Abstract
Islet transplantation is a promising treatment for diabetes. However, it faces several challenges including requirement of systemic immunosuppression. Indoleamine 2,3-dioxygenase (IDO), a tryptophan degrading enzyme, is a potent immunomodulatory factor. Local expression of IDO in bystander fibroblasts suppresses islet allogeneic immune response in vitro. The aim of the present study was to investigate the impact of IDO on viability and function of mouse islets embedded within IDO-expressing fibroblast-populated collagen scaffold. Mouse islets were embedded within collagen matrix populated with IDO adenovector-transduced or control fibroblasts. Proliferation, insulin content, glucose responsiveness, and activation of general control nonderepressible-2 kinase stress-responsive pathway were then measured in IDO-exposed islets. In vivo viabilities of composite islet grafts were also tested in a syngeneic diabetic animal model. No reduction in islet cells proliferation was detected in both IDO-expressing and control composites compared to the baseline rates. Islet functional studies showed normal insulin content and secretion in both preparations. In contrast to lymphocytes, general control nonderepressible-2 kinase pathway was not activated in islets cocultured with IDO-expressing fibroblasts. When transplanted to diabetic mice, syngeneic IDO-expressing composite islet grafts were functional up to 100 days tested. These findings collectively confirm normal viability and functionality of islets cocultured with IDO-expressing cells and indicate the feasibility of development of a functional nonrejectable islet graft.
Indoleamine 2,3-dioxygenase (EC 1.13.11.52) (IDO) is a cytosolic monomeric hemoprotein that catalyzes tryptophan, the least available essential amino acid in the human body, to N-formylkynurenine, which in turn is rapidly degraded to yield kynurenine.1 IDO has been proposed to have profound immunoregulatory activity.2 IDO-dependent T cell suppression by dendritic cells suggests that biochemical changes due to tryptophan catabolism have significant effects on T cell proliferation and function.3 The regulatory effect of IDO on T cells is probably due to providing a tryptophan-deficient microenvironment and/or accumulation of toxic metabolites of tryptophan. The stress-responsive kinase general control nonderepressible 2 (GCN2) has been identified as a signaling molecule that enables T cells to sense and respond to stress conditions created by IDO.4,5 The C/EBP homologous protein (CHOP) gene is a downstream target gene in GCN2 pathway and is considered as a well-accepted marker for GCN2 activation.6
It has been suggested that, due to its immunoregulatory effects, IDO may actively participate in down-regulating allogeneic immune responses in transplantation.7 Our research group has provided compelling evidence in support of the fact that the expression of IDO in bystander fibroblasts through IDO genetic modification or interferon-γ treatment suppresses immune cell proliferation.8,9,10,11,12,13 In addition, we also have shown that, by an unknown mechanism, only immune but not primary skin cells are sensitive to the IDO suppressive effect.8,14 In our recent study, we showed that bystander IDO-expressing syngeneic fibroblasts have the ability to suppress proliferation of lymphocytes stimulated by allogeneic mouse islets in vitro.15 This promising finding sets the stage for developing a nonrejectable composite graft consisting of islets and IDO-expressing fibroblasts embedded within a collagen scaffold. However, that study didn’t elucidate whether IDO by itself has any deleterious effect on viability and functionality of islets. Here, we therefore asked whether a) exposing mouse islets to an IDO-induced low tryptophan microenvironment compromises their viability and function, b) the GCN2 pathway is activated in islets exposed to IDO-expressing cells, and c) a three-dimensional fibroblast populated collagen scaffold is a favorable matrix for constructing a composite islet graft.
Materials and Methods
Mouse Islet Isolation and Culture
Islets were obtained from 6 to 8-week-old male BALB/c or C57BL/6 (B6) mice (The Jackson Laboratories, Bar Harbor, ME) as previously described.15 Briefly, mice were anesthetized and pancreases were distended through the pancreatic duct with 2.5 ml of Hanks’ balanced salt solution (Life Technologies, Gaithersburg, MD) containing 2.0 mg/ml of collagenase (Type V; Sigma Chemical Co., St Louis, MO). The distended pancreases were then removed and incubated at 37°C for 15 minutes. The islets were purified by discontinuous centrifugation on Ficoll (Sigma) gradients. After centrifugation, islets were handpicked and cultured in HAM’s F10 medium (Sigma) supplemented with 12 mmol/L HEPES, 2 mmol/L l-glutamine, 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in 95% air, 5% CO2 at 37°C. Care and maintenance of all animals were in accordance with the principals of laboratory animal care and the guidelines of institutional Animal Policy and Welfare Committee.
Cell Cocultures
Cell cocultures were set up using a two-chamber cell culture system (6-well plates, Corning incorporated, Corning, NY; cell culture inserts, Millicell, Millipore Corporation, MA) in which IDO-expressing fibroblasts were grown in the upper chambers while either lymphocytes, Jurkat cells, islets, or control fibroblasts were cultured in the lower chambers. To induce IDO expression, B6 mouse fibroblasts were infected with a recombinant IDO adenoviral vector carrying human IDO cDNA for 72 hours at an multiplicity of infection of 100, as previously described.15 Control fibroblasts were infected with a mock vector. Lymphocytes were isolated from peripheral lymph nodes of B6 mice by grinding lymph node tissues between the rough edges of glass slides and were stimulated with concanavalin A (2 μg/ml, Sigma) at the start of the culture. Specificity of the IDO effect was determined by addition of 1-methyl-tryptophan, an IDO inhibitor (Aldrich Chemical Co., Milwaukee, WI), to cocultures at the final concentration of 800 μmol/L.
Development of Islet-Fibroblast Composite in Collagen Gel Matrix
Mouse dermal fibroblasts were explanted from skin of B6 mice and transduced with a recombinant adenoviral vector carrying human IDO cDNA as described previously.15 Control fibroblasts were infected with a mock vector. Fibroblast-populated collagen gel matrices were prepared as described by Sarkhosh et al12 using IDO-expressing or control fibroblasts. Mouse islets were added to fibroblast-populated collagen gel before solidification in 24 well plates. The composites were maintained in 95% air, 5% CO2 at 37°C for up to 14 days.
CHOP, IDO, and Insulin Reverse Transcriptase-PCR
Total RNA was isolated using a RNeasy kit (Qiagen, Maryland). cDNA was synthesized using the ThermoScript reverse transcriptase (RT)-PCR System (Invitrogen, Carlsbad, CA). The primers used were as follows: CHOP: sense 5′-CATACACCACCACACCTGAAAG- 3′, antisense 5′- CCGTTTCCTAGTTCTTCCTTGC-3′; IDO: sense 5′-GGCACACGCTATGGAAAACT-3′, antisense 5′-CGGACATCTCCATGACCTTT-3′; mouse insulin 1: sense 5′- CCTGTTGGTGCACTTCCTAC-3′, antisense 5′-TGCAGTAGTTCTCCAGCTGG-3′; glyceraldehyde-3-phosphate dehydrogenase: sense 5′-TGGCACAGTCAAGGCTGAGA-3′ antisense 5′-CTTCTGAGTGGCAGTGATGG-3′. Amplified PCR products were separated by 2% agarose gel electrophoresis and visualized with ethidium bromide staining.
GCN2 and CHOP Immunoblotting
Cells were harvested and lysed in lysis buffer (50 mmol/L Tris-HCl, pH 7.4; 10 mmol/L EDTA; 5 mmol/L EGTA; 0.5% NP40; 1% Triton X-100, and protease inhibitor cocktail, Sigma). Equal amounts of total protein from cell lysates (30 μg) were separated by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore). The blots were probed with the following antibodies: anti phopho-GCN2 (Thr898, 1:1000 dilution, Cell Signaling Technology INC., Beverly, MA), anti GCN2 (1:1000 dilution, Cell Signaling), and anti-GADD153/CHOP-10 produced in rabbit (1:250 dilution, Sigma). Horseradish peroxidase conjugated goat anti-rabbit IgG served as a secondary antibody for the enhanced chemiluminescence detection system (Amersham Biosciences, UK).
IDO Immunoblotting and Kynurenine Measurement
Fibroblasts were harvested and lysed 72 hours postinfection. Equal amounts of cell lysate were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane as described above. The blots were then probed with polyclonal anti IDO antibody raised in rabbit by Washington Biotechnology Inc. (Baltimore, MD) at a final dilution of 1:5000. Horseradish peroxidase conjugated goat anti-rabbit IgG served as a secondary antibody for the enhanced chemiluminescence detection system (Amersham). The level of kynurenine was measured as previously described.15 Briefly, proteins in conditioned medium were precipitated by trichloroacetic acid. After centrifugation, 0.5 ml of supernatant was incubated with equal volume of Ehrlich’s reagent for 10 minutes at room temperature. Absorption of resultant solution was measured at 490 nm by spectrophotometer.
Methyl Thiazolyl Tetrazolium Proliferation Assay
A colorimetric methyl thiazolyl tetrazolium (MTT) [3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl tetrazolium bromide] assay was used to evaluate the effects of IDO on cell proliferation. At the indicated time points, MTT (Sigma) solution (5 mg/ml) was added to cell cultures and incubated at 37°C for 5 hours. The formazin crystals were solubilized in 500 μl of dimethyl sulfoxide at the end of incubation, and the optical density of the solutions was measured at 570 nm.
Insulin Immunostaining
Islets were recovered and fixed in Bouin’s solution for 2 hours. Islets were then washed three times with 70% ethanol and embedded in paraffin. Five micron sections of these samples were stained with guinea pig anti-insulin antibody (1:1000 dilution; Dako Laboratories, Mississauga, ON, Canada) for 30 minutes followed by the addition of biotinylated goat anti-guinea pig IgG secondary antibody (1:200 dilution; Vector Laboratories, Burlingame, CA). The avidin-biotin complex/horseradish peroxidase (Vector Laboratories) and 3,3-diaminobenzidinetetrahydrochloride (BioGenex, San Ramon, CA) was used to produce a brown positive reaction. All sections were counterstained with Harris’ hematoxylin and eosin (H&E).
β Cell Apoptosis
Cleaved caspase-3 was used as a marker for β-cell apoptosis. To estimate β cell apoptosis rates, islets were stained for cleaved caspase-3 together with insulin. β cell apoptosis rates were then estimated by calculating the frequency of the cells positively stained for both insulin and cleaved caspase-3. Insulin/cleaved caspase-3 dual immunofluorescence staining was accomplished as follows: islets were retrieved and fixed as described above. Five-micron sections were incubated overnight at 4°C with guinea pig anti insulin antibody (1:500 dilution, Dako) and rabbit anti-cleaved caspase-3 antibody (1:100 dilution, cell signaling, Beverly, MA). After three washing steps with PBS-Tween 20 for 5 minutes each, samples were incubated with fluorescein-conjugated donkey anti-rabbit antibody (1:200 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA) and rhodamine-conjugated anti-guinea pig antibody (1:200 dilution, Abcam, Cambridge, MA) for 45 minutes in the dark. Finally, after washing with PBS-Tween 20 three times for 5 minutes each, samples were mounted in Vectashield H-1200 (Vector Laboratories) containing 4,6-diamidino-2-phenylindole for nuclei staining. A Zeiss Axioplan 2 microscope and Northern Eclipse image analysis software were used to obtain the images.
Static Incubation Assay and Islet Insulin Content
Islet insulin secretory responsiveness was assessed using a static incubation assay as described by Korbutt et al.16 In brief, mouse islets were washed twice with Ham’s F10 medium and samples were taken for measurement of total cellular insulin content. Islets were cultured in 24-well plates and incubated in 1.5 ml of HAM’s F10 medium supplemented with 0.5% bovine serum albumin and either 2.8 or 20.0 mmol/L glucose for 120 minutes. At the end of the incubation, supernatants were collected for measurement of insulin release using radioimmunoassay. Insulin secretion was calculated by dividing the insulin released into the supernatant by the cellular insulin content of the islets (percentage of content). Stimulation indices were calculated by dividing the percentage of insulin released at 20.0 mmol/L glucose by the percentage released at 2.8 mmol/L glucose.
Determination of islet total insulin and DNA content was performed as described by Korbutt et al.16 In brief, samples were sonicated in 2 mmol/L acetic acid containing 0.25% bovine serum albumin and centrifuged (800 × g, 15 minutes). Insulin levels in the supernatants were measured in duplicate samples by radioimmunoassay (Diagnostic Products, Los Angeles, CA). The islet DNA content was quantified using PicoGreen kit (Molecular Probes, Eugene, OR) according to the manufacturer’s instruction.
Transplantation of Islet-Fibroblast Composite Grafts
Recipient B6 mice were rendered diabetic by a single intraperitoneal injection of 200 mg/kg streptozotocin (Sigma), and diabetes was defined as a minimum of two consecutive blood glucose measurements > = 20 mmol/L. Syngeneic islet plus IDO-expressing or control fibroblast composite grafts (approximately 500 islets) were transplanted under the left kidney capsule of isoflurane-anesthetized diabetic mice. After transplantation, blood from the tail vein of each recipient was collected two times a week between 7:00 and 9:00 AM to determine the normalization of blood glucose levels. Blood glucose levels were measured using a One Touch Ultra glucose meter (Lifescan, Milpitas, CA), and grafts were deemed functioning when blood glucose levels decreased to <10.0 mmol/L. Nephrectomy of the graft-bearing kidney was performed on recipients at the endpoint of the study (>100 days post-transplant) to confirm that hyperglycemia ensued, indicating that normal blood glucose was graft dependent. All animals were cared for according to the guidelines of the Institutional Animal Policy and Welfare Committee.
Intraperitoneal Glucose Tolerance Test
An intraperitoneal glucose tolerance test (IPGTT) was performed in mice transplanted with composite grafts 6 weeks after transplantation. After a 16-hour overnight fast, glucose (2 mg/g body weight) was injected i.p. into nonanesthetized mice. Blood samples were obtained from the tail vein at 0, 15, 30, 60, and 120 minutes. Area under the curve was determined using SigmaPlot software (Systat Software Inc., San Jose, CA).
Statistical Analysis
All data are reported as mean ± SD of three or more independent observations. Statistical significance was calculated using a two-tailed unpaired Students’ t-test or a one-way analysis of variance with post hoc test in case of multiple comparisons. P values less than 0.05 were considered to be significant.
Results
Selective Suppressive Effect of IDO on Immune Versus Islet Cells
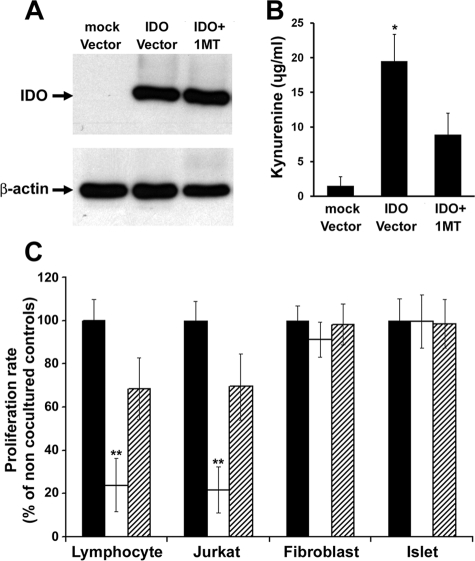
To confirm the differential suppressive effect of IDO on immune versus non-immune cells, we induced IDO expression in B6 mouse dermal fibroblasts using a recombinant adenoviral vector expressing IDO. Expression of IDO protein in infected cells was shown using Western blot analysis (Figure 1A). High levels of kynurenine—a tryptophan metabolite—in conditioned media of IDO vector infected cells further confirmed enzymatic activity of IDO (Figure 1B). IDO-expressing fibroblasts were then cocultured with stimulated mouse lymphocytes, CD4+ Jurkat cells, mouse fibroblasts, or islets using a two-chamber coculture system. The results of a MTT assay on cocultured cells after 72 hours showed significant reduction in cell proliferation rates in lymphocytes (23.9% ± 12.4) and Jurkat cells (21.8% ± 10.7) but not in fibroblasts and islets (Figure 1C). Proliferation of immune cells did not decrease when cocultured with control fibroblasts. Furthermore, addition of 1-methyl-tryptophan, a specific IDO inhibitor, resulted in partial recovery of immune cells proliferation (68.5% ±14.3 in lymphocytes and 69.4 ± 15.3 in Jurkat cells, Figure 1C).
Figure 1.
Selective suppressive effect of IDO on immune versus non immune cells. Mouse fibroblasts were transduced to express IDO using an adenoviral vector. A: IDO protein expression in mock and IDO vector infected cells. The level of kynurenine (the product of IDO mediated tryptophan degradation) was measured in the conditioned media (B). C: Stimulated mouse lymphocytes, Jurkat cells, mouse fibroblasts, and islets were cocultured in two-chamber culture plates with IDO-expressing (open bars) or control fibroblasts (solid bars) for 72 hours. A competitive IDO inhibitor, 1-methyl-tryptophan was added to one set of IDO-expressing cocultures (hatched bars). Cell proliferation rates were measured after 72 hours post-coculture using MTT assay. * denotes significant increase in kynurenine level in IDO vector infected cell conditioned medium compared to the control group (n = 3, P < 0.001); ** denotes significant difference in cell proliferation rate in comparison to the control group (n = 5, P < 0.001).
GCN2 Kinase Pathway Activation in Cells Exposed to IDO-Expressing Fibroblasts
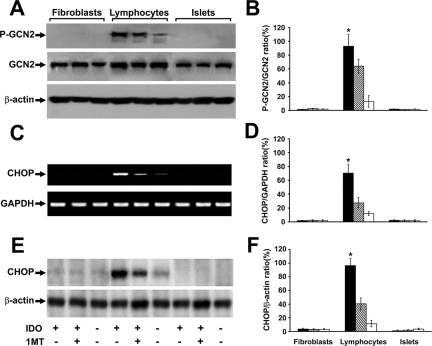
The activation of the GCN2 kinase pathway is suggested as the downstream mechanism for the suppressive effect of IDO. We therefore asked whether this stress-response mechanism is selectively activated in different cell strains when cocultured with IDO-expressing cells. To address this question, we examined phosphorylation of GCN2 and induction of intracellular CHOP in stimulated lymphocytes, fibroblasts, and islets cocultured with IDO-expressing fibroblasts. The results shown in the Figure 2 indicate a differential pattern of GCN2 pathway activation in immune versus islet cells in response to IDO. As shown in the Figure 2A, coculture with IDO-expressing cells promotes phosphorylation of GCN2 in mouse lymphocytes but not in islets and fibroblasts. Similarly, on exposure to IDO, CHOP was induced only in mouse lymphocytes at mRNA (Figure 2C) and protein (Figure 2E) levels. The quantitative analyses shown in Figure 2, B, D, and F indicated a significant and selective increase (more than sevenfold) in GCN2 phosphorylation and CHOP message and protein levels in lymphocytes cocultured with IDO-expressing cells that is partially reversed on IDO inhibition with 1-methyl-tryptophan.
Figure 2.
Selective activation of GCN2 and CHOP expression in immune cells as compared to islets and fibroblasts. Stimulated mouse lymphocytes, fibroblasts, or islets were cocultured with IDO-expressing or control fibroblasts for 48 hours. The phosphorylation of GCN2 and induction of CHOP was then measured. A competitive IDO inhibitor, 1-methyl-tryptophan was added to one set of IDO-expressing cocultures. A: phopho-GCN2 (upper row) and total GCN2 (middle row) Western blot. C: CHOP RT-PCR result. E: result of Western blot analysis for CHOP. Upper arrow shows a 29 kDa band corresponding to CHOP. B, D and F: the mean ratio of densities of phospho-GCN2, CHOP message, and protein bands to those of total GCN2, glyceraldehyde-3-phosphate dehydrogenase-1, and β-actin bands, respectively, in cells cocultured with either IDO expressing (solid bars), IDO expressing plus 1-methyl-tryptophan (hatched bars), or control (open bars) fibroblasts. * denotes significant difference in phospho-GCN2 and CHOP level between the IDO exposed and the control lymphocytes (n = 3, P < 0.001). P-GCN2: phosphorylated GCN2.
Viability of Islets Embedded within IDO-Expressing Fibroblast Populated Collagen Matrix
Embedding islets within an extracellular matrix generally improves islet survival and function. However, the impact of local IDO expression on enhanced viability of islets within three-dimensional matrix has not been elucidated. To investigate the effect of IDO on survival of islets within a collagen gel scaffold, we prepared three-dimensional composite cocultures embedded with mouse islets plus either IDO-expressing or control fibroblasts. These composites were then incubated in vitro for up to 2 weeks. On days 1, 7, and 14 post-coculture, the collagen matrices were digested and islets were retrieved and subjected to apoptosis and MTT assays. In parallel, one set of mouse islets was cultivated in a regular two-dimensional setting as a control.
Islet double-immunofluorescence staining of cleaved caspase-3 and insulin was used to estimate β-cell apoptosis rates. As shown in the Figure 3A–E, β cell apoptosis rates were significantly lower in matrix embedded islets and furthermore, being in close vicinity of IDO-expressing fibroblasts for 2 weeks did not increase β-cell apoptosis rate (5.3% ± 1.5 vs. 4.1% ± 1.2, P > 0.05). The MTT assay result indicated that, in contrast to two-dimensional culture, the islet cell proliferation rate was maintained near baseline level when embedded within the collagen matrix, regardless of being either alone, or cocultured with IDO-expressing or control fibroblasts. Additionally, MTT assays confirmed IDO did not significantly decrease the islet cell proliferation rate in composites containing IDO-expressing cells (96.8% ± 5.3) compared with control composites (99.2% ± 7.1; P > 0.05, Figure 3F).
Figure 3.
β cell apoptosis and proliferation rates of islets embedded within fibroblast populated collagen gel. Mouse islets were embedded within IDO-expressing or control scaffolds or cultivated in regular petri dishes as described in the Materials and Methods. On days 1, 7, and 14 of coculture, islets were harvested and subjected to insulin/cleaved caspase-3 dual immunofluorescence staining and MTT assay. The four upper panels show insulin (red)/cleaved caspase-3 (green) dual immunofluorescence staining in islets cultured in petri dishes (A), embedded within either acellular gel (B), control fibroblast gel (C), or IDO fibroblast gel (D) for 14 days. (E and F) show β cell apoptosis and islets proliferation rates in islets embedded in IDO-expressing (solid triangles) or control fibroblast (open triangles) populated or acellular (solid circles) collagen gel matrices and islets cultured in petri dishes using regular two-dimensional culture method (solid diamonds- dotted line) on days 1, 7, and 14 post-coculture. Islet proliferation rates are reported as the percentage of the optical densities of MTT assay at each time point adjusted to those of day 1. * denotes significant difference in apoptosis and proliferation rates on day 14 compared to day 1 (n = 3, P < 0.001). White arrows show representative islet cells stained for both insulin and cleaved capase-3. Scale bar = 50 μm.
Morphology, Insulin Content and Functional Capacity of Islets Embedded within an IDO-Expressing Fibroblasts Populated Collagen Matrix
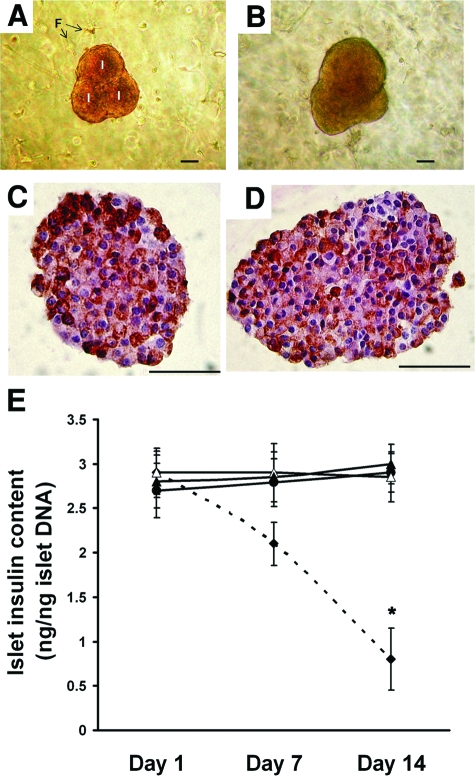
To gain perspective on the effect of IDO on islet functionality, the islets that were embedded within the three-dimensional composites were retrieved on days 1, 7, and 14 post-coculture and subjected to glucose-stimulated insulin secretion assay and insulin immunostaining. Total insulin content of islets was also measured. Figure 4, A–D shows photomicrographs of the composite islet-fibroblast-collagen matrix preparations, indicating normal spherical morphology of islets with smooth borders (Figure 4, A and B). Insulin immunostaining confirmed that the insulin content of islets exposed to IDO-expressing fibroblasts within a collagen scaffold for 2 weeks (Figure 4C) is comparable with islets cocultured with control fibroblasts under similar experimental condition (Figure 4D). Furthermore, total insulin content of islets exposed to IDO-expressing fibroblasts for 2 weeks was 3 ± 0.22 ng/ng islet DNA, which was not significantly different from insulin content of control islets (2.85 ± 0.28 ng/ng islet DNA, Figure 4E).
Figure 4.
Morphology, insulin immunostaining, and total insulin content of islets embedded within fibroblast populated collagen scaffold. Mouse islets were embedded in IDO-expressing or control fibroblast populated collagen gel matrices for up to 14 days. Upper panels show photomicrographs of composite islet cocultures in IDO fibroblast (A) and control fibroblast (B) populated collagen matrix. Islets cocultured with IDO fibroblasts (C) or control fibroblasts (D) were harvested after 14 days and immunostained for intracellular insulin. Panel (E) shows total insulin content of islets embedded within IDO-expressing (solid triangles) or control fibroblast (open triangles) populated or acellular (solid circles) collagen gel matrices and islets cultured in petri dishes using regular two-dimensional culture method (solid diamonds-dotted line) on days 1, 7, and 14 post-coculture. * denotes significant difference in total insulin content on day 14 compared to day 1 (n = 3, P < 0.001). I = islet, F = fibroblast. Scale bar = 50 μm.
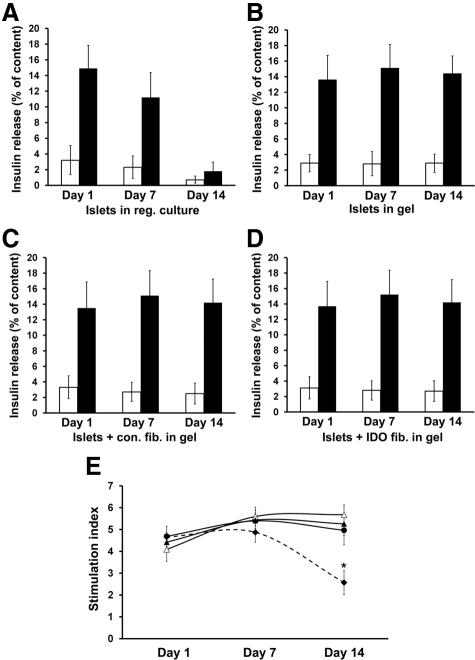
The insulin secretory capacity of islets was tested by comparing the percentages of cellular insulin released in low glucose (2.8 mmol/L) versus high glucose (20 mmol/L) media. The result showed a significant decrease in the insulin secretion ability of the islets cultured in the regular two-dimensional setting for 14 days (Figure 5A). However, glucose responsiveness and insulin secretory capacity remained at normal levels in the islets embedded within collagen matrix for 14 days, regardless of coculture conditions used (Figure 5, B–D). The calculated islet stimulation index did not significantly change in the islets cocultured with IDO-expressing fibroblasts for 14 days (5.26 ± 0.52) compared with the baseline (4.42 ± 0.45), whereas islet stimulation index significantly decreased in the islets cultivated in a two-dimensional setting from 4.66 ± 0.50 at the baseline to 2.57 ± 0.55 after 2 weeks (Figure 5E, n = 3, P < 0.001).
Figure 5.
Capacity of glucose-mediated insulin secretion in islets embedded within fibroblast populated collagen matrix. Mouse islets were embedded within IDO-expressing or control scaffolds or in regular petri dishes as described in the Materials and Methods. On days 1, 7, and 14 of coculture, islets were harvested and subjected to static incubation assay to test their glucose-stimulated insulin secretory capacity at low (2.8 mmol/L, open bars) versus high (20.0 mmol/L, solid bars) glucose concentrations. Insulin release rates were measured in islets cultured in petri dishes (A), embedded within either acellular gel (B), control fibroblast gel (C), or IDO fibroblast gel (D). The data are reported as the percentage of the released insulin to total insulin content of the islets. (E) shows islet stimulation indices, which were calculated by dividing the percentage of insulin released at high glucose by the percentage released at low glucose concentrations. Solid diamonds-dotted line, islets cultured in regular petri dishes; solid circles, islets embedded within acellular gel; open triangles, islets embedded within control fibroblast gel; solid triangles, islets embedded within IDO fibroblast gel. * denotes significant decrease in islet stimulation index on day 14 vs. day 1 (n = 3, P < 0.001).
Transplantation of a Syngeneic Islet-Fibroblast Composite Graft
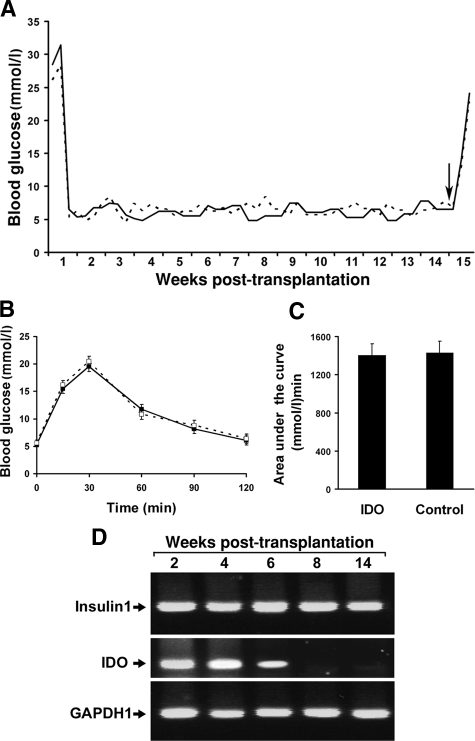
To confirm that islets embedded within the IDO-expressing fibroblast populated collagen scaffold are also viable and functional in a syngeneic transplantation model, composite grafts were prepared by embedding B6 mouse islets and IDO-expressing or control fibroblasts within the collagen matrix. These composite grafts were then transplanted beneath the renal capsule of chemically induced diabetic B6 mice. Blood glucose levels became normal in all graft recipient animals after 2 to 3 days post-transplantation and remained normal throughout the experiment (Figure 6A). On day 100 post-transplantation, removal of the graft bearing kidneys in graft recipient animals resulted in recurrence of hyperglycemia, which confirmed graft related euglycemia induction. To further investigate composite islet grafts function, an IPGTT was performed in graft recipient mice on week 6 post-transplantation. Blood glucose concentrations during IPGTT were similar in IDO composite graft recipients and controls (Figure 6B). Comparing the area under the IPGTT curve showed similar graft function in IDO group compared to the control group (1401 ± 120 (mmol/L) min vs. 1430 ± 135 (mmol/L) min, respectively; P > 0.05, Figure 6C).
Figure 6.
Syngeneic islet-fibroblast composite graft survival and intragraft IDO transgene expression in vivo. A: B6 mouse islets were embedded within IDO-expressing (solid line) or control fibroblast (dotted line) populated collagen matrices and transplanted to the kidney subcapsular spaces of chemically induced diabetic B6 mice (3 mice per group). Blood glucose levels of graft recipient animals were measured twice a week for 100 days. On day 100 post-transplantation (black arrow), graft bearing kidneys were removed, which resulted in recurrence of hyperglycemia (A). IPGTT was performed in graft recipients on week 6 post-transplantation. (B) and (C) show blood glucose concentrations during IPGTT and the area under the IPGTT curve, respectively. (D) shows result of intragraft insulin 1 (upper row) and IDO RT-PCR (middle row) at the end of weeks 2, 4, 6, 8, and 14 post-transplantation.
To examine the length of adenovirus-mediated IDO transgene expression in the composite grafts, IDO mRNA level was measured in the grafts using RT-PCR at different time points post-transplantation. As shown in Figure 6D, IDO is strongly expressed in the IDO composite grafts for up to 6 weeks after transplantation. High levels of insulin 1 expression were also detected in the grafts throughout the experiment (Figure 6D), which further confirmed maintenance of functional islets in the composite grafts during and after the IDO transgene expression period. These findings collectively indicate long term survival and normal functionality of islets exposed to IDO-expressing cells in a syngeneic islet transplantation model.
Discussion
In this study, we report that the viability and functionality of islets exposed to IDO-expressing fibroblasts are not compromised in vitro and in vivo, using different experimental approaches. The significance of this finding is more appreciated when the potential application of local expression of IDO is considered as a strategy for protecting islet grafts from immune rejection. IDO is a potent immunomodulatory enzyme that plays critical roles in regulation of T cell-mediated immune responses and has profound effects on T cell proliferation, differentiation, effector functions and viability.3 Considerable evidence now supports the importance of the immunoregulatory function of IDO, including studies of mammalian pregnancy,17,18,19 tumor resistance,20,21,22,23 chronic infections24,25,26 and autoimmune diseases.27 Based on these facts, it has been suggested that cells expressing IDO might be used to protect transplanted tissues and cells without the use of immunosuppressive drugs. We have recently introduced a model of a local immunosuppressive barrier to protect immune responses against islets in which syngeneic bystander IDO-expressing fibroblasts suppress lymphocyte proliferation induced by allogeneic mouse islets.15 Our model is based on development of a three-dimensional composite graft consisting of islets and IDO-expressing fibroblasts embedded within a collagen matrix.
Although the selective suppressive effect of IDO on T cells versus skin cells has already been noted,8,14 there was no evidence on the impact of low tryptophan microenvironment induced by IDO on other cell types including islets. We therefore, as shown in the Figures 1 and 3, tested the proliferation rates of mouse islets and fibroblasts when cocultured with IDO-expressing cells. The results of these experiments confirmed that exposure to IDO-expressing cells did not decrease islet cell proliferation or increase the β cell apoptosis rate in vitro.
The molecular mechanism(s) by which IDO suppresses T cells are still being investigated. It appears that some of the biological effects of IDO can be mediated via local depletion of tryptophan,28,29 whereas others are mediated via immunomodulatory tryptophan metabolites.30,31 As mentioned earlier, activation of the GCN2 kinase pathway, a nutrient deficiency stress-responsive mechanism, has been suggested as a potential mechanism responsible for the IDO-induced suppressive effect on T cells.4,5 Activation of the GCN2 kinase pathway can trigger cell-cycle arrest, differentiation, compensatory adaptation, or apoptosis, depending on the cell type and the initiating stress.32,33 CHOP (also known as GADD153) is a DNA damage-inducible, nuclear leucine zipper protein involved in differentiation and apoptosis. CHOP is a downstream target gene in GCN2 pathway and a well-accepted marker for GCN2 activation.6 Its expression is induced in a variety of stress responses such as endoplasmic reticulum stress,34 redox stress,35 and nutrient deprivation.36,37 Functional roles for CHOP have been well described in induction of apoptosis, and it has also been implicated in the pathogenesis of diabetes by promoting β cell destruction.38 This study for the first time showed that GCN2 activation and CHOP expression did not occur in mouse islets in response to IDO exposure whereas GCN2-mediated CHOP expression increased more than sevenfold in mouse lymphocytes under similar experimental condition. Thus, the selective unresponsiveness of mouse islets to IDO-induced GCN2 activation and CHOP expression suggests that islet cells are IDO resistant due to lack of GCN2 pathway responsiveness to IDO-induced low tryptophan environment.
Although the mechanism(s) underlying selective GCN2-CHOP pathway activation in immune versus islet cells needs to be further elucidated, at the present time, there are, at least, three potential explanations. First, activation of the GCN2 pathway is a nutrient deficiency stress response, and therefore actively dividing cells including highly proliferating T-cells, but not non-dividing islet cells, would be more sensitive to this environment. In fact, it has been shown that activated and proliferating T-cells, but not resting T cells, cultured in IDO-induced low tryptophan environment rapidly undergo apoptosis.39 Moreover, Mellor et al showed that suppression of T-cell proliferation in the presence of IDO-expressing cells occurred after the majority of T-cells entered the cell proliferation cycle.40 Second, there may be a compensatory mechanism by which islet cells suppress IDO mediated GCN2 activation. This suggestion is based on the fact that Pereira et al recently showed that the mouse protein IMPACT, which binds GCN1 and inhibits GCN2 activation, abolishes the expression of its downstream target genes ATF4 and CHOP.41 It should be emphasize that IMPACT is highly expressed in IDO-expressing tissues (eg, placenta and testis) and also in pancreas.42 As such, the expression of IMPACT in pancreatic islet cells might function as a protective mechanism against IDO-induced GCN2 activation. Finally, a different and high affinity transport system for tryptophan may exist in IDO resistant cells such as islet cells. Recently, a novel amino acid transport activity with high affinity and unusual selectivity for tryptophan has been described in IDO-expressing human monocyte-derived macrophages, which is up-regulated during monocyte-derived macrophage differentiation but not in T-cells.43 This selective transport system, if it exists in other IDO resistant cell types, can enhance tryptophan uptake in these cells and therefore overcome nutrient deficiency stress response in a low tryptophan environment.
The collagen matrix in our composite grafts plays a dual role since it works both as a scaffold to keep islets and fibroblasts together and therefore maintains the integrity of the graft and also works as an extracellular matrix, which improves the survival and function of islets. Extracellular matrix is one of the most important constituents of the islet microenvironment. It is well documented that when interrelationship between islets and extracellular matrix is disrupted following harsh process of islet isolation and purification, the function and survival of isolated islets are significantly compromised.44,45 It was also noted that islets cultivated in regular two-dimensional setting in petri dishes gradually disintegrate during culture in a time dependent manner.46 Therefore, it has been suggested that entrapment of islets in a three-dimensional collagen matrix would maintain satisfactory morphology, viability, and glucose-induced insulin secretory capability in islets.47 Our findings regarding improvement of cell survival and glucose stimulated insulin secretory capacity of the islets embedded within a collagen matrix, regardless of coculture conditions, are in concordance with previous studies48,49,50 and confirm that embedding islets in a scaffold consisting collagen will maintain integrity of islets and improve their viability and function.
Fibroblasts cocultured with islets in our proposed composite model can also improve islet cell viability. The essential role of islet-derived fibroblasts in islet physiological competence was previously reported, and it was shown that some fibroblast produced factors can promote islet survival in culture.51 Miki et al recently showed that coculture of the mouse, rat, and pig islets with islet-derived fibroblasts maintained islet viability, insulin secretion and glucose responsiveness.52 Cocultured fibroblast would not cause any concern in terms of overproliferation since it was shown that fibroblasts in floating collagen matrices have low levels of DNA synthesis and become quiescent.53,54,55 Thus, bystander fibroblasts will not have any negative impact on islet viability and function.
The findings shown in the Figure 6 further confirm the long term survival and functionality of syngeneic islets within the composite grafts in a diabetic animal model. The IDO transgene was strongly expressed in the composite grafts for up to 6 weeks post-transplantation, as expected following gene transfer using adenoviral vectors. The level of insulin mRNA was high in the IDO grafts throughout the experimental period. These data collectively demonstrate that none of key elements in our composite islet graft including IDO enzymatic activity, fibroblasts, and collagen matrix has a negative impact on long term viability and function of transplanted islets.
In conclusion, the findings of the present study suggest that mouse islets are selectively resistant to IDO-mediated activation of GCN2 kinase stress pathway and that coculture of mouse islets in a three-dimensional IDO-expressing fibroblast populated collagen matrix does not have any deleterious effect on viability and insulin secretory capacity of islets in vitro and in vivo. These findings together with the already proven immunosuppressive effect of IDO set the stage for developing a nonrejectable islet graft in the future.
Acknowledgments
We are grateful to Dr. Yunyuan Li (University of Alberta, Edmonton, Alberta, Canada) for constructing the IDO adenoviral vector.
Footnotes
Address reprint requests to Aziz Ghahary, PhD, Director, Burn and Wound Healing Research Lab, Rm 351, Jack Bell Research Centre, 2660 Oak Street, Vancouver, BC, Canada, V6H 3Z6, E-mail: aghahary@interchange. ubc.ca.
Supported by the Canadian Institutes of Health Research, the Edmonton Civic Employees’ Charitable Assistance Fund, and the C.F. “Curly” & Gladys MacLachlan Fund.
References
- Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7:31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor {zeta}-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Hainz U, Jurgens B, Heitger A. The role of indoleamine 2,3-dioxygenase in transplantation. Transpl Int. 2007;20:118–127. doi: 10.1111/j.1432-2277.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Ghahary A, Li Y, Tredget EE, Kilani RT, Iwashina T, Karami A, Lin X. Expression of indoleamine 2,3-dioxygenase in dermal fibroblasts functions as a local immunosuppressive factor. J Invest Dermatol. 2004;122:953–964. doi: 10.1111/j.0022-202X.2004.22409.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Tredget EE, Ghahary A. Cell surface expression of MHC class I antigen is suppressed in indoleamine 2,3-dioxygenase genetically modified keratinocytes: implications in allogeneic skin substitute engraftment. Hum Immunol. 2004;65:114–123. doi: 10.1016/j.humimm.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Tredget EE, Ghaffari A, Lin X, Kilani RT, Ghahary A. Local expression of indoleamine 2,3-dioxygenase protects engraftment of xenogeneic skin substitute. J Invest Dermatol. 2006;126:128–136. doi: 10.1038/sj.jid.5700022. [DOI] [PubMed] [Google Scholar]
- Sarkhosh K, Tredget EE, Li Y, Kilani RT, Uludag H, Ghahary A. Proliferation of peripheral blood mononuclear cells is suppressed by the indoleamine 2,3-dioxygenase expression of interferon-gamma-treated skin cells in a co-culture system. Wound Repair Regen. 2003;11:337–345. doi: 10.1046/j.1524-475x.2003.11505.x. [DOI] [PubMed] [Google Scholar]
- Sarkhosh K, Tredget EE, Karami A, Uludag H, Iwashina T, Kilani RT, Ghahary A. Immune cell proliferation is suppressed by the interferon-gamma-induced indoleamine 2,3-dioxygenase expression of fibroblasts populated in collagen gel (FPCG). J Cell Biochem. 2003;90:206–217. doi: 10.1002/jcb.10593. [DOI] [PubMed] [Google Scholar]
- Sarkhosh K, Tredget EE, Uludag H, Kilani RT, Karami A, Li Y, Iwashina T, Ghahary A. Temperature-sensitive polymer-conjugated IFN-gamma induces the expression of IDO mRNA and activity by fibroblasts populated in collagen gel (FPCG). J Cell Physiol. 2004;201:146–154. doi: 10.1002/jcp.20043. [DOI] [PubMed] [Google Scholar]
- Forouzandeh F, Jalili RB, Germain M, Duronio V, Ghahary A. Skin cells, but not T cells, are resistant to indoleamine 2, 3-dioxygenase (IDO) expressed by allogeneic fibroblasts. Wound Repair Regen. 2008;16:379–387. doi: 10.1111/j.1524-475X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- Jalili RB, Rayat GR, Rajotte RV, Ghahary A. Suppression of islet allogeneic immune response by indoleamine 2,3 dioxygenase- expressing fibroblasts. J Cell Physiol. 2007;213:137–143. doi: 10.1002/jcp.21100. [DOI] [PubMed] [Google Scholar]
- Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte AR. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97:2119–2129. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. The physiology of immune evasion during pregnancy; the critical role of placental tryptophan metabolism and transport. Pflugers Arch. 2001;442:639–641. doi: 10.1007/s004240100633. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Lee GK, Johnson T, Keskin DB, Lee J, Munn DH. Indoleamine 2,3-dioxygenase, immunosuppression, and pregnancy. J Reprod Immunol. 2002;57:143–150. doi: 10.1016/s0165-0378(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78:2632–2636. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakash JB, Byrne GI, Lichtman A, Libby P. Cytokines induce indoleamine 2,3-dioxygenase expression in human atheroma-associated cells: Implications for persistent Chlamydophila pneumoniae infection. Infect Immun. 2002;70:3959–3961. doi: 10.1128/IAI.70.7.3959-3961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NM, Rodrigues CV, Santoro MM, Reis LF, varez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: Induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RR, Ozaki Y, Datta SP, Borden EC, Sondel PM, Malone DG. Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv Exp Med Biol. 1991;294:425–435. doi: 10.1007/978-1-4684-5952-4_39. [DOI] [PubMed] [Google Scholar]
- Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KA, Zheng Y, Heidler KM, Mizobuchi T, Wilkes DS. CDllc+ cells modulate pulmonary immune responses by production of indoleamine 2,3-dioxygenase. Am J Respir Cell Mol Biol. 2004;30:311–318. doi: 10.1165/rcmb.2003-0268OC. [DOI] [PubMed] [Google Scholar]
- Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- Crosby JS, Chefalo PJ, Yeh I, Ying S, London IM, Leboulch P, Chen JJ. Regulation of hemoglobin synthesis and proliferation of differentiating erythroid cells by heme-regulated eIF-2alpha kinase. Blood. 2000;96:3241–3248. [PubMed] [Google Scholar]
- Yang L, Carlson SG, McBurney D, Horton WE., Jr Multiple signals induce endoplasmic reticulum stress in both primary and immortalized chondrocytes resulting in loss of differentiation, impaired cell growth, and apoptosis. J Biol Chem. 2005;280:31156–31165. doi: 10.1074/jbc.M501069200. [DOI] [PubMed] [Google Scholar]
- Tang JR, Nakamura M, Okura T, Takata Y, Watanabe S, Yang ZH, Liu J, Kitami Y, Hiwada K. Mechanism of oxidative stress-induced GADD153 gene expression in vascular smooth muscle cells. Biochem Biophys Res Comm. 2002;290:1255–1259. doi: 10.1006/bbrc.2002.6336. [DOI] [PubMed] [Google Scholar]
- Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–5297. doi: 10.1074/jbc.M311862200. [DOI] [PubMed] [Google Scholar]
- Friedman AD. GADD153/CHOP, a DNA damage-inducible protein reduced CAAT/enhancer binding protein activities and increased apoptosis in 32D cl3 myeloid cells. Cancer Res. 1996;56:3250–3256. [PubMed] [Google Scholar]
- Lawrence MC, McGlynn K, Naziruddin B, Levy MF, Cobb MH. Inaugural Article: differential regulation of CHOP-10/GADD153 gene expression by MAPK signaling in pancreatic beta-cells. Proc Natl Acad Sci USA. 2007;104:11518–11525. doi: 10.1073/pnas.0704618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- Pereira CM, Sattlegger E, Jiang HY, Longo BM, Jaqueta CB, Hinnebusch AG, Ronald C, Wek LE, Mello AM, Castilho, BA. IMPACT, a protein preferentially expressed in the mouse brain, binds GCN1 and inhibits GCN2 activation. J Biol Chem. 2005;280:28316–28323. doi: 10.1074/jbc.M408571200. [DOI] [PubMed] [Google Scholar]
- Okamura K, Hagiwara-Takeuchi Y, Li T, Vu TH, Hirai M, Hattori M, Sakaki Y, Hoffman AR, Ito T. Comparative genome analysis of the mouse imprinted gene impact and its nonimprinted human homolog IMPACT: toward the structural basis for species-specific imprinting. Genome Res. 2000;10:1878–1889. doi: 10.1101/gr.139200. [DOI] [PubMed] [Google Scholar]
- Seymour RL, Ganapathy V, Mellor AL, Munn DH. A high-affinity, tryptophan-selective amino acid transport system in human macrophages. J Leukoc Biol. 2006;80:1320–1327. doi: 10.1189/jlb.1205727. [DOI] [PubMed] [Google Scholar]
- Pinkse GGM, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E. Integrin signaling via RGD peptides and anti-{beta}1 antibodies confers resistance to apoptosis in islets of langerhans. Diabetes. 2006;55:312–317. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- Montesano R, Mouron P, Amherdt M, Orci L. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol. 1983;97:935–939. doi: 10.1083/jcb.97.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao SH, Peshwa MV, Sutherland DE, Hu WS. Entrapment of cultured pancreas islets in three-dimensional collagen matrices. Cell Transplant. 1992;1:51–60. doi: 10.1177/096368979200100109. [DOI] [PubMed] [Google Scholar]
- Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human {beta}-cells: Effect on insulin content, secretion, and gene transcription. Diabetes. 2006;55:2723–2729. doi: 10.2337/db06-0120. [DOI] [PubMed] [Google Scholar]
- Nagata N, Gu Y, Hori H, Balamurugan AN, Touma M, Kawakami Y, Wang W, Baba TT, Satake A, Nozawa M, Tabata Y, Inoue K. Evaluation of insulin secretion of isolated rat islets cultured in extracellular matrix. Cell Transplant. 2001;10:447–451. [PubMed] [Google Scholar]
- Perfetti R, Henderson TE, Wang Y, Montrose-Rafizadeh C, Egan JM. Insulin release and insulin mRNA levels in rat islets of Langerhans cultured on extracellular matrix. Pancreas. 1996;13:47–54. doi: 10.1097/00006676-199607000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A, Russell T, Mintz DH. Factors from fibroblasts promote pancreatic islet B cell survival in tissue culture. Diabetes. 1979;28:1108–1113. doi: 10.2337/diab.28.12.1108. [DOI] [PubMed] [Google Scholar]
- Miki A, Narushima M, Okitsu T, Takeno Y, Soto-Gutierrez A, Rivas-Carrillo JD, Navarro-Alvarez N, Chen Y, Tanaka K, Noguchi H, Matsumoto S, Kohara M, Lakey JR, Kobayashi E, Tanaka N, Kobayashi N. Maintenance of mouse, rat, and pig pancreatic islet functions by coculture with human islet-derived fibroblasts. Cell Transplant. 2006;15:325–334. [PubMed] [Google Scholar]
- Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Rep Regen. 2004;12:134–147. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- Fringer J, Grinnell F. Fibroblast quiescence in floating or released collagen matrices. Contribution of the erk signaling pathway and actin cytoskeletal organization. J Biol Chem. 2001;276:31047–31052. doi: 10.1074/jbc.M101898200. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt H, Grinnell F. Fibroblast quiescence and the disruption of ERK signaling in mechanically unloaded collagen matrices. J Biol Chem. 2000;275:3088–3092. doi: 10.1074/jbc.275.5.3088. [DOI] [PubMed] [Google Scholar]