Abstract
The purpose of this study was twofold: to reveal cellular events associated with the protective role of endogenous annexin A1 (AnxA1) in inflammation and to highlight the potential involvement of members of the formyl peptide receptor (Fpr) family in this process. We found that wild-type, AnxA1-null, and Fpr1-null mice all displayed an intense neutrophil recruitment into the peritoneal cavity as assessed 4 hours after carrageenin injection, and that this recruitment was most pronounced in AnxA1-null mice. In addition, this cell influx could be inhibited by the AnxA1 pharmacophore peptide, Ac2-26, in wild-type, AnxA1-null, and Fpr1-null mice, but was restored when co-treated with the pan-receptor antagonist Boc2. Using the LacZ gene reporter assay, an enhancement of AnxA1 gene promoter activity in extravasated neutrophils was evident in AnxA1-null mice; again this response was reduced after peptide treatment. The lack of functional involvement of Fpr1 prompted us to monitor the structurally related receptor Fpr2. We report, for the first time, the ultrastructural immunocytochemical co-localization of Fpr2 with AnxA1 in neutrophils that migrate into the mesenteric microcirculation and extravasate into the peritoneal fluid. Collectively, these data provide in vivo support to the hypothesis that endogenous AnxA1 is an essential effector of endogenous anti-inflammation and provide an ultrastructural indication that this mediator interacts with Fpr2 in murine neutrophils. We believe that these findings could significantly affect the development of novel therapeutics, which are modeled after the anti-migratory actions of AnxA1.
The annexin A1 (AnxA1) pathway in neutrophils is comprised of the ligand, receptor, and catabolic enzyme, the latter cleaving N-terminal peptides from the 346-amino acid native protein.1,2 We and others have demonstrated that the AnxA1 pathway is part of the complex response the host activates during inflammation to effect modulatory and homeostatic actions to keep cell activation and extravasation under a tonic inhibitory control. This model has been mostly based on pharmacological studies conducted with full-length AnxA1 (37-kDa protein), bioactive peptides derived from its N-terminal sequence (unique in the annexin superfamily of proteins)3 and passive immunization strategies.4,5,6,7,8
Studying AnxA1 expression and mobilization in human polymorphonuclear leukocytes (PMNs) has markedly contributed to understanding molecular and cellular mechanisms by which this endogenous inhibitory pathway can be activated. Resting PMNs express high levels of the protein in their cytosol, a large proportion of which (>50%) is in gelatinase granules.9 On PMN activation or adhesion, the protein is externalized to the cell surface10,11 where it activates downmodulating signals that inhibit PMN adhesion and transmigration.10,12,13
Generation of AnxA1-null mice has helped clarify the role of the AnxA1 pathway in inflammation: these mice are more prone to both acute and chronic inflammatory reactions.14,15 PMNs purified from AnxA1-null mouse blood exhibit greater degrees of activation and chemotaxis in response to distinct stimuli application.16 This behavior is reflected in a higher degree of emigration in the inflamed microcirculation, regardless of the vascular bed observed (cremaster or mesentery) or stimulus applied (platelet-activating factor or zymosan).16
A breakthrough in this field by Walther and colleagues11 demonstrated a direct interaction between AnxA1-derived peptide and the receptor for formylated peptides, FPR. Subsequent studies have demonstrated that the situation might be more complex, because all three human receptors of the FPR family [FPR, FPRL-1 (FPR-like 1, also called ALX because it is a functional transducer of the anti-inflammatory signal of lipoxin A4) and FPRL-2 (FPR-like 2)] could be activated with the peptide Ac2-26 and other N-terminal-derived sequences of AnxA1.17 In addition, comparison of the binding properties of full-length AnxA1 and the peptide Ac2-26 revealed distinct interactions with FPR and ALX/FPRL-1, such that the protein bound to and activated only the latter, whereas the peptide bound and activated both receptors.13 There are no data with respect to AnxA1 binding to, and/or activation of, FPRL-2. However, it is currently accepted that members of the FPR family transduce the cellular activities of AnxA1, at least at the level of monomyelocytic cells.18
We have previously used immunohistochemistry and ultrastructural analyses to shed light on components of the AnxA1 pathway during on-going inflammatory reactions in rats19 and mice.20,21 Collectively, these studies have shown that i) PMN-derived AnxA1 is mobilized on the cell surface of the adherent PMNs, probably subsequent to mobilization of gelatinase granules; ii) extravasated PMNs contain higher levels of AnxA1 compared with intravascular cells; iii) the AnxA1 gene promoter activity (monitored by expression of the reporter, the LacZ gene) is augmented in extravasated PMNs; and iv) AnxA1 cleavage appears to be more pronounced in extravasated PMNs so that cleaved (deprived on the N-terminal region) AnxA1-like immunoreactivity is more abundant in these cells, at least during the early hours of the inflammatory response.
One question that remains to be answered regards the identity of the receptor(s) responsible for the anti-inflammatory properties of AnxA1 and peptide Ac2-26 observed in experimental systems in rodents. To solve this problem in AnxA1 biology is not trivial, because FPR genes have undergone expansion in the mouse genome so that at least six different genes were initially described (Fpr, Fpr-related sequence 1 to 5),22 with two others being added later (Fpr-rs6 and Fpr-rs7).23 Here we have begun unraveling this question using the tools available, namely: i) the Fpr1-null mouse colony24; ii) a new antiserum to Fpr-rs2 (now known officially as Fpr2); iii) the pan-Fpr antagonist Boc2. In addition, we have extended our detailed analysis of the AnxA1 pathway in early inflammatory responses to the carrageenin-induced mouse peritonitis model, monitoring protein expression (and its cellular localization) as well as AnxA1 gene promoter activity (using the LacZ gene reporter borne by the AnxA1-null mice).20,21
Materials and Methods
Animals
Male wild-type (WT) littermate, AnxA1-null,14 and Fpr1-null mice22 (20 to 25 g of body weight) were used for all experiments. Founders of the Fpr-null colony were kindly donated by Ji Liang Gao and Philip Murphy (National Institutes of Health, Bethesda, MD).24 Animals were maintained on a standard chow pellet diet with tap water ad libitum and housed at a density of five animals per cage in a room with controlled lighting (lights on from 8:00 AM to 8:00 PM) in which the temperature was maintained at 21 to 23°C. Animal work was performed according to U.K. Home Office regulations (Guidance on the Operation of Animals, Scientific Procedures Act 1986).
Model of Inflammation
Experimental peritonitis was induced by an intraperitoneal injection of 1.5 mg/kg carrageenin (type λ; Sigma Chemical Co., Poole, UK) in phosphate-buffered saline (PBS). Vehicle animals were injected intraperitoneally with PBS rather than carrageenin. In all cases, mice (n = 5 per group) were sacrificed 1 and 4 hours later by CO2 exposure.
Drug Treatment
For this set of pharmacological experiments, mice (n = 5) were administered intravenously with 100 μg per mouse of the annexin 1 mimetic peptide Ac2-26 (Ac-AMVSEFLKQAWFIENEEQEYVQTVK)19 in PBS, 15 minutes before experimental peritonitis. As a control to Fpr receptors, the WT mice received intravenously 10 μg per mouse of the FPR antagonist Boc2 (N-t-butyloxycarbonyl-Phe-Dleu-Phe-Dleu-Phe) (ICN Pharmaceuticals, Basingstoke, UK) followed by peptide treatment.25
Real-Time Polymerase Chain Reaction (PCR) Analysis for Formyl Peptide Receptors and AnxA1 Protein
To quantify specific mRNA levels, RNA was isolated from total peritoneal neutrophils using spin column methodology according to the manufacturer’s guidance (RNeasy kit; Qiagen, Crawley, UK). Contaminating genomic DNA was removed by on-column pre-DNase digestion as per the manufacturer’s instructions (Qiagen). RNA was reverse-transcribed using 2 μg of oligo(dT) 15 primer (Promega, Southampton, UK), 10 U avian myeloblastosis virus (AMV) reverse transcriptase, 40 U ribonuclease inhibitor (Promega), and 1.25 mmol/L each deoxyribonucleoside triphosphate (dNTP) for 60 minutes at 42°C. The synthesized cDNA was used for real-time PCR. An equal amount of first-strand cDNA was amplified by PCR using platinum TaqDNA polymerase (Invitrogen, Carlsbad, California, USA). The forward and reverse primers for mouse Fpr1, Fpr-rs1, and Fpr2 are described by Wang and Ye.23,26 The PCR reaction was performed at 94°C for 5 minutes followed by 40 cycles of 94°C for 30 seconds, 61°C for 30 seconds, and 72°C for 30 seconds with a final extension of 72°C for 7 minutes. Amplification data are expressed as number of cycles whereby GAPDH is used as endogenous control.
Assessment of Cell Extravasation
Neutrophil migration was assessed both into the mesenteric tissue and into the peritoneal cavity. After sacrifice, 3 ml of PBS were injected into peritoneal cavity; then 100 μl of peritoneal fluid were collected and diluted 1/10 in Turk’s solution (0.1 crystal violet in 3% acetic acid). Quantification of neutrophils in the peritoneal fluids was performed with a Neubauer chamber (BOECO, Hamburg, Germany) using a ×40 objective.
Fixation, Processing, and Embedding for Light and Electron Microscopy
Peritoneal cells and fragments of mesentery were fixed in 4% paraformaldehyde, 0.5% glutaraldehyde, and 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 24 hours at 4°C, washed in sodium cacodylate, and dehydrated through graded percentages of ethanol, and embedded in LRGold (London Resin Co., Reading, UK). Sections (0.5 μm thick) were stained with 1% toluidine blue in 1% borax solution (TAAB Laboratories, Aldermaston, UK). Sections of mesentery were analyzed on an Axioskop 2-Mot Plus Zeiss microscope (Carl Zeiss, Jena, Germany) for quantification of neutrophils. Values are shown as mean ± SEM of cells number per mm2 of four sections (0.5 μm) per sample (five samples per animal). For electron microscopy, sections (∼90 nm) were cut on an ultramicrotome (Reichert Ultracut; Leica, Viena Austria) and placed on nickel grids for immunogold labeling.
AnxA1 Gene Expression by X-Gal Stain
AnxA1-null mice have a LacZ gene inserted into the targeting construct to facilitate measurement of gene expression. Cells and tissues from AnxA1-null mice were therefore stained with the histochemical X-Gal technique. In the presence of β-galactosidase, this staining produces a characteristic Prussian blue color. Samples were fixed in 4% paraformaldehyde, 0.1 mol/L phosphate buffer (pH 7.3) for 1 hour at 4°C, and washed with a rinse solution (0.1 mol/L phosphate buffer, pH 8, 2 mmol/L magnesium chloride, 0.1% Triton X-100), three times for 30 minutes each. Samples were stained overnight at 37°C using X-Gal staining solution (5 mmol/L potassium ferrocyanide in rinse buffer plus 1 mg/ml β-galactosidase in dimethylformamide). Fragments were then washed in PBS at room temperature and postfixed in 4% paraformaldehyde before embedding in LRGold resin. The embedded cellular and tissue samples were then cut (∼1 μm) on a microtome (RM2265; Leica, Wetzlar, Germany) and counterstained with hematoxylin for subsequent analysis. Densitometric analysis for X-Gal staining was done according to an arbitrary scale ranging from 0 to 255 by Axiovision software acopled on Zeiss Axioskop 2 light microscope.
Postembedding Immunogold Labeling
To detect the co-localization of endogenous AnxA1 protein with the Fpr2 receptor in neutrophils, ultrathin sections (∼90 nm) of mesentery and peritoneal fluid cell pellet were incubated in a step by step manner with the following reagents at room temperature: i) distilled water; ii) 0.1 mol/L phosphate buffer containing 1% egg albumin (PBEA); iii) 0.1 mol/L PBS containing 5% egg albumin (PBEA) for 30 minutes; iv) sheep polyclonal antibody termed LCS3, raised against intact and cleaved isoforms of AnxA1 (1:200 in PBEA) and rabbit polyclonal antibody Fpr2β (1:100 in PBEA) for 2 hours, normal sheep and rabbit sera were used as control (1:200); v) three washes (5 minutes each) in PBEA containing 0.01% Tween 20. To detect AnxA1, vi) donkey anti-sheep IgG (Fc fragment-specific) antibody (1:100 in PBEA) conjugated to 10-nm colloidal gold (British Biocell, Cardiff, UK) was added, and to detect Fpr2 receptor, goat anti-rabbit IgG (Fc fragment-specific) antibody (1:100 in PBEA) conjugated to 20 nm colloidal gold (British Biocell, Cardiff, UK) was added; vii) after 1 hour, sections were washed extensively in PBEA containing 0.01% Tween 20, then in distilled water. Sections were stained with uranyl acetate and lead citrate before examination using a Zeiss Leo 906 electron microscope. The density of immunogold, conjugated to AnxA1 and Fpr2, was calculated and expressed for plasma membrane of neutrophils. Values are reported as mean ± SEM of number of gold particles per μm.
Statistical Analysis
All data are mean ± SEM of n ≥ 5 mice per groups. In vitro analyses were repeated at least twice. Statistical differences between means were determined by analysis of variance followed, if significant, by the Bonferroni post-hoc test (on selected experimental groups). A probability value less than 0.05 was taken as significant.
Results
Effects of Peptide Ac2-26 and Boc2 on Carrageenin-Induced Neutrophil Migration
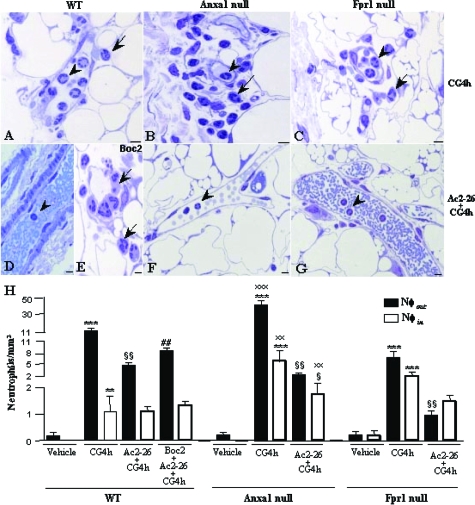
Initially, we used the carrageenin-induced mouse peritonitis model to study the function of the AnxA1 pathway. Figure 1 shows that carrageenin injection produced marked neutrophil infiltration into the mesenteric tissues. AnxA1-null mice displayed larger numbers of intravascular and extravasated neutrophils when compared with WT (Figure 1, A and B). Fpr1-null mice displayed attenuated cell recruitment in relation to WT mice; however, the difference was not significant (Figure 1C). Figure 1H reports the quantitative values, with a near doubling of neutrophils adherent in the vasculature in AnxA1-null mice, and a fourfold increase in the number of extravasated cells.
Figure 1.
Effects of the peptide Ac2-26 and the Fpr antagonist Boc2 treatment on carrageenin-induced neutrophil influx. WT, AnxA1-null, and Fpr1-null mice were administered with carrageenin (CG4h) and peptide Ac2-26 (Ac2-26 + CG4h) as described in the Materials and Methods. A–C: The carrageenin administration induced a high incidence of intravascular (arrowheads) and extravascular (arrows) neutrophils. D, F, and G: Few intravascular neutrophils were observed after peptide Ac2-26 treatment (Ac2-26 + CG4h). E: WT mice administered with Boc2 (Boc2 + Ac2-26 + CG4h) showed a high incidence of extravascular neutrophils. Sections were 0.5 μm. Staining: toluidine blue. H: Statistical analysis. Data are mean ± SEM of n = 5 mice per group. ***P < 0.001 versus respective vehicle group; §P < 0.05 and §§P < 0.01 versus respective CG4h group; ##P < 0.01 versus respective Ac2-26 + CG4h group; xxP < 0.01 and xxxP < 0.001 versus respective group from WT mice. Scale bars = 5 μm.
Treatment of mice with the peptide Ac2-26 exerted significant inhibitory properties on this inflammatory response, with a selective effect on extravasated cell numbers. This AnxA1 mimetic was equally effective in both WT and Fpr1-null mice, and produced a remarkable reduction of this inflammatory readout especially in the AnxA1-null mice, in which the response was very pronounced (Figure 1, D, F, and G). The antimigratory property of the peptide on intravascular adherent neutrophils was observed only in AnxA1-null mice (Figure 1H).
Finally, co-injection of the pan-receptor antagonist Boc2 prevented the inhibitory effect of peptide Ac2-26 on neutrophil extravasation, restoring values close to those measured in carrageenin-treated WT mice (Figure 1, E and H). Table 1 shows that carrageenin injection produced a marked neutrophil-infiltration, and details the antimigratory property of peptide Ac2-26 and Boc2 antagonist effect on neutrophil influx into the peritoneal fluid. These results justified the use of this model for monitoring Anxa1 gene promoter activity and the expression of AnxA1 protein in the neutrophils.
Table 1.
Effects of the Peptide Ac2-26 and the Fpr Antagonist Boc2 Treatment on Neutrophil Influx of Peritoneal Fluid
| Neutrophils × 105/ml in peritoneal fluid
| |||
|---|---|---|---|
| WT | Anxa1-null | Fpr1-null | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.03 |
| CG4h | 14.33 ± 1.76** | 30.67 ± 1.2*** | 16.33 ± 2.02** |
| Ac2-26 + CG4h | 8.0 ± 0.57§ | 6.33 ± 2.18§§ | 9.0 ± 0.57§ |
| Boc2 + Ac2-26 + CG4h | 14.0 ± 1.15# | ||
CG, Carrageenin. Data are mean ± SEM of n = 5 mice per group.
P < 0.01 and
P < 0.001 vs. respective vehicle group;
P < 0.05 and
P < 0.01 vs. respective CG4h group;
P < 0.05 vs. respective Ac2-26 + CG4h group. ANOVA plus Bonferroni test.
AnxA1 Gene Expression
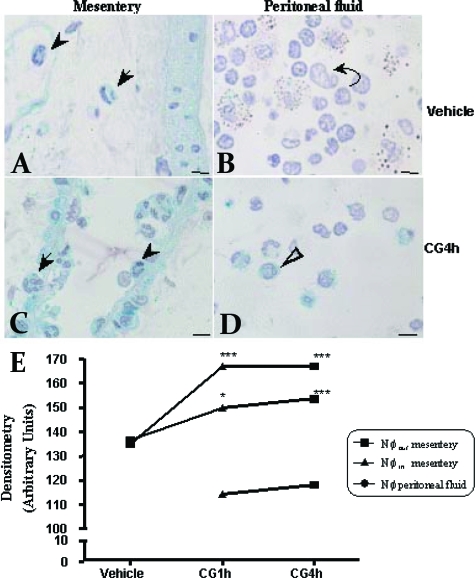
Activation of the AnxA1 gene promoter was monitored in the mesentery and peritoneal fluid neutrophils from AnxA1-null mice. As expected, mesenteric neutrophils from vehicle mice displayed negative reactivity for X-Gal staining assay (Figure 2A). We did not detect neutrophils in peritoneal fluid from vehicle mice (Figure 2B). Injection of carrageenin provoked intense gene promoter activation with marked staining both in intravascular and extravasated mesenteric neutrophils as well as transmigrated neutrophils in the peritoneal fluids (Figure 2, C and D). Figure 2E reports the densitometric analysis of this set of results: there was a time-dependent AnxA1 gene promoter activation, with a rapid response as early as 1 hour after carrageenin. In addition, extravasated neutrophils were more positive than intravascular cells.
Figure 2.
Analysis of LacZ gene expression on neutrophils from mesentery and peritoneal fluid. AnxA1 gene promoter activity was observed by LacZ gene expression as detailed in the Materials and Methods. Intravascular (arrowhead) and extravascular (arrow) neutrophils from mesentery (A) and macrophages (curved arrow) from peritoneal fluid (B) displayed LacZ-negative staining in vehicle mice. Higher staining after 4 hours of inflammation (CG4h) in intra- and extravascular neutrophils from mesentery (C) and neutrophils (open arrow) from peritoneal fluid (D). Counterstaining: hematoxylin. E: Statistical analysis. Data are mean ± SEM of n = 5 mice per group. ***P < 0.001 and *P < 0.05 versus respective vehicle group. Scale bars = 5 μm.
Ultrastructural Immunocytochemistry of Elements of the AnxA1 Pathway
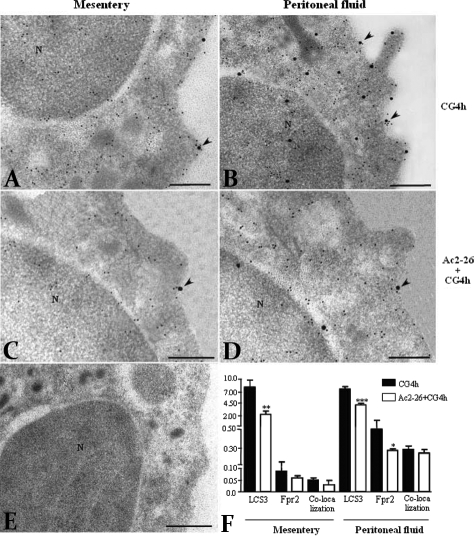
In the last section of the study we sought to extend the data produced with the LacZ gene reporter assay by monitoring the expression of the AnxA1 protein and its putative receptor Fpr2. To do so, we tested the expression of potential receptors responsible for the effects of peptide Ac2-26 to analyze the elements of the AnxA1 pathway. Real-time PCR of peritoneal neutrophils revealed presence of mouse Fpr1 and Fpr2 (Figure 3) but not Fpr-rs1 (data not shown). AnxA1 mRNA expression is shown for comparative purposes (Figure 3). Encouraged by these findings, we then performed ultrastructural immunocytochemistry in extravasated mesenteric neutrophils. These cells, which were induced with carrageenin, were strongly positive for AnxA1 and displayed reactivity also for Fpr2 (Figure 4A); the same held true for peritoneal neutrophils (Figure 4B). We did not detect ultrastructural immunocytochemistry co-localization of AnxA1 with Fpr2 in neutrophils treated with the antagonist Boc2, demonstrating that this antagonist blocks AnxA1 co-localization with Fpr2 (data not shown). Lower AnxA1 and Fpr2 immunoreactivity was observed in plasma membrane of mesenteric (Figure 4C) and peritoneal (Figure 4D) neutrophils after treatment of mice with peptide Ac2-26. No labeling was detected in sections incubated with the control nonimmune sheep or rabbit sera (Figure 4E).
Figure 3.
Real-time PCR analysis for mouse formyl peptide receptors and AnxA1 protein. Peritoneal neutrophils from WT mice were used to quantify specific mRNA levels. Primers for GADPH were used as controls. Data are presented as mean ± SEM of n = 5 mice per group.
Figure 4.
Co-localization and density of AnxA1 and Fpr2 immunogold particles on the plasma membrane of mesenteric and peritoneal neutrophils. Co-localized particles of AnxA1 and Fpr2 receptor (10-nm and 20-nm colloidal gold particles, respectively) were detected on plasma membrane (arrowheads) of neutrophils, from mesentery and peritoneal fluid, after 4 hours of carrageenin induction (A, B) and the peptide Ac2-26 treatment (C, D). E: Absence of gold labeling for AnxA1 and Fpr2 in neutrophils incubated with nonimmune sheep and rabbit serum. N, nucleus. F: Statistical analysis. Data are mean ± SEM of gold particles per μm of 10 distinct neutrophils analyzed at 4 hours after carrageenin-induced peritonitis and after peptide Ac2-26 treatment. *P < 0.05, **P < 0.01 and ***P < 0.001 versus CG4h group. Analysis of variance plus Bonferroni test. Scale bars = 0.5 μm.
Then, we noted that Fpr2 immunoreactivity was often associated with AnxA1 immunoreactivity; this was particularly evident on the neutrophil cells surface as well as in membrane protrusions (Figure 4B). Quantitative analyses of multiple cells, at 4 hours after the carrageenin time point, revealed that a high degree of co-localization (∼50%) was observed for AnxA1 and Fpr2 (Figure 4F). In addition, whereas there were no measurable differences between mesenteric and peritoneal neutrophil AnxA1, the latter cells expressed much higher amounts of Fpr2 protein (Figure 4F). As stated above, approximately half of this was co-localized with AnxA1.
Discussion
We demonstrate here that, in the carrageenin-induced peritonitis in the mouse, the endogenous AnxA1 system exerts tonic inhibitory properties on PMN migration. We also provide evidence for a rapid activation of the AnxA1 gene promoter, associated with an increased expression of the protein particularly in extravasated cells. The latter ultrastructural analysis was conducted, for the first time, in association with the determination of mouse Fpr2 protein expression, observing a high degree of co-localization with the putative ligand.
We began the present investigation by testing the role of endogenous AnxA1, and that of exogenously administered peptide Ac2-26, during carrageenin-induced acute peritonitis in WT and Anxa1-null mice. The rat carrageenin peritonitis model has been previously used to determine the pharmacological efficacy of AnxA1 and its peptidomimetics.19 Compared with WT mice, AnxA1-null mice displayed higher neutrophil extravasation at the 4-hour time point, confirming also in this model the importance of endogenous AnxA1 on the regulation of PMN migration.21 These findings are in line with a recent study in which the higher extent of AnxA1-null PMN migration in vivo, and chemotaxis in vitro, was highlighted.16 We propose that these alterations correlate with protein induction observed in neutrophils of WT mice. The AnxA1 gene activation in circulating neutrophils was augmented after carrageenin injection; the protein acts to down-regulate PMN migration across postcapillary venules. In the AnxA1-null mice, this braking mechanism is not operative and PMNs continue to extravasate even 4 hours after carrageenin. All of the changes observed as in WT as in AnxA1-null mice were rescued by administrating an anti-inflammatory dose of the AnxA1 mimetic peptide Ac2-26,27 confirming their genuine link to the absence of the protein. Next, we complemented these functional data by monitoring AnxA1 gene promoter activation by using the LacZ reporter gene.14 The observed increase in de novo protein synthesis after carrageenin was confirmed by intense activation of the AnxA1 gene promoter activity in circulating PMNs. Increased AnxA1 gene promoter activity was seen in extravasated PMNs, above the levels determined in intravascular PMNs. Therefore, in addition to recently published studies,20,21 PMN activation in the mouse mesenteric microcirculation in response to carrageenin is paralleled by a rapid activation of the AnxA1 gene promoter, as early as 1 hour after stimulus application. Such a response is also linked to the PMN transmigration process. It is plausible that outside-in signaling, generated by endothelial junction adhesion molecules and/or extracellular matrix proteins28 would be the stimulus responsible for up-regulating the AnxA1 gene promoter. Future studies will address these molecular mechanisms.
As often stated, the glucocorticoid-modulated protein AnxA1 is an endogenous mediator able to down-regulate the process of leukocyte extravasation, and therefore it is one of the mediators responsible for anti-inflammation,29,30 however, the molecular mechanism(s) responsible for AnxA1 anti-migratory activity has been elusive. Walther and colleagues11 were the first to suggest that human FPR might be an important target for AnxA1 peptides. However, subsequent findings in human PMNs indicated that FPR might not be the sole receptor for bringing about the homeostatic actions of AnxA1 and its peptides, such that other members of the FPR family may play a significant role.13,31,32,33 In the mouse, the FPR family is complex, with Fpr and seven Fpr-related receptors: Fpr-rs1 to Fpr-rs734; however, according to the latest annotation of the mouse genome project data (UCSC genome browser), the murine Fpr family has recently been again revised. The terminology of gene Fpr-rs1 has been changed. It is now thought to exist in two isoforms, one that is designated Fpr3 (now the official nomenclature) and the other comprises an exon from Fpr3 and one from Fpr2. So, it is clear that Fpr-rs1 does not exist as such.
We were interested in the biology of murine formyl peptide receptors because of the need to identify the receptor(s) responsible for the anti-inflammatory actions of AnxA1 and its peptides. Pharmacological studies were conducted with a Fpr-null mouse colony24,35 in combination with commercially available Boc derivatives (pan-antagonists). Using these tools we have shown that: i) the conventional inhibitory actions of peptide Ac2-26 in a model of murine peritonitis were absent in Fpr1-null mice whereas in the same study, AnxA1 retained a substantial anti-migratory effect.35 At the time this result was puzzling, however, we now know that indeed the peptide and its parent protein may activate distinct receptors, at least in human cells13; ii) analysis of the inflamed microcirculation of the mouse mesentery revealed that the detachment of leukocytes adherent to the postcapillary venules produced by peptide Ac2-26 was fully retained in Fpr1-null mice32; it was blocked by Boc antagonists and mimicked by a stable analogue of lipoxin A4 (but not by formyl-Met-Leu-Phe or the CXC chemokine KC)32; iii) the tissue-protective properties of peptide Ac2-26 in models of myocardial infarct36 and of brain stroke37 were maintained in Fpr1-null mice; however, they were blocked by Boc antagonists; in addition the injured tissues (heart or brain) displayed augmented expression of ALX/Fpr2; iv) finally, peptide Ac2-26 bound specifically to Fpr2-transfected HEK cells.37 Collectively, these studies indicate that peptide Ac2-26 activation of Fpr1 might be required for inhibition of leukocyte chemotaxis in the subendothelial tissue, in view of the data in the zymosan peritonitis mentioned above; however, the anti-adhesive and inhibitory actions of AnxA1 and peptide Ac2-26 on leukocyte/endothelium interaction in the inflamed microcirculation are Fpr1-independent.
In the present study the inhibition of carrageenin-induced PMN adhesion and extravasation exerted by peptide Ac2-26 was intact in Fpr1-null mice, yet susceptible to Boc2 antagonism. These new data corroborated previous findings and extended the evidence that a receptor other than Fpr1 is responsible for the vascular events modulated by peptide Ac2-26 and, likely, AnxA1. We directed our attention to Fpr2 in view of the expression data listed above, as well as by the fact that peptide Ac2-26 bound to this receptor when overexpressed in HEK293 cells with affinity similar to that reported for Fpr1 (∼1 μmol/L).37 In addition, we used samples of peritoneal neutrophils to demonstrate, by real-time PCR analysis, the mRNA expression of Fpr1 and Fpr2. Thus, we have used a novel antibody to monitor expression of Fpr2 on PMNs during an on-going vascular response. Therefore, the final part of the study yielded, perhaps, the most intriguing and thought-provoking observation. Because there are no published data of ultrastructural immunocytochemistry analysis for the mouse Fpr family, we used specific antibodies anti-AnxA1 and anti-Fpr-rs2 to demonstrate that the protein is in close vicinity to its putative receptor, in the microenvironment of an adherent and extravasated PMNs. Another interesting point was the attenuated expression of AnxA1 after Ac2-26 peptide treatment. We believe that after the peptide treatment there was a reduction of cytokine expression, which might affect the expression of endogenous AnxA1 in the neutrophils. Further studies will address this statement.
In line with current views on the role played by mediators of the innate protective response,38 we have investigated here some of the properties of endogenous AnxA1 and determined how this profile could be exploited by bioactive peptides, indicating that Fpr2 could be a possible new therapeutic target. The data presented here provides in vivo evidence that endogenous AnxA1 is an essential mediator for homeostasis during the inflammatory process, displaying a regulatory role on diapedesis of neutrophils, and that Fpr might be redundant for the inhibitory action of the AnxA1 and its mimetic Ac2-26 on PMN diapedesis during acute peritonitis. We propose that these experimental findings may impact the development of novel therapeutics based on the anti-migratory actions of AnxA1 and, more likely, peptide Ac2-26.
Footnotes
Address reprint requests to Sonia M. Oliani, Biology Department, Instituto de Biociências, Letras e Ciências Exatas, UNESP Rua Cristóvão Colombo, 2265, CEP 15054-000, São José do Rio Preto, São Paulo, Brazil; or Mauro Perretti, The William Harvey Research Institute, Bart’s and The London, Queen Mary School of Medicine and Dentistry, Charterhouse Square, London, EC1M 6BQ, UK. E-mail: smoliani@ibilce.unesp.br and m.perretti@qmul.ac.uk.
Supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (grants 03/11292-0 to S.M.O. and 04/03124-3 to T.S.G.); the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grant number 307920/2004-6 to S.M.O.); the Wellcome Trust (program grant 069234/Z/02/Z to M.P. and R.J.F.); and the William Harvey Research Foundation (to M.P. and R.J.F.).
S.M.O. and M.P. share senior authorship.
References
- Perretti M, Flower RJ. Annexin 1 and the biology of the neutrophil. J Leukoc Biol. 2004;76:25–29. doi: 10.1189/jlb.1103552. [DOI] [PubMed] [Google Scholar]
- Vong L, D'Acquisito F, Pederzoli-Ribeil M, Lavagno L, Flower RJ, Witko-Sarsat V, Perretti M. Annexin 1 cleavage in activated neutrophils: a pivotal role for proteinase 3. J Biol Chem. 2007;282:29998–30004. doi: 10.1074/jbc.M702876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signaling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol. 1993;150:992–999. [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A, Harris JG, Goulding NJ, Flower RJ. Lipocortin-1 fragments inhibit neutrophil accumulation and neutrophil-dependent edema in the mouse. A qualitative comparison with an anti-CD11b monoclonal antibody. J Immunol. 1993;151:4306–4314. [PubMed] [Google Scholar]
- Morand EF, Hall P, Hutchinson P, Yang YH. Regulation of annexin 1 in rheumatoid synovial cells by glucocorticoids and interleukin-1. Mediators Inflamm. 2006;2006:73835. doi: 10.1155/MI/2006/73835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand EF. Effects of glucocorticoids on inflammation and arthritis. Curr Opin Rheumatol. 2007;19:302–307. doi: 10.1097/BOR.0b013e32805e87d0. [DOI] [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A, Harris JG, Harris HJ, Wheller SK, Flower RJ. Acute inflammatory response in the mouse: exacerbation by immunoneutralization of lipocortin. Br J Pharmacol. 1996;117:1145–1154. doi: 10.1111/j.1476-5381.1996.tb16709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ. Annexin 1 is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 2000;24:163–174. doi: 10.1006/cbir.1999.0468. [DOI] [PubMed] [Google Scholar]
- Perretti M, Croxtall JD, Wheler SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- Zouki C, Ouellet S, Filep JG. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J. 2000;14:572–580. doi: 10.1096/fasebj.14.3.572. [DOI] [PubMed] [Google Scholar]
- Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buchingham JC, Flower RJ. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- Yang YH, Morand EF, Getting SJ, Paul-Clark M, Liu DL, Yona S, Hannon R, Buckingham JC, Perretti M, Flower RJ. Modulation of inflammation and response to dexamethasone by annexin 1 in antigen-induced arthritis. Arthritis Rheum. 2004;50:976–984. doi: 10.1002/art.20201. [DOI] [PubMed] [Google Scholar]
- Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ, Perretti M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol. 2005;78:639–646. doi: 10.1189/jlb.0405206. [DOI] [PubMed] [Google Scholar]
- Rescher U, Danielczyk A, Markoff A, Gerke V. Functional activation of the formyl peptide receptor by a new endogenous ligand in human lung A549 cells. J Immunol. 2002;169:1500–1504. doi: 10.4049/jimmunol.169.3.1500. [DOI] [PubMed] [Google Scholar]
- Perretti M. The annexin 1 receptor(s): is the plot unravelling? Trends Pharmacol Sci. 2003;24:574–579. doi: 10.1016/j.tips.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Paul-Clark MJ, Christian HC, Flower RJ, Perretti M. Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression. Am J Pathol. 2001;158:603–615. doi: 10.1016/S0002-9440(10)64002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damazo AS, Yona S, D'Acquisto F, Flower RJ, Oliani SM, Perretti M. Critical protective role for annexin 1 gene expression in the endotoxemic murine microcirculation. Am J Pathol. 2005;166:1607–1617. doi: 10.1016/S0002-9440(10)62471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damazo AS, Yona S, Flower RJ, Perretti M, Oliani SM. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JL, Chen H, Filie JD, Kozak CA, Murphy PM. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51:270–276. doi: 10.1006/geno.1998.5376. [DOI] [PubMed] [Google Scholar]
- Wang ZG, Ye RD. Characterization of two new members of the formyl peptide receptor gene family from 129S6 mice. Gene. 2002;299:57–63. doi: 10.1016/s0378-1119(02)01012-0. [DOI] [PubMed] [Google Scholar]
- Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La M, D'Amico M, Bandiera S, Di Filippo C, Oliani SM, Gavins FN, Flower RJ, Perretti M. Annexin 1 peptides protect against experimental myocardial ischemia-reperfusion: analysis of their mechanism of action. FASEB J. 2001;15:2247–2256. doi: 10.1096/fj.01-0196com. [DOI] [PubMed] [Google Scholar]
- Hartt JK, Barish G, Murphy PM, Gao JL. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med. 1999;190:741–747. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Perretti M. Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol Sci. 1997;18:418–425. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Takano T, Chiang N, Gronert K, Clish CB. Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis. Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am J Respir Crit Care Med. 2000;161:S95–S101. doi: 10.1164/ajrccm.161.supplement_1.ltta-19. [DOI] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins FNE, Yona S, Kamal AM, Flower RJ, Perretti M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- Ernst S, Lange C, Wilbers A, Goebeler V, Gerke V, Rescher U. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J Immunol. 2004;172:7669–7676. doi: 10.4049/jimmunol.172.12.7669. [DOI] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Perretti M, Getting SJ, Solito E, Murphy PM, Gao JL. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am J Pathol. 2001;158:1969–1973. doi: 10.1016/S0002-9440(10)64667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins FNE, Kamal AM, D'amico M, Oliani SM, Perretti M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005;19:100–102. doi: 10.1096/fj.04-2178fje. [DOI] [PubMed] [Google Scholar]
- Gavins FNE, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21:1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- Lim LHK, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]