Abstract
In rodents, the chemokine CXCL1 both induces the proliferation and inhibits the migration of oligodendrocyte precursor cells. We previously reported that in multiple sclerosis, the same chemokine is expressed by hypertrophic astrocytes, which associate with oligodendrocytes that express the receptor CXCR2. To investigate whether chemokines influence repair after autoimmune demyelination, we generated GFAP-rtTA × β-Gal-TRE-CXCL1 double-transgenic (Tg) mice that inducibly overexpress CXCL1 under the control of the astrocyte-specific gene, glial fibrillary acidic protein. Experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, was induced in these animals (and controls) by the subcutaneous injection of myelin oligodendrocyte glycoprotein, and after disease onset, CXCL1 production was initiated by the intraperitoneal injection of doxycycline. Double-Tg animals displayed a milder course of disease compared with both single (CXCL1 or glial fibrillary acidic protein)-Tg and wild-type controls. Pathologies were similar in all groups during the acute stage of disease. During the chronic disease phase, both inflammation and demyelination were diminished in double-Tg mice and Wallerian degeneration was markedly decreased. Remyelination was strikingly more prominent in double-Tg mice, together with an apparent increased number of oligodendrocytes. Moreover, cell proliferation, indicated by BrdU incorporation within the central nervous system, was more widespread in the white matter of double-Tg animals. These findings suggest a neuroprotective role for CXCL1 during the course of autoimmune demyelination.
Studies on multiple sclerosis (MS), and its animal model, experimental autoimmune encephalomyelitis (EAE), have suggested that chemokines might be key players in disease development. Th1-type effector cells expressing the chemokine receptors, CCR5 and CXCR3, appear to be recruited into central nervous system (CNS) parenchyma by the chemokines, CCL3, CCL5, and CXCL10,1,2,3,4,5 whereas CCL2, CCL3, and CCL5 are important in the recruitment of macrophages via interactions with CCR2, CCR1, and CCR5, respectively.6,7,8,9 Recently, CXCL13 has been linked to B-cell recruitment in MS10 and EAE,11 and immature dendritic cells might be attracted to MS lesions in response to CXCL12 and CCL20.12 Chemokine receptors have also been localized on resident CNS cell types but the role of these receptors is yet to be fully elucidated.13,14,15,16,17,18,19,20,21,22,23,24,25
In MS, oligodendrocytes are a primary target and are severely depleted in affected areas.26,27,28 However, to date, no clear mechanism underlying their demise has been elucidated.29,30,31 Although much evidence suggests MS to be an autoimmune disorder mediated by Th1-type T cells,32 there is still debate as to whether other pathways might be involved.31,32,33 Emphasis in MS has been applied to the study of oligodendrocytes and remyelination.34 Fueling this is the observation that in active lesions, oligodendrocytes are not only preserved, but might even exist in increased numbers.35,36 Moreover, remyelination (albeit limited in extent) has also been reported to take place during the active stage of the disease.37,38 The presence of oligodendrocyte precursor cells in normal and MS white matter is in accord with the generation of new oligodendrocytes.39,40,41,42,43 Recent studies report that remyelination can be extensive in MS patients with long-standing disease.44,45
In addition to their prime function as myelinating cells, oligodendrocytes are currently considered a more dynamic cell type, bearing (in humans) receptors normally associated with the immune system, suggesting that these cells might also be capable of responding to inflammatory signals.24,29,30,46 In the rat, oligodendrocytes have been found to express CXCR1 and CXCR218 and to show proliferative and migratory responses to the chemokine, CXCL1.47,48 It has also been reported that CXCR4, interacting with the ligand CXCL12, influences neuronal and oligodendrocyte precursor survival and migration.23 More recently, the chemokine, CCL11, has been shown to increase proliferation, inhibit migration and augment differentiation of primary rat oligodendrocyte precursor cells via CCR3.25 Astrocytes are a major source of chemokines within the CNS, and this cell type might function in the regulation of oligodendrocyte behavior in MS.24,49,50
To test the effects of astrocyte-produced CXCL1 on oligodendrocytes (known to express CXCR2), we generated a novel double-transgenic (Tg) mouse (GFAP-rtTA × β-Gal-TRE-CXCL1), which was then sensitized for EAE. Using this model, we were able to induce the production of CXCL1 by astrocytes in situ, after administration of doxycycline. Our findings demonstrate that double-Tg animals overexpressing CXCL1 displayed a milder form of EAE that was associated with reduced pathology [Wallerian degeneration (WD), demyelination] and more prominent remyelination. These findings suggest that CXCL1/CXCR2-mediated signals play a neuroprotective role during the course of CNS autoimmune demyelination.
Materials and Methods
Generation of GFAP-rtTA × β-Gal-TRE-CXCL1 Double-Tg Animals
We generated double-Tg mice that overexpress CXCL1 under the control of the promoter of the astrocyte-specific gene, glial fibrillary acidic protein (GFAP) on a C57BL/6 background. This was achieved by mating two strains of mice: mouse strain 1, containing the pTeton-GFAP construct, which induces the expression of the rtTA-VP16 (reverse tetracycline-controlled transactivator fusion protein) under the control of the GFAP promoter, and mouse strain 2, carrying the pTRE2-N51/CXCL1 construct, of which the TRE (tetracycline response element) promoter is made up of Tet operator (tetO) sequence concatemers fused to a minimal CMV promoter.51 The F1 cross between these two strains generated animals that express rtTA-VP16 in GFAP-positive cells. This in turn, and only in the presence of tetracycline or its derivative doxycycline, drives the expression of the CXCL1 and β-gal gene products in astrocytes (Figure 1). Tail DNA isolated from littermates was analyzed by polymerase chain reaction (PCR) for the presence of the transgenes using primers that amplify a 500-bp segment of the of the GFAP-rtTA/VP16 construct (GFAP forward: 5′-GCTCCACCCCCTCAGGCTATTCAA-3′; GFAP reverse: 5′-TAAAGGGCAAAA GTGAGTATGGTG-3′), and a 190-bp fragment of the TRE-N51/CXCL1 construct (CXCL1 forward: 5′-CTGGGATTCACCTCAAGAAC-3′; CXCL1 reverse: 5′-GGGGACAC CTTTTAGCATCT-3′).
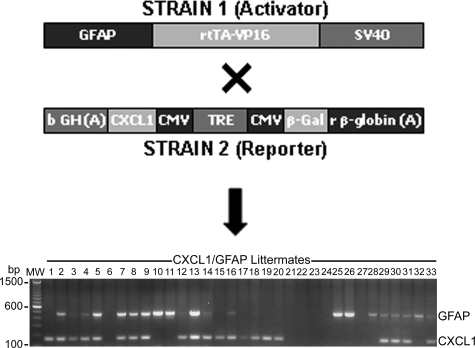
Figure 1.
Generation of double-Tg mice. Schematic representation of constructs in the mouse strains used to create the GFAP-rtTA × β-Gal-TRE-CXCL1 double-Tg mouse model. Strain 1 (activator), had the reverse-tetracycline transactivator fused to the VP16 viral gene (rtTA-VP16) under the control of the GFAP promoter, and strain 2 (reporter), had the CXCL1 and β-gal genes controlled by the tetracycline response element fused to a minimal CMV promoter (TRE/CMV) (top). Performing a F1 cross between these two strains generated animals that expressed rtTA-VP16 in GFAP-positive cells. This in turn, and only in the presence of tetracycline or its derivative doxycycline, drove the expression of the CXCL1 gene product in astrocytes. Representative PCR genotyping of littermates from animals set up to generate double-Tg mice is shown at the bottom (lanes 1 to 33). Lanes containing the 500-bp DNA fragment indicate the presence of GFAP transgene, and the 190-bp band represents CXCL1 gene amplification. Double-Tg animals display both bands, and lanes with no bands indicate animals carrying neither of the transgenes.
Tail DNA template (50 ng) was amplified in 25 μl of PCR mixtures containing 12.5 pmol of each primer added to PuReTaq Ready-To-Go PCR beads (GE Health Care Bio-Sciences Corp., Piscataway, NJ). DNA amplification was performed as follows: denaturation step at 94°C for 5 minutes, annealing step at 55°C for 1 minute, an extension step at 72°C for 3 minutes, followed by 39 cycles of 94°C denaturation for 30 seconds, 55°C annealing for 30 seconds, 72°C elongation for 45 seconds, and a final elongation step at 72°C for 7 minutes. A 10-μl sample of each PCR reaction was analyzed by electrophoresis on 2% agarose gels to distinguish double- and single-Tg animals by the presence or absence of the CXCL1 and GFAP DNA fragments (Figure 1).
Induction of CXCL1 in Double-Tg Mice
To determine the ability to induce CXCL1 production in the novel tetracycline-inducible Tg system, double-Tg animals at 8 to 12 weeks of age received intraperitoneal injections of 500 μg of doxycycline (Sigma, St. Louis, MO)51 for a period of 7 days (four injections). Animals were sacrificed 2 to 14 days after injection, and expression of CXCL1 was confirmed by immunohistochemistry using antibodies against CXCL1 and β-gal, as described below. Mice were also monitored for neurological signs during the 14-day period and analyzed for infiltration and demyelination to assess the global effects of regulated CXCL1 overexpression in the adult CNS. CXCL1 single-Tg and wild-type littermate mice were also treated with doxycycline for 7 days, and double-Tg animals receiving no doxycycline served as controls. A total of four animals were analyzed at each time point and processed for histopathology and immunohistochemistry analysis.
Myelin Oligodendrocyte Glycoprotein (MOG)-Induced EAE
The new inducible Tg model (described above) was used to test the effects of regulated overexpression of CXCL1 during the course of CNS inflammation. Double-Tg mice, 8 to 12 weeks of age, were sensitized for actively induced EAE by subcutaneous injection (two dorsal flank sites) of 200 to 400 μg of encephalitogenic MOG35-55 peptide in incomplete Freund’s adjuvant supplemented with Mycobacterium tuberculosis followed by 500 ng of pertussis toxin intraperitoneally on days 0 and 2 after injection.52 At initial signs of disease, doxycycline (500 μg) was injected into the peritoneal cavity throughout a period of 7 days (four injections) to cause overproduction of CXCL1 by astrocytes, and then removed (at which point CXCL1 production by astrocytes ceased). Animals were observed for up to 60 days after injection and scored on a clinical scale of 0 to 5 (grade 0, no abnormalities; grade 1, weak tail; grade 2, limp tail and weakness of hind-limbs; grade 3, hind-limb paraparesis; grade 4, tetraplegia; grade 5, moribund or death). For controls, CXCL1 and GFAP single-Tg, and wild-type littermates were similarly sensitized for EAE and treated with doxycycline. A total of 25 double-Tg, 23 single-Tg, and 10 wild-type animals were analyzed in EAE experiments.
Histopathology
For histopathology, animals were anesthetized with ether and perfused through the left cardiac ventricle with 20 ml of cold phosphate-buffered 2.5% glutaraldehyde. Tissue samples were taken from optic nerve, cerebrum, cerebellum, and several levels of spinal cord, and processed for embedding in plastic. Because EAE in this model is typically a disease of the lower spinal cord, we focused in large part on this region of the CNS. One-μm epoxy sections were cut and stained with toluidine blue for light microscope examination to assess levels of inflammation, demyelination, WD, and remyelination. Histopathology was scored on a scale of 0 to 5 in double- and single-Tg mice with MOG-induced EAE, by a method developed and routinely used in our laboratory.53 For ultrastructural analysis, ultrathin sections were cut and contrasted with lead and uranium salts. Tissue sections were then carbon-coated for transmission electron microscopy (TEM), and examined in a Hitachi (Tokyo, Japan) HS600 electron microscope. A total of four animals in each treatment group were sampled for each time point for histopathology, and one representative animal from each group was examined ultrastructurally.
Immunohistochemistry
For immunohistochemistry, anesthetized animals were perfused with cold phosphate-buffered saline (PBS). The brain and spinal cord were removed and blocks of tissue were embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and snap-frozen. Ten-μm frozen sections were fixed with ice-cold acetone, quenched for endogenous peroxidase activity (30 minutes in 3% H2O2), blocked for 1 hour with 10% normal goat or horse sera, and incubated with primary antibodies diluted in 2% normal sera overnight at 4°C. Immunoreacted sections were developed by incubation with secondary biotinylated antibodies, followed by the Vectastain avidin-biotin complex (ABC) solution (Vector Laboratories, Burlingame, CA), and the chromogen 3,3′-diaminobenzidine (KPL, Gaithersburg, MD). For immunofluorescent staining, after fixation, sections were incubated with 0.1% glycine for 20 minutes to quench autofluorescence before blocking with normal goat or horse sera. After incubation with primary antibodies, sections were treated with secondary Alexa-488- or Alexa-568-conjugated antibodies (Invitrogen Molecular Probes, Carlsbad, CA). In the case of CXCR2, sections were treated with a biotinylated secondary antibody followed by streptavidin-conjugated Alexa-488. Primary antibodies used include: anti-β-galactosidase (ProSci Inc., Poway, CA), anti-GFAP (Sigma), anti-CXCL1, anti-CXCR2 (both from R&D Systems, Minneapolis, MN), anti-Olig2 (Dr. C. Stiles, Harvard University, Boston, MA), and antibodies against T cells (CD4), monocytes/macrophages (F4/80), and neutrophils (all from AbD Serotec, Raleigh, NC), to assess infiltration.
Western Blotting
Western blotting was conducted to semiquantitate β-gal protein levels in animals after doxycycline treatment. Frozen spinal cord and brain tissue from each experimental group was homogenized on ice in lysis buffer and protein concentration determined using the Bio-Rad BCA assay (Bio-Rad, Hercules, CA). Protein samples (25 to 100 μg) were loaded in reducing sodium dodecyl sulfate buffer (1:5), separated on 10 to 15% sodium dodecyl sulfate-polyacrylamide gels (Mini-Protean, Bio-Rad), and transferred onto polyvinylidene difluoride membranes for 2 hours (Millipore, Bedford, MA). After blocking in 5% fat-free milk powder/PBS/0.05% Tween 20 (pH 7.4), membranes were incubated in diluted anti-β-gal antibody for 2 hours at room temperature. After washing and incubation in horseradish peroxidase-coupled secondary antibody (Vector Laboratories), immunoreactive bands for β-gal were visualized by enhanced chemiluminescence (SuperSignal West Pico chemiluminescent substrate; Pierce, Rockford, IL) on light-sensitive film (Biomax L, Sigma). Blots were then scanned and quantitated using Scion Image (Scion Corp., Frederick, MD). β-Gal protein expression levels within the CNS of doxycycline-treated double-Tg and control mice were normalized to the housekeeping protein, β-tubulin.
BrdU Proliferation Assay
CXCL1-generated signals have been reported to influence oligodendroglial proliferation. To assess this in double-Tg mice, 50 mg/kg of 5-bromo-2′-deoxyuridine (BrdU, Sigma), a pyrimidine analog of thymidine that is selectively incorporated into cell DNA at the S phase of the cell cycle, was injected into animals at the same time as doxycycline. This allowed it to be integrated into the DNA of cells undergoing division after induction of CXCL1 production by astrocytes. Proliferation, indicated by BrdU incorporation, was detected using an anti-BrdU antibody (LabVision, Fremont, CA). After fixation, frozen sections were pretreated with 2 N hydrochloric acid to denature the DNA, rinsed thoroughly in PBS after prerinsing in 0.1 mol/L sodium tetraborate (Na2B4O7/borax), incubated with primary antibody, and developed by standard immunohistochemistry techniques, as described above.
Data Acquisition and Analysis
Digital images of tissue sections were acquired on a Zeiss Axioskop epifluorescent microscope (Carl Zeiss, Thornwood, NY). Data from EAE experiments were subjected to analysis of variance to determine differences between treatment groups. Where differences were found, Student-Newman-Keuls all pairwise multiple comparisons were conducted to analyze individual differences against control groups. The Student’s t-test was applied to histopathological data to determine differences within treatment groups. Data were analyzed using SigmaStat v2.03 and graphs were generated on SigmaPlot 8.0 (SPSS Science, Chicago, IL).
Results
Generation of GFAP-rtTA × β-Gal-TRE-CXCL1 Double-Tg Mice
Generation of GFAP-rtTA × β-Gal-TRE-CXCL1 double-Tg mice was achieved by crossing two strains of mice, one strain containing the pTeton/GFAP construct, and the second, a pTRE2-N51/CXCL1 construct (Figure 1, top). Both Tg constructs were potentially expressed in every cell in the resulting double-Tg animals but because the Tet sequence was linked to a GFAP promoter, regulation was only effective in astrocytes when the animal was given tetracycline, or its derivative, doxycycline. At this point, the Tet transcription product from the GFAP/Tet construct activated the Tet response element on the second transgene to up-regulate expression of CXCL1 within the CNS. Representative genotyping of littermates is shown in Figure 1, bottom. Single-Tgs expressed the 500-bp DNA fragment indicating the presence of the GFAP transgene and the 190-bp band representing the CXCL1 gene, with double-Tg animals displaying both bands.
β-Gal Expression in Doxycycline-Treated Animals
β-Gal expression was analyzed in mice treated with doxycycline to confirm induced transgene expression in CNS tissue. Double-Tg animals and control mice received a total of four intraperitoneal injections of doxycycline (500 μg), administered every other day. Immunohistochemistry demonstrated an increase in β-gal up to 3 days after the last doxycycline injection (day 9), which appears to be decreasing by day 13 (Figure 2, A–C). In controls, weak β-gal reactivity was detected in CXCL1 single-Tg mice receiving the full compliment of injections (Figure 2D). Double-immunofluorescent staining confirmed expression of β-gal by astrocytes in Tg mice. More protein was detected in doxycycline-treated double-Tg mice (Figure 2E), compared with sterile PBS-treated double-Tg control mice (Figure 2I). At high magnification, the β-gal protein appeared to co-localize with the astrocytic marker, GFAP, in both treated and control animals (Figure 2, F, G, J, and K). CXCL1 expression was up-regulated in astrocytes and their processes in spinal cord tissue from doxycycline-treated normal double-Tg mice, but not in the CNS of double-Tg mice not receiving doxycycline (Figure 2, H and L).
Figure 2.
β-Gal expression in doxycycline-treated animals. β-Gal expression was analyzed in mice treated with doxycycline to confirm induced transgene expression in CNS tissue. Double-Tg and control mice received a total of four intraperitoneal injections of doxycycline (500 μg), administered every other day. A–C: Results show an increase in β-gal on astrocytes and their cell processes flanking the anterior fissure of the lumbar spinal cord up to 3 days after the last doxycycline injection (day 9), which appears to be decreasing by day 13. D: In controls, weak β-gal reactivity was detected in CXCL1 single-Tg mice receiving the full compliment of doxycycline injections. E and I: β-Gal expression was confirmed by double-immunofluorescent staining. Increased staining for β-gal was detected in doxycycline-treated double-Tg mice (E), whereas very little β-gal protein was present on anterior column astrocytes in control double-Tg mice receiving sterile PBS without doxycycline (I). β-Gal staining appeared to co-localize with the astrocytic marker, GFAP, in both doxycycline-treated (E–G) and control animals (I–K). In H, CXCL1 expression is shown in astrocyte cell bodies and their processes after doxycycline treatment (examples are highlighted by arrows). In L, note minimal expression of CXCL1 after no doxycycline treatment. Two double-Tg or control mice were sampled at each time point and immunohistochemistry and immunofluorescent images were taken from spinal cord sections of one representative animal. Scale bars: 55 μm (A–D, E–G, I–K); 70 μm (H, L).
Western blot analysis revealed a similar increase in β-gal protein starting 5 days after initiating doxycycline treatment, and up to a week after the last injection. Similar to immunohistochemistry results, low levels of β-gal protein were present in spinal cord tissue from control double-Tg animals not receiving doxycycline and in doxycycline-treated single-Tg mice (Figure 3A). In addition, levels of β-gal protein were analyzed in tissue samples from mice with MOG-induced EAE. Similar to disease-free animals, higher levels of β-gal were detected in spinal cord tissue from double-Tg mice after doxycycline injections, compared with tissue samples from single-Tg animals or wild-type controls (Figure 3B). Together, these results demonstrate specific induction of the β-gal and CXCL1 genes and suggested that CXCL1 overproduction was achieved in double-Tg mice.
Figure 3.
β-Gal protein levels analyzed by Western blotting. β-Gal protein expression was further analyzed by Western blotting in animals treated with doxycycline in the absence of disease (A), and in mice with MOG-induced EAE (B). In disease-free double-Tg mice, the highest levels of β-gal were present in spinal cord tissue of mice that had received all four doxycycline injections (days 9 and 13), compared with smaller amounts of β-gal detected in mice that had only received three injections of doxycycline (day 5). A: Low levels of β-gal protein were detected in control double-Tg mice not treated with doxycycline and single-Tg mice receiving the full compliment of doxycycline injections. B: In mice with EAE, the highest levels of β-gal were detected in double-Tg compared with single-Tg mice after doxycycline treatment, during the late acute phase of the disease. Bar graphs represent β-gal expression from spinal cord tissue (cervical, thoracic, and lumbar combined), of two animals from two separate experiments, presented as relative intensity compared with the internal standard β-tubulin.
EAE in Transgenic Mice
MOG-EAE was induced in double-Tg, and in control, single-Tg mice and wild-type, animals. At the onset of EAE, CXCL1 production by astrocytes was induced by injection of doxycycline. Single-Tg and wild-type animals developed moderate EAE (grade 3), whereas double-Tg animals displayed a milder course (grade 1 to 2) (Figure 4, A–C). Double-Tg mice recovered soon after the initial phase of EAE, by days 20 to 25 after injection, whereas the majority of control animals had more severe disease for the duration of the experiments (40 to 60 days). Differences in clinical EAE were statistically significant (P < 0.05) between double-Tg and control animals (wild-type and single-Tg), especially during the late acute phase of EAE. Introduction of the two transgenes had no significant effect on the induction of EAE.
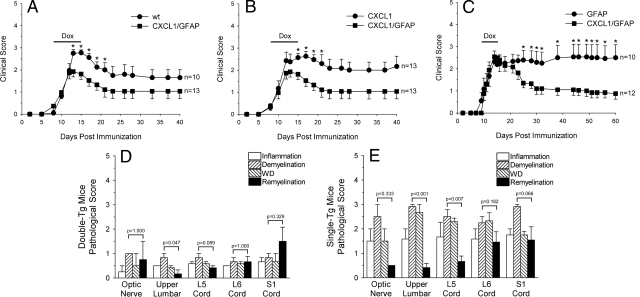
Figure 4.
EAE in double-Tg mice. A–C: Actively induced MOG-EAE is shown in double-Tg, single-Tg, and wild-type (wt) animals. After onset of signs, CXCL1 production by astrocytes was induced by intraperitoneal doxycycline injection (black bars). Although all three groups were treated with doxycycline and had a similar disease course during the acute phase of the disease, double-Tg animals displayed a milder chronic form of EAE compared with wt (A), CXCL1 (B), and GFAP (C) single-Tg littermate controls (*P < 0.05 compared with double-Tg mice). Histopathology was scored on a scale of 0 to 5 for inflammation, demyelination, axonal pathology (WD), and remyelination in double- and single-Tg mice with MOG-EAE. At 15 to 20 days after injection, there were similar levels of inflammation and pathology in mice with EAE (data not shown). However, beyond 40 days after injection, inflammation, demyelination, and WD were remarkably reduced in double-Tg mice (D), compared with single-Tg littermate controls (E). In addition, remyelination was more prominent relative to the overall pathology in the double-Tg group. Demyelination and remyelination were compared in double- and single-Tg mice at all CNS levels studied; P values are noted above each set of columns.
Histopathology was quantitated blindly under code by two investigators (K.M.O. and C.S.R.). CNS tissue from different regions, derived from double-Tg and control mice with EAE, was scored on a scale of 0 to 5 for inflammation, demyelination, axonal pathology (WD), and remyelination. During the acute phase of EAE (15 to 20 days after injection), there were similar amounts of myelin loss, axonal pathology, and inflammation in double-Tg animals and controls. However, in the chronic phase (40 to 60 days after injection), inflammation, demyelination, and WD were reduced in double-Tg mice, compared with CXCL1 or GFAP single-Tg littermate controls (Figure 4, D and E). Relative to the degree of myelin and axonal pathology, remyelination was more pronounced in the double-Tg group, a difference that was significant in the majority of regions of the CNS analyzed.
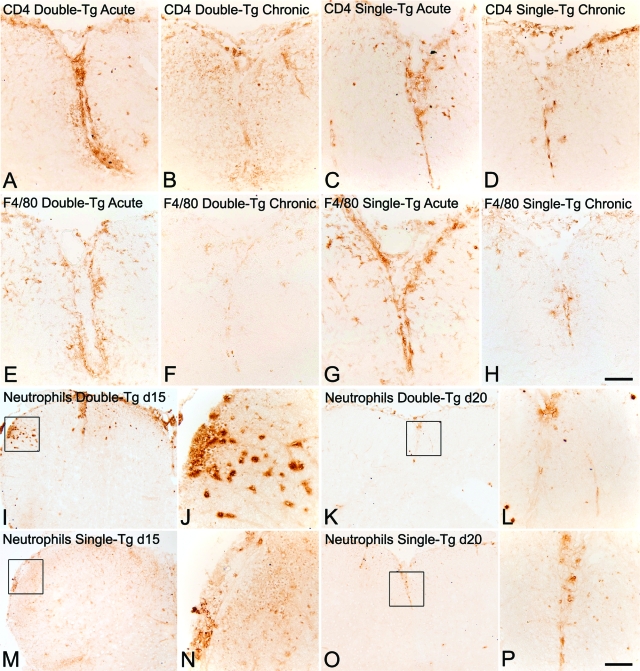
Immunohistochemistry revealed inflammatory cuffs containing T cells (CD4+) and macrophages/microglia (F4/80+), present in double-Tg, single-Tg, and wild-type animals during the late acute phase of EAE (15 and 20 days after injection). However, by 40 days after injection, few CD4+ and F4/80+ inflammatory cells were detected in lumbar anterior columns of double-Tg mice but were still prevalent in control animals (Figure 5, A–H). Immunostaining for neutrophils showed extensive infiltration in spinal cord sections from double-Tg mice at 15 days after injection compared with control single-Tg animals. However, by 20 days after injection, few neutrophils were present in both double-Tg and single-Tg mice (Figure 5, I–P). Because CXCL1 is a chemoattractant with the ability to influence neutrophil migration, these findings suggest that CXCL1 overproduction in mice with EAE resulted in increased neutrophil infiltration but numbers returned to control levels after doxycycline treatment ceased.
Figure 5.
Reduced inflammation and pathology in double-Tg mice with EAE. Inflammatory cell infiltration in mice with EAE was determined using antibodies against CD4 (A–D), F4/80 (E–H), and the anti-neutrophil antibody (I–P). Inflammatory cuffs containing CD4+ T cells (A, C) and F4/80+ macrophages/microglia (E, G) were present in lumbar spinal cords of both double-Tg and single-Tg animals during the late acute phase of EAE (15 and 20 days after injection). By 40 days after injection, few CD4+ and F4/80+ inflammatory cells were detected in lumbar anterior columns of double-Tg mice (B, F), but were still prevalent in control single-Tg animals (D, H). Immunostaining for neutrophils revealed extensive infiltration in spinal cord sections from double-Tg mice at 15 days after injection (I, J) compared with control single-Tg animals (M, N). However, by 20 days after injection, fewer neutrophils were present in both double-Tg (K, L) and single-Tg mice (O, P). Framed areas in I, K, M, and O are presented at higher magnification in the adjacent panels J, N, L, and P, respectively. Scale bars: 110 μm (A–H); 215 μm (I, K, M, O); 55 μm (J, N, L, P).
Reduced Demyelination and Axonal Damage in Double-Tg Mice with EAE
Histopathology revealed the presence of inflammation in spinal cord tissue from all groups (double-Tg, CXCL1 or GFAP single-Tg, and wild-type animals), at 15 days after injection, and demyelination and axonal pathology (WD) was also comparable in control mice (Figure 6, A–C). Samples from mice sacrificed after 40 days after injection showed less inflammation and WD in double-Tg animals, whereas single-Tg and wild-type mice still had extensive inflammation, demyelination, and axonal damage (Figure 6, D–F). This is in agreement with immunohistochemistry, which showed diminished inflammation in double-Tg animals compared with controls. During the chronic phase, in both double-Tg and control spinal cord tissue, numerous axons appeared to be thinly myelinated, indicative of remyelination (Figure 6, D and E). Similarly, in the optic nerve, demyelination and inflammation were reduced in double-Tg animals (Figure 6G), compared with extensive damage, gliosis, and inflammation in single-Tg and wild-type mice (Figure 6, H and I). Interestingly, there appeared to be increased numbers of oligodendrocytes in double-Tg mice, suggestive of proliferation (Figure 6, D and G).
Figure 6.
Toluidine blue-stained epoxy sections from doxycycline-treated mice with MOG-EAE. At 15 days after injection, inflammation is present around vessels (v) in spinal cord tissue of double-Tg (A), CXCL1 single-Tg (B), and wild-type (wt) animals (C), but demyelination and axonal pathology appear to be more prominent in the control mice (B, C). Asterisks indicate inset magnification point for A–I. Inset in A shows demyelinated axons and some remyelination; inset in B shows demyelination and WD; inset in C shows demyelinated axons and inflammatory cells. Tissue samples from mice sacrificed 40 days after injection reveal diminished inflammation and WD in double-Tg animals (D), whereas single-Tg (E) and wt (F) mice have extensive inflammation, demyelination, and axonal damage. Inset in D shows remyelination; inset in E demyelination and WD; and inset in F shows demyelination, WD, and infiltration. In both double-Tg and control spinal cord tissue, dotted lines outline zones of remyelination. In D, oligodendrocytes are more apparent and some are pointed out by small arrows. In the optic nerve, demyelination and inflammation were also reduced in double-Tg animals (G), compared with the extensive damage, gliosis, and inflammation observed in single-Tg (H) and wt mice (I). As in the spinal cord, there appeared to be an increased number of oligodendrocytes in double-Tg mice (G, small arrows), suggestive of an oligodendroglial response. Inset in G highlights remyelination and oligodendrocytes in double-Tg mice; inset in H shows demyelination and WD; inset in I shows inflammation and demyelination. Scale bar 30 μm (A–I); 60 μm (for insets in A–I).
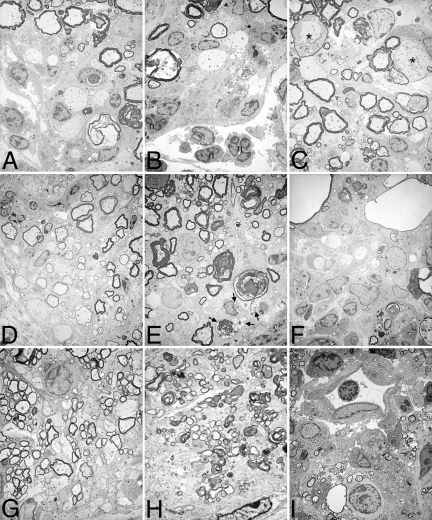
At the ultrastructural level, during the acute phase of EAE, inflammation and demyelination were prominent in spinal cord from double-Tg, single-Tg, and wild-type animals (Figure 7, A–C). During the chronic phase, remyelination was a major feature in double-Tg mice (Figure 7D). In contrast, although some remyelination was also evident in control animals, there was more ongoing demyelination, WD, and inflammation in double-Tg mice (Figure 7, E and F). Similar to spinal cord tissue, remyelination associated with oligodendrocytes was present in optic nerve from double-Tg animals (Figure 7G). Persistent axonal degeneration and inflammation were common in control animals at later stages (Figure 7, H and I). These findings suggest a role for CXCL1 in preventing widespread damage and in the sparing of axons during the course of EAE, which may in turn have a positive influence on oligodendrocyte proliferation/recruitment.
Figure 7.
Ultrastructural analysis of CNS tissue from mice with MOG-induced EAE. Plastic-embedded spinal cord (A–F) and optic nerve (G–I) tissue was processed for electron microscopy. Lumbar spinal cord from double-Tg (A, B) and single-Tg control mice (C) with acute EAE (15 to 20 days after injection) displayed classical features of EAE (infiltration, demyelination, WD). Neutrophils (multinucleated cells, n) appear to be part of the cellular infiltrate in double-Tg mice (B), and dystrophic axons (asterisk) were also observed (C). In animals with chronic EAE (>40 days after injection), remyelination was clearly evident in spinal cord tissue from double-Tg mice (D), indicated by the thinly myelinated axons. In contrast, demyelination and WD were still evident in control single-Tg (E) and wt (F) animals, despite some attempts to repair. Foamy macrophages with myelin debris were observed in sections from control mice (E, arrows). G: Similar to spinal cord tissue, remyelination was present in optic nerves from double-Tg mice with chronic EAE. Persistent neuronal degeneration, demyelination, and WD were found in optic nerves from single-Tg (H) mice, and inflammatory cells (lymphocytes and foamy macrophages) were found surrounding blood vessels in wt animals (I). Original magnifications: ×145 (A–C, I); ×110 (D–F); ×256 (G, H).
CXCL1/CXCR2 Expression and Cell Proliferation in Double-Tg Animals
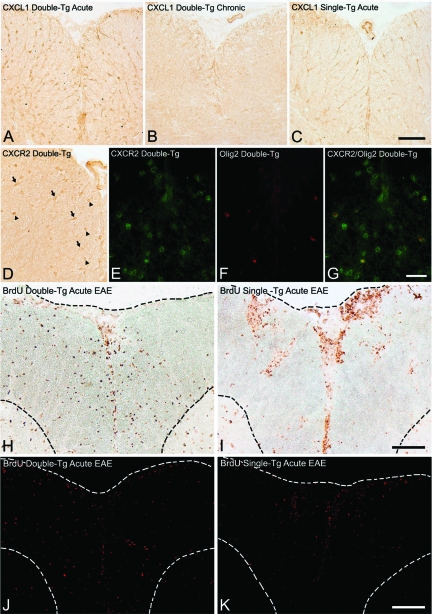
CXCL1 has been shown to be involved in oligodendrocyte proliferation and positioning in rodents.47,48 CXCL1 expression was examined in CNS tissue from mice with EAE. CXCL1 immunoreactivity was apparent during the late acute phase (15 to 20 days after injection) in both double-Tg (Figure 8A) and single-Tg (Figure 8C) mice, in cells with the morphology of reactive astrocytes. CXCL1 was more intense and widespread in double-Tg animals, compared with single-Tg controls, suggesting induced overproduction after administration of doxycycline. Similar areas from mice with chronic EAE revealed diminished CXCL1 reactivity on astrocytes in both double-Tg (Figure 8B) and single-Tg animals (data not shown). These results confirmed our ability to induce overproduction of CXCL1 within the CNS.
Figure 8.
Expression of CXCL1 and CXCR2 and cell proliferation in mice with EAE. CXCL1 expression was examined in spinal cord tissue from mice with EAE. Reactivity against CXCL1 was apparent during the late acute phase (15 to 20 days after injection) in both double-Tg (A) and single-Tg (C) mice, on cells with typical morphology of reactive astrocytes. CXCL1 expression appeared more intense and widespread in the double-Tg animals, compared with single-Tg controls, suggesting induced overproduction after doxycycline treatment. B: Staining of similar sections in mice with chronic EAE revealed diminished CXCL1 reactivity on astrocytes in double-Tg animals. D: Frozen sections from double-Tg mice were also stained for CXCR2, the receptor for CXCL1. Cells with typical oligodendroglial morphology were observed expressing CXCR2 (arrowheads) in spinal cord anterior columns. Astrocytes, with large nuclei and long processes (arrows), also stained positively for CXCR2. Double-immunofluorescence for CXCR2 (E, green) and Olig2 (F, red), demonstrated that CXCR2 was present on cells expressing the oligodendrocyte lineage marker (G, merge). To detect proliferation, BrdU was given to mice during doxycycline treatment and incorporation was analyzed in spinal cord sections. H: At 15 days after injection, BrdU was present in nuclei around inflammatory cuffs and was dispersed within white matter parenchyma of double-Tg mice. I: In contrast, BrdU was mainly found within cuffs in single-Tg mice, with limited BrdU uptake present in white matter regions. Immunofluorescence demonstrated the same pattern of staining with BrdU (red), staining more widespread in spinal cord parenchyma of double-Tg mice suggestive of oligodendrocyte proliferation (J), but more confined to inflammatory cuffs in single-Tg mice (K). Dotted lines in H–K outline the edge of spinal cord, and gray-white matter junction. Scale bars: 110 μm (A–C); 75 μm (D); 35 μm (E–G); 110 μm (H, I); 215 μm (J, K).
Frozen sections from double-Tg mice were also stained for CXCR2, the receptor for CXCL1. Immunohistochemistry showed small, round cells with a typical oligodendroglial morphology expressing CXCR2 in spinal cord anterior columns. Some astrocytes, with large nuclei and long processes also stained positively for CXCR2 (Figure 8D). Similar results were obtained by immunofluorescence with both oligodendrocytes and astrocytes labeling positively for CXCR2 (data not shown). Double immunofluorescence was performed to identify the cell types expressing CXCR2. Results demonstrated that some CXCR2 co-localized with the oligodendroglial cell lineage marker, Olig2 (Figure 8, E–G). Thus, CXCL1 overproduced by astrocytes in double-Tg occurred alongside receptors on both oligodendrocytes and astrocytes, was capable of initiating downstream effects in both these cell types (see below).
To determine whether CXCL1 production by astrocytes induced proliferation of oligodendrocytes during the course of EAE, BrdU was introduced into mice during doxycycline administration and incorporation was analyzed in spinal cord tissue. During the acute phase, BrdU was present in nuclei around inflammatory cuffs and was dispersed within white matter parenchyma of double-Tg animals (Figure 8, H and J). In contrast, BrdU was mainly found within inflammatory infiltrates in control mice, with limited uptake present in white matter regions (Figure 8, I and K). These results lend strength to the suggestion that CXCL1/CXCR2 interactions result in proliferation of cells within CNS white matter, which might explain the apparent increase in oligodendrocytes observed in the spinal cord and optic nerve in toluidine blue-stained sections of double-Tg animals (Figure 6, D and G).
Discussion
To further elucidate the role of chemokine receptors on human oligodendrocytes, and the role they might play during the course of MS, we generated and used a novel Tg mouse model in which CXCL1 expression was specifically induced within the CNS, under the control of the GFAP promoter. Analysis of spinal cord tissue from doxycycline-treated disease-free and EAE mice revealed an increase in β-gal expression in double-Tg mice but not in control single-Tg and wild-type animals. The increase in reporter gene product appeared to correlate with an increase in CXCL1 in astrocytes. CXCL1 binds to the chemokine receptor, CXCR2, which was found on mouse oligodendrocytes and some astrocytes in the same tissue. CXCR2-positive oligodendrocytes also expressed the oligodendrocyte lineage marker, Olig2.54 We recently reported the presence of CXC chemokine receptors (CXCR1, CXCR2, and CXCR3) on human oligodendrocytes, and the corresponding ligands, CXCL8, CXCL1, and CXCL10, on hypertrophic astrocytes (seen primarily in active MS lesions).24,55 Similar to our earlier results in human tissue, findings from the current study suggest that CXCL1/CXCR2-mediated signals might influence oligodendrocyte behavior during the course of inflammation in the CNS.
Double-Tg, CXCL1, or GFAP single-Tg and wild-type littermate mice all developed EAE when sensitized with MOG35-55 peptide, indicating that the two transgenes did not affect disease susceptibility. However, with the present novel Tg system, we could induce more CXCL1 production by astrocytes in double-Tg mice, demonstrated by the more increased intensity and widespread expression of the chemokine, compared with single-Tg and wild-type animals. CXCL1 overexpression in mice displaying initial signs of EAE resulted in a more attenuated disease course. In contrast, single-Tg and wild-type mice displayed a more severe course of EAE, which was significantly different from double-Tg mice. Histopathology and ultrastructural analysis showed that improved clinical scores of double-Tg mice were associated with reduced pathology, inflammation, and more remyelination. We also observed an apparent increase in small, round cells typical of oligodendrocytes after increased CXCL1 production by astrocytes. In single-Tg and wild-type animals, immune cells (T cells and macrophages) continued to infiltrate brain and spinal cord tissue. Moreover, demyelination and axonal pathology persisted in regions where there were also signs of repair (remyelination). The proliferative marker, BrdU, introduced during the course of doxycycline treatment, revealed cell proliferation within white matter parenchyma of double-Tg mice. In contrast, BrdU uptake was only associated with inflammatory cuffs in spinal cords of control animals.
Using the pTRE2-N51/CXCL1 mouse strain, Wiekowski and colleagues51 studied the effect of CXCL1 overexpression on the migration of neutrophils. They found that systemic overproduction of the chemoattractant results in receptor desensitization and consequently reduced neutrophil migration. A similar mechanism might explain the observed increase in neutrophil infiltration within the CNS immediately after doxycycline treatment, followed by reduced inflammation. CXCL1 has been detected in spinal cords of Theiler’s murine encephalomyelitis virus (TMEV)-infected SJL/J mice at the onset of clinical signs and has been proposed to act as a chemoattractant of neutrophils and macrophages in these mice.56 A recent study by Carlson and colleagues57 further supports a role of the chemokine CXCL1 in the recruitment of polymorphonuclear cells into the CNS. The authors demonstrate that disruption of the CXCL1/CXCR2 pathway during development and relapse of EAE prevents blood-brain barrier breakdown, CNS leukocyte infiltration, and diminishes clinical signs of the disease.57 In our study, neutrophils, known to influence the development on EAE,52,58 might have become desensitized to subsequent stimulation by chemokines (CXCL1), thus minimizing the extent of inflammation in double-Tg animals. In contrast, inflammatory infiltrates were present late in the chronic phase of EAE in single-Tg (CXCL1 or GFAP) and wild-type mice, which produced normal levels of CXCL1. Continued presence of CD4+ T cells and F4/80-expressing macrophages in control animals undoubtedly contributed to the ongoing pathology (demyelination, WD). Although the limited amount of inflammation in double-Tg mice might foster repair, ongoing pathology would probably prevent similar processes in the single-Tg and wild-type control animals. Although controlled CXCL1 overproduction might have beneficial effects during the course of EAE, unregulated overexpression of the same chemokine within the CNS has been shown to cause a neurodegenerative condition in mice.59 To what extent the improved outcome in the double-Tg group was related to a reduced inflammatory response or a direct neuroprotective effect remains to be elucidated.
Chemokines have been shown to influence inflammatory processes within the CNS. In particular, CCL2, CCL3, CCL5, and CXCL10 seem to play an important role in the recruitment of T cells and macrophages, acting via CCR2, CCR1, CCR5, and CXCR3, respectively.1,2,3,4,5,6,7,8,9 More recently, CXCL12 and CCL20 have been shown to attract immature dendritic cells to MS lesions,12 whereas CXCL13 appeared to direct B-cell recruitment into the CNS,10,11 and CXCL13-deficient mice have a mild, self-limiting form of EAE.60 In addition to a pro-inflammatory role, chemokines might also have regulatory effects within the CNS. Studies using CXCR3-deficient mice have demonstrated that CXC10/CXCR3 interactions reduce the severity of disease in mice with EAE.61,62 In addition, Elhofy and colleagues63 have reported that overproduction of the chemokine CCL2 in vivo down-regulates Th1 immune responses, resulting in mice with an attenuated form of EAE. Thus, our finding that double-Tg animals overproducing CXCL1 after administration of doxycycline display a milder course of MOG-EAE, compared with single-Tg and wild-type mice, is in line with the wide variety of effects that chemokines might have on CNS inflammation.
Our observation that CXCL1 overexpression in the presence of CNS inflammation might induce oligodendrocyte proliferation is in agreement with previous studies in rodents in which it was shown that immature CXCR2+ oligodendrocytes proliferated in response to CXCL1 (from astrocytes) in combination with PDGF-AA.47 Moreover, elevated levels of CXCL1 in the jimpy mutant correlated with increased proliferation of NG2+ oligodendrocyte progenitors.64 CXCL1 has also been shown to provide a migratory stop signal for oligodendrocyte precursor cells, thus influencing positioning of cells of the oligodendrocyte lineage during CNS development.48 Together with our earlier results that showed CXCR2+ oligodendrocytes closely associated with CXCL1+ hypertrophic astrocytes at the edge of MS lesions,24,55 the current findings suggest that CXCL1/CXCR2-mediated signals might lead to the accumulation or recruitment of oligodendrocytes at the lesion margin and potentially promote repair. However, the possibility also exists that the production of CXCL1 might prevent oligodendrocyte migration past the lesion edge, thus disrupting repair processes. Exploration of these pathways affords novel therapeutic avenues to enhance the limited remyelination typically seen in MS.
Acknowledgments
We thank Miriam Pakingan for expert technical assistance, and Drs. Stefanie Gaupp and Barbara Cannella for help with EAE experiments.
Footnotes
Address reprint requests to Dr. Cedric S. Raine, Ph.D., D.Sc., Department of Pathology (Neuropathology), Albert Einstein College of Medicine, 1300 Morris Park Ave., F-140, Bronx, NY 10461. E-mail: raine@aecom.yu.edu.
Supported in part by the National Multiple Sclerosis Society (grants FG 1422-A-1 to K.M.O. and RG 1001-K-11 and CA 1022-A-1 to C.S.R.), the National Institutes of Health (NS 07098, NS 08952, and NS 11920 to C.S.R.), and the Dana Farber Foundation (to S.A.L.).
Current address of K.M.O.: Phase Five Communications, Inc., New York, NY.
References
- Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran EH, Kuziel WA, Owens T. Induction of experimental autoimmune encephalomyelitis in C57BL/6 mice deficient in either the chemokine macrophage inflammatory protein-1alpha or its CCR5 receptor. Eur J Immunol. 2000;30:1410–1415. doi: 10.1002/(SICI)1521-4141(200005)30:5<1410::AID-IMMU1410>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Teleshova N, Pashenkov M, Huang YM, Soderstrom M, Kivisakk P, Kostulas V, Haglund M, Link H. Multiple sclerosis and optic neuritis: CCR5 and CXCR3 expressing T cells are augmented in blood and cerebrospinal fluid. J Neurol. 2002;249:723–729. doi: 10.1007/s00415-002-0699-z. [DOI] [PubMed] [Google Scholar]
- Fife BT, Kennedy KJ, Paniagua MC, Lukacs NW, Kunkel SL, Luster AD, Karpus WJ. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–7624. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst C, Sorensen TL, Kivisakk P, Cathcart MK, Hesselgesser J, Horuk R, Sellebjerg F, Lassmann H, Ransohoff RM. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am J Pathol. 2001;159:1701–1710. doi: 10.1016/s0002-9440(10)63017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, Meinl E. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:11–23. doi: 10.1016/j.jneuroim.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, O'Connor M, Hoxie JA, Gonzalez-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2000;26:133–142. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- Goldberg SH, van der Meer P, Hesselgesser J, Jaffer S, Kolson DL, Albright AV, Gonzalez-Scarano F, Lavi E. CXCR3 expression in human central nervous system diseases. Neuropathol Appl Neurobiol. 2001;27:127–138. doi: 10.1046/j.1365-2990.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Stangel M. Expression of the chemokine receptors CXCR1 and CXCR2 in rat oligodendroglial cells. Brain Res Dev Brain Res. 2001;128:77–81. doi: 10.1016/s0165-3806(01)00128-6. [DOI] [PubMed] [Google Scholar]
- Biber K, Dijkstra I, Trebst C, De Groot CJ, Ransohoff RM, Boddeke HW. Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience. 2002;112:487–497. doi: 10.1016/s0306-4522(02)00114-8. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Everall IP, Male DK. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS. Glia. 2002;37:64–75. doi: 10.1002/glia.1128. [DOI] [PubMed] [Google Scholar]
- Filipovic R, Jakovcevski I, Zecevic N. GRO-alpha and CXCR2 in the human fetal brain and multiple sclerosis lesions. Dev Neurosci. 2003;25:279–290. doi: 10.1159/000072275. [DOI] [PubMed] [Google Scholar]
- Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia. 2005;50:258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- Maysami S, Nguyen D, Zobel F, Heine S, Hopfner M, Stangel M. Oligodendrocyte precursor cells express a functional chemokine receptor CCR3: implications for myelination. J Neuroimmunol. 2006;178:17–23. doi: 10.1016/j.jneuroim.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Raine CS. The neuropathology of multiple sclerosis. Raine CS, McFarland HF, Tourtellotte WW, editors. London: Chapman & Hall,; Multiple SclerosisClinical and Pathogenetic Basis. 1997:pp 149–170. [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- D'Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–2370. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti B, Raine CS. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann Neurol. 1997;42:74–84. doi: 10.1002/ana.410420113. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Raine CS, Scheinberg L, Waltz JM. Multiple sclerosis. Oligodendrocyte survival and proliferation in an active established lesion. Lab Invest. 1981;45:534–546. [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Goldenberg PZ, Ilyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. [PubMed] [Google Scholar]
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121:2221–2228. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Solanky M, Menonna J, Chapin J, Li W, Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49:776–785. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Bruck W, Lucchinetti C, Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Cannella B, Raine CS. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation. Ann Neurol. 2004;55:46–57. doi: 10.1002/ana.10764. [DOI] [PubMed] [Google Scholar]
- Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH. The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. J Neurosci. 1998;18:10457–10463. doi: 10.1523/JNEUROSCI.18-24-10457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Frost E, To V, Robinson S, French-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sorensen T, Tani M, Wang J, Chien P, O'Bryan S, Bielecki B, Zhou ZL, Majumder S, Ransohoff RM. Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol Rev. 2000;177:52–67. doi: 10.1034/j.1600-065x.2000.17709.x. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- Wiekowski MT, Chen SC, Zalamea P, Wilburn BP, Kinsley DJ, Sharif WW, Jensen KK, Hedrick JA, Manfra D, Lira SA. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol. 2001;167:7102–7110. doi: 10.4049/jimmunol.167.12.7102. [DOI] [PubMed] [Google Scholar]
- Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GR, Traugott U, Farooq M, Norton WT, Raine CS. Experimental autoimmune encephalomyelitis. Augmentation of demyelination by different myelin lipids. Lab Invest. 1984;51:416–424. [PubMed] [Google Scholar]
- Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;54:35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- Omari KM, John G, Lango R, Raine CS. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia. 2006;53:24–31. doi: 10.1002/glia.20246. [DOI] [PubMed] [Google Scholar]
- Rubio N, Sanz-Rodriguez F. Induction of the CXCL1 (KC) chemokine in mouse astrocytes by infection with the murine encephalomyelitis virus of Theiler. Virology. 2007;358:98–108. doi: 10.1016/j.virol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- Tani M, Fuentes ME, Peterson JW, Trapp BD, Durham SK, Loy JK, Bravo R, Ransohoff RM, Lira SA. Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J Clin Invest. 1996;98:529–539. doi: 10.1172/JCI118821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagaeva LV, Rao P, Powers JM, Segal BM. CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176:7676–7685. doi: 10.4049/jimmunol.176.12.7676. [DOI] [PubMed] [Google Scholar]
- Liu L, Huang D, Matsui M, He TT, Hu T, Demartino J, Lu B, Gerard C, Ransohoff RM. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- Müller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, Campbell IL. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- Elhofy A, Wang J, Tani M, Fife BT, Kennedy KJ, Bennett J, Huang D, Ransohoff RM, Karpus WJ. Transgenic expression of CCL2 in the central nervous system prevents experimental autoimmune encephalomyelitis. J Leukoc Biol. 2005;77:229–237. doi: 10.1189/jlb.0804465. [DOI] [PubMed] [Google Scholar]
- Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the jimpy mutant. J Neurosci. 2000;20:2609–2617. doi: 10.1523/JNEUROSCI.20-07-02609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]