in the non-IBD tissue sections (C). However, co-staining for CCR7 revealed fewer CD83+ DCs that were CCR7+ in CD (B) compared with the non-IBD tissue sections, where CD83+ CCR7+ DCs were seen (D). Red = F-actin; blue = DC-SIGN or CD83; white = co-localization of DC-SIGN or CD83 with CCR6 or CCR7.
Table 2.
Quantification of the Percent of CD83+ DCs that Were Either CCR6+ or CCR7+ in the SED of CD and Non-IBD Control Tissues
| DC phenotype | Crohn’s disease (%) | Controls (%) |
|---|---|---|
| CD83+ CCR6+ | 71 (66 to 92)* | 8 (0 to 26) |
| CD83+ CCR7+ | 28 (27 to 42) | 92 (68 to 99)† |
In SED of CD tissues, a significantly greater percent of CD83+ CCR6+ DCs than CD83+ CCR7+ DCs (
P = 0.01, comparison done using Mann-Whitney U-test, n = 4) were found. In contrast, greater numbers of CD83+ CCR7+ DCs than CD83+ CCR6+ DCs were found in non-IBD controls (
P = 0.01, n = 5). Numbers presented as percent median (25th to 75th percentile).
CCL20 Is Expressed by the FAE of CD
Because the DCs present in the SED of the CD tissue sections were predominantly CCR6+, the presence of the recruiting chemokine CCL20 was examined. Indeed, the FAE of CD tissue sections did produce and release CCL20 into the SED and into the lumen (Figure 5A). Furthermore, DCs were found to be concentrated in areas where CCL20 was produced and released (Figure 5A, inset). Conversely, in none of the control sections stained did we find FAE producing CCL20 (Figure 5B).
Figure 5.
The FAE cells express and release CCL20. A: Staining for CCL20 (cyan) illustrated that it was expressed and released by the FAE into the SED and lumen of CD tissue sections. Magnification of the SED revealed the recruitment and clustering of DCs in areas where CCL20 release was more prominent (inset). B: In none of the non-IBD controls (stained) did we find FAE expressing CCL20 (representative of three slides from three different patients). Red = F-actin; blue = DC-SIGN; cyan = CCL20. Inset magnification, ×500.
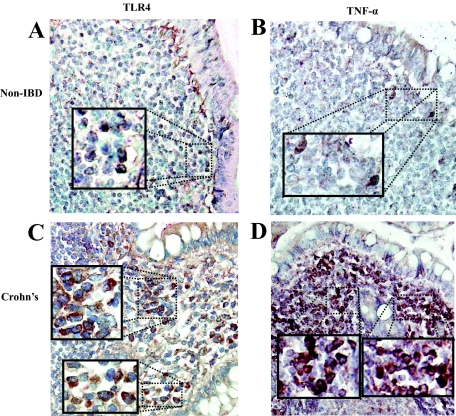
DCs in CD Tissue Sections are TLR4+ and TNF-α+
To identify whether DCs in the SED expressed TLR4 and released TNF-α, immunohistochemical staining of paraffin-embedded tissues was performed. Staining of CD tissue sections illustrated that DC-SIGN+ DCs in the SED expressed TLR4 (Figure 6C). Few DC-SIGN+ TLR4+ DCs could be seen in the control sections (Figure 6A). The double-stained DC-SIGN+ TLR4+ DCs (burgundy) were primarily found beneath the FAE. Staining the tissue sections for TNF-α revealed that DC-SIGN+ DCs were present in the SED of patients with CD (Figure 6D) and in control sections (Figure 6B).
Figure 6.
Immunohistochemical staining of DCs in CD tissues. Sections from archival tissues were co-stained for DC-SIGN (red) and TLR4 (brown), or DC-SIGN (red) and TNF-α (brown). A: Few DC-SIGN+ TLR4+ DCs (burgundy color; see inset) were found in the SED of non-IBD control sections but were more abundant in CD tissues (C, inset showing double-positive DC-SIGN+ TLR4+ cells). When tissue sections were stained for TNF-α (B, see inset) a similar observation with lower frequency of DC-SIGN+ TNF-α+ DCs were seen in controls (B) compared with CD tissue sections (D). Original magnifications, ×400. Inset magnification, ×600.
Discussion
Because the earliest endoscopic signs of relapsing CD are the minute mucosal erosions of the FAE, our group has focused attention on the potential initiating factors of recurrent local inflammation at these sites.17,18,23 We have recently found a defective epithelial barrier in the FAE of CD, leading to bacterial internalization into subepithelial DC.23 In the present study we expand on these findings by focusing on and characterizing the subepithelial DC population of the FAE in Peyer’s patch areas. Increased numbers of DCs were found in the ileal mucosa and SED of FAE in CD patients, by FACS analysis and immunofluorescence microscopy, respectively. Moreover, the DC phenotypes in the SED were characterized by their level of maturation as well as expression of migratory markers, leading to the identification of an unusual accumulation of mature CD83+ CCR7− DCs. These cells were in close interaction with bacteria and epithelial cells in the SED of CD; thus leading to increased internalization of translocated nonpathogenic E. coli HB101 compared with non-IBD control and UC tissues. In addition, DCs in the SED of CD expressed TLR4 and TNF-α.
The maturation and immunostimulatory properties of DCs are important in determining their dual role; tolerance versus immunity. The increased recruitment and accumulation of immature DCs seen in SED of CD may be as a consequence of a high amount of transported bacteria, as we have noted recently,23 and can be further explained by the expression and release of CCL20 by the ileal FAE. This is corroborated by a study that found the expression of CCL20 to be predominantly expressed by the FAE of human colonic tissues.33 Furthermore, CCL20 expression was found be to several folds higher in IBD than in non-IBD tissues,33 hence providing a possible explanation for the enriched DC population seen in SED. On the other hand, previous studies have demonstrated that maturated DCs expressing CCR7 migrate toward its ligand CCL21, which is normally expressed in T-cell-rich areas such as the lymph nodes.36,46 Therefore, the accumulation of CD83+ DCs found in the present study (by FACS analysis and confocal imaging) was unexpected and intriguing, and could be an indicator of altered functionality of mature DCs in CD. A similar observation, with accumulation of mature CD83+ DCs, was previously found in the colonic mucosa of CD patients.29 However, they found that the majority of the CD83+ DCs were CCR7+, maybe as a result of examining the entire intestinal wall. In addition, the expression of CCL21 by reticular cells and lymphatic vessels facilitated the entrapment of the DCs in the IFR and lymph nodes of CD tissues.29 In our present study, however, most of the accumulated CD83+ DCs were located in the SED area. Furthermore, we found in CD tissues that the majority of the CD83+ DCs were CCR7−. Simultaneously, more than 70% of the CD83+ DCs were still CCR6+. This, along with the release of CCL20 from the FAE, could result in further accumulation of DCs as seen in the SED of CD tissues. The number of CD83+ cells correlated to disease activity, which could be interpreted as the accumulation of CD83+ cells being secondary to inflammation because no correlation was found between DC-SIGN+ DCs and CDAI. Further studies looking at changes in DC populations after various types of anti-inflammatory therapy are needed to elucidate the implications of this finding.
The majority of DCs in CD were found to express TLR4 and produce TNF-α. Myeloid DCs are known to preferentially express TLR4 and respond to lipopolysaccharide.47 Our findings are consistent with a previous study in which myeloid DCs isolated from colonic sections of IBD patients had increased surface expression of TLR4.28 It is possible that the expression of TLR4 by DCs could facilitate and enhance the ability to internalize bacteria. As illustrated by de Baey and colleagues,32 a new class of DCs (M-DC8+) in the SED could contribute to the high levels of TNF-α production seen in individuals with CD. In addition, we recently found a significant release of TNF-α from FAE tissues of CD exposed to nonpathogenic E. coli compared with control tissues.23 In the present study, we show high amounts of TNF-α being produced by DCs, hence perturbing the cytokine balance in CD tissues.
In conclusion, the increased internalization of bacteria, TLR4 expression, and production of TNF-α, all implicate the trapped DCs in the SED to play a role in the relapse of ileal inflammation. One may speculate that a dysfunctional FAE barrier,23 and expression CEACAM 6 (carcinoembryonic antigen-related cell adhesion molecule 6) on epithelial cells48 in CD, increase the amount of bacteria entering the SED where they encounter accumulated DCs. As demonstrated by the present findings, these nonmigratory DCs are prone to interact with epithelial cells and to internalize bacteria. Future studies will aim at elucidating the molecular mechanisms involved in internalization of commensal and pathogenic bacterial strains and immune activation in these inflammatory DCs of the Peyer’s patches.
Supplementary Material
Acknowledgments
We thank Mrs. Ylva Braaf for skillful technical assistance and Dr. Florence Sjögren for assistance with FACS.
Footnotes
Address reprint requests to Johan D. Söderholm, M.D., Ph.D., Div. of Surgery, University Hospital of Linköping, 581 85, Linköping, Sweden. E-mail: johda@ibk.liu.se.
Supported by The Broad Medical Research Program of the Eli and Edythe L. Broad Foundation and The Swedish Research Council–Medicine.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Ardizzone S, Porro GB. Inflammatory bowel disease: new insights into pathogenesis and treatment. J Intern Med. 2002;252:475–496. doi: 10.1046/j.1365-2796.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Shanahan F. Crohn’s disease. Lancet. 2002;359:62–69. doi: 10.1016/S0140-6736(02)07284-7. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–416. doi: 10.1007/s11894-999-0023-5. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology. 2000;119:1740–1744. doi: 10.1053/gast.2000.20231. [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Peterson KH, Olaison G, Franzen LE, Westrom B, Magnusson KE, Sjodahl R. Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology. 1999;117:65–72. doi: 10.1016/s0016-5085(99)70551-2. [DOI] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Semin Immunol. 2005;17:284–294. doi: 10.1016/j.smim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Makala LHC, Suzuki N, Nagasawa H. Peyer’s patches: organized lymphoid structures for the induction of mucosal immune responses in the intestine. Pathobiology. 2002;70:55–68. doi: 10.1159/000067305. [DOI] [PubMed] [Google Scholar]
- Gebert A. The role of M cells in the protection of mucosal membranes. Histochem Cell Biol. 1997;108:455–470. doi: 10.1007/s004180050186. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Lugering N, Rautenberg K, Lugering A, Schmidt MA, Stoll R, Domschke W. Role of M cells in intestinal barrier function. Ann NY Acad Sci. 2000;915:171–183. doi: 10.1111/j.1749-6632.2000.tb05240.x. [DOI] [PubMed] [Google Scholar]
- Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- Sanders DS. Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease. J Clin Pathol. 2005;58:568–572. doi: 10.1136/jcp.2004.021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen RL. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer’s patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977;72:440–451. [PubMed] [Google Scholar]
- Gullberg E, Soderholm JD. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann NY Acad Sci. 2006;1072:218–232. doi: 10.1196/annals.1326.028. [DOI] [PubMed] [Google Scholar]
- Keita AV, Gullberg E, Ericson AC, Salim SY, Wallon C, Kald A, Artursson P, Soderholm JD. Characterization of antigen and bacterial transport in the follicle-associated epithelium of human ileum. Lab Invest. 2006;86:504–516. doi: 10.1038/labinvest.3700397. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer I, Costopoulos L. Early lesions of Crohn’s disease. Am J Roentgenol. 1978;130:307–311. doi: 10.2214/ajr.130.2.307. [DOI] [PubMed] [Google Scholar]
- Rickert RR, Carter HW. The “early” ulcerative lesion of Crohn’s disease: correlative light- and scanning electron-microscopic studies. J Clin Gastroenterol. 1980;2:11–19. [PubMed] [Google Scholar]
- Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita AV, Salim SY, Jiang T, Yang PC, Franzen L, Soderkvist P, Magnusson KE, Soderholm JD. Increased uptake of non-pathogenic E. coli via the follicle-associated epithelium in longstanding ileal Crohn’s disease. J Pathol. 2008;215:135–144. doi: 10.1002/path.2337. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–162. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AL, Al Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, Kamm MA, Stagg AJ. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–227. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MA, Lopez CB, Riverin F, Oligny L, Menezes J, Seidman EG. Characterization and distribution of colonic dendritic cells in Crohn’s disease. Inflamm Bowel Dis. 2004;10:504–512. doi: 10.1097/00054725-200409000-00003. [DOI] [PubMed] [Google Scholar]
- te Velde AA, van Kooyk Y, Braat H, Hommes DW, Dellemijn TA, Slors JF, van Deventer SJ, Vyth-Dreese FA. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12−IL-18− dendritic cell populations in the colonic mucosa of patients with Crohn’s disease. Eur J Immunol. 2003;33:143–151. doi: 10.1002/immu.200390017. [DOI] [PubMed] [Google Scholar]
- de Baey A, Mende I, Baretton G, Greiner A, Hartl WH, Baeuerle PA, Diepolder HM. A subset of human dendritic cells in the T cell area of mucosa-associated lymphoid tissue with a high potential to produce TNF-alpha. J Immunol. 2003;170:5089–5094. doi: 10.4049/jimmunol.170.10.5089. [DOI] [PubMed] [Google Scholar]
- Kaser A, Ludwiczek O, Holzmann S, Moschen AR, Weiss G, Enrich B, Graziadei I, Dunzendorfer S, Wiedermann CJ, Murzl E, Grasl E, Jasarevic Z, Romani N, Offner FA, Tilg H. Increased expression of CCL20 in human inflammatory bowel disease. J Clin Immunol. 2004;24:74–85. doi: 10.1023/B:JOCI.0000018066.46279.6b. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–826. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, Massacrier C, Vanbervliet B, Zlotnik A, Vicari A. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–369. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- Caux C, Vanbervliet B, Massacrier C, Ait-Yahia S, Vaure C, Chemin K, Dieu-Nosjean MC, Vicari A. Regulation of dendritic cell recruitment by chemokines. Transplantation. 2002;73:S7–S11. doi: 10.1097/00007890-200201151-00005. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent PL, Figdor CG, Barrera P, van Ginkel K, Sloetjes A, van den Berg WB, Torensma R. Expression of the dendritic cell-associated C-type lectin DC-SIGN by inflammatory matrix metalloproteinase-producing macrophages in rheumatoid arthritis synovium and interaction with intercellular adhesion molecule 3-positive T cells. Arthritis Rheum. 2003;48:360–369. doi: 10.1002/art.10786. [DOI] [PubMed] [Google Scholar]
- Bell SJ, Rigby R, English N, Mann SD, Knight SC, Kamm MA, Stagg AJ. Migration and maturation of human colonic dendritic cells. J Immunol. 2001;166:4958–4967. doi: 10.4049/jimmunol.166.8.4958. [DOI] [PubMed] [Google Scholar]
- Grass GM, Sweetana SA. In vitro measurement of gastrointestinal tissue permeability using a new diffusion cell. Pharm Res. 1988;5:372–376. doi: 10.1023/a:1015911712079. [DOI] [PubMed] [Google Scholar]
- Silva MA, Quera R, Valenzuela J, Salim SY, Soderholm JD, Perdue MH. Dendritic cells and toll-like receptors 2 and 4 in the ileum of Crohn’s disease patients. Dig Dis Sci. 2008;53:1917–1928. doi: 10.1007/s10620-007-0105-x. [DOI] [PubMed] [Google Scholar]
- Iking-Konert C, Wagner C, Denefleh B, Hug F, Schneider M, Andrassy K, Hansch GM. Up-regulation of the dendritic cell marker CD83 on polymorphonuclear neutrophils (PMN): divergent expression in acute bacterial infections and chronic inflammatory disease. Clin Exp Immunol. 2002;130:501–508. doi: 10.1046/j.1365-2249.2002.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Wang JM, Yang D, Gong WH, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–3963. [PubMed] [Google Scholar]
- Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.