Abstract
Mutations in the α7 integrin gene cause congenital myopathy characterized by delayed developmental milestones and impaired mobility. Previous studies in dystrophic mice suggest the α7β1 integrin may be critical for muscle repair. To investigate the role that α7β1 integrin plays in muscle regeneration, cardiotoxin was used to induce damage in the tibialis anterior muscle of α7 integrin-null mice. Unlike wild-type muscle, which responded rapidly to repair damaged myofibers, α7 integrin-deficient muscle exhibited defective regeneration. Analysis of Pax7 and MyoD expression revealed a profound delay in satellite cell activation after cardiotoxin treatment in α7 integrin-null animals when compared with wild type. We have recently demonstrated that the muscle of α7 integrin-null mice exhibits reduced laminin-α2 expression. To test the hypothesis that loss of laminin contributes to the defective muscle regeneration phenotype observed in α7 integrin-null mice, mouse laminin-111 (α1, β1, γ1) protein was injected into the tibialis anterior muscle 3 days before cardiotoxin-induced injury. The injected laminin-111 protein infiltrated the entire muscle and restored myogenic repair and muscle regeneration in α7 integrin-null muscle to wild-type levels. Our data demonstrate a critical role for a laminin-rich microenvironment in muscle repair and suggest laminin- 111 protein may serve as an unexpected and novel therapeutic agent for patients with congenital myopathies.
The α7β1 integrin is a major laminin receptor expressed in skeletal, cardiac, and vascular smooth muscle.1 At least six isoforms of the α7 integrin are produced by developmentally regulated RNA splicing in skeletal muscle. The cytoplasmic isoforms of the α7 integrin are designated α7A, α7B, and α7C, whereas the extracellular isoforms are designated α7X1 and α7X2.2,3,4,5 Whereas the α7 integrin cytoplasmic variants differ in their potential for signal transduction, the extracellular isoforms have different binding affinities for various laminin isoforms.4,6 Patients with mutations in the α7 integrin gene exhibit myopathy characterized by delayed developmental milestones and impaired mobility.7 Although mutations in the α7 integrin gene that lead to α7 congenital myopathy are rare, to date there is no treatment or cure for these patients. Mice that lack the α7 integrin also develop myopathy and vascular defects.8,9,10,11
Several studies indicate the α7β1 integrin is a major modifier of disease progression in various muscular dystrophies. Increased α7 integrin expression is observed in the skeletal muscle of patients with Duchenne muscular dystrophy and in dystrophin-deficient mdx mice.1,3 Transgenic overexpression of the α7 integrin in skeletal muscle has been demonstrated to alleviate muscle disease and improve the survival of a severely dystrophic mouse model of Duchenne muscular dystrophy.12,13,14 Finally, loss of the α7 integrin in γ-sarcoglycan-deficient or mdx mice results in severe muscle pathology and reduced survival.15,16,17
Laminin is a heterotrimeric protein composed of α, β, and γ chains, which, along with collagen IV, nidogen/entactin, agrin, biglycan, and perlecan, forms the basal lamina that surrounds muscle fibers.18,19,20,21,22 To date 5α, 3β, and 3γ laminin chains have been identified that form 16 distinct laminin heterotrimers.23 Laminin-211 (α2, β1, γ1) and laminin-221 (α2, β2, γ1) are the predominant laminin isoforms expressed in adult skeletal muscle that serve to anchor myofibers to the basal lamina via the α7β1 integrin and the dystrophin glycoprotein complexes.20 These attachments promote muscle cell integrity and survival.24,25 Mutations in the laminin-α2 gene result in congenital muscular dystrophy type 1A (MDC1A). Both MDC1A patients and laminin-α2-deficient mice have dramatically reduced levels of α7 integrin, which may contribute to the severe muscle pathology.3,25 In addition, laminin-α2 expression is decreased in α7 integrin-null muscle.17 These data suggest common pathways regulate the expression of α7 integrin and laminin-α2.
The regenerative capacity of skeletal muscle is dependent on satellite cells, a pool of myogenic cells located in close proximity to the myofiber under the basal lamina. These cells are quiescent in healthy uninjured muscle, but are rapidly activated in response to muscle damage, exercise, or disease. On activation, satellite cells proliferate and differentiate along a myogenic developmental pathway to repair damaged muscle.26,27,28,29 Several lines of evidence suggest the α7β1 integrin may modify disease progression by regulating myogenic cell function. MyoD transactivates α7 integrin gene expression in vitro, which would increase α7 integrin levels in myoblasts.30 Myoblast cell lines derived from satellite cells express high levels of α7 integrin.3 The α7β1 integrin associates with muscle-specific β1-integrin binding protein (MIBP), which regulates laminin deposition in myoblasts.31 The α7β1 integrin may regulate satellite cell transition into myofibers.32,33 Finally, enhanced expression of the α7 integrin in dystrophic skeletal muscle promotes satellite cell proliferation and activation.14
To test the hypothesis that the α7β1 integrin is important for skeletal muscle regeneration, we subjected α7 integrin-deficient muscle to cardiotoxin-induced damage. Our studies demonstrate for the first time that loss of the α7 integrin results in defective muscle regeneration in response to injury. Injection of laminin-111 (α1, β1, γ1) into α7 integrin-null muscle before cardiotoxin treatment restored muscle regeneration to wild-type levels. Results from this study indicate a critical role for the α7β1 integrin and laminin in muscle repair and suggest direct muscle injections of laminin may serve as an exciting novel therapy for patients with α7-integrin congenital myopathy and other muscle diseases.
Materials and Methods
Animals
Wild-type (C57BL/6), α7 integrin-null (C57BL/6 background), and Nestin-GFP mice (C57BL/6 background) were euthanized in accordance with protocols approved by the University of Nevada and University of Washington Institutional Animal Care and Use Committee.
Histology
Tibialis anterior (TA) muscles were embedded in OCT (Tissue-Tek; Sakura Finetek-USA Inc., Torrance, CA) and 10-μm cryosections were cut (≥50 μm apart) using a CM1850 cryostat (Leica, Wetzlar, Germany) and placed on Surgipath microscope slides (Surgipath Medical Industries, Richmond, IL). Tissue sections were stained with hematoxylin and eosin (H&E) as previously described.17 Central myonuclei in regenerating muscles were counted at ×630 magnification by bright-field microscopy. The number of central nuclei per muscle fiber was determined by counting a minimum of 1000 muscle fibers per animal. At least five animals from each genotype were analyzed. In addition the cross-sectional area was examined in a minimum of 5000 muscle fibers per group per time point. Results are reported as the average fiber cross-sectional area.
Immunofluorescence
TA muscles were embedded in Tissue-Tek OCT compound. Sections were cut at 10 μm using a Leica CM1850 cryostat and placed onto Surgipath microscope slides. Laminin-α2 chain was detected with a 1:500 dilution of rabbit anti-laminin-α2 (2G) polyclonal antibody (a kind gift from Peter Yurchenco, Department of Pathology, Robert Wood Johnson Medical School, Piscataway, NJ). The laminin-α1 chain was detected with a rat anti-laminin-α1 monoclonal antibody (MAB1903; Chemicon International, Temecula, CA). Primary rabbit antibodies were detected with a 1:500 dilution of fluorescein isothiocyanate-conjugated anti-rabbit secondary antibody and the rat monoclonal antibody was detected with 1:500 dilution of fluorescein isothiocyanate-conjugated anti-rat secondary antibody. In all immunofluorescence experiments, secondary only antibody controls were included to test for specificity.
For mouse monoclonal antibodies, endogenous mouse immunoglobulin was blocked with a mouse-on-mouse (MOM) kit (Vector Laboratories, Burlingame, CA). MyoD and Pax7 were detected using 5 μg/ml of anti-MyoD (Stratagene, La Jolla, CA) and 5 μg/ml of anti-Pax7 (Developmental Studies Hybridoma Bank, Iowa City, IA). eMyHC was detected as previously described.17 A 1-μg/ml concentration of tetramethylrhodamine-conjugated wheat germ agglutinin (WGA) (Molecular Probes, Eugene, OR) was used to define muscle fibers. To examine immune response, cytotoxic T cells were detected with fluorescein isothiocyanate-labeled rat anti-mouse CD8a (BD Pharmingen, San Diego, CA) and macrophages were detected with fluorescein isothiocyanate-conjugated anti-mouse F4/80 (Bioscience, San Diego, CA) at 1:1000. Immunofluorescence was performed on 10-μm sections from TA muscle from 5-week-old wild-type and α7−/− male mice. Images were captured at ×630 magnification. Fluorescence was observed with an Axioskop 2 Plus fluorescent microscope (Zeiss, Thornwood, NY) and images were captured with a Zeiss AxioCam HRc digital camera and Axiovision 4.1 software. Images for each experiment were captured with the same exposure time. Multiple adjacent sections were analyzed within 20 random, nonoverlapping microscopic fields per animal at ×630 magnification.
Single myofibers were isolated from the extensor digitorum longus muscles of 10-week-old nestin-GFP transgenic mice after collagenase digestion and cultured individually in Matrigel-coated wells as previously described.34,35 Adherent single myofibers were fixed in 4% paraformaldehyde and incubated with 1:1000 dilution of anti-α7 integrin rat monoclonal antibody (CA5.5) (Sierra BioSource, Morgan Hill, CA). The anti-α7 integrin rat antibody was detected with rhodamine-labeled anti-rat secondary antibody. Both GFP and rhodamine fluorescence were detected using an inverted fluorescent microscope (Eclipse, TE2000-S; Nikon, Tokyo, Japan) and images were acquired with a CoolSNAPES monochrome charge-coupled device camera controlled by MetaVue Imaging System (Universal Imaging Corporation, Sunnyvale, CA).
Evan’s Blue Dye (EBD) Assay
Mice were injected intraperitoneally with 50 μl of a 10-mg/ml solution of sterile EBD solution per 10 g of body weight. After 3 hours, the TA muscle was harvested and flash-frozen in liquid nitrogen. Ten-μm cryosections were placed on microscope slides and fixed in 4% paraformaldehyde. Muscle fibers were outlined by incubating tissue sections with Oregon Green-488-conjugated WGA (2 μg/ml, Molecular Probes). A minimum of 1000 fibers per animal were counted to determine the percentage of muscle fibers positive for EBD. At least four animals from each genotype were analyzed. Images were captured and counted at ×630 magnification.
Cardiotoxin-Induced Muscle Injury
Mice were anesthetized with avertin (0.25 μl/g of body weight). Using previously described methods,36 100 μl of a 10-μmol/L cardiotoxin solution (C3987; Sigma, St. Louis, MO) in phosphate-buffered saline (PBS) was injected using an insulin syringe into the left TA muscles of 5-week-old male wild-type and α7−/− mice. The right TA muscles were injected with 100 μl of PBS and used as controls. The mice were euthanized and muscles harvested at 4, 7, 10, and 28 days after cardiotoxin injection for analysis.
Laminin-111 Injections
Natural mouse laminin-111 (α1, β1, γ1) purified from Engelbreth-Holm-Swarm mouse sarcoma cells (Invitrogen, Carlsbad, CA) at 100 nmol/L in PBS was injected using an insulin syringe without exposing the muscle into the left TA muscles of anesthetized wild-type and α7−/− mice 3 days before cardiotoxin injection. The right TA muscles were injected with 100 μl of PBS and served as controls. The muscles were harvested at 0, 4, 7, 10 and 28 days after cardiotoxin injection for analysis.
Statistical Analysis
All averaged data are reported as the mean ± SD. Comparisons between multiple groups were performed by one-way analysis of variance for parametric data or by Kruskal-Wallis one-way analysis of variance on ranks for nonparametric data using SigmaStat 1.0 software (Jandel Corp., San Rafael, CA). P < 0.05 was considered statistically significant.
Results
The α7 Integrin Is Expressed in Quiescent Satellite Cells
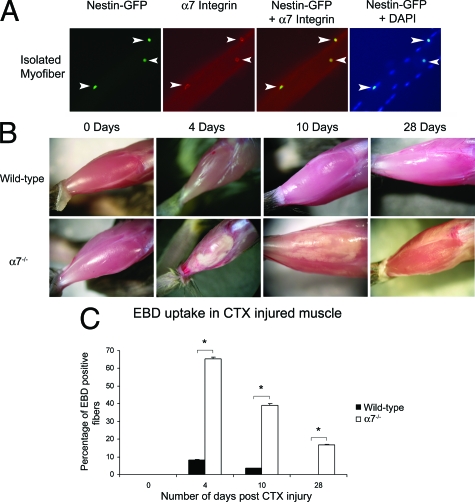
To determine whether the α7 integrin is expressed in satellite cells in vivo, isolated myofibers from nestin-GFP transgenic mice were subjected to immunofluorescence using an anti-α7 integrin antibody (Figure 1A). Nestin-GFP is specifically expressed in quiescent satellite cells.37 All nestin-GFP-positive cells on the myofiber were positive for the α7 integrin (Figure 1A). These data indicate that quiescent satellite cells express the α7 integrin.
Figure 1.
Loss of the α7 integrin results in defective muscle repair after cardiotoxin injury. A: Single myofibers isolated from nestin-GFP transgenic mice were used to identify satellite cells. All nestin-GFP satellite cells expressed the α7 integrin. The arrowheads indicate satellite cells on isolated muscle fibers, which are positive for nestin-GFP and a7 integrin. B: Wild-type muscle rapidly recovered after cardiotoxin-induced injury. In contrast α7 integrin-deficient muscle exhibited profound and prolonged muscle damage after injury. C: EBD uptake reveals that cardiotoxin-induced injury results in reduced sarcolemmal integrity in α7 integrin-null muscle compared with wild type (*P < 0.05).
α7 Integrin-Null Mice Exhibit Decreased Membrane Integrity and Delayed Muscle Repair
To examine if the α7β1 integrin is required for muscle repair, the TA muscles from wild-type and α7−/− mice were subjected to cardiotoxin injury and examined 4, 10, and 28 days later (Figure 1B). Four days after cardiotoxin injury, wild-type TA muscle appeared healthy and this appearance persisted for 28 days. In contrast, α7−/− muscle exhibited large white regions of damaged muscle at 4 and 10 days after injury. At 28 days, regions of muscle damage were still evident in α7−/− muscle. These data suggest loss of the α7 integrin in skeletal muscle results in a profound delay in muscle regeneration.
To examine membrane integrity after cardiotoxin damage, wild-type and α7−/− mice were injected with EBD. EBD uptake was absent in both groups before cardiotoxin injection (Figure 1C). At day 4 after injury, 8.5% of wild-type and 66% of α7−/− myofibers were EBD-positive. After 10 days, less than 4% of wild-type myofibers were positive for EBD uptake, whereas 40% of α7−/− myofibers were EBD-positive. At 28 days after cardiotoxin injection, 17% of α7−/− muscle fibers were still EBD-positive, whereas EBD was not observed in wild-type muscle. These results indicate loss of the α7 integrin results in increased membrane fragility after cardiotoxin treatment.
Muscle Repair Is Delayed in α7 Integrin-Null Mice
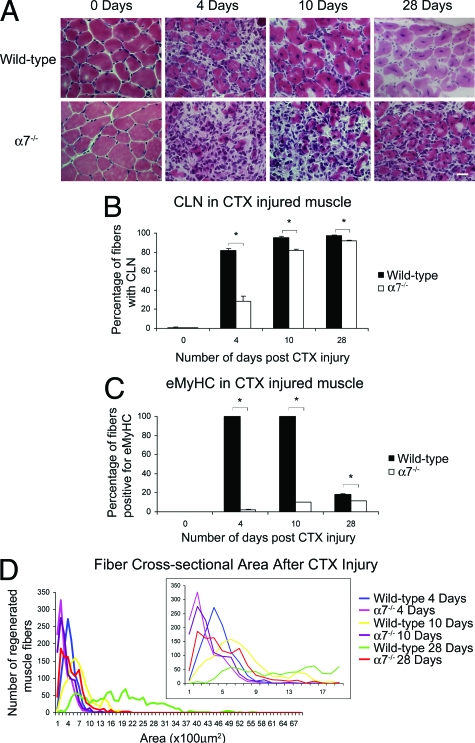
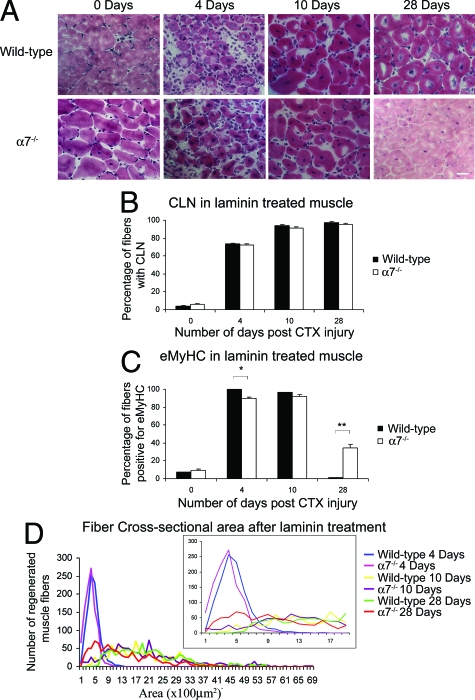
H&E staining was used to examine muscle pathology after cardiotoxin injury (Figure 2A). Four days after cardiotoxin injury, wild-type muscle exhibited mononuclear cell infiltrate and myofibers containing centrally located nuclei (Figure 2A). By day 10, wild-type muscle exhibited little mononuclear infiltrate, and most myofibers contained centrally located nuclei. By 28 days in wild-type muscle, most myofibers contained centrally located nuclei, and little mononuclear cell infiltrate was evident in wild-type muscle. In contrast, 4 days after cardiotoxin-induced damage, α7−/− muscle exhibited extensive mononuclear cell infiltrate and hypotrophic muscle fibers that persisted to 10 and 28 days after cardiotoxin injury (Figure 2A).
Figure 2.
Muscle regeneration is defective in α7 integrin-null mice. A: Cardiotoxin-induced injury resulted in pronounced and prolonged mononuclear cell infiltrate in α7 integrin-null muscle compared with wild-type as detected by H&E staining. B: Wild-type muscle treated with cardiotoxin contained more centrally located nuclei compared with α7 integrin-null muscle (*P < 0.05). C: Wild-type muscle injured with cardiotoxin contained more eMyHC-positive myofibers compared with α7 integrin-null muscle (*P < 0.001). Myofibers were delineated with WGA. D: α7 integrin-null muscle treated with cardiotoxin exhibited more hypotrophic myofibers compared with wild type. For clarity in the lower cross-sectional ranges the inset graph shows muscle cross-sectional areas from 0 to 19 μm. Scale bar = 20 μm.
To quantify muscle repair, the percentage of myofibers with centrally located nuclei was determined (Figure 2B). In wild-type mice, 81.8% of muscle fibers contained centrally located nuclei 4 days after cardiotoxin injury. In contrast only 28.1% of muscle fibers in α7−/− muscle were positive for centrally located nuclei (P < 0.05). By 10 and 28 days 95.5% and 97.5% of muscle fibers, respectively, in wild-type mice exhibited centrally located nuclei. By days 10 and 28, 82% and 95.5% of muscle fibers in α7−/− muscle, respectively, exhibited centrally located nuclei. These results indicate loss of the α7 integrin results in delayed muscle regeneration.
Embryonic myosin heavy chain (eMyHC) is transiently expressed after muscle repair and used as a marker for recent muscle regeneration. At day 0 there was an absence of eMyHC in both wild-type and α7−/− mice (Figure 2C). At 4 and 10 days after cardiotoxin treatment, expression of eMyHC was detected in more than 99% of wild-type muscle fibers (Figure 2C). In sharp contrast, only 2.2% and 9.9% of α7−/− muscle fibers expressed eMyHC at 4 and 10 days, respectively (Figure 2C). By day 28, only 11.3% of α7−/− myofibers were eMyHC-positive, whereas 18.5% of wild-type muscle was eMyHC-positive. These results confirm that loss of the α7 integrin results in defective muscle repair.
Cardiotoxin Injury Results in Hypotrophic Muscle Fibers in α7 Integrin-Null Mice
To determine if loss of the α7 integrin affected muscle repair after injury, myofiber cross-sectional areas were measured (Figure 2D). Regenerating muscle fibers in wild-type mice were 31% larger than α7−/− muscle fibers 4 days after cardiotoxin injury (Figure 2D). At day 10, regenerating wild-type myofibers were 45.1% larger compared with α7−/− muscle fibers (Figure 2D). By day 28, wild-type muscle displayed muscle fiber size variation; however, this was in contrast to the α7−/− muscle, which displayed small cross-sectional areas, with the vast majority of fibers between 100 to 600 μm2. These results indicate loss of the α7 integrin results in reduced regenerative capacity resulting in hypotrophic muscle fibers.
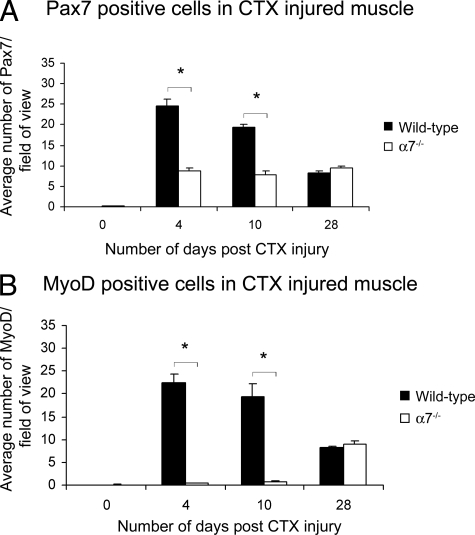
Myoblast Proliferation and Differentiation Are Impaired in α7 Integrin-Deficient Muscle
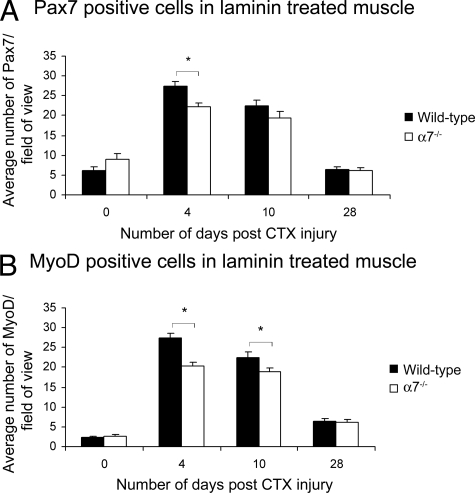
Pax7 is expressed in both quiescent and activated satellite cells, whereas MyoD is expressed in myoblasts.38,39,40 To examine if the developmental program regulating muscle repair was affected by the loss of the α7β1 integrin, we examined expression of Pax7 and MyoD (Figure 3, A and B). Compared with wild-type muscle, α7−/− mice exhibited twofold to threefold fewer Pax7-positive cells compared with wild type at days 4 and 10 after cardiotoxin injury (Figure 3A). By day 28, similar numbers of Pax7-positive cells were observed in wild-type and α7−/− mice. Analysis of MyoD expression showed α7−/− muscle contained 28- and 50-fold fewer MyoD-positive myoblasts compared with wild-type muscle at 4 and 10 days after cardiotoxin induced damage, respectively (Figure 3B). By day 28 similar numbers of MyoD-positive cells were observed in wild-type and α7−/− mice. Together these results indicate loss of the α7 integrin results in fewer activated satellite cells in injured skeletal muscle and a delay in myogenic differentiation.
Figure 3.
Satellite cell proliferation and differentiation are delayed in α7 integrin-null muscle after cardiotoxin treatment. A: Pax7 expression is reduced in α7 integrin-null satellite cells 4 and 10 days after cardiotoxin injury compared with wild type (*P < 0.05). B: MyoD expression is reduced in α7 integrin-null myoblasts at 4 and 10 days after cardiotoxin injury compared with wild type (*P < 0.05). Myofibers were delineated with WGA.
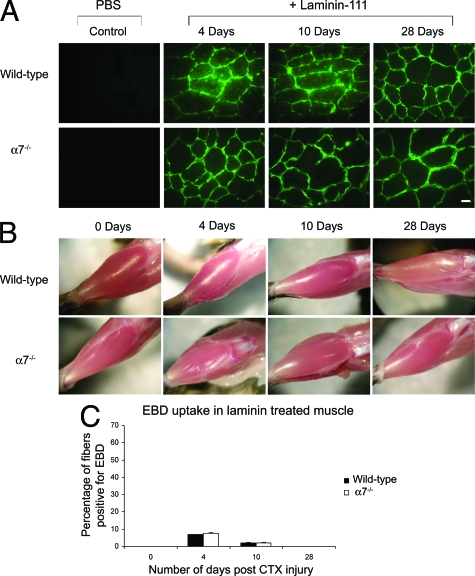
Laminin Treatment Restores Sarcolemmal Integrity in α7 Integrin-Null Mice
Recent studies have shown that the loss of the α7 integrin results in reduced laminin-α2 expression.17 To explore whether reduced laminin deposition could account for the defective muscle regenerative phenotype observed in α7 integrin-null mice, the TA muscle was injected with 100 μl of a 100 nmol/L solution of laminin-111 in PBS 3 days before cardiotoxin injury. Laminin-111 protein was used because a highly purified protein preparation of this laminin isoform was available from a mouse Engelbreth-Holm-Swarm sarcoma cell line. This would ensure minimal immunological response to the exogenously delivered laminin protein. Laminin-111 is not normally expressed in adult muscle, but studies indicate that it is functionally similar to laminin-211.41,42 The injected laminin-111 was detected with anti-laminin-α1-specific antibodies. Titration of laminin-111 in cultured myofibers revealed increased toxicity at 200 nmol/L and higher concentrations (data not shown).
Surprisingly, the injected laminin-111 permeated the entire TA muscle within 24 to 72 hours (Figure 4A and Supplemental Figure S1 available at http://ajp.amjpathol.org) and was maintained throughout the muscle for at least 28 days (Figure 4A). Secondary only antibody controls were negative at all time points analyzed indicating specificity of the rat monoclonal anti-laminin-α1 antibody for the injected laminin-111 protein (Supplemental Figure S2 available at http://ajp.amjpathol.org). At all time points analyzed after cardiotoxin injury, α7−/− muscle treated with laminin-111 appeared similar to wild-type muscle (Figure 4B).
Figure 4.
Laminin restores muscle repair and integrity in α7 integrin-null mice. A: Muscle cryosections were immunostained with anti-laminin-α1 antibody to detect injected laminin-111 protein. Laminin-111 protein was rapidly incorporated into the extracellular matrix surrounding muscle fibers after injection and persisted for more than 28 days. Muscle injected with PBS alone contained no laminin-111. B: Laminin-111 injection protected α7 integrin-null muscle from extensive injury from cardiotoxin treatment. C: Laminin-111 treatment restored sarcolemmal integrity after cardiotoxin injury in α7 integrin-null muscle to wild-type levels. Scale bar = 10 μm.
To examine if exogenously injected laminin-111 elicited an immunological response, wild-type and α7−/− muscle cryosections were subjected to immunofluorescence 3 days after laminin-111 injections using antibodies against CD8a and F8/40 to detect cytotoxic T cells and macrophages, respectively (Supplemental Figure S3 available at http://ajp.amjpathol.org). As a positive control α7−/− muscle was subjected to immunofluorescence using these antibodies 3 days after cardiotoxin treatment (Supplemental Figure S3 available at http://ajp.amjpathol.org). Analysis revealed muscle injected with mouse laminin-111 was negative for cytotoxic T cells and macrophages, indicating laminin-111 injections alone did not elicit an immunological response. Analysis of EBD uptake after cardiotoxin-induced injury revealed no difference in the percentage of EBD-positive myofibers between laminin-treated wild-type or α7−/− muscle at all time points (Figure 4C). These results demonstrate that injection of laminin-111 before cardiotoxin-induced injury restored sarcolemmal integrity to α7 integrin-null muscle.
Laminin Boosts Muscle Regeneration in α7 Integrin-Null Mice
To determine whether laminin-111 could improve muscle regeneration, 5-week-old wild-type and α7−/− TA muscle were injected with laminin and subjected to cardiotoxin-induced injury. Muscle sections were stained with H&E and mononuclear cell infiltrate and centrally located nuclei examined (Figure 5A). No differences in the myofiber size, centrally located nuclei, or mononuclear cell infiltrate were observed between wild-type and α7 integrin-null muscle treated with laminin-111 at 4, 10, or 28 days after injury (Figure 5A).
Figure 5.
Laminin restored muscle regeneration in α7 integrin-null mice to wild-type levels. A: α7 integrin-null muscle injected with laminin-111 protein before cardiotoxin injury exhibited a regenerative phenotype similar to wild type with less mononuclear cell infiltrate and fewer hypotrophic muscle fibers. B: Laminin-111 protein injection restored muscle repair and the percentage of myofibers containing centrally located nuclei to wild-type levels. C: Laminin-111 protein therapy restored eMyHC expression to wild-type levels at days 4 and 10 (*P < 0.05). At day 28 more α7 integrin myofibers expressed eMyHC compared with wild type (**P < 0.001). Myofibers were delineated with WGA. D: Laminin-111 protein treatment before cardiotoxin injury resulted in myofiber cross-sectional areas that were indistinguishable between α7 integrin-null and wild type. For clarity in the lower cross-sectional ranges the inset graph shows muscle cross-sectional areas from 0 to 19 μm. Scale bar = 20 μm.
Quantitation of centrally located nuclei confirmed that laminin-111 treatment restored regeneration in α7 integrin-null muscle to wild-type levels (Figure 5B). At all time points, the percentages of centrally located nuclei in laminin-treated wild-type and α7−/− mice were not significantly different from each other. These results indicate that laminin-111 restored muscle repair in α7 integrin-null muscle to wild-type levels.
The ability of laminin-111 to restore regenerative capacity in α7 integrin-null muscle was examined by assaying eMyHC expression (Figure 5C). At day 0, 7.3% of wild-type fibers and 9.7% of α7−/− fibers were eMyHC-positive as a result of injection with laminin (Figure 5C). At 4 and 10 days after cardiotoxin treatment, wild-type and α7−/− muscle exhibited similar levels of eMyHC expression (Figure 5C). At 28 days after injury, eMyHC was only present in negligible amounts in the wild-type muscle whereas 34.4% of myofibers in α7−/− muscle were positive for eMyHC (Figure 5C). These results demonstrate injection of laminin-111 greatly improved the regenerative capacity of α7 integrin-null muscle.
Laminin Therapy Restores Myofiber Size in α7 Integrin-Deficient Muscle
Myofiber cross-sectional area was examined in laminin-treated wild-type and α7 integrin-null mice before and after cardiotoxin-induced injury (Figure 5D). At 4 days after injury, the cross-sectional area of myofibers in wild-type mice was found to be only 13% larger compared with α7−/− muscle (Figure 5D). By days 10 and 28 after cardiotoxin injury, the cross-sectional area of myofibers in α7−/− muscle was similar to wild-type animals (Figure 5D). Together these data indicate treatment with laminin-111 restored muscle repair and myofiber size in α7 integrin-null muscle.
Laminin Restores Myoblast Proliferation and Differentiation in α7 Integrin-Null Muscle
To determine whether treatment with laminin-111 restored the myogenic repair program in α7−/− muscle, expression of Pax7 and MyoD was quantified (Figure 6, A and B). Before cardiotoxin-injury, wild-type and α7−/− muscle exhibited a few Pax7-positive cells, which could be attributed to the minor damage from the laminin injection (Figure 6A). At 4 days after cardiotoxin injury, there were 20% fewer Pax7-positive cells in laminin-treated α7−/− muscle compared with wild-type (Figure 6A). By 10 and 28 days after cardiotoxin injury levels of Pax7-positive cells in α7−/− muscle were similar to wild-type muscle (Figure 6A).
Figure 6.
Laminin restored satellite cell proliferation and differentiation in α7 integrin-null muscle. A: Laminin-111 treatment restored Pax7 expression in α7 integrin-null muscle to wild-type levels (*P < 0.05). B: Laminin-111 injection greatly improved MyoD expression in α7 integrin-null muscle after cardiotoxin-induced injury (*P < 0.05). Myofibers were delineated with WGA.
Analysis of MyoD revealed a few positive cells in laminin-treated wild-type and α7 integrin-null TA muscle at day 0 (Figure 6B). At days 4 and 10 after cardiotoxin injury, the number of MyoD-positive cells in laminin-treated α7−/− muscle was ∼25% lower than wild type (Figure 6B). However by day 28, wild-type and α7−/− muscle had similar numbers of MyoD-positive cells (Figure 6B). Laminin-111 treatment substantially restored the number of myogenic cells and promoted activation of the myogenic program involved in muscle repair in α7 integrin-null muscle.
Discussion
In this study we show that α7 integrin-null mice exhibit defective skeletal muscle regeneration after cardiotoxin-induced injury. Treatment with laminin-111 corrected the defective repair phenotype. Although many aspects of the myogenic developmental program have been elucidated, the mechanisms by which the extracellular matrix and integrin cell surface receptors participate in skeletal muscle repair after injury or during disease are not well understood.
Muscle damage is followed by the rapid activation of satellite cells.43 On activation, these cells proliferate and activate myogenic developmental programs to repair damaged muscle. Models suggest a subpopulation of satellite cells remain as stem cells to replace cells that have progressed down the myogenic lineage pathway.39,43,44 During activation satellite cells express the transcription factors Pax3, Pax7, MyoD, myogenin, and MRF4. In this study we show that loss of the α7 integrin leads to reduced satellite cell activation and myoblast differentiation in response to muscle injury. These data indicate the α7β1 integrin regulates a key transition early in muscle regeneration in which satellite cells switch to become myogenic cells capable of repairing muscle.
Increased EBD uptake in α7 integrin-null muscle after cardiotoxin treatment indicates that loss of the α7β1 integrin results in increased sarcolemmal fragility in response to cardiotoxin. Increased muscle damage may explain the delay in the capacity of α7 integrin-null muscle to undergo repair after cardiotoxin-induced injury. The observation that both wild-type and α7 integrin-null muscle exhibit comparable numbers of centrally located nuclei 28 days after cardiotoxin injections, however, indicates that similar numbers of myofibers must have been damaged by cardiotoxin and required repair. These observations indicate that a mechanism other than the extent of muscle damage produced by cardiotoxin injections is responsible for the delay in muscle repair observed in α7 integrin-null mice.
Laminin is normally produced by skeletal muscle and secreted into the surrounding basal lamina.45,46 Several studies suggest that laminin within the extracellular matrix promotes myoblast survival, proliferation, migration, and differentiation.24,47,48,49,50 Recently we demonstrated that loss of the α7 integrin leads to significantly reduced expression of laminin-α2 in skeletal muscle.17 Because the regenerative capacity of skeletal muscle is dependent on an intricate interplay between satellite cells and the extracellular matrix,26 absence of the α7 integrin may result in loss of an optimal laminin-rich microenvironment required for myogenic repair. To determine whether decreased laminin deposition contributes to the reduced muscle regenerative phenotype observed in α7 integrin-null mice, laminin-111 was injected into the muscle of mice before injury. Interestingly, within 48 to 72 hours, injected laminin-111 protein spread throughout the entire TA muscle and persisted for at least 31 days in the basal lamina. Injection of muscle with laminin-111 protein before cardiotoxin injury restored muscle regeneration in α7 integrin-null mice to wild-type levels.
Although laminin-211 and laminin-221 are expressed in adult muscle, laminin-111 is only present in embryonic skeletal muscle.20 One possible explanation for the improved muscle regeneration in laminin-treated α7 integrin-null muscle is that injection of laminin-111 may recapitulate an embryonic myogenic program in adult skeletal muscle. Activation of this embryonic program may result in enhanced satellite cell activation, proliferation, and improved muscle repair. These results suggest other laminin receptors may be expressed in satellite cells that can also promote myogenic repair or can act to compensate for the loss of α7 integrin in myoblasts.
This study suggests patients with α7 integrin mutations suffer from congenital myopathy as a result of decreased sarcolemmal integrity through loss of the α7β1 integrin linkage system and a reduced capacity for muscle repair after injury. Our data show direct injections of laminin-111 protein can improve sarcolemmal integrity and enhance regenerative repair after injury in α7 integrin-null muscle. Together our data suggest laminin-111 may potentially serve as a protein therapy for patients with α7 integrin congenital myopathy. Because loss of sarcolemmal integrity and regenerative capacity has been implicated in a variety of muscular dystrophies including MDC1A and DMD, we are currently investigating if laminin-111 protein therapy may also be beneficial in other forms of muscular dystrophy.
Supplementary Material
Acknowledgments
We thank Dr. Heather R. Burkin for critically reading this manuscript.
Footnotes
Address reprint requests to Dean J. Burkin, Ph.D., Department of Pharmacology MS/318, University of Nevada School of Medicine, Reno, NV 89557. E-mail: dburkin@medicine.nevada.edu.
Supported by the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR053697-01 and National Institute of Neurological Disorders and Stroke grant R21NS058429-01 to D.J.B. and National Institute on Aging grant R01AG021566-06 to Z.Y.R.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin alpha 7 beta 1 is developmentally regulated in skeletal muscle. J Biol Chem. 1993;268:19019–19024. [PubMed] [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110:2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of alpha 7 integrin cytoplasmic domains during skeletal muscle development: alternate forms, conformational change, and homologies with serine/threonine kinases and tyrosine phosphatases. J Cell Sci. 1993;106:1139–1152. doi: 10.1242/jcs.106.4.1139. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Vu MP, Waleh N, Crawford J, Lin CS, Kramer RH. Alternative extracellular and cytoplasmic domains of the integrin alpha 7 subunit are differentially expressed during development. J Biol Chem. 1993;268:26773–26783. [PubMed] [Google Scholar]
- von der Mark H, Williams I, Wendler O, Sorokin L, von der Mark K, Poschl E. Alternative splice variants of alpha 7 beta 1 integrin selectively recognize different laminin isoforms. J Biol Chem. 2002;277:6012–6016. doi: 10.1074/jbc.M102188200. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- Welser JV, Lange ND, Flintoff-Dye N, Burkin HR, Burkin DJ. Placental defects in alpha7 integrin-null mice. Placenta. 2007;28:1219–1228. doi: 10.1016/j.placenta.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welser JV, Lange N, Singer CA, Elorza M, Scowen P, Keef KD, Gerthoffer WT, Burkin DJ. Loss of the {alpha}7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res. 2007;101:672–681. doi: 10.1161/CIRCRESAHA.107.151415. [DOI] [PubMed] [Google Scholar]
- Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152:1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Burkin DJ, Kaufman SJ. Increasing alpha 7 beta 1-integrin promotes muscle cell proliferation, adhesion, and resistance to apoptosis without changing gene expression. Am J Physiol. 2008;294:C627–C640. doi: 10.1152/ajpcell.00329.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of {alpha}7{beta}1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol. 2005;166:253–263. doi: 10.1016/s0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikian MJ, Hack AA, Mewborn S, Mayer U, McNally EM. Genetic compensation for sarcoglycan loss by integrin alpha7beta1 in muscle. J Cell Sci. 2004;117:3821–3830. doi: 10.1242/jcs.01234. [DOI] [PubMed] [Google Scholar]
- Guo C, Willem M, Werner A, Raivich G, Emerson M, Neyses L, Mayer U. Absence of alpha 7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:989–998. doi: 10.1093/hmg/ddl018. [DOI] [PubMed] [Google Scholar]
- Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tiger CF, Velling T. Laminins during muscle development and in muscular dystrophies. Cell Mol Life Sci. 1999;56:442–460. doi: 10.1007/PL00000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F. Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci. 2005;62:809–823. doi: 10.1007/s00018-004-4510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schéele S, Nystrom A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Laminin isoforms in development and disease. J Mol Med. 2007;85:825–836. doi: 10.1007/s00109-007-0182-5. [DOI] [PubMed] [Google Scholar]
- Tunggal P, Smyth N, Paulsson M, Ott MC. Laminins: structure and genetic regulation. Microsc Res Tech. 2000;51:214–227. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vilquin JT, Engvall E. Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab Invest. 1999;79:1601–1613. [PubMed] [Google Scholar]
- Vachon PH, Xu H, Liu L, Loechel F, Hayashi Y, Arahata K, Reed JC, Wewer UM, Engvall E. Integrins (alpha7beta1) in muscle function and survival. Disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870–1881. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Kramer RH. Identification and characterization of the cell type specific and developmentally regulated alpha 7 integrin gene promoter. J Biol Chem. 1996;271:22915–22922. doi: 10.1074/jbc.271.37.22915. [DOI] [PubMed] [Google Scholar]
- Li J, Rao H, Burkin D, Kaufman SJ, Wu C. The muscle integrin binding protein (MIBP) interacts with alpha7beta1 integrin and regulates cell adhesion and laminin matrix deposition. Dev Biol. 2003;261:209–219. doi: 10.1016/s0012-1606(03)00304-x. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le GF, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki N, Lim M, Yao CC, Tolar M, Kramer RH. Alpha7 integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp Cell Res. 2006;312:4162–4180. doi: 10.1016/j.yexcr.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- Shefer G, Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 2005;290:281–304. doi: 10.1385/1-59259-838-2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102:2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Kim JE, Gu M, Kaufman SJ. Laminin and alpha7beta1 integrin regulate agrin-induced clustering of acetylcholine receptors. J Cell Sci. 2000;113:2877–2886. doi: 10.1242/jcs.113.16.2877. [DOI] [PubMed] [Google Scholar]
- Gawlik K, Miyagoe-Suzuki Y, Ekblom P, Takeda S, Durbeej M. Laminin alpha1 chain reduces muscular dystrophy in laminin alpha2 chain deficient mice. Hum Mol Genet. 2004;13:1775–1784. doi: 10.1093/hmg/ddh190. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, Smyth N. The role of laminins in basement membrane function. J Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi YK, Tezak Z, Momoi T, Nonaka I, Garcia CA, Hoffman EP, Arahata K. Massive muscle cell degeneration in the early stage of merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 2001;11:350–359. doi: 10.1016/s0960-8966(00)00203-0. [DOI] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J Clin Invest. 1998;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Engvall E. Disruption of the lama2 gene in embryonic stem cells: laminin alpha 2 is necessary for sustenance of mature muscle cells. Exp Cell Res. 1998;241:117–125. doi: 10.1006/excr.1998.4025. [DOI] [PubMed] [Google Scholar]
- Mrak RE. The pathologic spectrum of merosin deficiency. J Child Neurol. 1998;13:513–515. doi: 10.1177/088307389801301009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.