Abstract
Fibulin-5 is crucial for normal elastic fiber synthesis in the vaginal wall; more than 90% of fibulin-5-knockout mice develop pelvic organ prolapse by 20 weeks of age. In contrast, fibulin-1 and -2 deficiencies do not result in similar pathologies, and fibulin-4-knockout mice die shortly after birth. EFEMP1 encodes fibulin-3, an extracellular matrix protein important in the maintenance of abdominal fascia. Herein, we evaluated the role of fibulin-3 in pelvic organ support. Pelvic organ support was impaired significantly in female Efemp1 knockout mice (Fbln3−[supi]/−), and overt vaginal, perineal, and rectal prolapse occurred in 26.9% of animals. Prolapse severity increased with age but not parity. Fibulin-5 was up-regulated in vaginal tissues from Fbln3−[supi]/− mice regardless of prolapse. Despite increased expression of fibulin-5 in the vaginal wall, pelvic organ support failure occurred in Fbln3−[supi]/− animals, suggesting that factors related to aging led to prolapse. Elastic fiber abnormalities in vaginal tissues from young Fbln3−[supi]/− mice progressed to severe elastic fiber disruption with age, and vaginal matrix metalloprotease activity was increased significantly in Fbln3−[supi]/− animals with prolapse compared with Fbln3−[supi]/− mice without prolapse. Overall, these results indicate that both fibulin-3 and -5 are important in maintaining pelvic organ support in mice. We suggest that increased vaginal protease activity and abnormal elastic fibers in the vaginal wall are important components in the pathogenesis of pelvic organ prolapse.
Pelvic organ prolapse is a common pelvic floor disorder with substantial financial, social, and psychological implications.1,2,3 More than 11% of women will ultimately require surgical correction of prolapse or incontinence in their lifetimes.4 Despite the prevalence of prolapse, the true pathophysiology of the disease is not well understood although epidemiological studies indicate that aging and vaginal parity are major risk factors.1,5 Connective tissues of the pelvic floor are important for support of the pelvic viscera. Elastic fibers, which are abundant in connective tissues of the vaginal wall, confer resilience to stretching and expansive forces.6,7,8 The phenotype of pelvic organ prolapse in animals with defects in elastic fiber assembly and synthesis suggests that elastic fiber homeostasis pathways are involved in maintaining pelvic organ support.
Elastic fiber synthesis and assembly is a complex process that requires tropoelastin monomers to be cross-linked in the extracellular matrix to form a growing elastic fiber. Microfibrils are the scaffold on which tropoelastin is deposited before it is cross-linked by one or more of the copper-requiring lysyl oxidases.9 Fibulin-5 is a protein thought to participate in this elastic fiber building process by binding lysyl oxidase-like 1 (LOXL1) and possibly targeting it to fiber assembly sites in the extracellular matrix.10,11,12
Mice with null mutations in genes encoding either fibulin-5 (Fbln5−/−)10,12 or LOXL1 (Loxl1−/−)11 develop elastinopathies including emphysematous lungs; defective elastic laminae of the great vessels; and loose, stretchy skin. Both mouse models also develop pelvic organ prolapse remarkably similar to that seen in primates, ie, descent of the vagina, cervix, and bladder herniating through the pelvic floor musculature. Previously, we reported that aging alone, regardless of parity, results in pelvic organ prolapse in more than 90% of Fbln5−/− mice.13 Liu and colleagues14 reported that all parous Loxl1−/− mice developed prolapse after their first or second litters and that ∼50% of nulliparous animals ultimately progressed to prolapse after more than 1 year of age.
The phenotype of these mouse models has led us to predict that other matrix proteins involved in elastic fiber assembly or degradation may be involved in the pathophysiology of pelvic organ prolapse. The fibulin family of proteins has seven known members characterized by tandem repeats of calcium binding-epidermal growth factor-like motifs and a C-terminal fibulin module. Repeating calcium binding-epidermal growth factor motifs are thought to be involved in protein-protein interactions, and biochemical analyses have revealed many interacting binding partners for fibulin-1 and fibulin-2 including fibronectin, proteoglycans, and basement membrane proteins.15 Fibulins are divided into two subgroups by similar size and binding preferences: fibulins-1, -2, -6 and fibulins-3, -4, -5, and -7.15,16 Most have been shown to bind tropoelastin and are believed to contribute to elastic fiber assembly. There may be some redundancy in their roles, however, because absence of fibulin-1 or -2 does not appear to have effects on elastic fiber homeostasis in vivo.17 To date, only fibulin-4 and -5 have been found to be essential in elastic fiber assembly. Fbln4−/− mice die during or immediately after birth because of rupture of aortic aneurysms.10,12,18 Fbln5−/− mice are born with elastic fiber defects in the lungs, skin, and aorta, but do not develop prolapse until 12 weeks of age.13 The role of fibulin-3 (also known as EFEMP1, MBP1, H411, or UPH1) in elastic fiber assembly or pelvic organ prolapse is unknown. Among the seven known fibulins, fibulin-3 shares highest homology with fibulin-4 and -5.19,20,21,22 Recently, McLaughlin and colleagues23 reported that fibulin-3 has a specific effect on the integrity of elastic fibers in fascial connective tissues. Fbln3−/− mice develop early aging and herniation of the abdominal wall that increases progressively with age.23 In this study, we report the potential role of fibulin-3 in pelvic organ support by characterizing the gross and ultrastructural changes in the vaginal muscularis of mice deficient in fibulin-3 (Fbln3−/−). Further, studies were conducted to lend insight into potential mechanisms by which the lack of fibulin-3 led to pelvic organ prolapse.
Materials and Methods
Mice
All mice were studied and euthanized in accordance with the standards of humane animal care described by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Animals were housed under a 12-hour dark/light cycle at 22°C. Fbln3−/− mice and wild-type (WT, n = 81) mice were the same strain (C57BL/6x129Sv/J). At sacrifice, after disarticulation of the pubic symphysis, uterine horns together with the bladder, cervix, and vagina were dissected down to the perineal skin. The vaginal dissection included the entire vaginal muscularis and the connective tissue suspending the vaginal wall to the pubocaudalis. Using microinstruments and a dissection microscope, the uterus was removed at the utero-cervico junction. Perineal skin was removed and the bladder and urethra dissected from the anterior vaginal wall. Wet weight of the vagina was determined by subtracting the weight of the dissected cervix from that of the cervicovaginal complex. The intact vaginal wall (muscularis and epithelium) was snap-frozen in liquid N2 and stored at −80°C.
Prolapse Quantification
A validated mouse pelvic organ quantification (MOPQ) examination was used to serially measure the degree of vaginal, perineal, and rectal prolapse in 227 Fbln3−/− and 81 WT mice weekly for up to 75 weeks of age. Animals less than 11 weeks (n = 197) were not measured because prolapse was not observed before this age. The MOPQ was conducted with one investigator holding the animal by the scruff of the neck, which resulted in a prolonged, reflex valsalva (evidenced by defecation), whereas the other investigator performed the measurements using a caliper with the precision of 0.01 mm. Six assessments were performed in each animal: i) stage of perineal bulge; ii) magnitude of perineal bulge; iii) cervical descent; iv) anal prolapse; v) perineal body length, and vi) vaginal diameter.13 Perineal bulge (0, none; 1, detectable but small; 2, moderate size bulge; 3, huge; 4, vagina coming out), cervical descent (0, not visible; 1, visible on straining inside the introitus; 2, at the level of the introitus; 3, outside the introitus), and anal prolapse (0, none; 1, present but mild; 2, severe) were measured on an ordinal scale. Magnitude of perineal bulge was measured in mm from the point of insertion of inner thigh to maximal edge of perineum. Perineal body was measured in mm from the posterior fourchette to mid-anus. Vaginal diameter was measured in mm from the anterior to posterior vaginal walls at the level of the introitus.
Histology and Ultrastructural Morphology
WT, Fbln3−/−, and Fbln5−/− mice were anesthetized and perfused with 10% neutral buffered formalin (pH 7.4). Thereafter, the female urogenital tract was dissected en bloc. Serial transverse sections (5 μm) were obtained in 100-μm increments throughout the specimen, stained with hematoxylin and eosin or Hart’s stain, and analyzed with an Eclipse E1000N microscope (Nikon, Tokyo, Japan). For transmission electron microscopy, anesthetized animals were perfused first with ice-cold phosphate-buffered saline and then with 3% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4). Full thickness rings of vaginal wall from WT and Fbln3−/− mice were then dissected and fixed in fresh fixative overnight. Samples were then sequentially treated with 1% osmium tetroxide, 2% tannic acid, and 2% uranyl acetate before dehydration and Epon embedding. Thin sections (60 nm) were placed on formvar-coated grids and counterstained with 7% methanolic uranyl acetate followed by lead citrate. Sections were viewed using a Tecnai 12 (FEI Co., Hillsboro, OR) transmission electron microscope at 120 kV and images were digitally captured.
Immunohistochemistry and Antibody Production
Rabbit polyclonal antibody BSYN5128 was raised against mouse fibulin-3 polypeptide (78IVNNEHPQQETQPAAEASS95; Biosynthesis Inc., Lewisville, TX). Purified immunoglobulin-γ (IgG) and affinity-purified antibodies were prepared. Formalin-fixed, paraffin-embedded tissues from WT and Fbln3−/− animals were sectioned at 5 μm. After drying, slides were deparaffinized in xylene and rehydrated in graded alcohols to distilled water. Endogenous peroxidase activity was quenched for 10 minutes at room temperature, using 0.3% H2O2 with 0.1% sodium azide. Slides were subjected to steam-heat epitope retrieval in ethylenediaminetetraacetic acid buffer (1 mmol/L, pH 8.0) for 30 minutes. After rinsing in phosphate-buffered saline (PBS), slides were incubated in primary antibody (BSYN5128) for 30 minutes at 25°C using gentle orbital rotation. Negative control specimens were processed simultaneously in an identical manner, with the exception that PBS was used in place of primary antibody. After another rinse in PBS, slides were incubated with appropriate horseradish peroxidase-conjugated polymer (PowerVision reagent; ImmunoVision Technologies Co., Daly City, CA) for 30 minutes at 25°C. Finally, the slides were immersed for 5 minutes in 25°C diaminobenzidine (Invitrogen, Carlsbad, CA), enhanced with 0.5% copper sulfate in PBS for 5 minutes at 25°C, counterstained in hematoxylin, dehydrated in graded alcohols, cleared in xylene, and covered with a coverslip.
Gelatin Zymography
Vaginal tissues were thawed on ice, minced, washed in PBS, and homogenized in MMP2 assay buffer (EnzoLyte 520 MMP-2 assay kit; Anaspec, San Jose, CA) containing 0.1% Triton-X 100 (95× volume:tissue wet weight). Thereafter, homogenates were centrifuged at 10,000 × g for 15 minutes at 4°C and supernatants used for determination of protease activity. Protein concentrations were determined using a bicinchoninic acid protein assay (Pierce, Rockford, IL). Samples (5 μg per lane) were applied to gelatin polyacrylamide minigels (Invitrogen) (10%) in standard sodium dodecyl sulfate loading buffer containing 0.1% sodium dodecyl sulfate with no β-mercaptoethanol, and the samples were not boiled before loading. Gels were run at room temperature at 125 V. After electrophoresis, gels were soaked in renaturing buffer (2.7% Triton X-100 in distilled water) in a shaker for 30 minutes with one change after 30 minutes to remove sodium dodecyl sulfate. Next, gels were soaked in assay buffer (50 mmol/L Tris, 200 mmol/L NaCl, 10 mmol/L CaCl2, 0.05% Brij 35, pH 7.5) for 4 to 16 hours at 37°C and then stained with Coomassie Brilliant Blue-R 250 in 50% methanol and 10% acetic acid followed by destaining with 25% methanol and 7% acetic acid. Clear zones of lysis against a dark background indicated enzyme activity, and purified enzymes were used as positive controls (Anaspec). Conditions of zymography and analysis were quantitative because enzyme activity was linear with time of incubation and protein loading, and samples for each experiment were applied to the same gel to avoid intergel variation. Areas of lysis were quantified using a Fuji LAS 3000 image analysis system (Fujifilm Life Science, Stamford, CT). Side-by-side immunoblots for α-tubulin protein were conducted to ensure equal protein loading of the gel. MMP activity was expressed as relative units per unit α-tubulin.
Immunoblot Analysis
Relative amounts of fibulin-5 and tropoelastin in the vaginal wall from five virginal WT mice, six Fbln3−/− mice without prolapse, and five Fbln3−/− mice with prolapse were determined using immunoblot analysis as described previously using 10 μg of urea-extracted protein per lane.24 All mice were matched for age and estrus cycle (metestrus), except in some mice with advanced prolapse that were not cycling. Protein extracts from Fbln5−/− mice served as negative controls for fibulin-5.
Real-Time Polymerase Chain Reaction (PCR)
Quantitative PCR was used to determine the relative levels of Timp1, Timp2, and Timp3 in vaginal tissues as described previously.25 Primer sequences for amplifications were chosen using published cDNA sequences and chosen such that the resulting amplicons would cross an exon junction thereby eliminating the potential of false-positive signals from genomic DNA contamination. Primer sets for Timp3 (BC014713) were 5′-CACGGAAGCCTCTGAAAGTCTT-3′(513–534) and 5′-CATACACGCGCCCTGTCA-3′(592–575). SYBR Green was used for amplicon detection, and gene expression was normalized to that of the housekeeping gene β2-microglobulin (B2M). All assays included positive, negative, and no template controls.
Quantification of Desmosine
Elastic fiber content was assessed by radioimmunoassay for desmosine, an amino acid crosslink found only in elastin. After urea extraction, pelleted tissue extracts were hydrolyzed in 6 N HCl at 100°C for 24 hours. An aliquot was evaporated again to dryness and redissolved in 100 μl of H2O, vortexed, microfuged, and assayed for desmosine as previously described.26
Statistical Analysis
Differences in continuous variables of MOPQ scores, protease activities, and amounts of fibulin-5 and tropoelastin for Fbln3−/− mice with and without prolapse versus WT mice were assessed using analysis of variance with Student-Newman-Keuls method for pair-wise multiple comparisons. To determine whether age or parity were independent predictors of prolapse, a multiple logistic regression analysis was used with pelvic organ prolapse as the dependent variable and age and parity as independent variables. Vaginal weight comparisons between knockout and WT groups were made with the Student’s t-test. Statistical software used was SigmaStat version 2.03 (Jandel Scientific, San Rafael, CA). P values ≤0.05 were considered statistically significant.
Results
Pelvic Organ Support in Fbln3−/− Mice
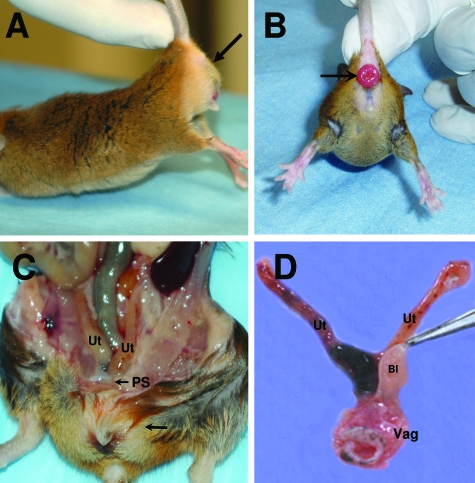
During the course of this investigation, overt pelvic organ prolapse was noted in a significant number of Fbln3−/− female mice (Figure 1A). Grossly, the phenotype of prolapse in Fbln3−/− knockout animals resembled that of Fbln5−/− and Loxl1−/− animals.14,24 Specifically, the perineal bulge was comprised of the bladder, vaginal vault, and descended uterine horns caudal to the pubic ligament (Figure 1C). Rectal prolapse and hernias were observed in the flank and inguinal areas (Figure 1B). Vaginal weight was increased in knockout animals compared with WT (mean ± SD): 77.6 ± 18.3 mg versus 44.2 ± 7.3 mg, respectively (P < 0.001). Prolapsed Fbln3−/− vaginas were thin and patulous (Figure 1D). Like Fbln5−/− mice, the connective tissue paravaginal and apical attachments (eg, the uterosacral ligaments) were either absent or attenuated in Fbln3−/− mice with prolapse. Rectal prolapse was a common finding among Fbln3−/− animals with concomitant perineal prolapse (18 of 53 or 34%) (Figure 1B). In contrast, rectal prolapse occurred in only 4 of 74 (5.4%) Fbln5−/− mice.
Figure 1.
Pelvic anatomy of Fbln3−/− mice with pelvic organ prolapse. Perineal bulge (stage 3; A, arrow) and rectal prolapse (B) are observed in Fbln3−/− females. C: Uterine horns (Ut) descend beneath the pubic symphysis (PS). The bulge (arrow) is comprised of enlarged bladder and vagina. D: Dissected female reproductive tract of Fbln3−/− mice with prolapse. Note patulous, enlarged vaginal wall (Vag). Ut, uterine horns; Bl, bladder.
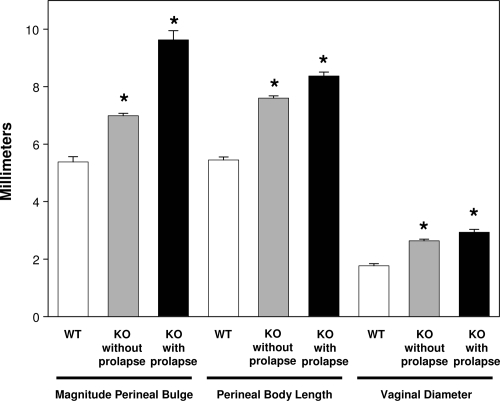
To study the effect of fibulin-3 on subtle increases (and waxing and waning) in perineal bulge, rectal prolapse, and cervical descent, a longitudinal observation of pelvic organ support was conducted in WT and Fbln3−/− mice. Knockout (n = 227) and C57BL/6 WT (n = 81) mice were examined weekly with MOPQ scores from birth; the oldest of which were greater than 1 year of age. The effects of pregnancy, parturition, and aging were noted. Continuous variable measurements of the MOPQ (magnitude of perineal bulge, perineal body length, and vaginal diameter) of WT and knockout animals with and without gross evidence of pelvic organ prolapse were compared and validated by examiners blinded to genotype (Figure 2). All parameters were increased significantly in knockout relative to WT animals (all P < 0.001, Figure 2), suggesting that pelvic organ support is abnormal in Fbln3−/− mice even in the absence of obvious prolapse.
Figure 2.
Pelvic organ support in Fbln3−/− with or without pelvic organ prolapse. Magnitude of perineal bulge, perineal body length, and vaginal diameter were measured in WT and Fbln3−/− mice without (KO without prolapse) and with (KO with prolapse) prolapse as part of the MOPQ scoring system. Prolapse was defined as the presence of ≥stage 1 perineal bulge (visually evident bulge). *P ≤ 0.05 compared with WT.
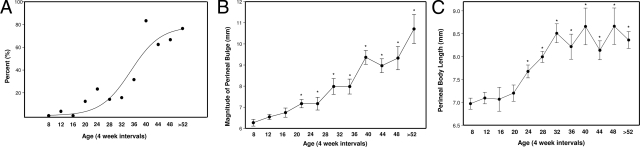
In agreement with our previous study involving WT mice of C3BL/6 strain,13,25 WT C57BL/6x129Sv mice did not develop perineal prolapse. In contrast, 53 of 197 knockout animals ≥11 weeks of age (26.9%) developed at least stage 1 perineal bulge. For these animals with prolapse, the average age at time of diagnosis was 37 weeks (range, 11.3 to 70.9 weeks). The majority of knockout animals (89%) developed prolapse after 20 weeks of age (n = 47, Figure 3). Of these older knockout mice with prolapse, 18 (34%) were parous and 35 (66%) were virginal. Using multivariate logistic regression analysis including age and parity as independent variables, prolapse was significantly related to age (P < 0.001; Figure 3, A–C) but not parity.
Figure 3.
Rate and severity of pelvic organ prolapse in Fbln3−/− mice. A: Percentage of Fbln3−/− mice with ≥stage 1 pelvic organ prolapse as a function of age. Magnitude of perineal bulge (B) and perineal body length (C) are increased significantly in Fbln3−/− mice with age. Measurements were derived from 197 animals >11 weeks of age. *P < 0.05 compared with 12 weeks.
As described by McLaughlin and colleagues,23 Fbln3−/− mice developed hernias in either one or both flanks or inguinal area. These hernias were distinct from the perineal bulge of pelvic organ prolapse. Herein, we found that of 129 animals older than 40 weeks of age, 53 (53.5%) developed hernias, and of the mice with pelvic prolapse, 32% had concomitant hernias. Using multivariate logistic regression analysis, hernias were associated with mouse age (P < 0.001) but not parity. Interestingly, the background strain altered the prevalence of hernias in Fbln3−/− mice.23 To determine whether strain may alter the prevalence of prolapse, Fbln3−/− mice on a BALB/c background were examined for 18 months. Of 36 animals younger than 1 year of age, none developed prolapse. Older BALB/c Fbln3−/− mice (>1 year of age), however, developed severe stage 3 pelvic organ prolapse involving the rectum, uterus, and cervix (3 of 12 animals, data not shown). Thus, the background strain appears to modify the time of onset of prolapse, but pelvic organ prolapse is a phenotype of both C57BL/6 and BALB/c Fbln3−/− mice.
Histology and Ultrastructural Morphology in Fbln3−/− Mice
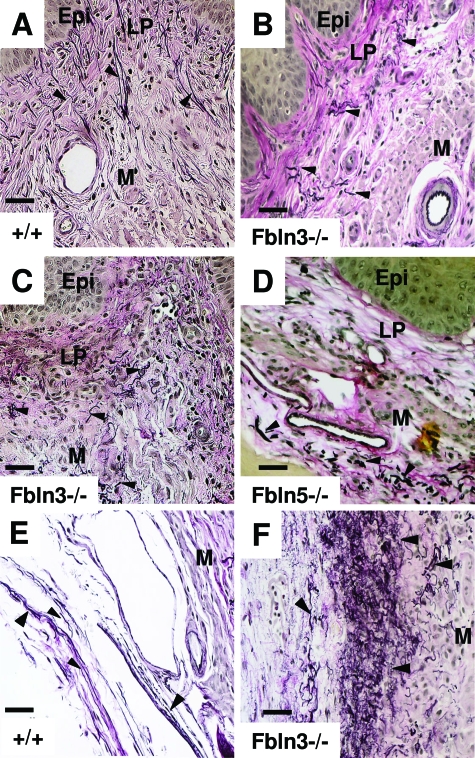
To examine the morphological changes in elastic fibers related to the loss of fibulin-3, vaginal tissues from Fbln3−/− mice with and without perineal prolapse were fixed in formalin and stained for elastic fibers using Hart’s stain. Transverse sections from the mid-vagina were analyzed. To control for potential changes induced by the estrus cycle, animals were sacrificed in metestrus, except in cases of stage 3 prolapse in which most animals were not cycling. In vaginal tissues from WT animals, elastic fibers were long and slender, with numerous fibers extending from the muscularis through the lamina propria branching just beneath the vaginal epithelium (Figure 4A). In vaginal tissues from Fbln3−/− mice (Figure 4, B and C), elastic fibers were abnormal and somewhat similar to those observed in vaginal tissues from Fbln5−/− mice (Figure 4D). Specifically, elastic fibers were blunt, thick, and fewer in number in the muscularis. Although normal in some areas near the subepithelium, elastic fibers were virtually always abnormal in the vaginal muscularis and adventitial layer in both Fbln5−/− (Figure 4D) and Fbln3−/− animals (Figure 4, B and C). The adventitial connective tissue is adjacent to the pubocaudalis muscle. In WT animals, this tissue is characterized by loose areolar tissue, neurovascular bundles, and long strands of intact elastic fibers that circumscribe the vaginal wall (Figure 4E). In Fbln3−/− animals, elastic fibers of the adventitial layer were grossly more abundant, fragmented, tortuous, and tangled (Figure 4F).
Figure 4.
Elastic fiber morphology in Fbln3−/− and Fbln5−/− mice. Transverse sections of mid-vagina were stained with Hart’s stain in WT mice (A, E), Fbln3−/− mice without prolapse (B), Fbln3−/− mice with prolapse (C, F), and Fbln5−/− mice with prolapse (D). Arrowheads indicate elastic fibers. A–D represent epithelium (Epi), lamina propria (LP), and muscularis (M). E and F represent deep muscularis and adventitial layers of the vaginal wall adjacent to the pubocaudalis. Scale bars = 20 μm.
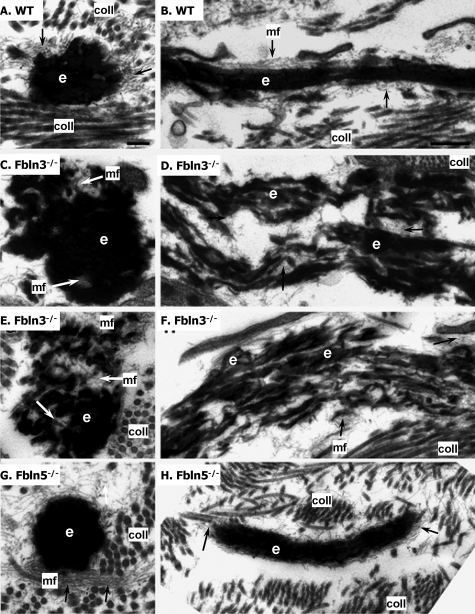
To gain further insight into the role of fibulin-3 and fibulin-5 in vaginal elastic fibers, vaginal tissues from 8-month-old WT, Fbln3−/− with and without prolapse, and Fbln5−/− mice with prolapse were analyzed by electron microscopy (Figure 5). In WT mice, vaginal elastic fibers were comprised of a solid core of elastin surrounded by microfibrils (Figure 5, A and B). Longitudinal sections of the fibers revealed that the fibers were long and slender with few disruptions in the elastin core region. In vaginal tissues from Fbln3−/− animals without prolapse, elastic fibers were disrupted and tortuous (Figure 5, C and D). The elastic core was not uniform in density and small clumps of globular elastin were surrounded by microfibrils (Figure 5, C and D). Longitudinal views of the fiber revealed microfibrils located in the regions of the disrupted elastin core (Figure 5D). Disruption of elastic fibers was more prominent in vaginal tissues from Fbln3−/− mice with prolapse with large areas of tangled microfibrils seen within the disrupted, tortuous, and frayed elastin core (Figure 5, E and F). Interestingly, although elastic fibers in the vaginal wall of Fbln5−/− mice were similar to those of Fbln3−/− by light microscopy, ultrastructural morphology of elastic fibers was unique between the two genotypes. In Fbln5−/− mice, vaginal elastic fibers consisted of large globules of solid elastin with displaced, more peripherally located microfibrils (Figure 5, G and H). Longitudinal sections of vaginal Fbln5−/− elastic fibers revealed that the fibers were shorter and thicker compared with WT, but that the fibers were not disrupted to the same degree as those in the vaginal wall of Fbln3−/− animals (Figure 5F).
Figure 5.
Ultrastructural morphology of elastic fibers in vaginal tissues from WT, Fbln3−/−, and Fbln5−/− mice at 6 to 8 months of age. A and B: Elastic fibers in vaginal wall from WT animals are comprised of a central elongated core of elastin (e) surrounded by numerous fine microfibrils (arrows, mf), and collagen fibers (coll) in the extracellular matrix. C and D: Elastic fibers in vaginal tissues from Fbln3−/− mice without prolapse. Elastic fibers are disrupted resulting in evident microfibrils within the elastin core. Longitudinal section reveals significant disruption of the elastic fiber, which is porous and frayed. E and F: Elastic fibers in vaginal tissues from Fbln3−/− mice with prolapse. Disrupted elastin core permits visualization of microfibrils throughout the elastin core. Severely disrupted and frayed elastic fibers are noted on longitudinal section. G and H: Elastic fibers in vaginal tissues from Fbln5−/− mice. Large amorphous elastin aggregates and displaced clusters of microfibrils. Scale bars: 0.2 μm (A, C, E, G); 0.5 μm (B, D, F, H).
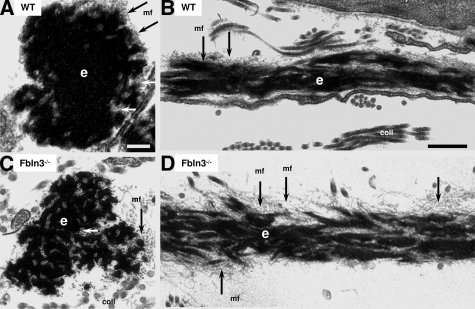
The abnormal morphology of elastic fibers in older (8 months) Fbln3−/− mice led us to investigate whether this morphology evolved with age, or whether abnormal vaginal elastic fibers were present in young knockout mice (Figure 6). Elastic fibers in the vaginal wall of young WT mice (5 weeks) were similar to those in older virginal animals (Figure 6, A and B, compared with Figure 5, A and B). Although disruption of vaginal elastic fibers was not as severe as that in the vagina of older Fbln3−/− mice, disruption of the elastin core was obvious even in young 5-week-old Fbln3−/− females several months before the onset of prolapse (Figure 6, C and D).
Figure 6.
Ultrastructural morphology of elastic fibers in vaginal tissues from young WT and Fbln3−/− mice. Elastic fibers from WT (A, B) and Fbln3−/− (C, D) mice at 5 weeks of age. The elastin core (e) is more fragmented in vaginal tissues from young Fbln3−/− females compared with WT. Microfibrils (mf) are noted with arrows. coll, collagen. Scale bars: 0.2 μm (A, C); 0.5 μm (B, D).
Localization of Fibulin-3 in the Vaginal Wall
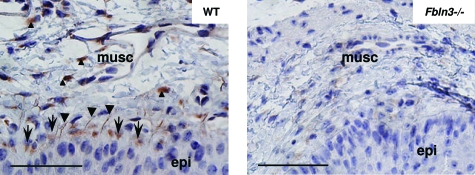
Using a polyclonal polypeptide antibody, immunohistochemistry was conducted to localize fibulin-3 in the vagina (Figure 7). Immunostaining was observed in cells juxtaposed to the basement membrane of epithelial cells, in the extracellular space lining subepithelial elastic fibers, and in stromal cells of the vaginal muscularis (Figure 7, left). Although immunostaining was completely absent in negative control sections (not shown), faint immunostaining could be detected in the vaginal wall of Fbln3−/− mice (Figure 7, right), suggesting that the antibody may recognize an epitope of another member of the fibulin family with much lower affinity because there is no evidence of fibulin-3 or fibulin-3 protein fragments in Fbln3−/− mice.23 Nevertheless, the results indicate that fibulin-3 protein is expressed in subepithelial cells of the vaginal wall and in the vaginal muscularis.
Figure 7.
Localization of fibulin-3 in the vaginal wall. Transverse sections of mid vagina from WT (left) and Fbln3−/− mice (right) at 6 months of age were immunostained with a polypeptide polyclonal antibody to fibulin-3. Immunostaining was visualized in subepithelial cells and basement membrane (arrows), subepithelial elastic fibers (arrowheads), and stromal cells of the vaginal muscularis (small arrowheads). Epi, epithelium; musc, muscularis. Scale bars = 20 μm.
Matrix Degradation in Fbln3−/− Mice
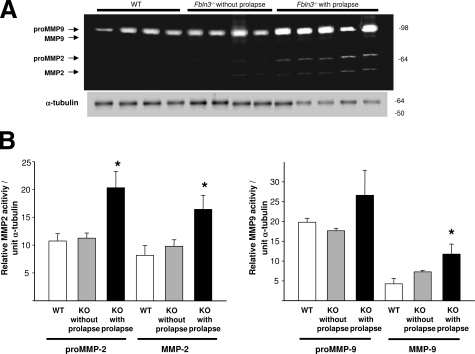
MMP2 and MMP9 enzyme activities were determined in vaginal tissues from Fbln3−/− mice with and without prolapse and virginal age- and estrus phase-matched WT controls using gelatin zymography (Figure 8). Pro-MMP2, active MMP2, and active MMP9 were increased significantly in vaginal tissues from Fbln3−/− mice with prolapse compared with WT (pro-MMP2, 1.9-fold, P = 0.013; MMP2, 2.0-fold, P = 0.039; total MMP9, 2.8-fold, P = 0.042) (Figure 8). MMP activity in Fbln3−/− without prolapse did not differ significantly from WT.
Figure 8.
MMP2 and MMP9 activity in vaginal tissues from Fbln3−/− mice. A: Gelatin zymogram indicating pro- and active MMP9 and MMP2 in vaginal tissue extracts from WT mice, Fbln3−/− mice without prolapse, and Fbln3−/− mice with prolapse. Immunoblot for α-tubulin is placed beneath the zymogram. B: Quantification of MMP2 and MMP9 in vaginal tissues from WT (n = 4) and Fbln3−/− (KO) mice without (n = 4) and with prolapse (n = 5). Enzyme activity is expressed in relative units and normalized to α-tubulin. *P ≤ 0.05 compared with WT.
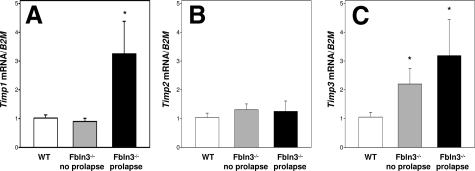
Tissue inhibitors of matrix metalloproteases (TIMPs) are extracellular matrix proteins that bind to and inhibit MMP activity. In addition to its role in elastogenesis, fibulin-3 has been shown to be a strong binding partner of TIMP3.27 Thus, a possible mechanism by which Fbln3−/− mice develop pelvic organ prolapse may be through increases in MMP activity mediated through changes in regulation or activity of TIMP3, TIMP2, or TIMP1. Expression of Timp1 mRNA was up-regulated in the prolapsed vagina from Fbln3−/− animals; Timp2 was not altered; and, Timp3 was increased in Fbln3−/− animals with or without prolapse (Figure 9). Thus, it does not appear that loss of tissue inhibitors (at least at the mRNA levels) is involved in up-regulation of MMP2 and MMP9 in vaginal tissues from Fbln3−/− mice.
Figure 9.
Expression of TIMPs in the vaginal wall. Relative levels of Timp1 (A), Timp2 (B), and Timp3 (C) mRNA were quantified in vaginal tissues from WT (n = 5) and Fbln3−/− mice without (n = 5) and with prolapse (n = 6) and expressed relative to that of β2-microglobulin (B2M). *P ≤ 0.05 compared with WT.
Elastic Fiber Synthesis in Vaginal Walls of Fbln3−/− Mice
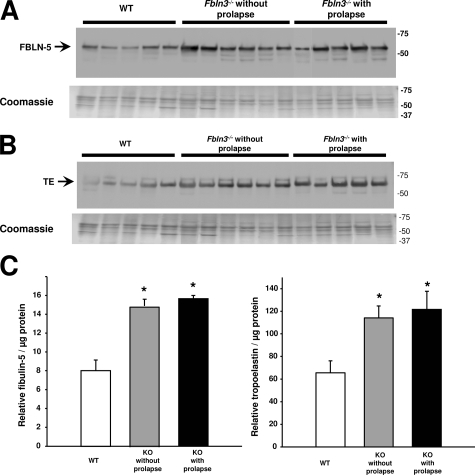
Fibulin-5 and tropoelastin proteins are both essential for assembly of mature elastic fibers, and both proteins are increased significantly in the vaginal wall postpartum.24 To determine whether increased MMP activity in knockout mice is counterbalanced by changes in elastic fiber synthesis, fibulin-5 and tropoelastin protein levels were quantified in vaginal tissues from virginal age- and estrus phase-matched WT (n = 5), Fbln3−/− mice without prolapse (n = 6), and Fbln3−/− mice with prolapse (n = 5). In agreement with previous studies,24 fibulin-5 and tropoelastin were highly expressed in urea extracts of vaginal tissues from nonpregnant WT mice (Figure 10). Fibulin-5 was increased significantly in vaginal tissues from Fbln3−/− with or without prolapse (P ≤ 0.01) (Figure 10, A and C). Tropoelastin was also increased in urea extracts from Fbln3−/− mice with or without prolapse (Figure 10, B and C). To address the possibility that fibulin-5 and tropoelastin may be more extractable from vaginal tissues from Fbln3−/− mice, we analyzed tropoelastin and fibulin-5 protein content in the initial salt/ethylenediaminetetraacetic acid extract. Although strong signals for each protein were present in urea extracts, tropoelastin and fibulin-5 were absent in all salt/ethylenediaminetetraacetic acid extracts (data not shown). The findings suggested that although increased expression of steady state levels of fibulin-5 may occur in response to the loss of fibulin-3 in the vaginal wall, it does not protect from failure of pelvic organ support.
Figure 10.
Expression of fibulin-5 (FBLN-5) and tropoelastin (TE) in vaginal tissues from Fbln3−/− mice. A: Immunoblot of FBLN-5 was conducted in urea extract proteins from vaginal tissues of WT and Fbln3−/− mice without and with prolapse. Coomassie staining was conducted in side-by-side gels to ensure even protein loading. B: Immunoblot of tropoelastin (TE) in urea extract proteins from vaginal tissues of WT and Fbln3−/− mice without and with prolapse. Coomassie staining was conducted in side-by-side gels to ensure even protein loading. C: Quantification of FBLN-5 and TE in urea extract proteins from vaginal tissues of WT and Fbln3−/− mice without and with prolapse. Data represent mean ± SEM of five to six tissues in each group. *P ≤ 0.05 compared with WT.
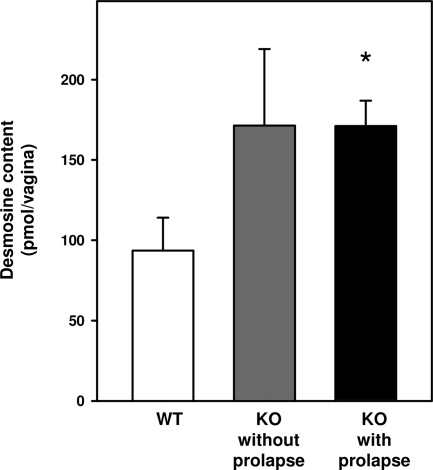
To quantify cross-linked elastin in the vaginal wall, desmosine levels were determined in vaginal tissues from age-, strain-, and cycle-matched WT and Fbln3−/− mice with or without prolapse (Figure 11). Desmosine levels were increased in vaginal tissues from knockout animals regardless of prolapse. Thus, the vaginal wall of knockout animals is characterized by increased levels of fibulin-5 and tropoelastin in the urea extract; increased desmosine content; and abundant, tortuous, and fragmented elastic fibers in the adventitial layer.
Figure 11.
Desmosine content in the vaginal wall. Desmosine levels were determined in vaginal tissues from age-, cycle-, and strain-matched WT (n = 5) and Fbln3−/− (KO) mice without (n = 5) and with prolapse (n = 7). Desmosine levels were expressed as the total amount of desmosine per vagina. *P ≤ 0.05 compared with WT.
Discussion
Role of Fibulin-3 in Pelvic Organ Support
Using a validated quantification tool for assessing prolapse, we found that 27% of mature Fbln3−/− knockout animals develop overt prolapse, which was concomitant with hernia formation in 32% and rectal prolapse in 34%. Compared with WT controls, perineal bulge, perineal body length, and vaginal diameter were increased significantly in Fbln3−/− mice even without obvious prolapse. As in women, the frequency of prolapse increased with age. Multivariate analysis did not indicate a significant association between parity and prolapse, suggesting that the lack of fibulin-3 does not impact the ability of pelvic floor connective tissue to recover after parturition but that fibulin-3 is necessary to maintain pelvic organ support during aging. These findings suggest that although elastic fiber assembly and synthesis are important in the pathophysiology of pelvic organ prolapse, biochemical pathways to maintain or recover pelvic organ support after parturition may be different from those during aging.
Development of Fbln5−/− and Loxl1−/− mouse models has led to the hypothesis that elastic fiber synthesis and assembly play important roles in the maintenance of pelvic organ support. The current study underscores this concept. Fibulin-3 has been shown to be important for elastic fibers in abdominal fascia such that absence of this protein leads to age-dependent development of abdominal and inguinal hernias.23 Herein, we show that absence of fibulin-3 also leads to age-dependent disruption of elastic fibers in connective tissue of the vaginal wall and development of pelvic organ prolapse.
Fibulin-3 Is Crucial for Normal Elastogenesis in the Vagina
Fibulin-5 and fibulin-4 have been shown to play crucial roles in elastic fiber development.10,12,28 Fibulin-5 preferentially binds monomeric tropoelastin29 and regulates self-aggregation of elastin, a process termed coacervation.30 Recent studies in vitro suggest that fibulin-5 promotes coacervation and alignment of tropoelastin on the microfibrillar scaffold for subsequent alignment and crosslinking.30,31,32 Our electron microscopy findings are consistent with this suggestion, in that large globules of elastin with displacement of microfibrils were observed in the vaginal wall of Fbln5−/− mice. In contrast, in the absence of fibulin-3, elastic fibers of the vaginal wall were disrupted such that microfibrils were easily visualized within the elastin core. Disruption of elastic fibers was more severe with age and prolapse.
Age-related modifications in elastic fibers (extensively described in virtually all organs and tissues) may be primarily interpreted as progressive degradation of elastin polymers produced early in life. During aging, elastic fibers become tortuous, frayed, and porous.33 In the vaginal wall, however, elastic fiber degradation was not observed during aging. Unlike other adult organs, elastic fiber turnover appears to be continuous in the female reproductive tract.6,34 This unique adaptation to synthesize and assemble new elastic fibers may allow the vagina to expand during pregnancy, recover from childbirth, and cope with prolonged gravitational forces associated with aging. Unlike skin,23 fibulin-3 deficiency may lead to progressive and severe deterioration of elastic fibers in an organ such as the vagina that undergoes continuous remodeling with cyclic degradation and synthesis of elastic fibers.
Phenotypic Differences in Fbln3−/− and Fbln5−/− Females
The magnitude or severity of prolapse in Fbln3−/− mice was similar to that of Fbln5−/− animals. Nonetheless, a smaller percentage of Fbln3−/− mice developed the disease, and animals were much older at the onset of prolapse. The lack of fibulin-3 was associated with progressive degradation of elastic fibers in the vaginal wall with aging. Despite increases in fibulin-5, tropoelastin, and desmosine levels, elastic fibers became disrupted with age and failure of pelvic organ support occurred in Fbln3−/− animals, suggesting that other factors related to aging may have led to pelvic organ prolapse. One of these factors may be increased vaginal MMP activity. Because the age of prolapse was not predictable in Fbln3−/− mice, it is unclear whether increased vaginal protease activity preceded the development of prolapse or if increased MMP activity resulted from the mechanical stress on the prolapsed vaginal tissue. Nevertheless, progressive elastin degradation in the vaginal wall may lead to accumulation of elastin degradative peptides, which activate proteases in a number of tissues.35 Overall, the results indicate that development of prolapse in Fbln3−/− mice involves both increased production and degradation of elastic fibers in the vaginal wall, a situation not unlike solar elastosis of photoaged skin in which production of matrix macromolecules by dermal fibroblasts is accompanied by significant increases in matrix-degrading enzymes.36
Fbln3−/− animals develop abdominal and inguinal hernias whereas Fbln5−/− mice do not. On the other hand, Fbln5−/− animals develop tortuosity of the great vessels whereas Fbln3−/− mice do not. Both animals develop pelvic organ prolapse. The importance of each fibulin in different tissues is probably related not only to compensatory roles of other family members, but also to tissue-specific levels of expression. Overall, the results in this investigation indicate that both fibulin-3 and -5 are important in maintaining pelvic organ support in mice. Absence of either protein results in altered elastic fibers in the connective tissue of the vaginal wall and in failure of pelvic organ support with age. Fibulin-5 and tropoelastin are increased in the vaginal wall of Fbln3−/− mice with or without prolapse, suggesting a redundant and compensatory mechanism for elastic fiber assembly in the vagina. MMP2 and MMP9 enzyme activities, however, are increased only in vaginal tissues of Fbln3−/− mice with prolapse. The difference in penetrance of pelvic organ prolapse between the two mouse models may be explained by the significant up-regulation of other components of elastic fiber assembly in Fbln3−/− mice, possible differences in the relative abundance of each fibulin in connective tissues of the pelvic floor, differences in localization throughout the pelvic floor connective tissues, and the magnitude and timing of protease activation in the vaginal wall.
Acknowledgments
We thank Dr. Hiromi Yanagisawa for advice during the course of these experiments and for reading the manuscript; Jiwon Choi for his assistance and expertise in obtaining electron microscopy data; and Dr. Barry Starcher (Department of Biochemistry, University of Texas Health Science Center, Tyler, TX) for conducting the desmosine assays.
Footnotes
Address reprint requests to R. Ann Word, M.D., Division of Urogynecology and Reconstructive Surgery, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd., Dallas, TX 75390-9032. E-mail: ruth.word@utsouthwestern.edu.
Supported by the National Institutes of Health (grants AG028048 to R.A.W. and EY013847 to L.Y.M.), Research to Prevent Blindness (career development award to L.Y.M.), and the Canadian Institutes of Health Research (operating grant MOP-57663 to E.C.D.).
E.C.D. is a Canada Research Chair.
References
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106:615–634. doi: 10.1097/01.AOG.0000175832.13266.bb. [DOI] [PubMed] [Google Scholar]
- Jelovsek JE, Barber MD, Paraiso MFR, Walters MD. Functional bowel and anorectal disorders in patients with pelvic organ prolapse and incontinence. Am J Obstet Gynecol. 2005;193:2105–2111. doi: 10.1016/j.ajog.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Woodman PJ, Swift SE, O'Boyle AL, Valley MT, Bland DR, Kahn MA, Schaffer JI. Prevalence of severe pelvic organ prolapse in relation to job description and socioeconomic status: a multicenter cross-sectional study. Int Urogynecol J. 2006;17:340–345. doi: 10.1007/s00192-005-0009-2. [DOI] [PubMed] [Google Scholar]
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25:723–746. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- Starcher B, Percival S. Elastin turnover in the rat uterus. Connect Tissue Res. 1985;13:207–215. doi: 10.3109/03008208509152400. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Rahn DD, Ruff MD, Brown S, Tibbals HF, Word RA. Biomechanical properties of the mouse vagina: changes seen in pregnancy and with elastinopathy. Am J Obstet Gynecol. 2008;198:590e1–590e6. doi: 10.1016/j.ajog.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Wachi H, Ishida M, Nonaka R, Onoue S, Urban Z, Starcher BC, Seyama Y. Distinct steps of cross-linking, self-association, and maturation of tropoelastin are necessary for elastic fiber formation. J Mol Biol. 2007;369:841–851. doi: 10.1016/j.jmb.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- Wieslander CK, Acevedo JF, Drewes PG, Yanagisawa HK, Word RA. Pelvic organ prolapse severity increases with age in fibulin-5 knockout mice. Int Urogynecol J. 2006;17:S371–S372. [Google Scholar]
- Liu X, Zhao Y, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorders. Am J Pathol. 2006;168:519–528. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kostka G, Garbe JH, Keene DR, Bächinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T. A comparative analysis of the fibulin protein family: biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- de Vega S, Iwamoto T, Nakamura T, Hozumi K, McKnight DA, Fisher LW, Fukumoto S, Yamada Y. TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J Biol Chem. 2007;282:30878–30888. doi: 10.1074/jbc.M705847200. [DOI] [PubMed] [Google Scholar]
- Sicot FX, Tsuda T, Markova D, Klement JF, Arita M, Zhang RZ, Pan TC, Mecham RP, Birk DE, Chu ML. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol Cell Biol. 2008;28:1061–1067. doi: 10.1128/MCB.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4:1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher WM, Currid CA, Whelan LC. Fibulins and cancer: friend or foe? Trends Mol Med. 2005;11:336–340. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Chu ML, Tsuda T. Fibulins in development and heritable disease. Birth Defects Res C Embryo Today. 2004;72:25–36. doi: 10.1002/bdrc.20003. [DOI] [PubMed] [Google Scholar]
- Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, Uitto J, Chu ML. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet. 2003;72:998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, Marmorstein AD, Marmorstein LY. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum Mol Genet. 2007;16:3059–3070. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- Drewes PG, Yanagisawa H, Starcher B, Hornstra IK, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170:578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander CK, Marinis SI, Drewes PG, Keller PW, Acevedo JF, Word RA. Regulation of elastolytic proteases in the mouse vagina during pregnancy, parturition, and puerperium. Biol Reprod. 2008;78:521–528. doi: 10.1095/biolreprod.107.063024. [DOI] [PubMed] [Google Scholar]
- Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31:133–140. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1): implications for macular degenerations. J Biol Chem. 2004;279:30469–30473. doi: 10.1074/jbc.M403026200. [DOI] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78:1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Davis EC, Richardson JA, Starcher BC, Li T, Gerard RD, Yanagisawa H. Molecular analysis of fibulin-5 function during de novo synthesis of elastic fibers. Mol Cell Biol. 2007;27:1083–1095. doi: 10.1128/MCB.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi H, Nonaka R, Sato F, Shibata-Sato K, Ishida M, Iketani S, Maeda I, Okamoto K, Urban Z, Onoue S, Seyama Y. Characterization of the molecular interaction between tropoelastin and DANCE/fibulin-5. J Biochem. 2008;143:633–639. doi: 10.1093/jb/mvn014. [DOI] [PubMed] [Google Scholar]
- Wachi H, Sato F, Nakazawa J, Nonaka R, Szabo Z, Urban Z, Yasunaga T, Maeda I, Okamoto K, Starcher BC, Li DY, Mecham RP, Seyama Y. Domains 16 and 17 of tropoelastin in elastic fibre formation. Biochem J. 2007;402:63–70. doi: 10.1042/BJ20061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Takema Y, Yorimoto Y, Tsukahara K, Kawai M, Imayama S. Degree of ultraviolet-induced tortuosity of elastic fibers in rat skin is age dependent. J Invest Dermatol. 1995;105:254–258. doi: 10.1111/1523-1747.ep12317607. [DOI] [PubMed] [Google Scholar]
- Sharrow L, Tinker D, Davidson JM, Rucker RB. Accumulation and regulation of elastin in the rat uterus. Proc Soc Exp Biol Med. 1989;192:121–126. doi: 10.3181/00379727-192-42965. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Moran M, Kochevar IE. Chronic photodamage in skin of mast cell-deficient mice. Photochem Photobiol. 1999;70:248–253. [PubMed] [Google Scholar]