Abstract
We have extensively analyzed the biochemical and histochemical profiles of the tau protein from the rTg4510 transgenic mouse model in which the animals uniquely develop forebrain tau pathologies similar to those found in human tauopathies. Levels of several soluble phosphorylated tau species were highest at 1 month relative to later time points, suggesting that certain tau hyperphosphorylation events were insufficient to drive tangle formation in young mice. Despite a robust, pre-tangle-like accumulation of phospho-tau in 1-month-old mice, this material was cleared by 3 months, indicating that the young mouse brain either fails to facilitate tau insolubility or possesses an enhanced ability to clear tau relative to the adult. We also found that while heat shock protein expression increased with normal aging, this process was accelerated in rTg4510 mice. Moreover, by exploiting an exon 10 (−) specific antibody, we demonstrated that endogenous mouse tau turnover was slowed in response to human tau over-expression, and that this endogenous tau adopted disease-related properties. These data suggest that a younger brain fails to develop lasting tau pathology despite elevated levels of phosphorylated tau, perhaps because of reduced expression of stress-related proteins. Moreover, we show that the active production of small amounts of abnormal tau protein facilitates dysfunction and accumulation of otherwise normal tau, a significant implication for the pathogenesis of patients with Alzheimer’s disease.
Cerebral accumulation of the microtubule associated protein tau into punctate fibrillar aggregates is a hallmark of a class of disorders termed tauopathies. Tau within these aggregates exhibits a significant amount of posttranslational modifications, the most common being hyperphosphorylation of the more than 20 phosphorylation sites found on the protein.1 There are 17 known neurodegenerative diseases that exhibit postmortem tau pathology, the most common of which is Alzheimer’s disease.2 Several of these diseases arise from mutations within the MAPT gene itself, including frontotemporal dementia with parkinsonism linked to chromosome 17 and progressive supranuclear palsy.3,4 While these mutations are often very close in proximity, the clinical presentation and the pathological profile of each disorder can be quite distinct. For example, the P301L mutation causes the clinical and pathological presentation of frontotemporal dementia with parkinsonism linked to chromosome 17, while the G303V mutation causes progressive supranuclear palsy.4 Most tau mutations modify the alternative splicing of tau pre-mRNA, such that splicing out of exon 10 is reduced. This alters the typical 1:1 ratio of exon 10+ (4R) and exon 10− (3R) tau seen in normal adults and is thought to be a key event in tau pathogenesis. The discovery of tau mutations has facilitated the generation of several mouse models of tauopathy, which have become important tools for our understanding of the neurodegenerative mechanisms elicited by tau aggregation.5,6,7
Recently, the rTg4510 mouse model was developed in an effort to generate a model with significant forebrain pathology, a feature that previous models had failed to reliably produce.8 Santacruz and colleagues used a CaMKIIalpha promoter driven tetracycline operator to focus human mutant P301L tau over-expression in the forebrain (ie, hippocampus and higher cortical layers). These inducible transgenic mice developed robust forebrain tangle pathology, cognitive deficits, significant neuron loss, and cortical thinning in τ-associated areas. Once neurofibrillary tangles had begun, suppression of tau with doxycycline in this model partially reversed memory deficits; however, tangles persisted and continued to increase. This rTg4510 model has led to a number of investigations studying how mutant tau facilitates neuronal dysfunction.9,10,11 These mice have also been used to address very topical questions for the field such as the role of caspase cleavage of tau in tangle formation.12,13 A large repertoire of immunological agents is available for various tau species, particularly those that recognize distinct phospho-tau species, each of which has unique properties; however, only a handful of these have been investigated in this model. In our current report, we endeavored to extensively evaluate the biochemical and histological properties of these distinct tau species cross-sectionally. We emphasized several epitopes in these studies; 1) pS262/S356 tau, which has unique KXGS consensus sites in the microtubule binding domain and is thought to be an initiating event for tau pathogenesis; 2) pS202/T205 tau, which is one of the earliest phospho-tau epitopes and occurs on endogenous mouse tau; and 3) MC1/Alz50 tau, which are two similar conformational epitopes that are formed when the N-terminal folds back on itself and interacts with the microtubule binding domain.14 Our findings led to several novel observations, particularly with regard to the mechanisms of tau processing and the stress response that seem to vary between juvenile and adult mice.
Materials and Methods
Mouse Breeding and Tissue Handling
The rTg4510 mice and parental mutant tau and tTA lines were generated and maintained for this study as previously described in SantaCruz et al.8 We harvested brain tissue from 1-, 3-, 5.5-, and 9-month-old rTg4510 mice and non-transgenic littermates. Each group consisted of 5 to 6 animals per genotype. Mice were weighed, overdosed with pentobarbital (200 mg/kg) and perfused with 25 ml of 0.9% normal saline solution. Brains were collected from the animals immediately following perfusion and hemisected down the sagittal midline. Half of the brain was frozen on dry ice for biochemical studies and half was immersion fixed in 4% paraformaldehyde for 24 hours.
Antibodies (Clonal Names in Parentheses)
Mid-domain 218–225 (Tau5), N-terminal 2–18 (Tau12), and C-terminal 401–441 (Tau46) antibodies were provided by Dr. Lester Binder, Northwestern University School of Medicine; pS202/T205 (CP13), pS396/S404 (PHF1), MC1, and Alz50 tau antibodies were provided by Dr. Peter Davies, Albert Einstein College of Medicine; pS262/356 (12E8) tau antibody was provided by Dr. Peter Seubert, Elan Pharmaceuticals15,16; 3R-specific tau antibody was provided by Dr. Rohan de Silva, Reta Lila Weston Institute of Neurological Studies, United Kingdom; N-terminal 19–33 tau and carboxy terminus of Hsc70 interacting protein (CHIP) antibodies were provided by the Dr. Leonard Petrucelli, Mayo Clinic Jacksonville; P23 (JJ3) and Hop (F5) antibodies were provided by Dr. David Toft, Mayo Clinic Rochester; pT231 tau antibody was from Abcam, Cambridge, MA; pS199/S202, pS212, and pS262 rabbit polyclonal antibodies were from Anaspec, San Jose, CA; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody was from Biodesign International, Saco, ME; HSP (heat shock protein) 70 antibody was from Stressgen, Ann Arbor, Michigan; Akt antibody was from Cell Signaling Technologies, Beverly, MA; Hsp40 and Hsp90 antibodies were from BD Biosciences, San Jose, CA; Hsp27 antibody was from Santacruz Biotech, Santacruz, CA. Horseradish peroxidase- and biotin-conjugated secondary antibodies were obtained from Southern Biotech, Birmingham, AL and Vector Laboratories, Burlingame, CA.
Centrifugal Fractionation of Brain Extracts, Cell Culture, and Western Blot Analysis
After brain tissue was harvested as described above, each sample was homogenized as previously described.17 HEK293 and CHO cells were transfected with pcDNA3.1 vectors containing wild-type 3R0N, wild-type 4R0N, or P301L 4R0N tau using lipofectamine 2000 as previously described.18 Stably expressing cell lines were established using G418 selection. Cells were lysed in a Tris buffer containing 1% SDS and sonicated. Samples were run on 10% Tris-Glycine gels or 18-well 10% Criterion gels (BioRad, Hercules, CA). Protein expression was visualized by electrochemiluminescence treatment and exposure to film. Bands were quantified using Scion Image. A semiquantitative analysis was performed by densitometry, correcting protein levels for GAPDH. For P3 fractions, 3 μl of sample were added to 3 μl of Laemmli buffer, heated and 5 μl loaded into wells. For fragmentation study, films were exposed for 20 to 30 minutes.
Immunoprecipitation with RD3 and Alz50 Antibodies
Immunoprecipitation (IP) studies from rTg4510 mice were performed as previously described.19 RD3 IP blots were probed with rabbit polyclonal phospho-tau antibodies and Alz50 IP blots were probed with RD3 antibody using mouse IgG-specific secondary detection to avoid capture antibody heavy chain interference.
Histochemical Studies and Quantification
Fixed mouse brains were cryoprotected in successive 24 hours incubations of 10%, 20%, and 30% solutions of sucrose and then sectioned as previously described.20 One series of sections was mounted on slides and stained with Gallyas silver stain using a protocol for non-paraffinized tissue adapted from previously described methods (Lewis et al, 2000).
Stained sections were imaged using an Olympus BX51 microscope rig at original ×40, ×100, or ×200 final magnification. For quantification, images (original ×100 magnification) of cornu ammonis (CA)1, CA3, and the entorhinal cortex were taken using spatial orientation cues. Quantification of positive staining product was determined using Image-Pro Plus (Media Cybernetics, Silver Springs, MD). analysis of variance statistical analysis was performed using StatView version 5.0.1 (SAS Institute, Raleigh, NC).
Results
Age-Dependent Changes in Distinct Soluble Tau Species
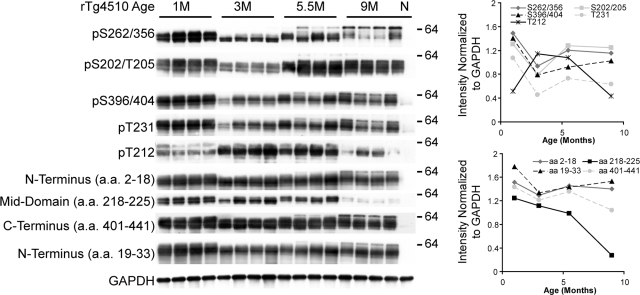
Western blot analysis of the soluble (S1) protein fraction from brain homogenates of rTg4510 mice demonstrated that human tau was expressed at the highest levels at 1 month of age (Figure 1). Levels of tau phosphorylation were not concurrent across different epitopes. Multiple phospho-tau species (S262/S356, T202/S205, S396/S404, and T231) were elevated at 1 month, which sharply declined at 3 months followed by a gradual increase at 5.5 and 9 months. Conversion to a 64-kDa soluble tau species was observed as early as 5.5 months for each of these epitopes. In particular, tau phosphorylated at S262/S356 was predominantly in the 64kDa species at 9 months. A component of the AT100 epitope, pT212, was the only epitope tested that was initially low at 1 month, followed by a substantial increase at 3 and 5.5 months, with a sharp decline at 9 months. Similar to antibodies against p-tau epitopes other than pT212, antibodies targeting specific portions of the tau protein were also most reactive with tau from 1-month-old rTg4510; however, only minor reductions in both N- and C-terminal tau were observed at 3 months. N-terminal tau specific immunoreactivity was recovered at 5.5 months and maintained thru 9 months (Figure 1). C-terminal reactivity was recovered at 5.5 months but decreased again by 9 months. Most noticeable was the unique profile of the mid-domain antibody, tau5 that recognizes aa218–225; this epitope was the only species to gradually decrease at each time point with a dramatic drop between 5.5 and 9 months.
Figure 1.
Cross-sectional analysis of soluble tau species in the rTg4510 mice. Representative immunoblots of soluble tau species from whole brains of 1-, 3-, 5.5-, and 9-month-old rTg4510 mice and 9-month-old non-transgenic (N) mouse as a control were normalized to GAPDH levels and quantified using pixel density. With the exception of pT212 tau, soluble phospho-tau species were at maximum levels at 1 month. In aged mice, a 64kDa tau species (as indicated on the right) appeared in the soluble fraction using antibodies against pS262/S356, pS202/T205, pS396/S404, pT231, and N-terminal tau.
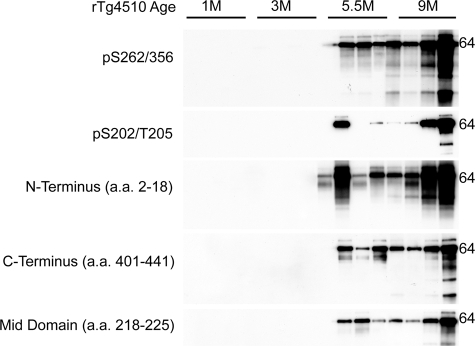
Age-Dependent Increases in Sarkosyl-Insoluble Tau
The cross-sectional sarkosyl-insoluble tau profiles for the rTg4510 mice were similar to what have been previously described.9 Most noticeable of these results was the absence of insoluble tau in the 1-month-old cohort despite the high amount of soluble material (Figure 2). Interestingly, while mouse-to-mouse levels of soluble tau species were quite consistent, considerable variation in levels of the insoluble tau fraction for different tau epitopes within cohorts was observed. This could indicate that the rate of conversion to insoluble tau is quite variable between animals. The presence of insoluble tau correlates with the 64kDa species in the soluble fractions in the 5.5-month-old cohort (Figure 1). For example, the first two mice in the 5.5-month-old cohort are female siblings, but the second mouse has considerably more insoluble tau and also has more of the 64-kDa soluble tau species, particularly as detected with the pS262/356 antibody. This variance among individual mice only allowed us to qualitatively assess tau insolubility among the cohort as a whole, as it appears that the total amount of insoluble tau across cohorts increases with age.
Figure 2.
Cross-sectional analysis of insoluble tau species in the rTg4510 model. Representative immunoblots of sarkosyl insoluble tau species from whole brains of 1-, 3-, 5.5-, and 9-month-old rTg4510 mice. 64-kDa sarkosyl insoluble tau species was apparent at 5.5- and 9-month-old in rTg4510. Interestingly, considerable variability within age groups was observed with insoluble tau fractions.
Robust Soluble Tau Accumulation Only Leads to Persistent Pathology in Adult Mice
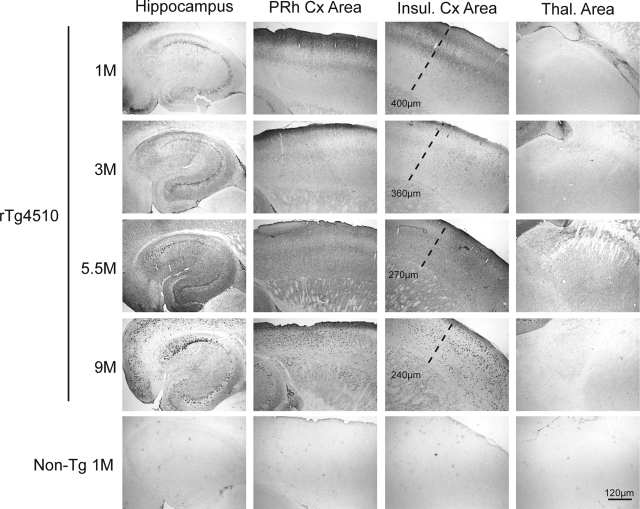
We subsequently performed histochemical studies and quantitative image analysis (previously described20) to evaluate the accumulation of abnormal tau species in rTg4510 mice compared with non-transgenic littermates. Tau phosphorylated at S262/S356 was abundant, particularly in higher cortical layers and the piriform cortex (Figure 3), in 1-month-old rTg4510 mice. Similar to the biochemical profile for this antibody, cortical immunostaining for phosphorylation at S262/S356 was reduced by the 3 month time point and steadily increased with subsequent time points. Cortical thinning was notable by 5.5 months in rTg4510 mice. Thalamic staining for abnormally phosphorylated tau was not observed at any age. Hippocampal levels of S262/S356 phospho-tau were also slightly elevated in 1-month-old rTg4510 mice compared with 3-month-old mice. Higher magnification images of pS262/S356 tau in 1-month-old rTg4510 mice showed tau accumulation in molecular layer I, which predominantly harbors apical dendrites and axon terminals, as well as in the external granular layers, as evidenced by the perikaryal tau accumulation reminiscent of pre-tangles (see Supplemental Figure S1 at http://ajp.amjpathol.org). This tau species also decorated long axonal processes throughout the cortical layers. Perikaryal tau was also observed in CA1 pyramidal neurons of 1-month-old rTg4510 mice. Some less intense, perinuclear staining was observed with what appeared to be occasional tangles. By 5.5 months, S262/S356 phosphorylated tau accumulated in both CA1 and the subiculum with abundant immunostaining of the perikarya and processes, which continued to progress through the 9-month time point.
Figure 3.
pS262/S356 tau staining and progressive cortical thinning rTg4510 mice. Neocortical sections show abundant immunoreactivity with 12E8 (pS262/S356) tau. Early (1-month-old) cortical accumulation of 12E8 tau was seen in higher cortical layers, including the molecular layer. Granular staining was also evident in CA and the subiculum of the hippcampus, however, minimal staining was observed in the dentate gyrus (DG). By 3 months, staining in all regions was reduced although reactivity was still apparent in the molecular layer of the cortex. At 5.5 months, staining was more prevalent in neuronal cell bodies of CA1 and the subiculum. Diffuse staining was also elevated throughout the neocortex. By 9 months, punctuate perikaryal tau was seen in neurons throughout CA and DG, with particularly robust staining in CA1. Neuronal staining was also observed throughout the neocortex. Cortical thinning was progressive, as measured by the distance from the outer cortical layer to the lateral edge of the caudate putamen. Staining in thalamic areas was not observed and non-transgenic mice showed no reactivity with this antibody. 1 cm is equivalent to 120 μm in all images for this figure.
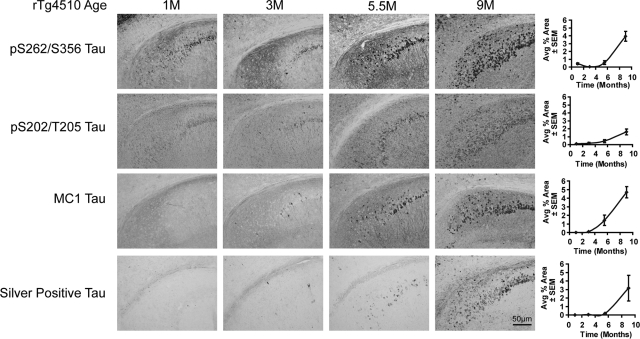
Levels of pS202/T205 tau were similar between 1 and 3 months, rTg4510 mice in CA1 (Figure 4), entorhinal cortex (EnCx; see Supplemental Figure S2 at http://ajp.amjpathol.org), and CA3 (see Supplemental Figure S3 at http://ajp.amjpathol.org), except that more neurites were stained at 3 months. Staining progressively increased at 5.5 and 9 months, although staining intensity was reduced at 9 months relative to the other tau species investigated. Staining with MC1, an antibody that recognizes a conformation adopted by tau that is thought to nucleate filament formation was first observed in the cortex and hippocampus at 3 months and progressed in an age-dependent manner (Figure 4, and Supplemental Figures S2 & 3 at http://ajp.amjpathol.org). Occasional argyrophilic tangles were observed in the cortex and hippocampus of rTg4510 mice at 3 months with steady increases at 5.5 and 9 months (Figure 4, and Supplemental Figures S2 & 3 at http://ajp.amjpathol.org). Quantitation was performed for CA1, CA3, and EnCx and the combined values for these regions are represented graphically in Figure 4. Together with the biochemical analyses described above, these results provided a foundation for more detailed studies into the mechanisms of the robust and progressive tau accumulation occurring in these mice.
Figure 4.
Progressive changes in tau phosphorylation, conformation, and argyrophilia in CA1 of rTg4510 mice. Matched sections serially collected were stained with 12E8 (pS262/S356), CP13 (pS202/T205), and MC1 antibodies and Gallyas silver stain. Perikaryal phospho-tau was apparent in 1-month-old mice throughout CA1 and into the subiculum. This was decreased by 3 months of age. 5.5- and 9-month-old mice showed progressive increases for both phospho-tau species analyzed with more apical staining at 5.5 months. MC1 tau only emerged in 3-month-old mice and stained apical dendrites at 3 and 5.5 months. Argyrophilic tau was occasional observed in the CA1 at 3 months, which progressively increased with age. Quantitation of each stain was performed for CA1, CA3 (not shown), and entorhinal cortex (S Figure 1) and expressed as tau burden. Overall levels of pS202/T205 tau were lowest in 9-month-old mice compared with the other species investigated. 1 cm is equivalent to 50 μm in all images for this figure.
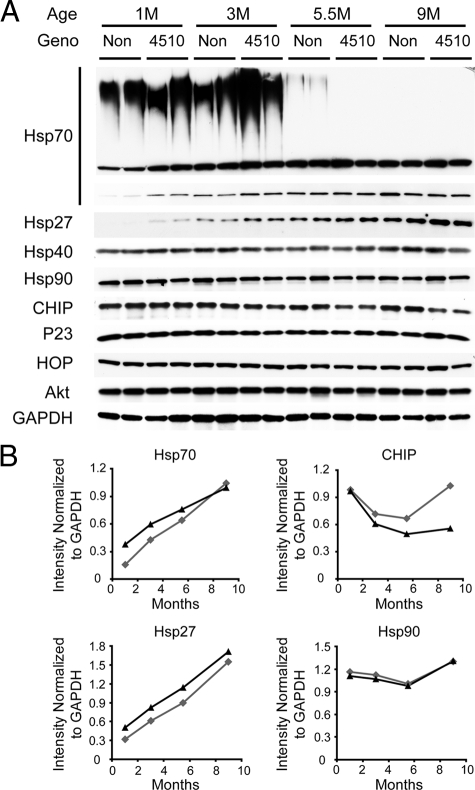
Age- and Tau-Associated Alterations in Chaperone Proteins
Given our recent work in chaperone regulation of tau biology, we evaluated whether tau accumulation affected the levels and activity of heat shock proteins and other proteins involved in protein homeostasis. Young mice, regardless of transgenic status, had a high molecular weight (HMW) smear reactive with anti-Hsp70 antibody, suggestive of polyubiquitination (Figure 5A). This HMW smear was reduced, but not absent, in 5.5-month-old non-transgenic mice; however, it was ablated in rTg4510 mice of the same age. Age-dependent increases in Hsp70 and Hsp27 levels were clearly observed in non-transgenic mice. Interestingly, this increase was modestly accelerated in rTg4510 mice compared with age-matched non-transgenic mice, an effect that appeared to plateau by 5.5 months (Figure 5B). No changes were observed in Hsp90 (Figure 5B). CHIP levels, which were elevated in 1-month-old mice, decreased at 3 and 5.5 months, and then increased again at 9 months in non-transgenic mice; however rTg4510 mice had reduced levels of CHIP from 3 months onward and they failed to return to higher levels at 9 months (Figure 5B). Levels of other chaperone-related proteins such as P23, HOP, Hsp40, and Akt were equivalent across genotypes.
Figure 5.
Levels of Hsp70 and Hsp27 increased with age and were accelerated by tau overexpression. A: Soluble tau was extracted from whole brains of 1-, 3-, 5.5- and 9-month-old rTg4510 mice (2 per group) and 1-, 3-, 5.5- and 9-month-old non-transgenic littermates (Non; 2 per group). Antibodies used are indicated to the left. B: Densitometry was used to analyze levels of Hsp27, Hsp70, CHIP, and Hsp90 normalized to GAPDH and these ratios are shown as a function of time. NonTg mice are indicated in gray and rTg4510 mice are shown in black.
Overexpression of Mutant Human Tau Facilitates Persistence of Endogenous Mouse Tau
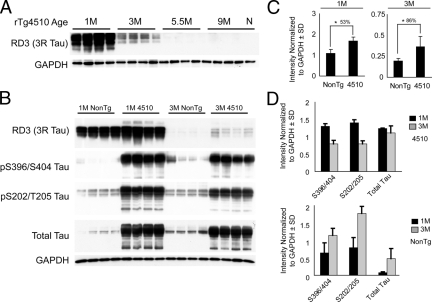
We had previously demonstrated that insoluble tau in P301L 4R0N transgenic mice did not contain mouse tau,5 suggesting that mouse tau was not incorporated into tangles; however, the impact that human tau expression may have on soluble mouse tau species remained unclear. Using the rTg4510 mice, we investigated this impact by exploiting a unique property of mouse tau that distinguishes it from human tau. In fetal/juvenile brains from both humans and rodents, the tau is predominantly exon 10−; however, exon 10+ tau mRNA increases with age. In normal adult humans, there is a 1:1 ratio of exon 10+ (4R) and exon 10− (3R) tau. In normal adult mice, however, murine tau exclusively becomes 4R.21 Antibodies have been previously generated to specifically recognize 3R tau species22,23 and we used these antibodies to determine whether 3R tau was present at different time points in the rTg4510 in response to high levels of 4R human tau.
Using the 3R specific antibody RD3 on soluble tau fractions, we found that 3R tau was still detectable at 3 months of age, but was no longer apparent at 5.5 or 9 months in rTg4510 mice (Figure 6A). In comparison with non-transgenic mice, 3R tau was ∼50% higher in rTg4510 mice at 1 month of age (Figure 6, B & C). Interestingly, a higher molecular weight species emerged, suggesting increased phosphorylation of 3R mouse tau in 1-month-old rTg4510 mice. More striking was the finding that rTg4510 mice maintained nearly double the level of 3R mouse tau at 3 months of age (∼86%) compared with nearly undetectable levels in non-transgenic age-matched mice (Figure 6, B & C). Also of interest, pS396/S404, pS202/T205, and total tau were elevated in 3-month-old non-transgenic mice relative to their 1-month-old counterparts (Figure 6, B & D). In contrast, these species appeared reduced between the 1-month and 3-month time points in rTg4510 mice. Lighter exposure profiles for these epitopes in the same transgenic mice are shown in Figure 1.
Figure 6.
Endogenous mouse tau is preserved in rTg4510 mice. A: Soluble tau was extracted from whole brain of 1-, 3-, 5.5-, and 9-month-old rTg4510 mice (four per group) and one non-transgenic 9-month-old mouse as a control (N). 3R tau (RD3 antibody) was highest at 1 month and absent at 5.5 months in the rTg4510 mice. B: 3R tau was higher in rTg4510 mice compared with non-transgenic littermates at 1 and 3 months. Other phospho-tau epitopes were consistent with data from Figure 1; however, non-transgenic mice showed increases in phospho-tau between 1 and 3 months. C: Densitometry was used to analyze levels of RD3-positive tau in 1-month-old and 3-month-old mice after GAPDH normalization. These values are plotted as a function of genotype. D: Densitometry was used to analyze levels of indicated tau species tau in 1-month-old and 3-month-old mice after GAPDH normalization ± 1 SD. These values are plotted as a function of tau species. Black bars indicate 1-month-old mice and gray bars indicate 3-month-old mice.
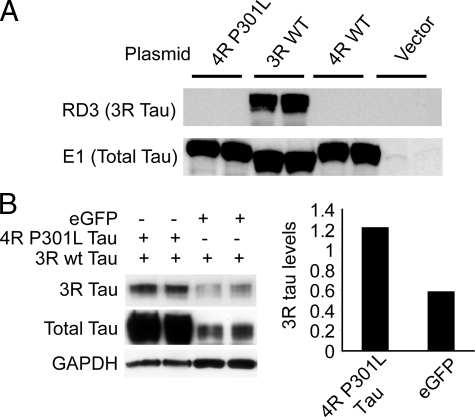
To ensure that the RD3 antibody did not recognize 4R tau, we over-expressed 3R and 4R tau clones in HEK293 cells and tested cross-reactivity by Western blot. Although total tau was detected in all tau transfected HEK293 cells, regardless of transfected tau isoform, RD3 only recognized tau from the cell line transfected with 3R tau (Figure 7A). To prove that overexpression of 4R tau could impair the turnover of 3R tau, CHO cells stably expressing wild-type (wt) 3R tau were transfected with enhanced green fluorescent protein (eGFP) or 4R human tau harboring the P301L mutation, the same form used to make the rTg4510 mouse model. After 24 hours, levels of 3R tau were substantially higher in those transfected with 4R P301L tau compared with those transfected with eGFP (Figure 7B).
Figure 7.
Turnover of 3R tau is impaired by over-expression of 4R P301L tau. A: RD3 only recognized 3R tau. E1, an antibody targeting total human tau (a.a. 19–33), confirmed over-expression of all tau plasmids. B: CHO cells stably expressing human 3R wild-type (wt) tau were transfected with either human 4R P301L tau or eGFP and harvested 24 hours later. 3R tau levels were ∼50% higher in 4R P301L tau transfected cells relative to eGFP transfected cells. Total tau levels were determined using E1. GAPDH was used as a protein loading control. The histogram shows the average optical density for 3R tau as a ratio of GAPDH for both 4R P301L tau and eGFP transfected cells.
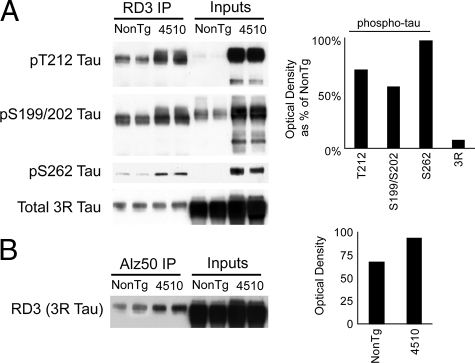
We then immunoprecipitated 3R tau from 1-month-old rTg4510 mice and non-transgenic littermates and found that the 3R tau from rTg4510 was phosphorylated at T212, S199/S202, and S262 to a greater extent than in non-transgenic age-matched mice (Figure 8A). Levels of 3R tau that were captured by IP were consistent across non-transgenic and rTg4510 mice. We then used the Alz50 antibody to immunoprecipitated tau from these same 1-month-old mice. The Alz50 antibody recognizes a similar disease-related conformational epitope to MC1, but is an IgM rather than an IgG. We wanted to determine whether endogenous 3R mouse tau was capable of assuming a disease-specific conformation. Since the RD3 antibody is an IgG, we were able to use isotype specific secondary detection to avoid heavy chain contamination from the IP process by using Alz50 as the capture antibody and RD3 with an IgG-specific secondary for detection. We found that 3R tau was capable of forming the Alz50 epitope and this was enhanced in the rTg4510 mice when compared with non-transgenic age matched mice (Figure 8B).
Figure 8.
Endogenous 3R mouse tau adopts disease-associated properties in rTg4510 mice. A: Immunoprecipitation (IP) with RD3 antibody of soluble tau in 1M rTg4510 and non-transgenic littermates followed by detection with phospho-tau antibodies (indicated on left) showed that rTg4510 mice had higher levels of phosphorylated 3R tau than non-transgenic mice. Inputs indicate whole brain homogenate. Optical densitometry for rTg4510 mice as a percentage of the non-transgenic mice is shown for all tau species analyzed in the RD3 IP lanes. B: IP with Alz50, an IgM that recognizes a specific disease-associated tau conformation, from the same samples followed by RD3 detection using IgG showed that more 3R tau was also in the Alz50 conformation in rTg4510 mice compared with non-transgenics. Optical densitometry for rTg4510 and non-transgenic mice is shown for RD3 in the Alz50 IP lanes.
These data showed that turnover of endogenous mouse tau was causing the preservation of endogenous 3R tau in the rTg4510 mice. To determine whether 3R tau could be processed via the typical degradation pathway, we investigated 3R tau levels in the same 1-month-old CHIP knockout mice that we previously described, which have no transgenic over-expression of human tau.17 Since CHIP knockout mice lack a critical tau ubiquitin ligase,17,24 we were able to ascertain whether 3R tau turnover was indeed impaired in 1-month-old CHIP−/− mice. 3R tau levels were significantly elevated in CHIP null mice compared with wild-type littermates (see Supplemental Figure S4 at http://ajp.amjpathol.org). This data also suggested that 3R tau is susceptible to accumulation when the degradation machinery is compromised either by increased usage demands, such as in the rTg4510 mice, or malfunctioning components of the degradation cascade, such as in the CHIP knockout mice.
Discussion
The tau protein can undergo many posttranslational modifications, the most common being phosphorylation and protease cleavage. However, other changes have also been reported, including nitration, glycosylation, and folding. Adding further to this complexity is posttranscriptional alternative splicing that promotes production of six tau isoforms, each of which is expressed in the human adult brain. The large number of different tau species has made it difficult to ascertain the precise role of each form of the tau protein in disease development and progression, thereby fuelling the generation of antibodies to detect these tau species, particularly those recognizing certain phospho-tau epitopes. These immunochemicals have been critical for allowing us to extensively characterize both the biochemical and histochemical profiles in the rTg4510 mouse model of tauopathy. The robust tau over-expression in the rTg4510 model also provided an opportunity to investigate the impact of age on the management of elevated tau levels.
In the rTg4510 mice, we found that most soluble tau species were at their highest levels at 1 month compared with other time points. Soluble phospho-tau in rTg4510 mice, in general, showed greater reductions between 1- and 3-month-old mice than did phospho-independent tau epitopes (ie, N- and C-terminal; Figure 1). This was consistent with work done nearly 15 years ago showing high levels of phosphorylated tau in the developing brain in comparison with aged mice.25 This high level of phospho-tau in 1-month-old mice was attributed to the increased demand for neurite outgrowth in the developing brain, a role that phosphorylated tau has been linked to in the past.26 In the rTg4510 model, phosphorylation of epitope T212, a site that is critical for formation of the filament specific AT100 epitope,6 was increased between 1- and 3-month-old (Figure 1). In addition to being a target of GSK3ß and protein kinase A-directed phosphorylation, T212 has been shown to be a target for stress activated kinases.27 Taken together with our data suggesting that the stress response accelerates with age (Figure 6), phosphorylation of tau at T212 in the rTg4510 model may be a stress-responsive event.
We also observed an age-dependent decline in immunoreactivity of tau for the mid-domain (a.a.218–225) of tau in the rTg4510 mice using the tau 5 antibody. This was the only soluble tau epitope, including the N- and C-termini, that we found to consistently decrease with age, as the others typically “rebounded” at 5.5 months. Since the mid-domain antibody, tau 5, recognizes a span in close proximity to the AT100 epitope (Figure 1), increased phosphorylation at T212, S214 and T217 (the AT100 epitope), or other nearby sites such as pT231, may progressively impair access to a.a. 218–225. This explanation, however, does not fully account for the dramatic reduction of the tau 5, pT212, and C-terminus immunoreactivity in 9-month-old rTg4510 mice, suggesting that other modifications such as ubiquitination or conformation may hinder antibody access to these domains.
We also noted the conversion of soluble tau to a 64-kDa species that coincided with tau insolubility as previously described9 (Figures 1 and 2). This was particularly evident for pS262/S356 tau, further emphasizing the significance of this epitope in disease pathogenesis and the novel role that MARK2/PAR1 plays in not only priming tau for subsequent phosphorylation, but also in shielding tau from degradation.28,29,30,31 Histological analyses with this antibody revealed that this particular phospho-tau species was prevalent in higher cortical layers at 1 month, staining cell bodies and neurites throughout (Figures 3 & Supplemental Figure S1 at http://ajp.amjpathol.org). This suggested the important role for pS262/S356 tau in early brain development, perhaps indicating activity of kinases that can phosphorylate KXGS motifs, ie, MARKs (microtubule affinity regulating kinases) and protein kinase A, each of which have been implicated in cell polarity during development.29,32,33 Of particular note, perikaryal tau appeared at this early age in structures resembling the pathology at later time points. Unlike the neurofibrillary tangles that aggregate in older rTg4510 mice, this pS262/S356 tau was cleared by 3 months, suggesting that the adolescent brain either lacks activity of a necessary component that facilitates pathological tau stability or that the adolescent brain is more efficient than the adult brain in removing phosphorylated tau.
Our past studies on the role of chaperones and heat shock proteins in tau biology led us to evaluate the levels of several key regulators of these protein families in our time course study here. The HMW Hsp70 species that we observed in young mice was perhaps the most surprising result as this has not previously been reported (Figure 5); however, Hsp70 is indeed ubiquitinated and a recent study demonstrated that this ubiquitination can be mediated by CHIP.34 While this HMW species decreased between 3 and 5.5 months, it was still present in 5.5-month-old non-transgenic animals, but not in age-matched rTg4510 mice. Thus this HMW Hsp70 species may be indicative of CHIP activity in these mice, suggesting that CHIP activity not only decreases or changes with age, but that this feature is actually accelerated by tau pathology. Coinciding with this result was our finding that CHIP levels were slightly reduced in older rTg4510 mice compared with non-transgenic littermates.
Constitutive Hsp70 and Hsp27 levels increased between adolescence and adulthood, but this was accelerated by tau over-expression (Figure 5). Previous work has shown that older animals have an impaired heat shock response35,36; however these data could suggest that stress-related protein expression may actually facilitate tau accumulation and pathology. Indeed, the role of chaperones is to prevent unfolded proteins from being unnecessarily degraded. Perhaps the absence of inducible chaperone expression is allowing for the clearance of tau that we observed between 1 month and 3 months. This could, in fact, be a critically timed developmental necessity to allow for the removal of hyperphosphorylated 3R tau from the juvenile brain while 4R tau replaces it. But as the mice age the increased activity of stress-inducible chaperones prevents tau degradation and may actually facilitate filament formation. The fact that tau over-expression may be facilitating stress protein expression further implicates their role in facilitating pathogenesis. If heat shock proteins are facilitating filament formation, in light of recent evidence from our group and others suggesting that the tangles themselves might not be toxic, but rather soluble intermediates,8,12,17 then it still is possible that chaperones are protective. While, it has yet to be definitively demonstrated how chronically elevated expression of stress proteins will affect the neurodegenerative disease process in the mammalian brain, these data certainly are suggestive that they are intimately involved.
The adolescent rodent brain produces exclusively 3R tau, which eventually is converted to exclusively 4R tau.37,38 Since the human tau over-expressed in the rTg4510 mice is 4R tau, we were able to investigate what impact over-expressed mutant human tau has on endogenous 3R tau from the juvenile mouse brain. Mouse 3R tau was elevated in the rTg4510 mice at 1 month and 3 month, as compared with non-transgenic mice. It was possible that human 4R tau might be altering endogenous tau splicing leading to elevation of 3R tau in rTg4510 mice; however, using a cell culture model of constitutively expressed 3R tau, we were able to prove that over-expression of 4R P301L tau was in fact capable of impairing 3R tau turnover, since each tau variant was being independently expressed in this cell model (Figure 7). Endogenous 3R mouse tau also had elevated levels of disease-associated phosphorylation and adopted the disease-associated Alz50 conformation in the rTg4510 mice compared with non-transgenic littermates39 (Figure 8). This is the first time that evidence has been presented to suggest that simply by accumulating, abnormal tau can facilitate dysfunction of a distinct, normal tau population. Therefore, the accumulation of tau that occurs in tauopathies may be self-perpetuating by slowing the removal of endogenous tau and preserving toxic tau intermediates within the neurons for a longer period than normal.
The consequences of abnormal tau production in Alzheimer’s disease and other tauopathies could be that the production and accumulation of aberrant tau actually slows the turnover of normal tau within the same cell, suggesting that the threshold required for abnormal tau to facilitate neuronal proteotoxicity is lower than one might think. It also provides evidence for the theory proposed by Trojanowski and Lee40 that while there may indeed be some gain of toxic function required for initiating tau dysfunction, only a small amount of this material may be required to promote loss of normal function for the remaining pool of tau, leading to its accumulation, giving abnormal tau a prion-like capacity. This point was also suggested by Oddo et al when they demonstrated that removing Aß by immunotherapy was sufficient to also stop tau accumulation early in the life of 3xTg AD mice; however, if removal of Aß was initiated later in the life of these mice, tau accumulation continued unabated.41 Additionally, our previous work with the rTg4510 model indicates that early removal of mutant tau slows tau aggregation; however, later reduction of mutant tau levels fails to reduce tau aggregation. Thus, clinical trials designed for AD aimed at removing Aß should perhaps be initiated much earlier in the course of the disease. In fact these anti-amyloid strategies may be most effective even before symptoms manifest, making the need for pre-phenotype biomarkers for AD prognosis of critical importance for the success of these strategies. Our data also suggests that therapies aimed at removing abnormal tau could be critical for stopping the disease in later Braak-staged individuals. Certainly this approach was highlighted by the recent clinical success of the anti-tau drug, methylthionium chloride, in mild-to-moderate AD patients.42
Supplementary Material
Acknowledgments
Dr. Leonard Petrucelli (Mayo Clinic Jacksonville) contributed the CHIP antibody and Dr. Peter Seubert (Elan Pharmaceuticals) provided the 12E8 antibody. Dr. Cam Patterson (University of North Carolina-Chapel Hill) provided the CHIP knockout mice. Dr. Peter Davies (Albert Einstein College of Medicine) provided multiple tau antibodies.
Footnotes
Address reprint requests to Chad Dickey, Assistant Professor, University of South Florida, Tampa, FL 33612. E-mail: Cdickey1@health.usf.edu.
Supported by NIA K99/R00-AG31291 Alzheimer’s Association and CurePSP (C.D.); NIH/NINDS R01 NS046355 (J.L.); Reta Lila Weston Trust for Medical Research and a research grant (G0501560) from the UK Medical Research Council (R.D.).
J.L. and Mayo Clinic hold the patent associated with the rTg4510 mice, have a financial interest associated with the rTg4510 mice and have received annual royalties from the licensing of the first technology of greater than the federal threshold for significant financial interest.
References
- Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging. 1995;16:355–362. doi: 10.1016/0197-4580(95)00025-a. discussion 362–353. [DOI] [PubMed] [Google Scholar]
- Hardy J, Orr H. The genetics of neurodegenerative diseases. J Neurochem. 2006;97:1690–1699. doi: 10.1111/j.1471-4159.2006.03979.x. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ros R, Thobois S, Streichenberger N, Kopp N, Sanchez MP, Perez M, Hoenicka J, Avila J, Honnorat J, de Yebenes JG. A new mutation of the tau gene. G303V, in early-onset familial progressive supranuclear palsy. Arch Neurol. 2005;62:1444–1450. doi: 10.1001/archneur.62.9.1444. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Takashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol. 2006;168:1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, de Calignon A, Matsui T, Zehr C, Pitstick R, Wu HY, Osetek JD, Jones PB, Bacskai BJ, Feany MB, Carlson GA, Ashe KH, Lewis J, Hyman BT. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J Neurosci. 2008;28:862–867. doi: 10.1523/JNEUROSCI.3072-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho RM, Viana RS, Castro RE, Steer CJ, Low WC, Rodrigues CM. Apoptosis in transgenic mice expressing the P301l mutated form of human tau. Mol Med. 2008;14:309–317. doi: 10.2119/2007-00133.Ramalho. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GV, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ, Lee VMY. Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- Sun L, Liu SY, Zhou XW, Wang XC, Liu R, Wang Q, Wang JZ. Inhibition of protein phosphatase 2A- and protein phosphatase 1-induced tau hyperphosphorylation and impairment of spatial memory retention in rats. Neuroscience. 2003;118:1175–1182. doi: 10.1016/s0306-4522(02)00697-8. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Yue M, Lin WL, Dickson DW, Dunmore JH, Lee WC, Zehr C, West G, Cao S, Clark AM, Caldwell GA, Caldwell KA, Eckman C, Patterson C, Hutton M, Petrucelli L. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci. 2006;26:6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Patterson C, Dickson DW, Nahman NS, Jr, Hutton M, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M. CHIP and Hsp70 regulate tau ubiquitination, degradation, and aggregation. Hum Mol Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- Gordon MN, Holcomb LA, Jantzen PT, DiCarlo G, Wilcock D, Boyett KW, Connor K, Melachrino J, O'Callaghan JP, Morgan D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. ExpNeurol. 2002;173:183–195. doi: 10.1006/exnr.2001.7754. [DOI] [PubMed] [Google Scholar]
- Collet J, Fehrat L, Pollard H, Ribas de Pouplana L, Charton G, Bernard A, Moreau J, Ben-Ari Y, Khrestchatisky M. Developmentally regulated alternative splicing of mRNAs encoding N-terminal tau variants in the rat hippocampus: structural and functional implications. Eur J Neurosci. 1997;9:2723–2733. doi: 10.1111/j.1460-9568.1997.tb01701.x. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Petersen RC, Knopman DS, Boeve BF, Smith GE, Geda YE, Johnson KA, Cha R, Delucia MW, Braak H, Dickson DW, Parisi JE. Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:602–609. doi: 10.1097/01.jnen.0000225312.11858.57. [DOI] [PubMed] [Google Scholar]
- de Silva R, Lashley T, Gibb G, Hanger D, Hope A, Reid A, Bandopadhyay R, Utton M, Strand C, Jowett T, Khan N, Anderton B, Wood N, Holton J, Revesz T, Lees A. Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol. 2003;29:288–302. doi: 10.1046/j.1365-2990.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion JP, Octave JN, Couck AM. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994;63:895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Caceres A, Kosik KS. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990;343:461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38delta or JNK2 in the presence of heparin generates the AT100 epitope. J Neurochem. 2006;99:154–164. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Yang Y, Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, Mandelkow EM. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell. 2002;13:4013–4028. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Dunmore J, Lu B, Wang JW, Lee WC, Kamal A, Burrows F, Eckman C, Hutton M, Petrucelli L. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J. 2006;20:753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Koren J, Zhang YJ, Xu YF, Jinwal UK, Birnbaum MJ, Monks B, Sun M, Cheng JQ, Patterson C, Bailey RM, Dunmore J, Soresh S, Leon C, Morgan D, Petrucelli L. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA: 2008;105:3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, Benton R, St Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell. 2000;101:377–388. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- Struhl G, Barbash DA, Lawrence PA. Hedgehog acts by distinct gradient and signal relay mechanisms to organise cell type and cell polarity in the Drosophila abdomen. Development. 1997;124:2155–2165. doi: 10.1242/dev.124.11.2155. [DOI] [PubMed] [Google Scholar]
- Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavani MA, Harris MD, Moore SA, Richardson A. Expression of heat shock protein 70 in rat spleen lymphocytes is affected by age but not by food restriction. J Nutr. 1996;126:2069–2075. doi: 10.1093/jn/126.9.2069. [DOI] [PubMed] [Google Scholar]
- Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13:2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C, Gorath M. Developmental regulation of alternatively spliced isoforms of mRNA encoding MAP2 and tau in rat brain oligodendrocytes during culture maturation. J Neurosci Res. 1999;56:259–270. doi: 10.1002/(SICI)1097-4547(19990501)56:3<259::AID-JNR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nothias F, Boyne L, Murray M, Tessler A, Fischer I. The expression and distribution of tau proteins and messenger RNA in rat dorsal root ganglion neurons during development and regeneration. Neuroscience. 1995;66:707–719. doi: 10.1016/0306-4522(94)00598-y. [DOI] [PubMed] [Google Scholar]
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Pathological tau: a loss of normal function or a gain in toxicity? Nat Neurosci. 2005;8:1136–1137. doi: 10.1038/nn0905-1136. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Opar A. Mixed results for disease-modification strategies for Alzheimer’s disease. Nature Reviews Drug Discovery. 2008;7:717–718. doi: 10.1038/nrd2676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.