Abstract
During skeletal remodeling, pre-osteoclasts and pre-osteoblasts are targeted to critical sites of the bone to resorb and reconstruct bone matrix, respectively. Coordination of site-specific recruitment of these two cell types is a prerequisite to maintain the specific architecture of each bone within strict limits throughout adult life. Here, we determined that the bone marrow microanatomy adjacent to remodeling areas is a central player in this process. By using histomorphometry and multiple immunostainings, we demonstrated in biopsies exhibiting coupled bone resorption and formation that osteoclasts and osteoblasts on the bone surface were always covered by a canopy of flat cells expressing osteoblast markers. In contrast, in biopsies in which this canopy was disrupted, bone formation was deficient. Three-dimensional visualizations revealed that this canopy covered the entire remodeling site and was associated with capillaries, thereby forming a previously unrecognized microanatomical entity. Furthermore, pre-osteoclasts were positioned along these capillaries. These findings led to a model that implicates vasculature in the site-specific recruitment of osteoclasts and osteoblasts and embraces the current knowledge on the molecular mechanism of bone remodeling.
Bone matrix is subjected throughout adult life to a series of resorption and formation events. These processes allow the bone architecture to be modeled according to the current mechanical demands and also the bone matrix to be remodeled, thereby replacing possibly damaged matrix. A remarkable property of bone remodeling is that it restitutes bone structure and keeps the specific shape of each bone within strict limits despite repeated resorption and formation. This is achieved through strict coordination of these two events, determining not only how much bone is resorbed and reconstructed, but also precisely where resorption and reconstruction should occur. Excess of resorption over formation results in loss of bone mass and architecture, and leads to fragility, vertebra collapses, fractures, and disabled mobility, as seen in osteoporosis or cancer-induced bone disease.1
Cells responsible for these events, osteoclasts (OCs) and osteoblasts (OBs), respectively, work in concert at specific points of the bone matrix in so-called bone remodeling units.2 Key factors regulating these cells have been identified. They include systemic hormones, nerve signals, vascular agents, acidosis and hypoxia status, and importantly also a diversity of local growth factors, cytokines, chemokines, cell adhesion molecules, extracellular matrix molecules, and proteinases, generated not only by cells positioned on the bone surface, but also by osteocytes embedded in the bone matrix and cells positioned in the bone marrow.1,3,4,5,6,7,8,9,10 It remains to be elucidated how the interplay of these many diverse regulators contributes to direct both OCs and OBs to the critical sites of the bone and to coordinate the respective activities of these cells.
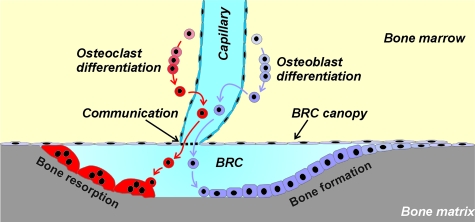
OCs and OBs are believed to originate from progenitors that differentiate in specific niches of the bone marrow.11 The recruitment mechanism, therefore, should not merely be based on the levels of molecular regulators controlling the differentiation of progenitors, but also on a directional determinant defining a route that brings both OC and OB progenitors to the specific points of the bone matrix that will be removed and thereafter will need reconstruction. Chemoattractants are believed to play a role in this process, but the mechanism supporting their spatial organization has not been investigated.12,13 Nearby capillaries were also proposed to contribute to the guidance mechanism,13,14,15 based on the histological analysis of the remodeling areas. Furthermore, a study conducted on bone sections of hyperparathyroid patients led to the proposal that OCs and OBs exert their activities in so-called bone-remodeling compartments (BRC), separated from the bone marrow cavity by a monolayer of flat cells that show OB-like cell markers.16 This observation led to the hypothesis that physical constraints may contribute to coordinating resorption and formation at specific sites of the bone surface. However, in this primary hyperparathyroidism study, virtually all of the bone-remodeling activity occurred inside BRCs, and bone resorption and formation were always tightly coupled. Hence, it could not be assessed whether the absence of BRC would indeed result in impaired coupling of bone resorption and formation. Furthermore, it was not assessed whether the BRC is really a compartment when analyzed in three-dimension, nor how OC and OB progenitor cells access the BRC.17 Despite the interest raised by BRCs and capillaries, their role as site-specific determinants of bone resorption and formation remained speculative.17,18,19,20
The present study of bone remodeling areas in bone marrow biopsies of multiple myeloma (MM) patients gave us the opportunity to demonstrate the effective role of these structures. MM is a disease in which bone tissue-bone marrow interactions are greatly disturbed, in which bone resorption is often increased, and in which, in contrast with hyperparathyroidism, bone resorption tends to be uncompensated by bone formation, thereby leading to generation of osteolytic lesions.21,22 Our analysis showed that MM patients exhibit typical BRCs like the hyperparathyroid patients, but also disrupted BRCs, thereby allowing to investigate whether there is a link between BRCs and the control of the resorption/formation balance.
Furthermore, to identify the route of OC and OB progenitors to the BRCs, we combined multiple-immunostainings of serial sections with three-dimensional reconstructions. We demonstrated BRCs in their three-dimensional reality and showed that they are part of a previously unrecognized microanatomical structure that involves microcapillaries and mediates communications between niches of the bone marrow and bone surfaces undergoing remodeling. These observations lead to a model for position-specific coordination of OC and OB activities on the bone surface.
Materials and Methods
Patients and Histological Material Used for This Study
Bone marrow biopsies (n = 32) were selected from 150 biopsies of MM patients, and 9 bone marrow biopsies were selected from 30 biopsies of control patients with no sign of cancer or any other bone disease (Danish Ethical Committee approval, journal no. 19980185 and 20010082). The selection was based on the morphological quality of the biopsies and the number of Tartrate Resistant Acid Phosphatase 5b (TRAcP) positive OCs within the biopsy (more than 10 OCs per section). The patients were between 53 and 84 years of age. The MM patients were stage I to III in the Durie-Salmon scale. The bone marrow biopsies were decalcified and embedded in paraffin and consecutive 5-μm adjacent sections were obtained and processed for histological and immunohistochemical staining procedures.
Immunohistochemistry
Sections were treated overnight at 60°C in citrate buffer (pH 6.0) or in TE buffer (pH 9.0) to expose the antigens. Multiple antigens were detected in the same section through a sequential protocol. First the sections were incubated with a mouse anti-TRAcP antibody (Zymed, South San Francisco, CA), which was detected with an alkaline-phosphatase polymer conjugated to goat anti-mouse IgGs (PowerVision; Immunovision, Springdale, AZ) followed by fuchsin+ staining. Secondly the sections were incubated with one of the following antibodies: mouse IgG1 antibodies against the N-terminal propeptide of collagen type 1 (PINP, clone mAb 1912; Chemicon, Temecula, CA), osteocalcin (clone mAb 8H12; a gift from professor Kalervo Väänänen, Department of Anatomy, University of Tuku, Finland), osteonectin (clone ON1-1, Zymed), fibronectin (clone FBNII; Lab Vision, Fremont, CA), CD3 (clone F7.2.38; DAKO, Glostrup, Denmark), CD9 (clone 72F6; Novocastra, Newcastle upon Tyne, UK), CD38 (clone SPC32, Novocastra), CD56 (clone 56C05, Lab Vision), CD138 (clone B-B4; Serotec, Raleigh, NC), MMP-9 (clone 2C3, Lab Vision), or Ki-67 (clone MIB-1, DAKO), which were detected with biotin-conjugated IgG1 subtype-specific antibody (Jackson Immunovision, West Grove, PA). Mouse IgG2a antibodies against CD14 (clone 7, Novocastra) or CD20 (clone L26, DAKO) were detected with biotin-conjugated IgG2a subtype-specific antibody (Jackson Immunovision). The rabbit antibodies against the N-terminal propeptide of collagen type 3 (PIINP, a gift from professor Juha Risteli, Department of Clinical Chemistry, University of Oulu, Finland) were detected with biotin-conjugated anti-rabbit antibody (Jackson Immunovision). The biotin was detected with a gold-conjugated anti-biotin antibody (Aurion, Wageningen, The Netherlands). The gold particles were visualized by silver enhancement (Aurion). Finally the sections were incubated with fluorescein isothiocyanate-labeled mouse anti-CD34 antibody (clone QBend10, DAKO), which was detected with horseradish peroxidase-conjugated FAB anti-fluorescein isothiocyanate (Roche, Basel, Switzerland) followed by DAB+ staining.
Microscopy and Image Analysis
Light microscopic analysis was performed on an upright DMRXAZ microscope (Leica, Wetzlar, Germany) with ×2.5 to ×100 objectives. Pictures were obtained using a DC500 charge-coupled device camera controlled by the IM500 (v1.2) software (Leica, Wetzler Germany). The final figures were assembled using the CorelDraw package (version 9) (Corel Corp., Ottawa, Canada).
Three-Dimensional Reconstruction Based on Histological Images
Images were obtained from the same area of 40 to 70 consecutive triple-immunostained bone marrow sections. The images were stacked and aligned based on the adipocytes within the marrow compartment using the Amira v3.1 software (Mercury Computer Systems, Merignac, France). Different features such as the bone surface, OCs, pre-OCs, bone (and bone marrow) lining cells, BRC wall, blood vessels, adipocytes, and OBs were marked within the images, allowing the Amira program to reconstitute the three-dimensional structure of the different elements and their interrelations. They were viewed from different angles, and movies of the rotating three-dimensional reconstructions were generated.
Bone Histomorphometry
Paraffin sections from the bone marrow biopsy of each patient were stained with a modified Masson trichrome stain for light microscopy. The sections were analyzed using a light microscope in which the eyepiece was equipped with an integrated Mertz graticule with a grid of sinus curves. The conventional bone histomorphometric parameters in trabecular bone, ie, the fraction of eroded surface (ES/BS), osteoid surface (OS/BS), and OC covered surface (Oc.S/BS) surfaces relative to total trabecular bone surface (BS), were determined both inside and outside intact BRCs. This was assessed at the intersection points between the sinus curves and the bone surfaces. The sections were blinded and 300 to 700 intersection points on sections from each patient included were assessed independently by two scientists.
Statistical Analysis
The control group and the two myeloma subgroups were compared for the different histomorphometric parameters using the Mann-Whitney test. P < 0.05 was defined as statistically significant.
Assessment of Blood Flow
Anesthetized rabbits (1 to 1.3 kg) were injected in the ear vein with 62 mg of ferritin (Sigma, St. Louis, MO) per 100 g of body weight or with a 0.9% NaCl solution as a control. The rabbits were sacrificed 5 minutes after injection, and the tibia, femur, and vertebra were immediately dissected. Each bone was cut longitudinally into 2- to 3-mm slices and fixed in Karnovsky fixative (3% glutaraldehyde and 3% paraformaldehyde in phosphate-buffered saline) for 18 hours, and stained in Perls’ Prussian Blue (2% potassium ferrocyanide and 2% HCl) overnight. They were thereafter decalcified in formic acid, paraffin-embedded, and sectioned. After hydration, they were counterstained with neutral red, and mounted for light microscopy.
Results
Basic Histological Characteristics of the BRC and Its Peculiarities in MM
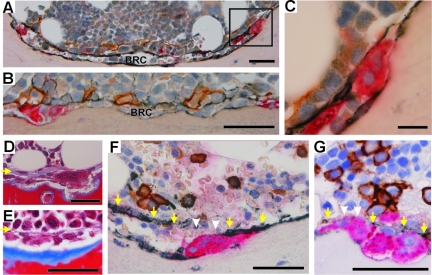
First, we investigated whether the BRCs reported in bones of hyperparathyroid patients could also be identified in bones of MM and control patients. Both MM and control patients showed the typical BRCs as reported in hyperparathyroidism. In two dimensions, BRCs appear as a space interposed between the bone surface undergoing remodeling and the bone marrow. The space of the BRC is separated from the bone marrow cavity by a layer of elongated cells that very tightly line the bone marrow and appear to form a canopy over the bone-remodeling cells (Figure 1, A–C). These canopy cells are positive for osteocalcin and osteonectin as in previous studies,16,17 but also for procollagen type I (PINP) and even more strongly for procollagen type III (PIIINP) and neural cell adhesion molecule-NCAM (CD56), all of which are typical products of OB lineage cells. They are negative for Ki-67, a proliferation marker; CD34, an endothelial cell marker; and for CD3, CD14, CD20, CD38, CD138, MMP-9, and TRAcP, which are typical of the lymphocytic and monocytic lineages. Abundant capillaries at the bone marrow side of the canopy are revealed by CD34. Bone surfaces inside BRCs show erosion and TRAcP+ OCs. They also show frequent osteoid and bone forming OBs, as well as flat NCAM-positive cells, reminiscent of so-called bone-lining cells involved in the reversal phase between resorption and formation.23 Contacts between OCs and these cells were easily seen, as expected,23,24 but were also seen between OCs and BRC canopy cells. All cells within the BRC are negative for Ki-67. The very distinct morphology of the features composing the BRCs renders their detection straightforward through Masson’s trichrome staining (Figure 1, D and E), as mentioned earlier.16 Thus, our observations extend the previous two-dimensional identification of BRCs in hyperparathyroid patients to control and myeloma patients, and further stress the OB characteristics of the BRC canopy.
Figure 1.
The histological appearance of the BRC. A: Cross section of a complete BRC. The wall of the BRC is made of NCAM-positive (black) elongated cells lining the bone marrow. The bone-lining cells are also NCAM-positive. B: Another cross section of a BRC showing PIIINP immunoreactivity (black) in the elongated cells of the wall and in the adjacent CD34-positive capillaries (brown). C: High magnification of a TRAcP+ (red) OC, positioned at the periphery of the BRC (inset in A) and between the bone surface and an elongated NCAM-positive cell of the BRC canopy. D and E: The morphological appearance of BRCs as revealed by Masson’s trichrome, containing eroded (D) or osteoid (E) bone surfaces. The yellow arrows point to the BRC canopy. F and G: Two examples of disrupted BRC canopies. The canopy is not detected in the area highlighted by the white arrowheads, but is seen along the yellow arrows. Scale bars: 25 μm (C); 50 μm (A, B, D–G). BRC: bone remodeling compartment.
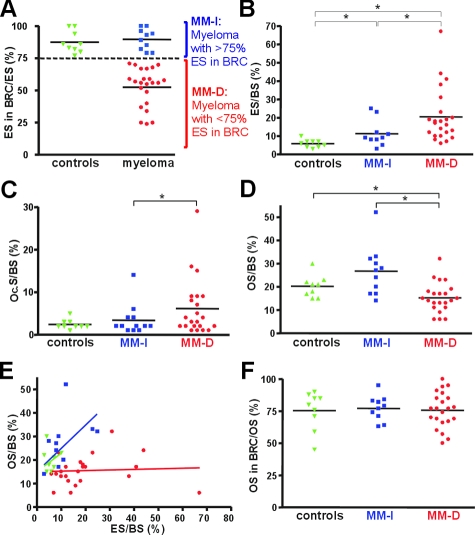
Interestingly however, our study revealed an important difference in some BRC canopies of MM patients compared with those seen in hyperparathyroidic and control patients. In MM, some BRCs are disrupted and may be almost completely absent (Figure 1, F and G). To evaluate the prevalence of intact/disrupted BRC canopies, we quantified for each biopsy what percentage of their erosion surface appeared under intact BRC canopies (Figure 2A). All control biopsies show more than 75% of erosion under intact BRC canopies (87% on average). The same holds true in 10 of the 32 MM biopsies, but the other 22 showed only from 20 to 75% of erosion surface under intact BRC canopies (∼50% on average).
Figure 2.
Correlation between the presence of intact BRC canopies and coupled bone erosion/formation. A: The proportions of erosion surfaces (ESs) in and out of BRCs were measured in bone biopsies from 9 control and 32 myeloma patients. Note that all control bones (green) show more than 75% ES in intact BRCs, whereas myeloma samples show values both above (blue) and less than 75% below (red). Therefore our analysis considered separately these two myeloma subpopulations, indicated as MM-I(ntact) and MM-D(isrupted), respectively. B–D: In the biopsies of each of these two MM groups and of controls, the extent of eroded surfaces (ES) (B), OC surfaces (Oc.S) (C), and osteoid surfaces (OS) (D) was measured and related to the total bone surface (BS). Note that the MM-D population shows the largest ES (B) and the smallest OS (D). E: Plotting osteoid surface (OS) versus eroded surface (ES) for each biopsy shows coupling between these two events in both the control and MM-I biopsies and uncoupling in the MM-D biopsies. F: The proportion of osteoid surfaces (OS) in and out of BRCs was measured in biopsies from each of the three groups. Note that all show the same average of ∼77% OS in intact BRCs. A: This is in contrast with erosion that may occur outside of intact BRCs. The statistical significances were calculated by using the Mann-Whitney test: *P < 0.05.
Relation between the Integrity of the BRC and Magnitude of Bone Erosion/Formation
Well-known characteristics of MM bone are deep osteolytic lesions and lack of bone formation. To investigate whether there is a relation between the integrity of the BRC canopy and the magnitude of bone resorption/formation activities, we compared the extent of erosion and osteoid surfaces i) in the control bones, ii) in the MM biopsies showing more than 75% of the total erosion under intact BRC canopies (MM-I), and iii) in those with at least 75% erosion under disrupted BRC canopies (MM-D). Figure 2, B–D, reveals that MM-I biopsies show increased erosion surface, OC surface, and osteoid surface compared to controls. MM-D biopsies show even more increased erosion surface and OC surface compared to MM-I biopsies, but in contrast, their osteoid surface falls below control levels, thereby indicating lack of bone formation despite increased bone resorption. When directly plotting osteoid surface versus erosion surface for each of the three groups, the importance of intact BRC is even more clear (Figure 2E). In control and MM-I biopsies, increased osteoid surface parallels increased erosion surface, indicating coupling between bone formation and resorption. In contrast, in MM-D biopsies, erosion surface increases strongly without corresponding increase in osteoid surface, indicating absence of coupling between bone formation and resorption. Thus, bone formation responds commensurately to bone resorption only when the BRC canopy is continuous. The same conclusion holds true if the analysis is based on OC surface and if the MM biopsies are grouped according to the proportion of OC surface in intact BRCs (see Supplemental Figure S1 at http://ajp.amjpathol.org). The fact that bone formation occurs very preferentially in intact BRCs is also clear when analyzing the proportion of osteoid in intact BRC (Figure 2F): this proportion averages 75% in all three groups of biopsies, despite their differences in overall extent of osteoid surface. This is in marked contrast with erosion, which proceeds whether BRCs are intact or not (Figure 2A) and becomes even higher in the latter case (Figure 2, B and E). We deduce from these observations that there is a close link between the integrity of BRC canopies and the magnitude of OC and OB activities. When the BRC is disrupted, bone resorption tends to increase, and bone formation to be prevented.
Communication of the BRC with the Vascular Space and the Bone Marrow
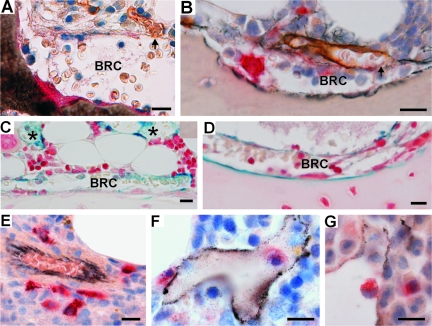
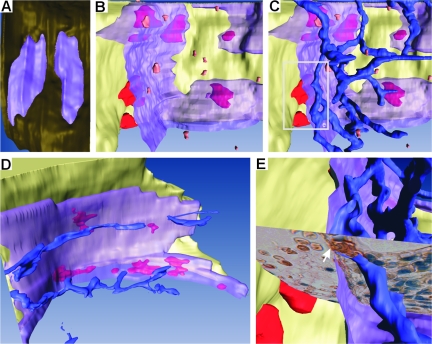
It is in the marrow cavity that OCs and OBs mature and that key cells regulating bone remodeling are localized.18 To understand in which way the BRC may be critical for OC and OB activities and their coupling, one should thus determine how the BRC communicates with the bone marrow cavity. Our approach was based on a combination of double/triple immunostaining of serial sections and three-dimensional reconstructions, allowing to relate bone surface events with the features of the bone marrow. In three-dimension, the bone remodeling site appears bounded, as predicted by Hauge and colleagues,16 by a continuous canopy that covers the OCs and OBs sitting on the bone surface and that joins the bone-lining cells at the periphery. Capillaries are abundant at the bone marrow side of this canopy. Serial sections allowed the detection of points where capillaries appeared connected to the BRC canopy, (Figure 1B; Figure 3, A and B; Figure 4, A–C, and E; and see Supplemental Figure S2 and Movies S1 to S6 at http://ajp. amjpathol.org). We regularly noticed erythrocytes in the BRC. They were sometimes distributed in continuation of the lumen of the associated capillary, which is compatible with a communication of BRCs with the systemic circulation (Figures 3A and 4E). Vascular communication was confirmed by injecting ferritin into rabbits as a tracer and establishing its presence in BRCs and related capillaries 5 minutes later (Figure 3, C and D). TRAcP+ pre-OCs were detected in the bone marrow (Figure 4B). When staining simultaneously for capillaries, it appeared that these pre-OCs were positioned at their proximity (Figure 3E), and three-dimensional reconstructions revealed clear correlations between the position of pre-OCs and capillaries (Figure 4C, and see Supplemental Movie S2 at http://ajp.amjpathol.org). Therefore these capillaries appear to be the likely route of pre-OCs to the BRC. This view is supported by the presence of pre-OCs in the capillary lumen (Figure 3, F and G). Furthermore, the position of the mature OCs on the bone surface correlated strikingly with the connection points between the capillaries and the BRC canopy (Figure 4D). Thus the BRC is part of a larger microanatomical structure allowing communication with the bone marrow cavity and the systemic circulation.
Figure 3.
Histology revealing the physical relation between the BRC canopy and capillaries, and between capillaries and OC progenitors. A and B: Two histological sections passing through a contact point (arrows) between a CD34+ capillary (brown) and a BRC canopy. A: Erythrocytes can be clearly distinguished in the lumen of the capillary and inside the BRC. C and D: Ferritin was detected in the capillaries (asterisk) and in the BRCs of rabbits 5 minutes after intravenous injection, as visualized with Perls’ Prussian blue. E–G: TRAcP+ pre-OCs (red) were observed around (E) and in rare occasions within (F, G) CD34+ capillaries (black) of the bone marrow. Scale bars = 25 μm.
Figure 4.
Three-dimensional reconstructions of BRCs and related microanatomical structures. A: Top view of two nearby BRCs allowing appreciation of the continuity of the BRC canopy (purple) and its sealing with the bone-lining cells (brown), thereby forming a closed compartment. B: Semilateral view of a BRC shown open to visualize the space comprised between the bone surface (pale yellow) bearing large mature OCs (red) and the BRC canopy (transparent purple). Small pre-OCs (orange) are visible at the bone marrow side of the BRC canopy C: The same BRC as in B, shown with the capillary network (blue). Note the position of pre-OCs along the capillaries. D: Another BRC. Large OCs (red) are visible on the bone surface through the BRC canopy (transparent purple). Note the correlation between the positions of these OCs and those of the capillaries at the bone marrow side of the BRC wall. E: Focus on the area framed in C, with superimposed histological picture (see Figure 3A), to illustrate the connection point of a capillary with the BRC canopy (arrow). The three-dimensional information contained in A–E can best be appreciated in the supplemental movies available at http://ajp.amjpathol.org. Supplemental Figure S2 at http://ajp.amjpathol. org also shows a low-magnification view of a piece of bone with BRCs and related (pre-) OCs, indicating the in situ position of the BRCs shown in A and B.
Discussion
So far, the only study in which OB lineage canopies covering bone-remodeling sites were identified and analyzed in relation with the extent of bone resorption/formation was performed in hyperparathyroid patients. It revealed these canopies over virtually all bone remodeling sites and perfect coupling between bone resorption and formation.16 Hence, BRC canopies were proposed to be critical for reconstruction of resorbed bone, but this remained a hypothesis because of the lack of negative controls showing uncoupled resorption/formation in the absence of BRC canopies.16,17,18,19,20 It was thus interesting to extend the previous study to MM, a disease in which resorption and formation are often uncoupled and in which we regularly found bone resorption beneath disrupted BRC canopies. The resulting conclusion is that bone formation responds commensurately to bone resorption only underneath intact canopies, ie, in one third of the MM biopsies, in our control bones, and in the previous analysis of hyperparathyroid patients. Bone formation was deficient in the other two thirds of the MM biopsies, which had disrupted BRC canopies. These results hold true whether resorption is evaluated through the extent of erosion surfaces or OC surfaces, thus showing that it concerns active resorption. It remains to be investigated whether this relation between coupling of resorption/formation and BRC canopies exists in other bone diseases characterized by uncoupling and where the systematic presence of BRCs was not analyzed, such as postmenopausal and glucocorticoid-induced osteoporosis.1,25
An important question that remains is whether disruption of BRC canopies in MM is the direct cause of deficient bone formation at bone-remodeling sites. Indeed, absent repair of osteolytic lesions in MM is classically ascribed to the production of MM-induced inhibitors of bone formation.21 Such a molecular mechanism is, however, not sufficient to explain why osteolytic lesions may persist without repair for several years in patients who are in remission and virtually devoid of MM cells producing bone formation inhibitors.26 These data point to additional factors responsible for the absence of reconstruction. Conversely, MM in itself is not sufficient to cause inhibition of bone formation (our study),22 because MM bone shows commensurate resorption and formation as long as BRC canopies are intact, even when the extent of bone remodeling is far greater than control levels (our study). Thus intact BRC canopies may well be critical for reconstruction of resorbed bone. But how can BRC canopies contribute to this process?
Insight in this mechanism is provided by our three-dimensional analysis. It indicates that BRC canopies are closely associated with capillaries, and that these two structures may function as a unit allowing a connection between the bone resorption/formation events on the bone surface with the recruitment process of both OC and OB progenitors from the bone marrow. Our three-dimensional analysis demonstrates definitively that the BRC canopy forms a continuous roof that completely seals-off the OCs and OBs on the bone surface, from the bone marrow, as predicted by Hauge and colleagues.16 The only possible communication of this closed BRC with the rest of the body is through the cell layer of the canopy or through the connections with capillaries revealed in our study. Whether these connections represent cell juxtapositions of BRC canopies and capillaries,27 where diapedesis of OC and OB progenitor cells may occur, or open communications awaits morphological analysis at higher resolution. However, the presence of a tracer inside BRCs as soon as 5 minutes after injection into rabbits demonstrates a rapid exchange between the blood stream and the BRC. It is likely that these capillaries are the routes that direct the pre-OCs to the BRCs, because pre-OCs identified in the bone marrow were associated with the capillaries and because the connection point of the capillaries with the BRC canopy corresponds with the position of the OCs on the bone surface. The occasional finding of a pre-OC inside the lumen of a capillary further supports this view. This position of OC progenitors along capillaries provides histological support for the proposal that endothelial cells play a role in OC differentiation.28,29,30
Still, the microanatomical position of OB progenitors in this system should be clarified.31 OB differentiation may be promoted by direct contacts between OCs and OB lineage cells.32,33 Such contacts are easy to notice in the case of OCs and so-called bone-lining cells involved in the reversal phase between bone resorption and formation (our study).23,24 We now extend this situation to contacts between OCs and the BRC canopy cells, which are also OB lineage cells, as demonstrated by Hauge and colleagues16 and as strengthened by several additional OB lineage markers in the present study. Importantly, it has also been stressed that OB differentiation is promoted by endothelial cells, including during remodeling.14,34,35,36,37 Furthermore, OB precursor cells have been reported in perivascular niches of the bone marrow.38 A privileged site for endothelial-OB interactions is at the connections between capillaries and the BRC canopy itself. Another possible position of OB precursors is further away from the canopy, along the capillaries, and next to differentiating pre-OCs.11 They may initially promote differentiation of these pre-OCs through RANKL expression, and be programmed later to become bone-forming cells.39,40 OB progenitors may also originate from more remote sites because they were reported in the circulation of patients showing enhanced bone formation.41 It is of interest that each of these origins fit our model, which implies that OB progenitors may reach the remodeling site through the same capillary-BRC canopy structures as OC progenitors, ie, exactly where bone resorbed by OCs has to be reconstructed (Figure 5). Worth noting, this microanatomical structure may provide guidance to progenitor cells through multiple mechanisms: by exerting physical constraints on the cells; through site-specific anchorage of regulators of their differentiation along the route toward remodeling sites; or by building-up a high concentration of chemoattractants in the sealed BRC, because chemoattractants for OBs are believed to be released by OCs.12,13,42
Figure 5.
Model for coordinating site-specific recruitment of OC and OB progenitors to bone remodeling sites. The model summarizes the main features recognized in our study (see text). A bone remodeling site involves joint OC and OB activities on the bone surface [bone remodeling unit (BRU) or basic multicellular unit (BMU)2]. These activities were proposed to occur in a BRC separated from the bone marrow by an OB lineage cell canopy.16 According to our study, this BRC is part of a more complex entity because the OB lineage cell canopy is connected to capillaries. This structural entity directs OC and OB progenitors to the bone remodeling site, as evidenced, respectively, by direct observations on OC progenitors and by lack of bone formation when the BRC canopy is disrupted in diseased bone. It remains to be elucidated where OB progenitors are positioned and how the capillaries communicate with the BRC.
An important aspect of our model is that it further strengthens the link between vascular and bone pathophysiology4,13,19,43 and their molecular control.3,4,5,6,7,8,9,43,44 Our study shows that this link is reflected by structural interactions between the vascular system and bone remodeling sites. A limitation of our study is that it does not show the dynamic perspective showing how this structure originates and evolves during the different steps of bone remodeling. Bone remodeling is triggered by hypoxia-related signals from osteocytes in a diversity of situations including (micro)-fractures and immobility.1,8,45,46,47,48 A typical scenario could be that hypoxic bone matrix induces growth of capillaries toward itself. As explained above, OC progenitors and OB progenitors would then differentiate along these capillaries and be directed to the hypoxic bone surface (Figure 5). OCs have a propensity to position themselves underneath layers of OB-like cells.49 This gives insight in how they may migrate underneath bone-lining cells thereby lifting them away from the bone and creating the BRC canopy. Our observations show that once initiated, bone resorption can continue and is even enhanced if the BRC is disrupted, whereas bone formation does not follow, as discussed above. The mechanism of disruption of the BRC canopy by myeloma cells, and the possible involvement of Dkk1 or other bone formation inhibitors21 in this disruption requires further investigations.
Another unanswered question that deserves attention is whether the so-called bone-lining cell involved in the reversal phase represents an intermediate differentiation stage between bone forming OBs and less mature stages. These bone-lining cells are still poorly characterized, but were reported to prepare the bone surface for bone formation both by cleaning matrix left over by OCs and by depositing thin collagen fibers.23 These cells were also called peri-OC cells to emphasize their close association with OCs, and they express high levels of MMP-13 diffusing at the OC-bone interface.24 Activity of these cells proved to be a prerequisite for subsequent bone formation.23 These cells might well represent the early OB lineage cell population recruited to BRCs as discussed above, and they may later differentiate into bone-forming OBs. Notably, it is unlikely that these bone-lining cells originate by proliferation of local bone-lining cells, because all cells on the bone surface prove to be Ki-67-negative.
This vascular model has potentially additional roles. These include a fast clearance of resorbed bone constituents including phosphate and calcium ions; a fast access of hormones such as calcitonin and PTH, known to affect quickly OCs50; and regulation of bone remodeling through alterations of the blood flow.51 One may speculate that the present model for the role of vasculature in trabecular bone remodeling is also relevant to the capillaries of the Haversian canal and cortical bone remodeling.16
In conclusion, to date, the efforts for understanding the recruitment mechanism of OCs and OBs to bone-remodeling sites have focused on molecular regulators, without taking anatomical structures into account. Here we recognized that microanatomical arrangements of capillaries and BRC canopies contribute critically to control bone resorption and formation at remodeling sites of human bone, as had been speculated earlier.16,17,19 These microanatomical arrangements allow integration of the current knowledge on the bone-remodeling mechanism into a single model. Furthermore, this model draws the attention to the BRC canopy and associated capillaries as possible new cellular targets for treatment of diseases characterized by imbalanced bone resorption-formation. Drugs targeting the coupling mechanism could indeed be more efficient for treating diseases characterized by coupling deficiency, compared to drugs targeting either osteoclasts or osteoblasts.
Supplementary Material
Acknowledgments
We thank Tinna Herløv Jensen and Birgit MacDonald for excellent technical assistance, Kalervo Väänänen for supplying osteocalcin binding antibody, and Juha Risteli for supplying PIIINP binding antibody.
Footnotes
Address reprint requests to Thomas Levin Andersen, Department of Clinical Cell Biology (KCB), Vejle Hospital, IRS-CSFU, University of Southern Denmark, 7100 Vejle, Denmark. E-mail: thomas.levin.andersen@slb.regionsyddanmark.dk.
Supported by The West-Denmark Research Forum for Health Science and the Danish Cancer Society.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7:379–408. doi: 10.1210/edrv-7-4-379. [DOI] [PubMed] [Google Scholar]
- Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, Meghji S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Karsdal M, Delaisse JM, Engsig MT. RANKL and vascular endothelial growth factor (VEGF) induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. J Biol Chem. 2003;278:48745–48753. doi: 10.1074/jbc.M309193200. [DOI] [PubMed] [Google Scholar]
- Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312:1693–1702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Curr Top Dev Biol. 2005;65:169–187. doi: 10.1016/S0070-2153(04)65006-X. [DOI] [PubMed] [Google Scholar]
- Arnett TR, Dempster DW. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology. 1986;119:119–124. doi: 10.1210/endo-119-1-119. [DOI] [PubMed] [Google Scholar]
- Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000;26:319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- Burkhardt R, Bartl R, Frisch B, Jäger K, Mahl C, Hill W, Kettner G. The structural relationship of bone forming and endothelial cells of the bone marrow. Arlet J, Ficat RP, Hungerford DS, editors. Baltimore: Williams and Wilkins,; Bone Circulation. 1984:pp 2–14. [Google Scholar]
- Laroche M, Barbier A, Ludot I, Vernhet C, Thiechart M, Viguier G, Mazieres B. Effect of ovariectomy on intraosseous vascularization and bone remodelling in rats: action of tiludronate. Osteoporos Int. 1996;6:127–129. doi: 10.1007/BF01623935. [DOI] [PubMed] [Google Scholar]
- Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Eghbali-Fatourechi GZ, Khosla S. Remodeling and vascular spaces in bone. J Bone Miner Res. 2007;22:1–6. doi: 10.1359/jbmr.060910. [DOI] [PubMed] [Google Scholar]
- Compston JE. Bone marrow and bone: a functional unit. J Endocrinol. 2002;173:387–394. doi: 10.1677/joe.0.1730387. [DOI] [PubMed] [Google Scholar]
- Mödder UI, Khosla S. Skeletal stem/osteoprogenitor cells: current concepts, alternate hypotheses, and relationship to the bone remodeling compartment. J Cell Biochem. 2008;103:393–400. doi: 10.1002/jcb.21423. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res. 2001;16:1583–1585. doi: 10.1359/jbmr.2001.16.9.1583. [DOI] [PubMed] [Google Scholar]
- Esteve FR, Roodman GD. Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol. 2007;20:613–624. doi: 10.1016/j.beha.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple-myeloma. J Clin Invest. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 2002;17:77–90. doi: 10.1359/jbmr.2002.17.1.77. [DOI] [PubMed] [Google Scholar]
- Andersen TL, Ovejero MD, Kirkegaard T, Lenhard T, Foged NT, Delaisse JM. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004;35:1107–1119. doi: 10.1016/j.bone.2004.06.019. [DOI] [PubMed] [Google Scholar]
- De Nijs RN. Glucocorticoid-induced osteoporosis: a review on pathophysiology and treatment options. Minerva Med. 2008;99:23–43. [PubMed] [Google Scholar]
- Epstein J, Walker R. Myeloma and bone disease: “the dangerous tango.”. Clin Adv Hematol Oncol. 2006;4:300–306. [PubMed] [Google Scholar]
- Miller SC, Jee SS. The microvascular bed of fatty bone marrow in the adult beagle. Metab Bone Dis Rel Res. 1980;2:239–246. [Google Scholar]
- Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Miner Res. 2006;21:193–206. doi: 10.1359/JBMR.051027. [DOI] [PubMed] [Google Scholar]
- Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350:557–561. doi: 10.1016/j.bbrc.2006.09.079. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 1992;275:287–299. [PubMed] [Google Scholar]
- Decker B, Bartels H, Decker S. Relationships between endothelial cells, pericytes, and osteoblasts during bone formation in the sheep femur following implantation of tricalciumphosphate-ceramic. Anat Rec. 1995;242:310–320. doi: 10.1002/ar.1092420304. [DOI] [PubMed] [Google Scholar]
- Guillotin B, Bourget C, Remy-Zolgadri M, Bareille R, Fernandez P, Conrad V, Amedee-Vilamitjana J. Human primary endothelial cells stimulate human osteoprogenitor cell differentiation. Cell Physiol Biochem. 2004;14:325–332. doi: 10.1159/000080342. [DOI] [PubMed] [Google Scholar]
- Stahl A, Wenger A, Weber H, Stark GB, Augustin HG, Finkenzeller G. Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal coculture model. Biochem Biophys Res Commun. 2004;322:684–692. doi: 10.1016/j.bbrc.2004.07.175. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res. 2003;18:1088–1098. doi: 10.1359/jbmr.2003.18.6.1088. [DOI] [PubMed] [Google Scholar]
- Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/endo.141.12.7840. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Neutzsky-Wulff AV, Dziegiel MH, Christiansen C, Henriksen K. Osteoclasts secrete non-bone derived signals that induce bone formation. Biochem Biophys Res Commun. 2008;366:483–488. doi: 10.1016/j.bbrc.2007.11.168. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- Dodd JS, Raleigh JA, Gross TS. Osteocyte hypoxia: a novel mechanotransduction pathway. Am J Physiol. 1999;277:C598–C602. doi: 10.1152/ajpcell.1999.277.3.C598. [DOI] [PubMed] [Google Scholar]
- Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Saltel F, Chabadel A, Zhao Y, Lafage-Proust MH, Clezardin P, Jurdic P, Bonnelye E. Transmigration: a new property of mature multinucleated osteoclasts. J Bone Miner Res. 2006;21:1913–1923. doi: 10.1359/jbmr.060821. [DOI] [PubMed] [Google Scholar]
- Baron R, Vignery A. Behavior of osteoclasts during a rapid change in their number induced by high doses of parathyroid hormone or calcitonin in intact rats. Metab Bone Dis Rel Res. 1981;2:339–346. [Google Scholar]
- Reeve J, Arlot M, Wootton R, Edouard C, Tellez M, Hesp R, Green JR, Meunier PJ. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rate. J Clin Endocrinol Metab. 1988;66:1124–1131. doi: 10.1210/jcem-66-6-1124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.