Abstract
Nicotinamide dinucleotide phosphate oxidase-deficient (p47phox−/−) mice are a model of human chronic granulomatous disease; these mice are prone to develop systemic infections and inflammatory diseases. The use of antibiotic (Bactrim) prophylaxis in a specific pathogen-free environment, however, impedes infection in the majority of p47phox−/− mice. We examined infection-free p47phox−/− mice between 1 and 14 months of age and found that they developed proliferative macrophage lesions containing Ym1/Ym2 protein and crystals in lung, bone marrow, lymph nodes, and spleen. Here, we show that the lung lesions progressed from single macrophages with intracellular Ym1/Ym2 protein crystals to severe diffuse crystalline macrophage pneumonia without histological evidence of either granulation tissue or pulmonary fibrosis. Ym1/Ym2 is a chitinase-like secretory protein that is transiently induced in alternatively activated macrophages during T-helper (Th)2-biased pathogenesis and during chemical and traumatic inflammation. Bronchoalveolar lavage from p47phox−/− mice contained significantly higher levels of Th-1 (interferon-γ), Th-2 (interleukin-4), and Th-17 (interleukin-17)-associated cytokines than wild-type mice, as well as copious amounts of interleukin-12, indicating that Ym1-secreting p47phox−/− macrophages are also integrated into classically activated macrophage responses. These results suggest that p47phox−/− macrophages are extremely pliable, due in part to an intrinsic dysfunction of macrophage activation pathways that allows for distinct classical or alternative activation phenotypes.
Macrophages are adaptable cells that are skewed toward distinct activation phenotypes in response to environmental stimuli. Responsiveness to varied endogenous and exogenous stimuli drives macrophage heterogeneity toward classical or alternative activation. Classically activated macrophages are responsive to inflammatory stimuli such as lipopolysaccharide (LPS) and T helper (Th)-1 cytokines such as interferon-gamma (IFN-γ).1 Classically activated macrophages produce reactive oxygen species and pro-inflammatory cytokines and are also bacteriocidal and tumoricidal.2 Alternatively activated macrophages are responsive to Th-2 cytokines, such as interleukin (IL)-4 or IL-13 and to Th-2-mediated infection and allergy.3 Alternatively activated macrophages produce anti-inflammatory cytokines and metabolic factors such as arginase to block inducible nitric oxide synthase.3 In contrast to classically activated macrophages, alternatively activated macrophages drive toward the resolution of inflammation, tissue repair, and angiogenic remodeling.3,4
We studied the pathology of p47phox (Ncf1) deficient (p47phox−/−) mice, which are deficient in nicotinamide dinucleotide phosphate (NADPH) oxidase,5 and present a description of spontaneous sequential age-related changes in these mice. p47phox−/− mice are a model for human chronic granulomatous disease (CGD) and are prone to develop systemic bacterial and fungal infections, as well as acute and chronic inflammatory diseases.6,7 However, with the use of antibiotic prophylaxis in a specific pathogen-free environment, the majority of p47phox−/− mice remain infection free for a significantly longer period than mice not on antibiotics. Sixty-four male and female p47phox−/− mice on antibiotics were sacrificed at various time points from 1 to 14 months of age. As the mice aged, they developed proliferative macrophage lesions in lung, bone marrow, lymph nodes, and spleen in the absence of infection. Consistently, and with increasing age, p47phox−/− mice without evidence of infection spontaneously develop progressive crystalline macrophage pneumonia as the major cause of mortality. We found that the crystalline material secreted by proliferative p47phox−/− macrophages is Ym1/Ym2 protein, a chitinase-like lectin typically produced by alternatively activated macrophages during Th-2 biased immune responses.8 However, bronchoalveolar lavage (BAL) from p47phox−/− mice contained two- to threefold more Th1, Th2, and Th17 cytokines: IFN-γ, IL-4, and IL-17 respectively, than wild-type mice, indicating that the inflammatory pulmonary microenvironment of p47phox−/− mice is not Th-2 biased. Importantly, BAL from p47phox−/− mice also contained copious amounts of IL-12, indicating that Ym1/YM2 secreting p47phox−/− macrophages are also integrated into classically activated macrophage responses. These results suggest that p47phox−/− macrophages are extremely pliable and that classical and alternative macrophage activation programs may be triggered simultaneously in response to heterogeneous microenvironmental factors.
Materials and Methods
Mice
NADPH oxidase p47phox deficient (p47phox−/−) mice have been described.5 Congenic p47phox−/− on a C57BL/6NTac background were generated by backcrossing over 10 generations with wild-type C57BL/6NTac. Both congenic p47phox−/− and wild-type control mice (C57BL/6NTac) were obtained from Taconic Farms, Inc (Hudson, NY). An extensive National Institutes of Health (NIH) mouse sentinel program revealed no serum antibodies to known murine viral pathogens, and no ecto- or endoparasites, Helicobacter sp. or Salmonella sp. infection. In addition, although testing for P. carinii is not performed as part of the NIH sentinel screening because it is presumed to be ubiquitous, health testing for mouse specific Pneumocystis murina is performed at Taconic Farms, Inc using a genus specific set of primers. The p47phox−/− colony has been screened twice annually since inception for pneumocystis and has been documented to be free of Pneumocystis murina. p47phox−/− mice were housed in aseptic conditions and given water containing Bactrim (0.13 mg/ml trimethoprim and 0.67 mg/ml sulfamethoxazole, Actavis MidAtlantic LLC, Columbia, MD). Animal care was provided in accordance with Institutional Animal Care and Use Committee procedures approved by National Institute of Allergy and Infectious Diseases/NIH. Tissues showing gross evidence of infection or inflammatory disease were discarded and not used for the laboratory investigations reported. They were always used, however, for pathology studies.
Pathology
All mice were euthanized and necropsied at the ages noted, whether healthy or sick. All tissues were examined grossly and many were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were stained with H&E. Special stains were performed, including Gram stain (Brown-Hopps), modified Steiners, Gomori methenamine silver, acid fast, Giemsa, and periodic acid-Schiff, on sections of lung, spleen, or lymph nodes to screen for the presence of bacterial or fungal agents. No organisms were identified in any sections of the lung, spleen, or lymph nodes for the investigations reported here.
Immunohistochemistry and Electron Microscopy
Immunohistochemistry was performed on formalin-fixed tissues embedded in paraffin. Slides were incubated with rabbit anti-Ym1/Ym2 at dilutions of 1:1000.9 Signal was visualized using biotinylated anti-rabbit IgG made in goat (Vector Laboratories, Inc., Burlingame, CA) and VECTASTAIN Elite ABC reagent (Vector Laboratories, Inc., Burlingame, CA). Additionally, slides were also labeled for macrophage mannose receptor (MMR; R&D Systems, Inc., Minneapolis, MN), incubated with goat anti-mouse serum MMR IgG, and evaluated at dilutions of 1:100 to 1:200 (R&D Systems, Inc., Minneapolis, MN). Signal was visualized using a Goat horseradish peroxidase Polymer kit (Biocare, Concord, CA) with diaminobenzidine as the chromogen.
BAL
BAL was performed after euthanasia by terminal exsanguination under deep ketamine/xylazine anesthesia (80 mg/kg ketamine and 20 mg/kg xylazine). Lungs were lavaged with 0.5 ml PBS to collect supernatant. A second lavage with 0.7 ml of PBS was added to the tubes from the first collection to collect cell pellets. Cell pellets were stored at −80°C until needed.
Macrophage Preparation and Culture
Peritoneal macrophages were harvested from resting or thioglycollate (Sigma Aldrich, St. Louis, MO) stimulated p47phox−/− mice and wild-type mice. Mice received 2 ml 3% W/V thioglycollate i.p. 3 days before macrophage harvest. For macrophage harvest 10 ml of cooled PBS was injected into the peritoneal cavity. After gentle massage of the abdominal wall, fluid was collected from the peritoneal cavity via a 10-ml syringe with an 18G needle. Approximately 1 × 106 cells per sample were seeded into 24-well plates in a volume of 1 ml. After a 3-hour incubation, the nonadherent cells were removed by washing with PBS. The adherent cells were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco/Invitrogen Corp, Carlsbad, CA) supplemented with 10% fetal bovine serum (ATCC, Manassas, VA), and stimulated as indicated.
Bone Marrow-Derived Macrophages
Briefly, bone marrow was flushed with PBS, resuspended in 1 ml ACK Lysing Buffer (BioWhittaker, Walkersville, MD) and incubated at room temperature for 5 minutes to eliminate red cells. The single-cell suspension was filtered though a 40-μm cell strainer (BD Falcon/BD Biosciences, San Jose, CA), resuspended at a density of 3 × 106/ml in DMEM (Gibco/Invitrogen Corp) supplemented with 10% fetal bovine serum (ATCC) and 10 ng/ml granulocyte macrophage-colony stimulating factor (R&D Systems) then plated and incubated in a 5% CO2 incubator for 5 days. Non-adherent cells were decanted, and the remaining adherent cells were washed with fresh media and stimulated as indicated.
T-Cell Isolation
Lymph node single-cell suspensions were prepared from peripheral mouse lymph nodes. CD4+ and CD8+ T cells were negatively selected using the Dynal bead mouse CD4 or CD8 negative isolation kit (Invitrogen Corp) according to the manufacturer’s protocol. The purity of each cell population was >98% as determined by flow cytometry.
T-Cell Culture and Stimulation
Lymph node purified CD4 or CD8 T lymphocytes, as indicated, were cultured in Iscove’s modified Dulbecco’s medium complete: Iscove’s modified Dulbecco’s medium (Gibco/Invitrogen Corp) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 2.0 mmol/L l-glutamine (Hyclone), 50 mol/L β-mercaptoethanol (βME, Sigma Aldrich), 100 units/ml of penicillin and 10 units/ml streptomycin (Gibco/Invitrogen Corp). Cells were cultured at a concentration of 1 × 106 cell/ml in Iscove’s modified Dulbecco’s medium complete supplemented with 10-units/ml rhIL-2 (Rohmann-LaRoche Inc, Nutley, NJ), and stimulated with plate-bound anti-CD 3 mg/ml (Harlan), anti-CD28 3 mg/ml (Harlan) as indicated.
Cytokine Assessment
Peritoneal macrophages from p47phox−/− and normal C57BL/6 mice were incubated for 2 to 3 hours before stimulation or were rested in vitro for 24 hours. Cells were then washed, given fresh medium, and incubated with 1 μg/ml LPS, 1 μg/ml LPS + 100 ng/ml INF or 1300 units/ml IL-4 overnight. CD4+ or CD8+ lymphocytes from p47phox−/− and wild-type mice at 1 × 106 cells/ml were incubated ± anti-CD3 3 mg/ml (Harlan)/anti-CD28 3 mg/ml (Harlan) overnight. Culture supernatants were assessed for cytokines using the mouse Th1/Th2 10 plex for IL-4, IFN-γ, IL-17, IL-1, IL-6, Il-10, and tumor necrosis factor (TNF)α (Bender Medsystems, Burlingame, CA), and IL-1β, IL-12p40 (R&D Systems, Inc.) All enzyme-linked immunosorbent assays (ELISA)s were performed according to the manufacturers’ instructions.
Western Blot Analysis
Cell lysates were prepared in Mammalian-Protein Extraction Reagent (M-PER) Buffer (Pierce Biotechnology) supplemented with 0.01 volume of a protease inhibitor cocktail stock solution (Pierce Biotechnology, Rockford IL). Cell debris was removed by centrifugation at 10,000 × g for 5 minutes, and the protein concentrations were determined by the Bradford assay. Proteins were resolved by NuPage 4 to 12% Bis-Tris Gel (Invitrogen), transferred to nitrocellulose membranes by iBlot Gel Transfer (Invitrogen), and nonspecific binding sites were blocked by incubation in Tris-buffered saline containing 0.05% Tween 20 and 5% (w/v) nonfat dry milk. Immunoblotting analyses were performed in the blocking buffer with Ym1/Ym2 (a gift from Dr. Shioko Kimura, National Cancer Institute/NIH), eosinophilic chemotactic factor-L (R&D Systems), or Arginase-1 (BD transduction) antibodies. Immunocomplexes were reacted with enhanced chemiluminescence horseradish peroxidase-conjugated goat anti-rat or sheep anti-mouse IgG (GE Health care) and SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology). Proteins were then visualized by exposure to BioMax light film (Kodak). For the relative quantification of the proteins, scanned images were analyzed using ImageJ (Rasband WS, ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2007).
Statistical Analysis
Means and SEM for cytokine concentrations were determined. Differences between the group means were analyzed by the Student’s t. (Prisim 5, GraphPad Software, Inc. San Diego, CA).
Results
p47phox−/− Mice Develop Progressive Crystalline Macrophage Pneumonia
Histopathology
p47phox−/− mice without evidence of infection develop single hypertrophied alveolar macrophages as early as 5 weeks of age. In addition, these young p47phox−/− mice also develop foci of pulmonary macrophage hypertrophy with intracellular stellate or needle-like eosinophilic hyaline crystals (Figure 1). Between 12 and 16 weeks the pulmonary lesions progress to early foci of macrophage pneumonia with crystals. The macrophages in these lesions are larger than normal macrophages, contain abundant pale pink cytoplasm, and are described as large hypertrophic and proliferative macrophages. This lesion progressed to severe diffuse crystalline macrophage pneumonia as the major cause of mortality in p47phox−/− mice by 14 months. The amount of lung involvement varied and was generally milder in p47phox−/− mice treated with antibiotics; however, all mice had a tendency to develop pulmonary lesions with increasing age. The predominant inflammatory infiltrate within the lungs consisted of macrophages admixed with lymphocytes, plasma cells and neutrophils. In addition, p47phox−/− mice also develop systemic hypertrophic and proliferative macrophages in lymph nodes, spleen and bone marrow.
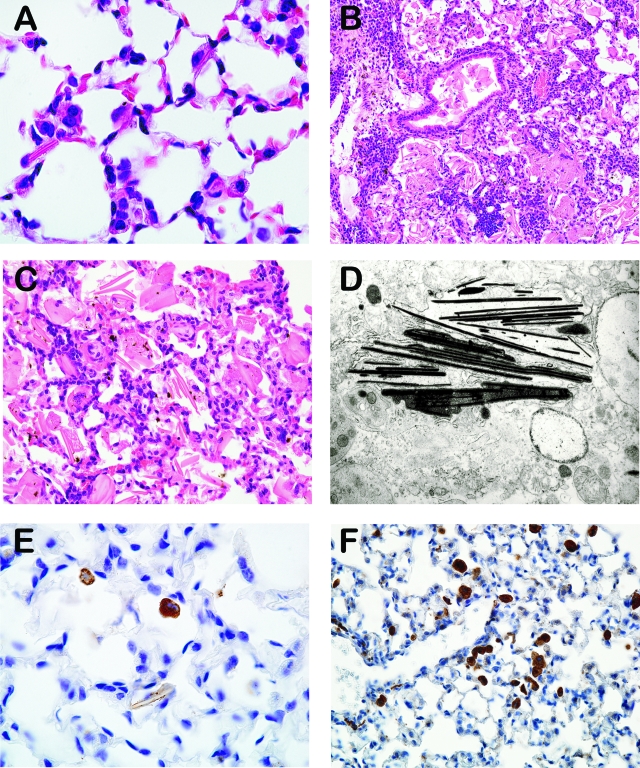
Figure 1.
A–F: Histology of macrophage crystalline pneumonia. A: Lung, early crystalline pneumonia, 4-month-old p47phox−/− mouse, H&E, 1000x magnification. B: Lung, severe lesions with abundant crystalline material within alveoli and bronchioles, 14-month-old p47phox−/− mouse, H&E, original magnification ×200. C: Lung, higher magnification demonstrating intra and extracellular giant crystals admixed with macrophages, 14-month-old p47phox−/− mouse, H&E, original magnification ×400. D: Spleen, electron microphotograph of crystalline material showing electron-dense spicules, >12-month-old p47phox−/− mouse (original magnification ×11,880). E: Lung, immunostaining against Ym1/Ym2 protein visualized in pulmonary macrophages with few crystals, 9-week-old p47phox−/− mouse, IHC, original magnification ×1000. F: Lung, immunostaining against macrophage mannose receptor, 9-week-old p47phox−/− mouse, IHC, original magnification ×400.
Pulmonary lesions in p47phox−/− mice consisted of variable degrees of macrophage pneumonia; macrophages within alveolar spaces had abundant foamy pale eosinophilic cytoplasm and occasionally contained rhomboidal eosinophilic crystalline material (Figure 1, A–C). Ultrastructural evaluation of the crystalline material demonstrated clusters of angular electron-dense spicules within the cellular matrix (Figure 1D). The majority of animals between 4 and 9 months demonstrated some degree of macrophage crystalline pneumonia accompanied by mild to moderate lymphoplasmacytic cuffing of smaller airways and vasculature. There was also mild to moderate hyperplasia of type II pneumocytes within the alveolar walls in these lesions. Nearly all p47phox−/− mice over 9.5 months developed multifocal to coalescing accumulations of foamy macrophages containing both intracellular and an increasingly higher incidence of extracellular crystalline material that effectively reduced alveolar airspace by 40% to 60% within lung lobes. The crystals were often several times larger than the macrophages (giant crystals) and free within the bronchiolar lumen or alveolar space (Figure 1, B and C). Occasionally crystals were surrounded, lined or, less frequently, engulfed by macrophages or multinucleated giant cells. In the oldest mice evaluated (>14 months), the pulmonary lesions consisted of moderate to marked accumulations of predominantly extracellular crystals admixed with abundant foamy macrophages and scattered small aggregates of lymphocytes and plasma cells. There was prominent perivascular and peribronchiolar cuffing by lymphocytes and plasma cells with normal to minimally affected bronchiolar lining epithelium. In some mice, entire lung lobes were largely obliterated by myriad crystals within alveolar spaces or free within the bronchiolar lumen, interspersed with multifocal to coalescing aggregates of neutrophils, lymphocytes and degenerate cellular debris within bronchioles. Pulmonary edema, characterized by pale eosinophilic, homogenous material filling alveolar spaces, was moderate in affected lungs, and bronchiolar lymph nodes occasionally had inflammatory infiltrates (adenitis).
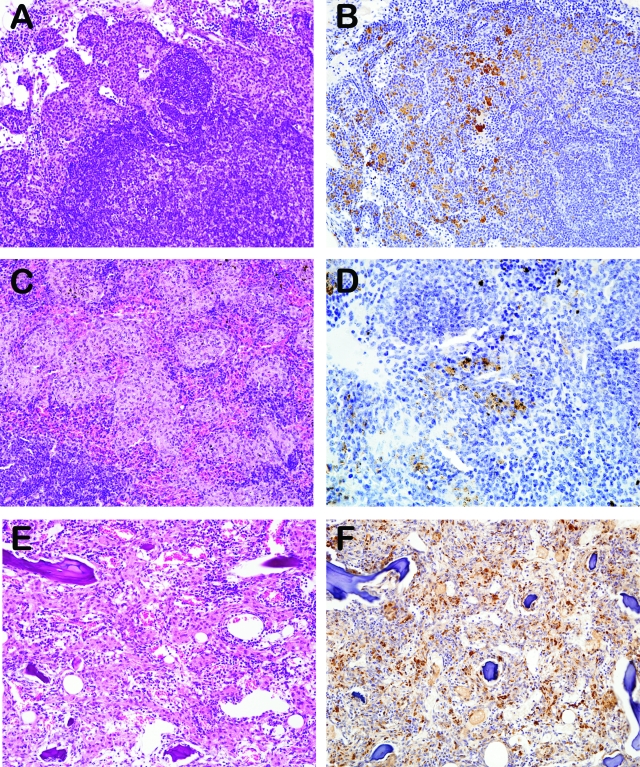
Extrapulmonary lesions occurred most often within medullary sinuses of lymph nodes, red pulp of the spleen and in the bone marrow (sternum and femur) and consisted primarily of many hypertrophic histiocytes (Figure 2, A–F).
Figure 2.
A–F: H&E histology and immunohistochemical labeling for Ym1/Ym2 in extrapulmonary lesions with histiocytic proliferation in p47phox−/− mice. A, B: Lymph node, original magnification ×200. C, D: Spleen, original magnification ×200, ×400, respectively. E, F: Bone marrow, original magnification ×200.
Immunohistochemical evaluation revealed that hypertrophic p47phox−/− macrophages in the lung, splenic, lymph node and bone marrow lesions expressed Ym1/Ym2 (Figure 2), a secretory protein produced by activated macrophages and also normal alveolar macrophages (Figure 1E,10,11). Pulmonary macrophages in affected p47phox−/− lungs also expressed MMR (Figure 1F). Finally, there was no histological evidence of granulation tissue or early pulmonary fibrosis, indicating that the spontaneous severe diffuse crystalline macrophage pneumonia found in p47phox−/− mice is due to a progressive inflammatory cellular infiltrate accompanied by the crystalline material deposition that obliterates alveolar spaces.
The Inflammatory Pulmonary Microenvironment of p47phox−/− Mice Contains Multiple T-helper Cytokines
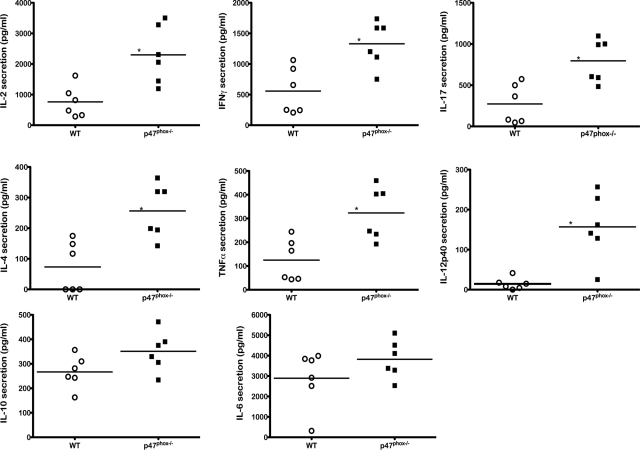
Large hypertrophic p47phox−/− macrophages secrete Ym1/Ym2 protein, a chitinase-like lectin typically produced by alternatively activated macrophages during Th-2-biased host responses.3,10 However, Ym1 is also transiently induced during normal developmental hematopoiesis and during inflammatory responses by wild-type mouse macrophages following chemical or traumatic stimuli.8,11 To discern whether the pulmonary microenvironment of p47phox−/− mice was Th-2 biased, we examined cytokine secretions from BAL of p47phox−/− and wild-type mice during the early stage of large hypertrophic macrophage development (12 to 16 weeks). Multiple cytokines were detected in the BAL of both wild-type and p47phox−/− mice. As shown in Figure 3, BAL from p47phox−/− mice contained high levels of cytokines that are typically secreted by T cells as well as macrophages. Unexpectedly, we found that BAL from 12- to 16-week-old p47phox−/− mice contained significantly higher levels of T cell secreted cytokines IL-2, IFN-γ, IL-4, and IL-17 than wild-type mice. However, IL-5, which is also a Th-2 associated cytokine, was not detected in BAL from p47phox−/− or wild-type mice. BAL from p47phox−/− mice also contained significantly more IL-12 than BAL from wild-type mice, indicating classic macrophage activation. The amount of TNFα, which is secreted by both T cells and macrophages, was also significantly higher in BAL from p47phox−/− mice. In contrast, the levels of IL-6 and IL-10, which are also secreted by T cells and macrophages, were comparable in BAL from p47phox−/− and wild-type mice. Thus, BAL from p47phox−/− mice contained high levels of Th1, Th2, and Th17 cytokines: IFN-γ, IL-4, and IL-17 respectively, indicating that the inflammatory pulmonary microenvironment of p47phox−/− mice is not Th-2 biased.
Figure 3.
p47phox−/− bronchoalveolar lavage (BAL) cytokine milieu. BAL was obtained from wild-type (WT) and p47phox−/− mice. The amount of IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-17, IFN-γ, and TNFα in the BAL was quantified using specific ELISA. Scatter plots show the responses of six individual WT (open circle) or p47phox−/− (filled squares) mice. Horizontal bars represent mean cytokine levels. *P < 0.005.
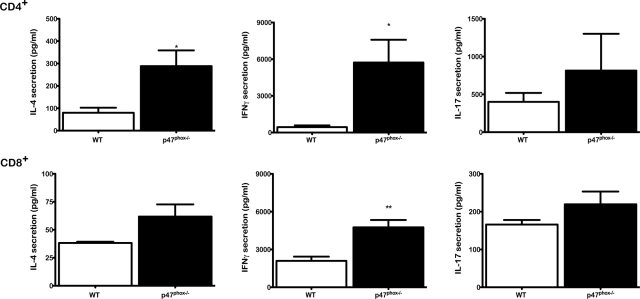
Next, we examined cytokine secretions by peripheral LN T cells from p47phox−/− and wild-type mice. For these investigations, we stimulated fractionated CD4+ and CD8+ T cells in vitro with immobilized anti-CD3-anti-CD28 (CD3-CD28). CD4+ lymphocytes from 12 to 16 week old p47phox−/− mice secreted significantly more IL-4 and IFN-γ than wild-type CD4+ lymphocytes, and p47phox−/− CD8+ lymphocytes produced significantly more IFN-γ than wild-type CD8+ lymphocytes (Figure 4). Furthermore, while p47phox−/− CD4+ lymphocytes produced 3 times more IL-4 than wild-type lymphocytes, they also produced 12 times more IFN-γ than wild-type CD4+ lymphocytes following αCD3-CD28 stimulation. We also found that p47phox−/− and wild-type T cells produced comparable amounts of IL-5 (data not shown) and IL-17 (Figure 4). Collectively these data indicate that CD3-CD28 activated p47phox−/− peripheral LN T cells produce high levels of multiple cytokines and are not Th-2 biased. Furthermore, as we previously reported12 CD3-CD28 activated p47phox−/− T cells secrete higher levels of IFN-γ, TNFα, and IL-2 than wild-type T cells, indicating a Th-1 bias.
Figure 4.
Cytokine production by lymph node T cells stimulated with anti-CD3/anti-CD28. Purified lymph node CD4+ and CD8+ lymphocytes from WT (open histograms) or p47phox−/− (filled histograms) were stimulated with plate-bound anti-CD3/anti-CD28 in the presence of IL-2 for 18 hours. Concentrations of IL-4, IL-17, and IFN-γ were analyzed using specific ELISA. Each histogram shows the mean (± SEM) results obtained for four independent experiments with pooled cells from three mice per experiment. *P < 0.05, **P = 0.007.
p47phox−/− Macrophages Are Not Polarized toward Distinct Classical or Alternative Activation Programs
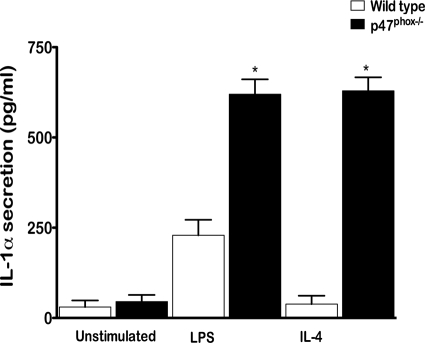
Histology findings and BAL from p47phox−/− mice reveal an ongoing and progressive inflammatory reaction predominately mediated by macrophages that highly expressed Ym1/Ym2 protein. We also found that p47phox−/− alveolar macrophages express MMR, further indicating that p47phox−/− macrophages may be alternatively activated. Immunostaining of in vivo activated peritoneal macrophages showed that both wild-type and p47phox−/− macrophages express Ym1/Ym2 and MMR proteins. However, as indicated in Figure 3 compared with levels found in wild-type mice, BAL from p47phox−/− mice contained 11-times more IL-12, indicating that p47phox−/− macrophages are classically activated macrophages. To further characterize the p47phox−/− macrophage phenotype we examined resting peritoneal cells and thioglycollate induced in vivo activated peritoneal macrophages. For these investigations, we stimulated resting peritoneal cells and in vivo activated peritoneal macrophages with IFN-γ/LPS, LPS alone, or IL-4 in vitro and measured cytokine release (IL-1α, IL-1β, IL-6, IL-10, IL-12, and TNFα, Table 1). We found that using IFN-γ/LPS to simulate classical macrophage activation comparably enhanced release of each of the cytokines from resting and activated wild-type and p47phox−/− macrophages (data not shown). Resting unstimulated p47phox−/− macrophages secreted significantly more IL-6 and IL-12p40 than wild-type macrophages (Table 1). The toll-like receptor 4 ligand LPS alone comparably enhanced IL-6 and TNFα production from resting and in vivo activated wild-type and p47phox−/− macrophages. In addition, LPS induced significantly more IL-12p40 release from resting and in vivo activated p47phox−/− peritoneal cells than wild-type cells (Table 1), and significantly more IL from in vivo activated p47phox−/− macrophages than wild-type macrophages (Table 1). LPS also induced significantly more IL-1 release from in vivo activated p47phox−/− macrophages than wild-type macrophages (Table 1, Figure 5). Similarly, stimulation with IL-4 to simulate alternative macrophage activation also induced significantly more IL-1α release from in vivo activated p47phox−/− macrophages than wild-type macrophages (Figure 5). Comparison of cytokine secretion elicited by LPS verses IL-4 showed that for each of the other cytokines, IL-4 stimulation inhibited or marginally enhanced secretion compared with the unstimulated cytokine release for both resting and in vivo activated wild-type and p47phox−/− macrophages (Table 1). This is most evident for IL-6 secretion, which shows that both resting and IL-4 stimulated p47phox−/−macrophages express essentially the same level of IL-6, which is 3 to 5 times more than wild-type macrophages. Thus IL-4 stimulation elicited a relative anti-inflammatory response compared with stimulation with LPS alone or LPS/IFN-γ for wild-type and p47phox−/− macrophages.
Table 1.
Resting and Thioglycollate-Activated Peritoneal Macrophage Cytokine Secretion
| Thioglycollate-activated peritoneal macrophages
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation | IL-6 (ng/ml)
|
TNFa (pg/ml)
|
IL-1b (pg/ml)
|
IL-12p40 (pg/ml)
|
IL-10 (pg/ml)
|
|||||
| p47phox−/− | Wild-type | p47phox−/− | Wild-type | p47phox−/− | Wild-type | p47phox−/− | Wild-type | p47phox−/− | Wild-type | |
| None | 0.53 ± 0.25 | 0.4 ± 0.14 | 150 ± 53 | 150 ± 87 | 7.2 ± 1.1 | 8.9 ± 2.4 | 44 ± 16 | 16 ± 4 | 130 ± 50 | 130 ± 44 |
| LPS | 39 ± 4.4 | 56 ± 0.91 | 1100 ± 420 | 510 ± 47 | 36 ± 6.4** | 17 ± 4 | 810 ± 290* | 110 ± 28 | 140 ± 50 | 180 ± 63 |
| IL-4 | 0.7 ± 0.052 | 1.1 ± 0.34 | 250 ± 24 | 130 ± 77 | ND | ND | 15 ± 6.4 | ND | 200 ± 16 | 120 ± 49 |
| Resting peritoneal macrophages
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation | IL-6 (ng/ml)
|
TNFa (pg/ml)
|
IL-1b (pg/ml)
|
IL-12p40 (pg/ml)
|
IL-10 (pg/ml)
|
|||||
| p47phox−/− | Wild-type | p47phox−/− | Wild-type | p47phox−/− | Wild-type | p47phox−/− | Wild-type | p47phox−/− | Wild-type | |
| None | 13 ± 2* | 3.7 ± 1.7 | 220 ± 120 | 61 ± 23 | 8.3 ± 2.7 | 26 ± 9.4 | 15 ± 5.4 | ND | 27 ± 16 | 32 ± 18 |
| LPS | 25 ± 2.4 | 29 ± 2.5 | 650 ± 270 | 670 ± 160 | 26 ± 3.8 | 29 ± 6.8 | 36 ± 12¶ | 6.6 ± 2.5 | 260 ± 150 | 200 ± 82 |
| IL-4 | 18 ± 3.1** | 3.7 ± 1 | 65 ± 65 | 100 ± 81 | ND | ND | ND | ND | 110 ± 48 | 84 ± 84 |
P = 0.015,
P = 0.0041,
P = 0.025.
Mean cytokine secretion ± SEM.
ND – Below limit of detection.
Figure 5.
Enhanced IL-1α production by classically and alternatively activated p47phox−/− macrophages in vitro. Thioglycollate activated peritoneal macrophages were stimulated with media alone, LPS, or IL-4 for 18 hours. The concentration of IL-1 was analyzed using specific ELISA. Each histogram shows the mean (± SEM) results obtained for four independent experiments with pooled cells from three to four mice per experiment. *P < 0.0005.
To further investigate whether activated p47phox−/− macrophages follow distinct classical or alternative activation programs, we used immunoblotting to examine Ym1 expression in activated peritoneal macrophages. Interestingly, we found that although immunostaining of in vivo activated peritoneal macrophages showed that both wild-type and p47phox−/− macrophages express Ym1/Ym2 protein, Ym1 was not consistently detected by Western analysis in thioglycollate elicited macrophages from either wild-type or p47phox−/− macrophages immediately following isolation (baseline expression, Figure 6). We also discerned that IL-1 secretion was only enhanced in vitro from in vivo activated macrophages p47phox−/− that also expressed Ym1 at baseline on western analysis. In addition, IL-4 induced nearly twofold more Ym1 expression in in vivo activated p47phox−/− macrophages than wild-type macrophages (2.4 ± 0.4, P = 0.027, Figure 6A). However, LPS did not induce Ym1 expression in in vivo activated wild-type or p47phox−/− macrophages in vitro. In additional experiments, we examined Ym1 expression in macrophages derived from unfractionated bone marrow (BMMφ) cells cultured in granulocyte macrophage-colony stimulating factor (see Materials and Methods). Similar to the response of in vivo activated peritoneal macrophages, LPS did not induce Ym1 expression in wild-type or p47phox−/− BMMφ, whereas IL-4 induced Ym1 expression in both (Figure 6B). Ym1 was transiently expressed in wild-type BMMφ following IL-4 stimulation. However, IL-4 stimulated p47phox−/− BMMφ continued to express significantly more Ym1 during a 24-hour withdrawal of IL-4 stimulation than wild-type BMMφ. Interestingly, unlike wild-type BMMφ, IL-4 stimulated p47phox−/− BMMφ also continued to express Ym1 on restimulation with LPS (Figure 6B). These results indicate that unlike wild-type macrophages, p47phox−/− macrophages that are induced to express Ym1 may not be polarized toward a distinct alternative activation program, and that once activated, both classical and alternative activation pathways maybe turned on in the absence of p47phox.
Figure 6.
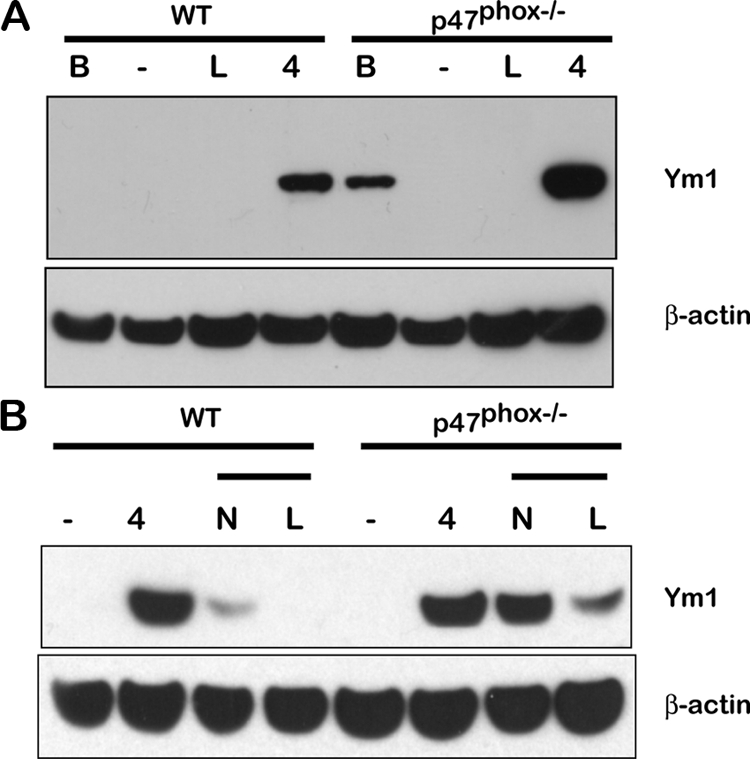
Enhanced Ym1 expression in p47phox−/− macrophages. Thioglycollate induced peritoneal and bone marrow derived macrophages were incubated with 1 μg/ml LPS or 1300u/ml IL-4 for 18 hours. Equal amount of proteins extracted from the cells were subjected to Western blot analysis using rat antibody against eosinophilic chemotactic factor-L and mouse antibody against actin. A: Thioglycollate induced peritoneal macrophage Ym1 expression at baseline (B), and following stimulation with media alone (–), LPS (L), or IL-4 (4). One representative experiment of four is shown. B: Bone marrow derived macrophage Ym1 expression following stimulation with media alone (–) or IL-4.4 For the chase Ym1 response, IL-4 stimulated macrophages were washed out of the IL-4 containing media and restimulated with media alone (N) or LPS (L). One representative experiment of two is shown.
Discussion
Immune responses are primarily orchestrated for eradicating pathogens. However, patients with primary immunodeficiencies are incapable of mounting adequate immune responses. As a consequence, prolonged and sustained immune reactions result, ultimately producing local or systemic chronic inflammation due to the infiltration of phagocytes and effector lymphocytes. p47phox−/− mice are a mouse model of human CGD, a genetically heterogeneous immunodeficiency syndrome caused by defective or absent NADPH oxidase enzymatic function.6 The underlying defect is an inability of phagocytic cells to reduce molecular oxygen. Consequently CGD phagocytes fail to generate superoxide and its derivatives, hydrogen peroxide and hydroxyl radicals, which facilitate the intracellular killing of microorganisms.13,14 CGD patients also are prone to develop inflammatory and autoreactive diseases, such as inflammatory bowel disease, juvenile rheumatoid arthritis, sarcoidosis, lupus-related syndromes, and granulomatous obstructive lesions of the gastrointestinal and genitourinary tracts.7,15 Recurrent pulmonary infection with fungal organisms is now the most common clinical manifestation of CGD. Other infectious manifestations of CGD include suppurative adenitis, subcutaneous abscess, osteomyelitis, liver abscess, and sepsis.16 Identical to human CGD patients, p47phox−/− mice spontaneously develop systemic bacterial and fungal infections.5 While abscesses are readily discerned as lymphadenitis or gross cutaneous lesions, deep tissue infections involving lung, liver, bone, and spleen are generally found on necropsy of morbid p47phox−/− mice (ie, mice found with ruffled fur, hunched posture, and labored breathing). Using Bactrim prophylaxis in specific pathogen free environments, the majority of p47phox−/− mice remain infection-free and can survive for greater than 14 months. Here we report that aging p47phox−/− mice without evidence of infection develop proliferative macrophage lesions. Although the macrophage lesions are systemic, involving bone marrow, lymph node, and spleen, the predominant lesion producing clinical signs and death is progressive diffuse crystalline macrophage pneumonia.
Several other descriptions of similar crystalline macrophage pneumonia lesions have been described in C57BL/6 and 129Sv strains naturally, as well as in knockout mice on C57BL/6 and 129 backgrounds. The lung lesions in aging p47phox−/− mice resembled those seen in SHP1 null (motheaten) mice, aging mice of various mouse strains9,17,18,19 and in some immunologically induced lung disease.20 The lesion in p47phox−/− mice, however, had a sequence of lesion development not usually seen in those mice. The earliest lesions in p47phox−/− mice were seen in young mice (2 to 6 months) with individual alveolar macrophages containing intracytoplasmic crystals that expressed Ym1/Ym2. As the lesions expanded to involve individual lung lobes and eventually the entire lung, numerous macrophages with intracellular crystals led to many extracellular crystals that obliterate aerated p47phox−/− lung spaces. In addition, although pulmonary fibrosis was not found in p47phox−/− mice or in the other lung models noted above, the inflammatory lung infiltrates in p47phox−/− mice were less severe than in other mouse models. This finding may be related to the hodgepodge cytokine milieu in p47phox−/−BAL, which contains Th-1, Th-2, and Th-17 cytokines, which may prevent the mounting of a more severe inflammatory or fibrotic lesion.
The crystalline material secreted by proliferative p47phox−/− macrophages is Ym1/Ym2 protein, a chitinase-like lectin that is transiently induced during developmental hematopoiesis, inflammatory responses to chemical or traumatic stimuli and by alternatively activated macrophages during T helper-2 biased immune responses.8,9,10,11 Ym1, also called eosinophilic chemotactic factor-L, is a chitinase-related protein and is highly related to Ym2, an eosinophilic chemotactic factor with stomach specific expression.9 Although Ym1 secretion has been associated with tissue damage as well as tissue repair and remodeling11,21 a definitive function of Ym1 has not been determined. Our findings indicate that p47phox−/− macrophages have an intrinsic dysfunction of macrophage activation pathways that allow for distinct classical or alternative activation phenotypes. Additionally, in response to environmental stimuli, Ym1 expression by p47phox−/− macrophages may be a targeted activation response and independent of either classical or alternative macrophage programs of activation. We plan to examine Ym1 expression and function in future investigations in our laboratory.
Previous studies have shown the extensive pulmonary lesions of infected NADPH oxidase deficient mouse models including crystalline macrophage pneumonia22,23; however, this is the first report describing spontaneous systemic proliferative macrophage lesions in NADPH oxidase mice without evidence of infection. The pulmonary inflammation in p47phox−/− mice contained copious amounts Th-1, Th-2, and Th-17 cytokines as well as signature proteins for classical (IL-12) and alternatively (Ym1 and MMR) activated macrophages. In addition, in vitro investigations revealed that the pattern of response to alternative pathway stimuli is not restricted for p47phox−/− macrophages (Table 1, Figure 6). Instead, unlike wild-type macrophages, once activated Ym1 expressing p47phox−/− macrophages can exhibit both classical and alternative activation programs.
Our findings implicate a role for NADPH oxidase p47phox as a mediator of signaling networks that regulate macrophage activation pathways, and indicate that NADPH oxidase p47phox influences additional critical processes in macrophage function that extend beyond its recognized role in innate phagocyte immunity. The mechanisms appear to involve the dysregulation of cytokine and protein expression in macrophages. Furthermore, we found that p47phox−/− mice develop spontaneous systemic and organ specific autoreactive disease, which is consistent with the observation that NADPH oxidase deficient CGD patients are prone to develop various autoimmune-like diseases such as inflammatory bowel disease, rheumatoid arthritis, and sarcoidosis. While the molecular basis for these complex disorders in an NADPH oxidase reactive oxygen species-deficient host remains unclear, these findings raise interesting questions about how the local redox environment and intercellular, NADPH oxidase dependent- reactive oxygen species or NADPH oxidase reactive oxygen species independent, processes promote autoimmune-like inflammation. Thus, our targeted investigations to characterize molecular pathways that are controlled by NADPH oxidase p47phox and/or NADPH oxidase derived reactive oxygen species may reveal novel targets for the development of immunomodulatory therapies.
Acknowledgments
We gratefully acknowledge the excellent histotechnology support of Elizabeth M. Williams and Lawrence J. Faucette and Histoserv, Inc. We also thank Mitzi Donaldson for careful review of this manuscript.
Footnotes
Address reprint requests to Sharon H. Jackson, Laboratory of Host Defenses, NIAID, NIH, CRC Bldg 5-West Labs, Room 5-3942, 10 Center Dr. MSC 1456, Bethesda, MD 20892-1456. E-mail: sjackson@niaid.nih.gov.
Supported by the Division of Intramural Research of the National Institutes of Health/National Institute of Allergy and Infectious Diseases. This work was also partially supported by the National Center on Minority Health and Health Disparities/National Institutes of Health.
References
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–106. [PubMed] [Google Scholar]
- Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari T. Chronic granulomatous disease; fundamental stages in our understanding of CGD. Med Immunol. 2006;5:4. doi: 10.1186/1476-9433-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig SD. Inflammatory manifestations in chronic granulomatous disease (CGD). J Clin Immunol. 2008;28:Suppl 1:S67–S72. doi: 10.1007/s10875-007-9160-5. [DOI] [PubMed] [Google Scholar]
- Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- Ward JM, Yoon M, Anver MR, Haines DC, Kudo G, Gonzalez FJ, Kimura S. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am J Pathol. 2001;158:323–332. doi: 10.1016/S0002-9440(10)63972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DC, McKenzie AN, Foster PS. Expression of the Ym2 lectin-binding protein is dependent on interleukin (IL)-4 and IL-13 signal transduction: identification of a novel allergy-associated protein. J Biol Chem. 2001;276:41969–41976. doi: 10.1074/jbc.M106223200. [DOI] [PubMed] [Google Scholar]
- Hung SI, Chang AC, Kato I, Chang NC. Transient expression of Ym1, a heparin-binding lectin, during developmental hematopoiesis and inflammation. J Leukoc Biol. 2002;72:72–82. [PubMed] [Google Scholar]
- Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- Karupiah G, Hunt NH, King NJ, Chaudhri G. NADPH oxidase. Nramp1 and nitric oxide synthase 2 in the host antimicrobial response. Rev Immunogenet. 2000;2:387–415. [PubMed] [Google Scholar]
- Verhoeven AJ. The NADPH oxidase: lessons from chronic granulomatous disease neutrophils. Ann NY Acad Sci. 1997;832:85–92. doi: 10.1111/j.1749-6632.1997.tb46239.x. [DOI] [PubMed] [Google Scholar]
- De Ravin SS, Naumann N, Robinson MR, Barron KS, Kleiner DE, Ulrick J, Friend J, Anderson VL, Darnell D, Kang EM, Malech HL. Sarcoidosis in chronic granulomatous disease. Pediatrics. 2006;117:e590–595. doi: 10.1542/peds.2005-1349. [DOI] [PubMed] [Google Scholar]
- Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Ward JM. Pulmonary pathology of the motheaten mouse. Vet Pathol. 1978;15:170–178. doi: 10.1177/030098587801500203. [DOI] [PubMed] [Google Scholar]
- Tsui FW, Martin A, Wang J, Tsui HW. Investigations into the regulation and function of the SH2 domain-containing protein-tyrosine phosphatase SHP-1. Immunol Res. 2006;35:127–136. doi: 10.1385/IR:35:1:127. [DOI] [PubMed] [Google Scholar]
- Hoenerhoff MJ, Starost MF, Ward JM. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet Pathol. 2006;43:682–688. doi: 10.1354/vp.43-5-682. [DOI] [PubMed] [Google Scholar]
- Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci USA. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem. 2000;275:8032–8037. doi: 10.1074/jbc.275.11.8032. [DOI] [PubMed] [Google Scholar]
- Harbord M, Novelli M, Canas B, Power D, Davis C, Godovac-Zimmermann J, Roes J, Segal AW. Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. J Biol Chem. 2002;277:5468–5475. doi: 10.1074/jbc.M110635200. [DOI] [PubMed] [Google Scholar]
- Bingel SA. Pathology of a mouse model of x-linked chronic granulomatous disease. Contemp Top Lab Anim Sci. 2002;41:33–38. [PubMed] [Google Scholar]