Abstract
Inherited tooth enamel hypoplasia occurs due to mutations in genes that encode major enamel components. Enamel hypoplasia also has been reported in junctional epidermolysis bullosa, caused by mutations in the genes that encode type XVII collagen (COL17), a component of the epithelial-mesenchymal junction. To elucidate the pathological mechanisms of the enamel hypoplasia that arise from the deficiency of epithelial-mesenchymal junction molecules, such as COL17, we investigated tooth formation in our recently established Col17−/− and Col17 rescued mice. Compared with wild-type mice, the incisors of the Col17−/− mice exhibited reduced yellow pigmentation, diminished iron deposition, delayed calcification, and markedly irregular enamel prisms, indicating the presence of enamel hypoplasia. The molars of the Col17−/− mice demonstrated advanced occlusal wear. These abnormalities were corrected in the Col17 rescued humanized mice. Thus, the Col17−/− mice clearly reproduced the enamel hypoplasia in human patients with junctional epidermolysis bullosa. We were able to investigate tooth formation in the Col17−/− mice because the Col17−/− genotype is not lethal. Col17−/− mouse incisors had poorly differentiated ameloblasts that lacked enamel protein-secreting Tomes’ processes and reduced mRNA expression of amelogenin, ameloblastin, and of other enamel genes. These findings indicated that COL17 regulates ameloblast differentiation and is essential for normal formation of Tomes’ processes. In conclusion, COL17 deficiency disrupts the epithelial-mesenchymal interactions, leading to both defective ameloblast differentiation and enamel malformation.
Mesenchymal-epithelial interactions are thought to play essential roles in development of epithelial organs including the epidermis, hair follicles, and teeth. A variety of soluble factors, cell surface markers, and signal molecules have been reported to be involved in mesenchymal-epithelial interactions.1,2 The hemidesmosome is a subcellular junctional adhesion structure overlying the basement membrane between the mesenchyme and epithelial cells that binds the epithelial cells to the underlying mesenchymal tissue.3 Type XVII collagen (COL17) previously called “bullous pemphigoid antigen 2” or “BP180,” is a transmembrane glycoprotein expressed in stratified and complex epithelia, such as the skin, the mucous membrane, and the eye, where it plays a crucial role in hemidesmosome stability and epithelial-mesenchymal attachment.4
Non-Herlitz junctional epidermolysis bullosa (nH-JEB) caused by COL17 deficiency shows the abnormal tooth formation of amelogenesis imperfecta.5,6,7 We therefore hypothesized that COL17 in hemidesmosomes also plays an important role in mesenchymal-epithelial interactions in tooth formation.
Enamel formation is easily disrupted and enamel defects may reflect more than just genetic abnormalities. Enamel defects can also be attributed to environmental factors that cause chronological hypoplasia of the enamel during the enamel formation period.7 It is important to study the pathomechanisms of enamel malformation in mice with defects in hemidesmosome components. There are several model mice with epithelial mesenchymal junction (EMJ) component deficiencies.3,8 Among them, only laminin332-deficient mice are expected to have tooth malformation. However, the laminin332 knockout mice are lethal in their early development and tooth abnormality in adult mice has not been examined sufficiently.8
The Col17 knockout (Col17−/−) mice that we established recently are not lethal at birth; thus, we can use them to investigate the pathomechanisms of enamel defects that arise from hemidesmosome component deficiency.
To clarify the roles of COL17 in tooth formation, we studied the detailed process of tooth formation in Col17 knockout (Col17−/−) mice, which we recently established.9 We show that COL17 has a critical role in tooth formation, especially in the differentiation of ameloblasts and enamelization, suggesting the importance of junction structure in mesenchymal-epithelial interaction during tooth formation.
Materials and Methods
Generation of Col17−/− Mice and Rescued COL17-Humanized Mice
The procedure for generating COL17−/− mice has been described.9 Briefly, we cloned a 14.7-kb mouse genomic DNA COL17 fragment from the mouse 129Sv/Ev genomic library (Stratagene, La Jolla, CA). We subcloned a 11.5-kb NheI to NotI fragment to make the targeting vector. We inserted the PGK/Neo cassette between 6-bp upstream of the ATG start codon in exon 2 and 1.2-kb downstream in intron 2. We transfected the targeting vector by electroporation into 129 Sv/Ev embryonic stem cells, then microinjected the correctly targeted embryonic stem cell line into blastocysts obtained from C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) to generate chimeric mice, which we then mated with C57BL/6J females. We crossed F1 heterozygotes with C57BL/6J for more than four generations and then intercrossed them to generate Col17−/− mice. The procedures for screening Col17−/− mice by PCR, reverse transcription (RT)-PCR, Northern and Western blotting, histology, electron microscopy, and immunofluorescence are described elsewhere.9
The phenotypic features of the Col17 knockout (Col17−/−) mice closely resembled those seen in nH-JEB (OMIM: 226650) caused by null mutations in the COL17A1 gene, as previously described.9 The Col17−/− mice had skin blisters and erosions from mild trauma. Col17−/− mice skin showed subepidermal blistering associated with a lack of COL17 and poorly formed hemidesmosomes.
Procedures for generating COL17-rescued mice have been described elsewhere.9 Briefly, we crossed transgenic mice (C57BL/6 background) expressing the squamous epithelium-specific K14 promoter and a human COL17 cDNA (Col17m+/+,COL17h+) with heterozygous Col17m+/− mice. Mice that carried both the heterozygous null mutation of Col17 and the transgene of human Col17 (Col17m+/−,COL17h+) were bred to produce rescued Col17m−/−, COL17h+ COL17-humanized mice.
The rescued mice showed almost none of the abnormal manifestations seen in the Col17−/− mice.9
Structural Analysis of Mouse Dentition
Tissue samples of mice were incubated in hot (approximately 90°C) distilled water for several minutes, and soaked in 10% Tasinase (Kyowa-hakkou, Tokyo, Japan) at 37°C for 6 hours. Incisors and first molars were taken from maxillomandibular tissue by removal of soft tissue. The teeth were carefully cleaned and were observed macroscopically. After air-drying overnight, the teeth were sputter-coated with carbon CC-40F (Meiwa-shouji, Osaka, Japan), and were observed with a Hitachi S-4000 scanning electron microscope (Hitachi Electronics, Tokyo, Japan) operated at 15 kV. For the observation of enamel rod inclination, sagittal sections of maxillary incisors were etched by a grinder for 30 seconds in 0.1N hydrochloric acid and were observed similarly.
Chemical and Mineralization Analyses
Qualitative and distributive elemental analysis was performed in sagittal sections of maxillary incisors prepared with a grinder and in the labial side of maxillary incisors with a Hitachi S-2380 scanning electron microscope (Hitachi, Tokyo, Japan) operated at 15kV and energy dispersive X-ray spectrometry (EDX).
To demonstrate the patterns of mineralization, radio transparencies of the contact microradiographs were examined as previously described.10 Maxillary incisors were dehydrated by passage through a series of ascending concentrations of ethanol solutions and embedded in polyester resin (Rigolic, Ouken Co., Tokyo, Japan). Longitudinal labio-lingual ground sections of 100-μm thickness were prepared with a rotary diamond saw (Speadrap ML521; Maruto, Tokyo, Japan) and emery papers. Microradiographs of the ground sections were recorded on Kodak SO-181 high-resolution film (Eastman Kodak, Rochester, NY) using a cabinet X-ray apparatus (CSM-2; Softex, Tokyo, Japan) at 15 kV, 4 mA for 20 minutes. The films were developed, fixed, and observed under a light microscope.
Preparation of Tissue Sections and Immunohistochemistry
Under anesthesia with ether inhalation, intracardiac perfusions for 2-week-old mice were performed with a fixative solution containing 4% paraformaldehyde in PBS, pH 7.4. Postfixation was ensured by immersion of dissected maxilla and mandible in the fixative solution overnight at 4°C.
The maxillae and mandibles with incisors were processed for histological analysis by decalcification at 4°C for up to 2 weeks in a pH 7.4 PBS solution containing 10% EDTA. After extensive washing in PBS, the samples were dehydrated in increasing concentrations of ethanol and lemosol (Wako, Osaka, Japan), and were finally embedded in paraffin. Serial longitudinal and frontal sections of the incisors of the paraffin-embedded specimens (5 μm) were processed for H&E staining.
For immunohistochemistry, neonatal mice (day-1) were sacrificed and the tissue samples were embedded in optimal cutting temperature compound (Sakura Finetechnical Co., Tokyo, Japan) for frozen sectioning. Frozen tissue sections were cut at a thickness of 6 μm sagittally until incisors were exposed, or coronally until molars were exposed. Sections were fixed with acetone for 10 minutes at −20°C, and washed in PBS, incubated with a primary antibody, anti-mouse COL17 monoclonal antibody (NC-16A, final dilution, 1:2500), at 37°C for 30 minutes. Then, the sections were incubated with a secondary antibody, fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (H+L; Jackson ImmunoResearch Laboratories, Suffolk, UK; final dilution, 1:50), at 37°C for 30 minutes, and incubated with 10 μg/ml of propidium iodide at 37°C for 10 minutes for nuclear counterstaining. Sections were observed under an Olympus Fluoview confocal laser-scanning microscope (Olympus, Tokyo, Japan).
Ultrastructural Analysis during Tooth Formation
As above, from the maxillomandibular tissue fixed with modified Karnovsky’s fixative (at a final concentration of 2% paraformaldehyde and 2.5% glutaraldehyde in 0.05 mol/L cacodylate buffer solution, pH 7.4), 2-week-old mice incisors were obtained and decalcified in 10% EDTA pH 7.4, at 4°C for 2 weeks. After decalcification, samples were postfixed in 1% osmium tetroxide at 4°C for 2 hours and stained en bloc with 1% uranyl acetate at 4°C for 20 minutes. The samples were dehydrated through a graded series of ethanol and embedded in Epon 812 (TAAB Laboratories, Berkshire, UK). Ultrathin sections were cut in the sagittal direction to include both the separated enamel organ and the dental papilla. Sections were stained with uranyl acetate and lead citrate, and observed under a Hitachi H-7000 transmission electron microscope (Hitachi, Tokyo, Japan).
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Staining
For the detection of apoptotic cells in the ameloblast layer by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, paraffin sections were processed with in situ apoptosis detection kits (Apoptag; Chemicon International, Temecula, CA).11 The number of apoptotic ameloblasts at each stage was calculated based on the criterion that an apoptotic body of more than 2 μm in diameter could be defined as a count; these numbers were compared between Col17+/+ and Col17−/−.
Cell Cultures and Immunolabeling
For dental epithelial cell cultures, maxillary and mandibular incisors from 2-week-old mice were dissected, and the distal part of the incisors was removed. Tooth samples were treated with 0.25% trypsin for 10 minutes and pipetted up and down intensely. The dental epithelial cells, dental mesenchymal cells, and various other cells were isolated from incisors. To separate dental epithelial cells from the other cells, cells were cultured in epidermal keratinocyte medium containing a small amount of bovine pituitary extract (CNT-57; CELLnTEC Advanced Cell Systems, Bern, Switzerland) for 7 days. After obtaining a sufficient number of dental progenitor epithelial cells, we changed the culture medium to epidermal keratinocyte medium containing 0.07 mmol/L calcium (CNT-02; CELLnTEC Advanced Cell Systems, Bern, Switzerland) to induce differentiation, and cultured it for 10 days.
For fluorescence staining, the cells were fixed with 70% ethanol for 10 minutes and washed with PBS. The cells were incubated with a primary antibody anti-mouse amelogenin polyclonal antibody (Hokudo, Sapporo, Japan), final dilution of 1:100 or with anti-mouse ameloblastin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), final dilution, 1:50, at 37°C for 30 minutes. Then, the cells were incubated with the secondary antibody FITC-conjugated goat anti-rabbit IgG (H+L; Jackson ImmunoResearch Laboratory, West Grove, PA), final dilution, 1:50, or with FITC-conjugated donkey anti-goat IgG (H+L; Jackson ImmunoResearch Laboratory, West Grove, PA), final dilution, 1:50, at 37°C for 30 minutes and incubated with 10 μg/ml of propidium iodide at 37°C for 10 minutes to visualize the nucleus. The cells were observed under an Olympus FluoView confocal laser-scanning microscope (Olympus, Tokyo, Japan).
RT-PCR Analysis
To study Col17 mRNA expression in dental epithelial cells and ameloblasts, total RNA from incisors or cultured dental epithelial cells was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Extracted RNA was used for cDNA synthesis in SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The following primers specific for mouse Col17 sequence (NM: 007732) were used for RT-PCR: 5′-AGAAGAAAA GCATCCGAGGG-3′ (RT-F); and 5′-TGGTTGAAGAAGAGGCGAGT-3′ (RT-B). As a control, we used the primers for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH; NM: 001001303): 5′-TTAGCCCCCCTGGCCAAGG-3′ (mGAPDH-F) and 5′-CTTACTCCTTGGAGGCCATG-3′ (mGAPDH-B), which amplified a 541-bp fragment.
Real-Time RT-PCR Analysis
To quantitatively analyze mRNA expression levels of tooth-formation-associated proteins, amelogenin, ameloblastin, enamelin, tuftelin, enamelysin, and dentin sialophosphoprotein (DSPP), in teeth from the Col17+/+ and Col17−/− mice, cDNA samples were analyzed using the ABI prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers and probes specific for amelogenin, ameloblastin, enamelin, tuftelin, enamelysin, DSPP, and control housekeeping genes, GAPDH and β-actin, were obtained from the TaqMan gene expression assay (Applied Biosystems, Foster City, CA; Probe ID; Mm00711644_g1, Mm00477485_m1, Mm00516922_m1, Mm00449139_m1, Mm00600244_m1 and Mm00515666_m1, Mm99999915_gl, Mm00607939_sl).
Differences between the mean CT values of mRNA expressions of tooth-formation-associated proteins and those of GAPDH or ß-actin were calculated as ▵CT Col17−/− mice = CTtooth protein − CTGAPDH (or other housekeeping genes) and those of ▵CT for the Col17+/+ incisors as CTcalibrator = CTtooth protein − CTGAPDH (or other housekeeping genes). Final results for Col17−/− incisor samples/Col17+/+ incisor samples (%) were determined by 2−(CT Col17−/− − CTcalibrator).
Using similar methods, we quantitatively analyzed the tooth-formation-associated protein mRNA expression levels in the dental epithelial cells cultured from the Col17+/+ and Col17−/− mice.
Results
COL17 Expression Pattern in the EMJ of Teeth in Col17−/− Mice
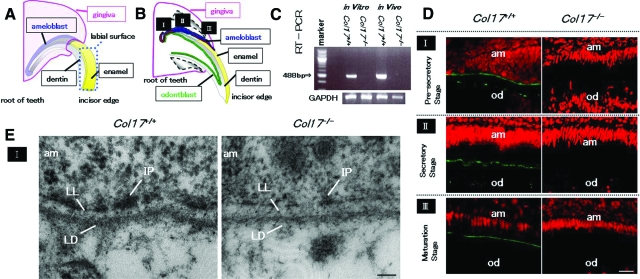
We observed the expression of Col17 at each of the three stages of enamel formation: pre-secretory, secretory, and maturation (Figure 1, A and B). The 488-bp fragments of mouse Col17 mRNA were detected in Col17+/+ mouse incisors in vivo and in cells cultured from Col17+/+ mouse incisors in vitro, although mouse Col17 mRNA was detected in neither incisors nor cultured cells from Col17−/− mice (Figure 1C).
Figure 1.
COL17 expression in the tooth of Col17+/+ mice and COL17 absence in the tooth of Col17−/− mice. A, B: Mouse incisors are continuously elongating teeth. In the root of these incisors, ameloblasts (blue) and odontoblasts (green) secrete enamel matrix and dentin, respectively, during the secretory stage (II). I: the pre-secretory stage; II: the secretory stage; III: the maturation stage. C: A RT-PCR assay revealed that Col17 mRNA (488 bp band) was expressed in cultured ameloblasts from Col17+/+ mice (left lane) and Col17+/+ mouse teeth (second right). Col17 mRNA was not expressed in cultured ameloblasts from Col17−/− mice (second left lane) or Col17−/− mouse teeth (right hand lane). D: Immunofluorescence staining for COL17 (green) revealed that COL17 was expressed in the EMJ between ameloblasts and odontoblasts at the pre-secretory stage of a Col17+/+ mouse (upper, left), between ameloblasts and enamel matrix in the secretory stage (middle, left) and in the maturation stage (lower, left) of a Col17+/+ mouse. At the secretory stage, COL17 expression was weak, intermittent, or absent. In Col17−/− mice, no COL17 staining was observed in the EMJ at any stage (right column). am: ameloblast; od: odontblast. Scale bar = 20 μm. E: Ultrastructural features of the basement membrane zone at the pre-secretory stage. Normal hemidesmosomes were seen in the Col17+/+ mouse (left), but hypoplastic, malformed hemidesmosomes were observed in the Col17−/− mice (right). am: ameloblast; LL: lamina lucida; IP: inner attachment plaques; LD :lamina densa. Scale bar = 60 nm.
To clarify COL17 expression during tooth formation, we immunostained tissue sections of maxillary incisors in which we could observe all differentiation stages of tooth formation. COL17 was expressed in the EMJ between ameloblasts and odontoblasts at the pre-secretory stage. Due to elongation of Tomes’ processes, the basement membrane became discontinuous and COL17 expression was reduced and in places became intermittent at the secretory stage. COL17 expression reappeared at the maturation stage (Figure 1D).
In the Col17−/− mice, COL17 expression was not observed in the EMJ under the ameloblasts at any stage during tooth development.
The basement membrane on the basal surface of the ameloblasts separates the ameloblasts from mesenchymal tissue/pre-odontoblasts. Hemidesmosomes are observed in the EMJ, and they are composed of prominent inner plaques, outer plaques, and sub-basal dense plates, similar to those in the dermo-epidermal junction in the skin. Anchoring filaments cross the lamina lucida, and anchoring fibrils anchor lamina densa to the mesenchymal tissue in the Col17+/+ mice (Figure 1E).
In the Col17−/− mice, there were a reduced number of hypoplastic inner and outer hemidesmosomal attachment plaques with poor keratin filament association and less prominent anchoring filaments, whereas anchoring fibrils and the lamina densa were both normally preserved (Figure 1E).
Dental Phenotype in the Col17−/− Mice
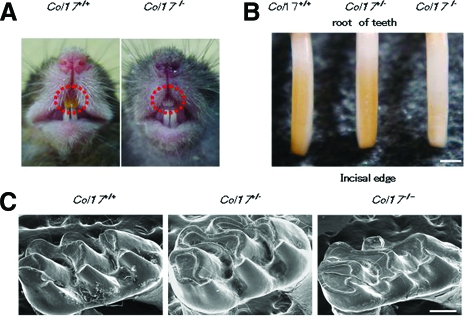
The incisors of wild-type (Col17+/+) and heterozygous (Col17+/−) mice exhibit yellow pigmentation on the surface. The incisors of the Col17−/− mice had a chalky, whitish appearance (Figure 2, A and B). The Col17-rescued mice (mouse Col17−/−, human COL17+/+) had yellowish incisors, as did the wild-type Col17+/+ mice (data not shown). By scanning electron microscopy, the enamel surface of the Col17+/+, Col17+/− and Col17−/− mice appeared smooth and unpitted (data not shown). Molar wear was more advanced in the Col17−/− mice than in the Col17+/+ and Col17+/− mice. This tooth wear became more severe with age, although it failed to extend to loosen the molar crown (Figure 2C). In sagittal sections of the Col17−/− mice maxillary incisors, the enamel rod inclination was irregularly oriented and disrupted and had lost its normal network arrangement seen in that of Col17+/+ and Col17+/− mice (Figure 3 A−D, F−H). In the COL17-rescued mouse Col17−/−, human COL17+/+ mice, the maxillary incisors showed normal enamel rod formation (Figure 3, E and I) confirming that the enamel changes were caused by a Col17 deficiency.
Figure 2.
Dental phenotype of Col17−/− mice. A: At 4 weeks of age, a Col17−/− mouse (right) had whitish incisors. B: Incisors from Col17+/+ and Col17+/− mice showed yellowish color, although an incisor from a Col17−/− mouse seemed whitish (right). Scale bar = 500 μm. C: In the molars, tooth wear was more advanced for the Col17−/− mice (right) than for the Col17+/+ (left) and Col17+/− (center) mice. Scale bar = 250 μm.
Figure 3.
Scanning electron microscopy of the sagittal section of maxillary incisors. A: A model of an upper incisor. The enamel layer indicated by an upper blue rectangle and by a lower red rectangle is enlarged in B, C, D and E, and in F, G, H and I, respectively. In the Col17−/− mouse, irregular inclinations of enamel rods without a normal network arrangement are observed (D, H), in contrast to the regular network of enamel rods observed in the Col17+/+ incisor (B, F) and in the Col17+/− incisor (C, G). The normal, regular network of enamel rods has been restored in the COL17 humanized mouse (E, I). en: enamel; de: dentin. Scale bar = 20 μm.
Chemical and Mineralization Analysis of the Teeth
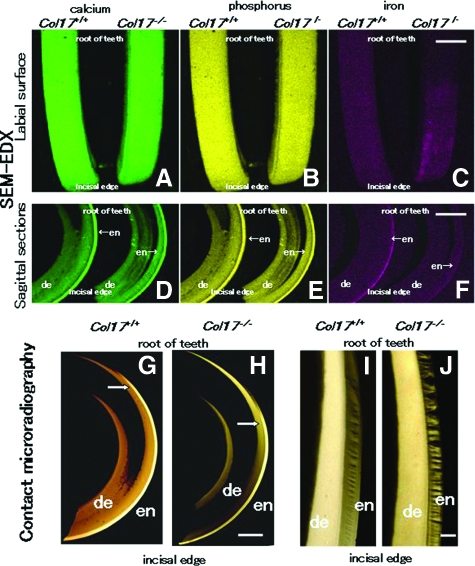
Backscatter electron images of the labial surface and the sagittal sections of the maxillary incisors in the Col17+/+, Col17+/−, and Col17−/− mice revealed that calcium and phosphorus were homogeneously distributed from incisal edge to apical root in all samples (Figure 4A, B, D, E). In the Col17+/+ and Col17+/− mice, iron was lightly but uniformly distributed from incisal edge to the middle of teeth, and the density corresponded with the yellow pigmentation. In the Col17−/− mice, iron was irregularly distributed (Figure 4, C and F).
Figure 4.
Difference in enamel formation between Col17+/+ and Col17−/− mice incisors. The labial surface (see Figure 1A) is featured in A, B, and C. A sagittal section is shown in D, E, and F. A, B: The labial surface of the maxillary incisors in both Col17+/+ (left) and Col17−/− (right) mice was scanned for calcium (green) and phosphorus (yellow) with EDX spectrometry. No obvious difference was observed in elemental distribution mapping. C: The same surfaces scanned in EDX for iron (red). In Col17−/− mice (right), the distribution of iron was irregular, compared with that of a Col17+/+ mice (left). Scale bar: (A, B, C) = 500 μm. D, E, F: The sagittal sections of the maxillary incisors of both Col17+/+ (left) and Col17−/− (right) mice scanned in EDX for calcium (green), phosphorus (yellow), and iron (red). No obvious difference is observed in the distribution of calcium or phosphorus between the Col17+/+ (left) and Col17−/− (right) mice. In the Col17−/− mice (right), the iron concentration in the enamel is lower than that in the Col17+/+ mouse (left; F). en: enamel; de: dentin. Scale bars in (D, E, F) = 1000 μm. G, H: Microradiographs of maxillary incisors in Col17+/+ (G) and Col17−/− (H) mice. The position (arrows) where sufficient mineralization occurred in the enamel judged from the low radio-opacity signal, moved toward the incisal edge in maxillary incisors of a Col17−/− mouse (G), compared with that in incisors of a Col17+/+ mouse (H). en: enamel; de: dentin. Scale bar: (G, H) = 500 μm. I, J: Microradiographs showing the mineralization pattern of the developing enamel at the maturation stage from Col17+/+ (I) and Col17−/− (J) mice. As compared with Col17+/+ (I), the mineralization demonstrated by radio-opacity of the enamel was irregular in both stages in Col17−/− mice (J), although there were no differences in the radio-opacity of dentine between Col17+/+ (I) and Col17−/− (J) mice. en: enamel; de: dentin. Scale bar: (I, J) = 100 μm.
To compare the mineralization patterns of teeth between the Col17+/+ and Col17−/− mice, radio transparencies of the microradiographs were examined in maxillary incisors. The radio-opacity of enamel decreased gradually toward the incisal edge, from the enamel secretory stage to the maturation stage. Mineralization reached its maximum during the late maturation stage (Figure 4G). To objectively evaluate the mineralization level in the enamel layers, we set up a marker-point for enamel matrix sufficiently completed mineralization using image analysis. The point exhibited 90% or more saturation levels in the completely mineralized incisal edge-side of enamel layer. We then assessed each image for the area that showed this or higher saturation signals. The mineralization marker-points that we defined were at 1.7 mm and 2.7 mm from the incisor root in the Col17+/+ mice and Col17−/− mice, respectively (Figure 4H). These findings indicated that, in the Col17−/− incisors, mineralization of enamel was delayed by 1.0 mm toward the incisal edge compared with that of the Col17+/+ incisors. Mineralization of the enamel matrix, at the maturation stage, was irregular and discontinuous in the Col17−/− mice (Figure 4I) compared with the Col17+/+ mice (Figure 4J).
Defective Amelogenesis in Col17−/− Mice
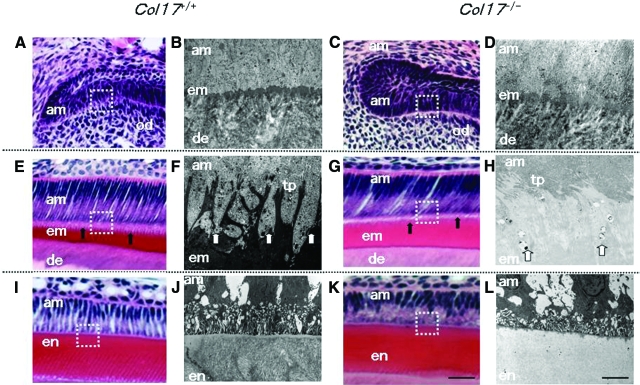
Ameloblast size and the enamel matrix thickness in the Col17−/− mice were similar to those in the Col17+/+ mice. The Tomes’ processes of the Col17+/+ mice were triangular and arranged in order. However, the processes of the Col17−/− mice were deformed and difficult to clearly visualize in H&E-stained sections (Figure 5A, C, E, G, I, K).
Figure 5.
Malformed Tomes’ processes and defective amelogenesis in Col17−/− mice A–D: At the pre-secretory and early secretory stages, the EMJ separates pre-ameloblasts and pre-odontoblasts. A, C: The overall structures of pre-ameloblasts and pre-odontoblasts were similar in the Col17+/+ (A) and Col17−/− (C) mice in the pre-secretory to the early secretory stages at the light microscopic level. B, D: Ultrastructurally, from the pre-secretory to the early secretory stages, the basement membrane between ameloblasts and odontoblasts was blurred in the Col17−/− mouse (D), compared with more obvious, intact basement membrane structures in a Col17+/+ mouse (B). E–H: At the secretory stage, Tomes’ processes are formed and enamel matrix is produced by ameloblasts. E, G: In the secretory stage, the processes of ameloblasts were malformed and blurred (arrows) in the Col17−/− mouse (G), compared with well-organized lattice-like structures of the Tomes’ processes (arrows) in the Col17+/+ mice (E). The thickness of the enamel matrix seemed similar both in Col17+/+ (E) and Col17−/− (G) mice. At the secretory stage, Tomes’ processes were apparently hypoplastic in the Col17−/− mouse (H), compared with normal Tomes’ processes in the Col17+/+ mouse (F). I–L: In the maturation stage, disruption of the processes of ameloblasts (am) was more advanced in the Col17−/− mouse (K), compared with regular processes in the Col17+/+ mice (I). At the maturation stage, the electron density of the enamel matrix is remarkably lower in the Col17−/− mouse (L) than that in the Col17+/+ mouse (J). In addition, enamel rod structures are blurred in the enamel matrix of the Col17−/− mouse (L). am: ameloblast; em: enamel matrix; en: enamel; de: dentin; od: odontoblast; tp: Tomes’ processes. Scale bars: (A, C, E, G, I, K) = 30 μm; (B, D, F, H, J, L) = 3 μm.
Furthermore, we observed enamel formation of the incisors of the Col17+/+, Col17+/−, and Col17−/− mice ultrastructurally. Secretory ameloblasts were tall columnar cells with intact Tomes’ processes producing enamel matrix in the Col17+/+ and Col17+/− mice (Figure 5, B and D).
In the Col17−/− mice, the Tomes’ processes were thin, fragmented and disorganized, showing a wavy, villous appearance. There was no obvious difference in the other structural components of the ameloblasts (Figure 5, F and H).
Mature ameloblasts were columnar cells and could be divided into ruffle-based ameloblasts and smooth-ended ameloblasts by the presence of a ruffled border. Rough endoplasmic reticulum, lysosomes, mitochondria, small vacuoles and Golgi apparatus were seen in the apical and mid portions of mature ameloblasts. The cell structure and organelles of Col17−/− mature ameloblasts appeared normal, but the enamel rods were malformed and irregularly distributed. The electron density of the enamel matrix was remarkably low during the secretory and maturation stages in the Col17−/− mice, compared with the high electron density of the enamel matrix in the Col17+/+ and Col17+/− mice (Figure 5, J and L).
Assay of Ameloblast Proliferation and Differentiation
Colony-forming analysis revealed there was no significant difference in colony-forming ability of cultured ameloblasts between the Col17+/+ and Col17−/− mice (data not shown). As for apoptosis, TUNEL staining did not reveal excessive apoptosis of ameloblasts at the pre-secretory to secretory stages in either the Col17+/+ or the Col17−/− mice (data not shown).
TUNEL assays showed that some apoptotic cells appeared from the late secretory stage to the early maturation stage (called the “transitional stage”) of the Col17+/+ and Col17−/− mice (data not shown). However, there was no significant difference in the number of TUNEL-positive cells between Col17+/+ and Col17−/− mice; in the numbers of apoptotic ameloblasts per sagittal incisor section, 7.5 ± 0.7 cells/sagittal section in Col17+/+ incisors and 7.0 ± 1.0 cells/sagittal section in Col17−/− incisors.
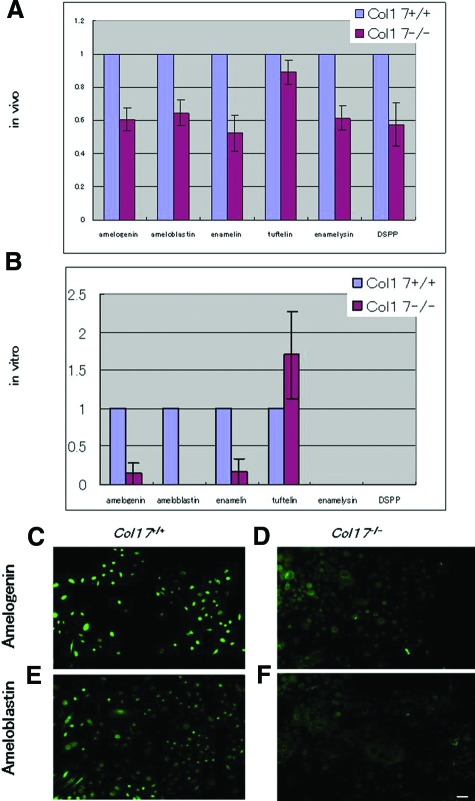
We examined the expression of enamel proteins in the incisors in vivo and in cultured dental epithelial cells in vitro using real-time RT-PCR analysis.12,13,14 mRNA expression of the major enamel proteins produced by ameloblasts, including amelogenin, ameloblastin, enamelin, enamelysin, and DSPP, was significantly decreased in the Col17−/− incisors, except for the expression of tuftelin (Figure 6A). Tuftelin expression was only slightly reduced in Col17−/− mice incisors. In dental epithelial cells cultured from the Col17+/+ mice, mRNA expression of amelogenin, ameloblastin, enamelin, and tuftelin was confirmed, although mRNA expression of enamelysin and DSPP was absent. In the Col17−/− mice, mRNA expression of amelogenin, ameloblastin and enamelin in cultured cells were remarkably lower than in the Col17+/+ mice. Tuftelin expression was higher than that in the cells cultured from the Col17+/+ mice (Figure 6B). Immunocytologically, strong expression of amelogenin and ameloblastin was seen in the ameloblasts cultured from the Col17+/+ mice, although expression of both proteins was remarkably weak in cells cultured from the Col17−/− mice (Figure 6, C−F).
Figure 6.
Expression of enamel proteins in Col17−/− ameloblasts. A: mRNA expression of all of the enamel proteins examined (amelogenin, ameloblastin, enamelin, tuftelin, enamelysin, and DSPP) was down-regulated in ameloblasts of incisors of the Col17−/−mice in vivo. B: In vitro ameloblasts cultured from incisors of the Col17−/− mice showed down-regulated mRNA expression of amelogenin, ameloblastin and enamelin, although tuftelin expression was up-regulated relative to tuftelin expression of the cultured ameloblasts from the Col17+/+ mice. Neither enamelysis nor DSPP was expressed in ameloblasts cultured from the Col17+/+ and Col17−/− mice. C: Protein expression (FITC, green) of amelogenin and ameloblastin was decreased in ameloblasts cultured from the Col17−/− mice (D, F), relative to that in ameloblasts cultured from the Col17+/+ mice (C, E). (C, D) amelogenin staining; (E, F) ameloblastin staining; (C, E) cells from Col17+/+ mice; (D, F) cells from Col17−/− mice. Scale bar = 20 μm.
Discussion
nH-JEB is a hereditary blistering skin disease with tissue separation occurring within the lamina lucida of the epidermal basement membrane zone. nH-JEB is characterized by generalized blistering, alopecia, reduced axillary and public hair, dystrophic nails, and dental abnormalities.4,15 Molecular genetic studies revealed that nH-JEB is caused by mutations in the genes encoding COL17 or laminin 332.16 Most nH-JEB patients exhibit enamel hypoplasia, and pitting and coarsening of the tooth surface enamel.6,7
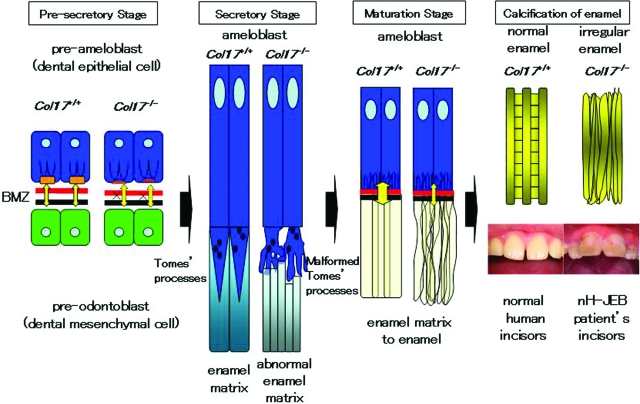
The present study revealed that the secretory ameloblasts of the Col17−/− mice lacked Tomes’ processes and exhibited disturbed enamel matrix secretion, which resulted in imperfect amelogenesis demonstrated by malformed enamel rods and irregular enamel matrix (Figure 7).
Figure 7.
Schemes of normal enamel formation in Col17+/+ mice and defective enamel formation in Col17−/− mice. In the Col17+/+ incisors (left), normal enamel matrix is formed by Tomes’ processes, resulting in intact enamel formation. In the Col17−/− incisors (right), disruptive Tomes’ processes produce disturbed enamel matrix, leading to irregular enamel formation.
Mice only have one set of dentition whereas the human disease nH-JEB affects both primary and secondary dentition. Due to these differences, the tooth abnormalities demonstrated in Col17−/− mice are unlikely to be pathophysiologically relevant to the nH-JEB human disease. However, the physiological processes of enamel formation are identical both in human and mouse dentition.17,18 Thus, we believe that the present Col17−/− mice are a practical and useful model in which to study nH-JEB dental abnormalities.
We studied the developmental processes of the teeth in Col17−/− mice. The teeth develop through the pre-secretory, secretory, and maturation stages.19 At pre-secretory stage, hypoplasia of hemidesmosomes is the only apparent abnormality in Col17−/− mice teeth. In ameloblasts in the secretory stage, disturbed Tomes’ process formation was observed in the Col17−/− mice, although enamel matrix was seen around the disrupted Tomes’ processes. The Tomes’ processes are known to be involved in the secretion of enamel matrix.19
Ameloblasts at the maturation stage showed no apparent abnormality, although the crystal structure of the enamel matrix was disturbed in the Col17−/− mice. Scanning electron microscopy revealed that enamel rods were malformed and irregular in the enamel matrix of the Col17−/− mice. These morphological abnormalities were not observed in the rescued COL17-humanized mice and thus it was confirmed that the abnormalities were direct effects of the COL17 deficiency.
Contact microradiography demonstrated that enamelization of the enamel matrix and calcification were delayed in the Col17−/− mice. In addition, reduced iron deposition was revealed in the enamel of Col17−/− incisors from their whitish color and scanning electron microscopy-EDX findings. Iron deposition is known to occur according to the maturation of enamel matrix and mineralization. Thus, reduced iron deposition in the Col17−/− mouse incisors suggests defects in enamel maturation and/or mineralization. These results clearly indicate that tooth malformation (amelogenesis imperfecta) in Col17−/− mice and probably in COL17-deficient nH-JEB patients is caused by aberrant differentiation of ameloblasts. These abnormal ameloblasts lacked Tomes’ processes and secreted reduced amounts of enamel matrix irregularly, resulting in disturbed enamel matrix, irregular enamelization and calcification (Figure 7).
We ultrastructurally examined teeth from an adult patient with nH-JEB due to COL17 deficiency using scanning electron microscopy. Enamel rods were malformed and the enamel rod inclination was irregularly oriented and disrupted in the enamel layer of the patient’s teeth (data not shown). These abnormalities are most likely a consequence of a lack of COL17 causing aberrant ameloblast differentiation, similar to the Col17−/− mice, although we cannot completely exclude the possibility that the morphological changes in the nH-JEB patient’s teeth were non-specific abnormalities caused by secondary bacterial infection, etc.
It is reported that heterozygous carriers of glycine substitutions in COL17A1 show dental abnormalities,7,20 although such dominant negative mutations in COL17A1 fail to manifest with a blistering skin phenotype.20 It is considered that abnormal dentition in the heterozygous carriers is a direct result of dominantly inherited glycine substitutions in COL17A1 with dominant interference between the wild-type and mutant protein causing ameloblast dysfunction and disruption of enamel deposition.20 In addition, dental abnormalities were seen both in individuals heterozygous for a COL17A1 nonsense mutation p.Arg1226X21 and in heterozygous carriers of a COL17A1 deletion mutation c.823delA.7 By contrast, in the present study, Col17+/− mice showed no apparent tooth abnormality, probably because the critically disruptive Col17 allele carried by the mice had no dominant negative effect against wild-type COL17 protein.
Ameloblasts cultured without interaction with mesenchymal tissue cannot differentiate sufficiently to form columnar epithelium.22 Such insufficiently differentiated ameloblasts express tuftelin, but not other enamel proteins, including amelogenin and ameloblastin.
Ameloblasts in Col17−/− mice express tuftelin to an extent similar to that of Col17+/+ mice, Col17−/− ameloblasts express reduced amounts of amelogenin and ameloblastin. Tuftelin is known to be expressed by epithelial cells at a very early stage (the pre-secretory ameloblast stage) of odontogenesis,23,24 although other major enamel proteins are expressed at the secretory stage.25 Thus, the results of the present enamel protein expression study further support the idea that ameloblast differentiation from the pre-secretory stage to the secretory stage is disturbed in Col17−/− mice.
In the Col17−/− mice, ameloblast differentiation was retarded, resulting in malformation of Tomes’ processes. The present results in Col17−/− mice clearly demonstrated that COL17, a component of the hemidesmosome involved in basement membrane adhesion, also regulates differentiation of odontogenic epithelial cells in ameloblasts and plays and essential role in enamelization.
Laminin 332 is known to be an important component of hemidesmosomes and another causative molecule underlying the JEB phenotype. Remarkable abnormalities, including disturbance of ameloblast differentiation and reduced enamel deposition, have also been reported in the incisors of laminin 332-disrupted mice.8 These facts further support the idea that interactions between ameloblasts and mesenchymal tissue via hemidesmosomes are crucial for ameloblast differentiation and function.26,27 Ultrastructural changes of Tomes’ processes were not described in laminin 332-disrupted LAMA3−/− mice. However, the reduced size of secretory ameloblasts reported in LAMA3−/− mice suggest absence or hypoplasia of Tomes’ processes in LAMA3−/− mice, similar to that observed in Col17−/− mice. During the maturation stage, tissue organization was completely disrupted in the enamel epithelium of LAMA3−/− mice,8 but not of Col17−/− mice. These findings suggest that a lack of COL17 and a lack of laminin 332 have similar negative effects on ameloblast differentiation and enamel formation, although laminin 332 deficiency appears to have more severe disruptive effects on enamel epithelium, compared with COL17 deficiency.
Our results show that disruption of the Col17 gene leads to abnormal interaction between enamel epithelium and the underlying mesenchyme via the EMJ, resulting in defective ameloblast differentiation. Consequently, the Col17−/− mice exhibit ameloblasts with malformed Tomes’ processes and the secretion of enamel matrix was diminished at the secretary stage. At the maturation stage, the Col17−/− mice show delayed calcification and reduced iron deposition in the enamel. We consider that these mechanisms contribute to the immature and irregular enamel formation seen in Col17−/− mice. In conclusion, epithelial-mesenchymal interactions via the EMJ are important for tooth morphogenesis, and hemidesmosome components are thought to regulate the proliferation and differentiation of tooth forming cells including ameloblasts.
Acknowledgments
We thank Prof. James R. McMillan and Dr. Heather A. Long for their revisions and comments and Dr. Yoshinobu Nodasaka, Mr. Yoshiyuki Honma, and Ms. Kaori Sakai for their fine technical assistance on this project.
Footnotes
Address reprint requests to Masashi Akiyama, M.D., Ph.D., Department of Dermatology, Hokkaido University Graduate School of Medicine, North 15 West 7, Kita-ku, Sapporo 060-8638, Japan. E-mail: akiyama@med.hokudai.ac.jp.
Supported in part by Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan to M. Akiyama (Kiban 20390304).
References
- Maas R, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol Med. 1997;8:4–39. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Gatalica B, Christiano AM, Li K, Owaribe K, McMillan JR, Eady RA, Uitto J. Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat Genet. 1995;11:83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- Kirkham J, Robinson C, Strafford SM, Shore RC, Bonass WA, Brookes SJ, Wright JT. The chemical composition of tooth enamel in recessive dystrophic epidermolysis bullosa: significance with respect to dental caries. J Dent Res. 1996;75:1672–1678. doi: 10.1177/00220345960750090901. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Sawamura D, Goto M, Nakamura H, Kida M, Ariga T, Sakiyama Y, Tomizawa K, Mitsui H, Tamaki K, Shimizu H. Analysis of the COL17A1 in non-Herlitz junctional epidermolysis bullosa and amelogenesis imperfecta. Int J Mol Med. 2006;18:333–337. [PubMed] [Google Scholar]
- Murrell DF, Pasmooij AM, Pas HH, Marr P, Klingberg S, Pfendner E, Uitto J, Sadowski S, Collins F, Widmer R, Jonkman MF. Retrospective diagnosis of fatal BP180-deficient non-Herlitz junctional epidermolysis bullosa suggested by immunofluorescence (IF) antigen-mapping of parental carriers bearing enamel defects. J Invest Dermatol. 2007;127:1772–1775. doi: 10.1038/sj.jid.5700766. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Lee K, Miyashita Y, Carter WG. Targeted disruption of the LAMA3 gene in mice reveals abnormalities in survival and late stage differentiation of epithelial cells. J Cell Biol. 1999;145:1309–1323. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan J, Sakai K, Nakamura H, Olasz E, Yancey K, Akiyama M, Shimizu H. Humanization of autoantigen. Nat Med. 2007;13:378–383. doi: 10.1038/nm1496. in-90. [DOI] [PubMed] [Google Scholar]
- Tung K, Fujita H, Yamashita Y, Takagi Y. Effect of turpentine-induced feverduring the enamel formation of rat incisor. Arch Oral Biol. 2006;51:464–470. doi: 10.1016/j.archoralbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Osawa M, Kenmotsu S, Masuyama T, Taniguchi K, Uchida T, Saito C, Ohshima H. Rat wct mutation induces a hypo-mineralization form of amelogenesis imperfecta and cyst formation in molar teeth. Cell Tissue Res. 2007;330:97–109. doi: 10.1007/s00441-007-0452-0. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Yamada A, Nonaka K, Yamada Y. Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation. Cells Tissues Organs. 2005;181:189–195. doi: 10.1159/000091380. [DOI] [PubMed] [Google Scholar]
- Masuya H, Shimizu K, Sezutsu H, Sakuraba Y, Nagano J, Shimizu A, Fujimoto N, Kawai A, Miura I, Kaneda H, Kobayashi K, Ishijima J, Maeda T, Gondo Y, Noda T, Wakana S, Shiroishi T. Enamelin (Enam) is essential for amelogenesis: eNU-induced mouse mutants as models for different clinical subtypes of human amelogenesis imperfecta (AI). Hum Mol Genet. 2005;14:575–583. doi: 10.1093/hmg/ddi054. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, de Jong MC, Heeres K, Pas HH, van der Meer JB, Owaribe K, Martinez de Velasco AM, Niessen CM, Sonnenberg A. 180-kD bullous pemphigoid antigen (BP180) is deficient in generalized atrophic benign epidermolysis bullosa. J Clin Invest. 1995;95:1345–1352. doi: 10.1172/JCI117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki R, Sadowski S, Pfendner E, Uitto J. Epidermolysis bullosa. I. Molecular genetics of the junctional and hemidesmosomal variants. J Med Genet. 2006;43:641–652. doi: 10.1136/jmg.2005.039685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich I, Sharpe PT. Normal and abnormal dental development. Hum Mol Genet. 2003;12:R69–R73. doi: 10.1093/hmg/ddg085. [DOI] [PubMed] [Google Scholar]
- Fleischmannova J, Matalova E, Tucker AS, Sharpe PT. Mouse models of tooth abnormalities. Eur J Oral Sci. 2008;116:1–10. doi: 10.1111/j.1600-0722.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Gatalica B, Li K, Dunnill MG, McMillan JR, Christiano AM, Eady RA, Uitto J. Compound heterozygosity for a dominant glycine substitution and a recessive internal duplication mutation in the type XVII collagen gene results in junctional epidermolysis bullosa and abnormal dentition. Am J Pathol. 1996;148:1787–1796. [PMC free article] [PubMed] [Google Scholar]
- Floeth M, Bruckner-Tuderman L. Digenic junctional epidermolysis bullosa: mutations in COL17A1 and LAMB3 genes. Am J Hum Genet. 1999;65:1530–1537. doi: 10.1086/302672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi T, Kawano S, Toyono T, Kitamura C, Terashita M, Uchida T, Toyoshima K, Harada H. In vitro differentiation of dental epithelial progenitor cells through epithelial-mesenchymal interactions. Arch Oral Biol. 2005;50:695–705. doi: 10.1016/j.archoralbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Deutsch D, Leiser Y, Shay B, Fermon E, Taylor A, Rosenfeld E, Dafni L, Charuvi K, Cohen Y, Haze A, Fuks A, Mao Z. The human tuftelin gene and the expression of tuftelin in mineralizing and nonmineralizing tissues. Connect Tissue Res. 2002;43:425–434. doi: 10.1080/03008200290001186. [DOI] [PubMed] [Google Scholar]
- Leiser Y, Blumenfeld A, Haze A, Dafni L, Taylor AL, Rosenfeld E, Fermon E, Gruenbaum-Cohen Y, Shay B, Deutsch D. Localization, quantification, and characterization of tuftelin in soft tissues. Anat Rec. 2007;290:449–454. doi: 10.1002/ar.20512. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamada Y. Extracellular matrix regulates tooth morphogenesis. Connect Tissue Res. 2005;46:220–226. doi: 10.1080/03008200500344017. [DOI] [PubMed] [Google Scholar]
- Yoshiba K, Yoshiba N, Aberdam D, Meneguzzi G, Perrin-Schmitt F, Stoetzel C, Ruch JV, Lesot H. Expression and localization of laminin-5 subunits during mouse tooth development. Dev Dyn. 1998;211:164–176. doi: 10.1002/(SICI)1097-0177(199802)211:2<164::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, Hoffman MP, Yamada Y. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J Biol Chem. 2006;24;281:5008–5016. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]