Abstract
Protein kinase CK2 (CK2) is a serine/threonine kinase that participates in important cellular processes. We have recently demonstrated that CK2 plays a role in resistance to TRAIL/Fas-induced apoptosis in endometrial carcinoma (EC) by regulating FLIP. Here, we assessed the immunohistochemical expression of CK2β in EC and checked its role in cell proliferation and anchorage-independent cell growth. CK2β immunostaining was assessed in two tissue microarrays, one constructed from paraffin-embedded blocks of 95 ECs and another from 70 samples of normal endometrium. CK2β expression was correlated with histological type; grade and stage; cell proliferation (Ki-67) and apoptotic index; immunostaining for cyclin D1, PTEN, AKT, β-catenin, and FLIP. Moreover, the Ishikawa EC cell line was subjected to down-regulation of CK2 by shRNA. CK2β expression was frequent in EC (nuclear, 100%; cytoplasmic, 87.5%). The staining was more intense in EC than in normal endometrium (P = 0.000), and statistically correlated with AKT, PTEN, β-catenin, and FLIP. In EC, CK2β expression correlated with cell proliferation. Knock-down of CK2β blocked colony formation of EC in soft agar, and also resulted in decreased expression of cyclin D1 and ERK phosphorylation. The results confirm that CK2β is widely expressed in EC, and suggest a role in cell proliferation and anchorage-independent cell growth.
Endometrial carcinoma (EC) is one of the most common gynecological malignant tumors in Europe and the United States.1 EC can be divided into two main clinicopathological variants. Type I ECs are endometrioid EC; they are estrogen-related tumors, frequently well differentiated and developing mostly in pre- and perimenopausal women. Type I ECs are associated more frequently with four main molecular alterations: microsatellite instability and mutations of K-RAS, PTEN, and β-catenin. Type II ECs are nonendometrioid EC (papillary serous and clear cell carcinomas); they tend to occur in older women, and are estrogen-unrelated tumors, frequently aneuploid, associated with p53 mutations, and are clinically more aggressive tumors.2
Protein kinase CK2 (previously known as casein kinase II) is a serine/threonine kinase that has been implicated in cell growth, differentiation, proliferation, and apoptosis. CK2 has been shown to be deregulated in several kinds of tumors.3 CK2 is an enzyme with extensive homology across species that consists of two catalytic subunits (α, α’) and the regulatory subunit (β). CK2 exists as α2β2, αα’β2, or α’2β2 configurations. In the β subunit, certain cysteine residues may play a role in anchoring the kinase to nuclear structures. CK2 activity may have a role in cell growth through its signaling to key sites in nuclear matrix and chromatin structures.4 Several growth stimuli can enhance CK2 nuclear shuttling, so that higher nuclear localization is observed in tumor cells compared with normal cells.5,6 Moreover, CK2 dysregulation in tumor cells may influence the apoptotic activity and to enhance cell survival.7,8
We have recently demonstrated a role for CK2β in apoptosis resistance to TRAIL in EC.9 We first showed that pharmacological inhibition or silencing of CK2β sensitized EC cell lines to TRAIL and Fas-induced apoptosis, by down-regulating FLIP. Moreover, we found that forced expression of FLIP restored resistance to TRAIL and Fas in cells lacking CK2 activity or CK2β expression.
In the present study, we assessed the expression of CK2β in a large series of ECs by using an anti-CK2β antibody in two tissue microarrays that included 95 ECs and 70 samples of normal endometrium (NE). CK2β immunostaining was correlated with histological type, grade and stage, proliferation cell (Ki-67), and apoptotic index. Moreover, CK2β immunoreactivity was correlated with immunostaining for PTEN, AKT, β-catenin, and FLIP, to check the relationship of CK2β with the PI3K and Wnt signaling pathways, as well as to confirm the association between CK2β and FLIP. Finally, the role of CK2 on cell proliferation and anchorage-independent cell growth was assessed in one EC cell line, which was subjected to CK2β knock-down.
Materials and Methods
Tissue Microarray
Two tissue microarrays (TMAs) were constructed. One contained paraffin-embedded blocks of 95 ECs that were classified by following the World Health Organization criteria, and were staged and graded according to the International Federation of Gynecology and Obstetrics. The cases included 25 endometrioid carcinomas grade I, 34 endometrioid carcinomas grade II, 19 endometrioid carcinomas grade III, 10 serous carcinomas, 4 clear cell carcinomas, and 3 mixed Müllerian malignant tumors; 65 cases were stage I, 4 were stage II, 14 were stage III, and 1 was stage IV. Staging information was incomplete in 11 cases. A second TMA was constructed from 70 paraffin-embedded samples of NE in different phases of the menstrual cycle: 20 in proliferative phase, 40 in secretory phase, and 10 menstrual endometria. A tissue arrayer device (Beecher Instruments, Sun Prairie, WI) was used to construct the TMA. Briefly, all of the samples were histologically reviewed and representative areas were marked in the corresponding paraffin blocks. Two selected cylinders (0.6 mm in largest diameter) from two different areas were included in each case. Control normal tissues from the same specimens were also included. The study was approved by the local ethical committee. Specific informed consent was obtained.
Immunohistochemical Study
TMA blocks were sectioned at a thickness of 3 μm, dried for 16 hours at 56°C before being dewaxed in xylene and rehydrated through a graded ethanol series, and washed with phosphate-buffered saline (PBS). Antigen retrieval was achieved by heat treatment in a pressure-cooker for 2 minutes in ethylenediaminetetraacetic acid (pH 8.9). Before staining the sections, endogenous peroxidase was blocked. The antibodies used were: anti-CK2β (monoclonal, 6D5, 1:400 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), Ki-67 (monoclonal, MIB-1, 1:100 dilution; DAKO, Glostrup, Denmark), cyclin D1 (monoclonal, DCS6, 1:25 dilution; DAKO), antiphosphorylated AKT (polyclonal, 1:25 dilution; Cell Signaling, Beverly, MA), PTEN (monoclonal, 6H2.1, 1:300 dilution; Cascade Bioscience, Winchester, MA), β-catenin (monoclonal, clone 14, 1:300 dilution; Transduction Laboratories, Lexington, KY), anti-FLIP (polyclonal H-202, 1:10 dilution; Santa Cruz Biotechnology). After incubation, the reaction was visualized with the EnVision Detection Kit (DAKO) using diaminobenzidine chromogen as a substrate. Sections were counterstained with hematoxylin. Immunohistochemical results were evaluated by two pathologists, by following uniform pre-established criteria. CK2β, AKT, PTEN, and FLIP immunoexpressions were graded semiquantitatively by considering the percentage and intensity of the staining. A histological score was obtained from each sample, which ranged from 0 (no immunoreaction) to 300 (maximum immunoreactivity). The score was obtained by applying the following formula, Hscore = 1× (% light staining) + 2× (% moderate staining) + 3× (% strong staining). The reliability of the scoring system in TMA evaluation in EC has been demonstrated previously.10,11,12,13 Because each TMA included two different tumor cylinders from each case, immunohistochemical evaluation was done after examining both samples. To assess the cellular proliferation (Ki-67) and the cyclin D1 expression, we used the percentage of positive nuclei in each case, and finally for β-catenin we noted the membranous or nuclear staining. The specificity of the CK2β antibody was confirmed by obtaining a negative staining in cell blocks from Ishikawa cells infected with lentiviruses carrying CK2β shRNA; whereas, Ishikawa cells infected with lentiviruses carrying the empty vector were positive.
Cell Lines and Culture Conditions
The Ishikawa 3-H-12 cell line (IK) was obtained from the American Type Culture Collection (Manassas, VA). IK cells were grown in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Invitrogen, Inc., Carlsbad, CA), 1 mmol/L HEPES (Sigma), 1 mmol/L sodium pyruvate (Sigma), 2 mmol/L l-glutamine (Sigma), and 1% of penicillin/streptomycin (Sigma) at 37°C with saturating humidity and 5% CO2.
Lentiviral Production and Infection
Oligonucleotides to produce plasmid-based shRNA were cloned into the FSV vector using AgeI-BamHI restriction sites. shRNA target sequence was: Ck2β, 5′-TGGTTTCCCTCACATGCTCT-3′. To produce infective lentiviral particles, 293T cells were co-transfected by the calcium phosphate method with the virion-packaging elements (VSV-G and Δ8.9) and the shRNA-producing vector (FSV) on 293T human embryonic kidney. 293T cells were allowed to produce lentiviral particles for 3 to 4 days in same culture medium used for endometrial cell lines. Culture medium was collected, centrifuged for 5 minutes at 1000 rpm, and filtered through a 0.45-μm filter (Millipore, Bedford, MA). The medium was diluted 1:2 to 1:4 with fresh medium, and added to growing cell lines or primary explants. Cells were incubated for 24 to 48 hours in the presence of medium containing lentiviral particles. After this period, medium was replaced with fresh medium and the cells were incubated for an additional 2 to 3 days to allow endogenous protein knock-down.
Western Blot Analysis
The EC cell line was washed with cold phosphate-buffered saline (PBS) and lysed with lysis buffer (2% sodium dodecyl sulfate, 125 mmol/L Tris-HCl, pH 6.8). Protein concentrations were determined with a protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore). Nonspecific binding was blocked by incubation with TBST (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Tween-20) plus 5% of nonfat milk. Membranes were incubated with the primary antibodies overnight at 4°C. Signal was detected with ECL Advance (Amersham-Pharmacia, Buckinghamshire, UK). The antibodies used were: anti-CK2β (monoclonal, 6D5; Santa Cruz Biotechnology), CK2α (monoclonal, 1AD9; Santa Cruz Biotechnology), FLIP (monoclonal, NF6; Alexis Biochemicals, Lausen, Switzerland), anti-phospho ERK-1/2 MAPK (Thr202/Tyr204) (polyclonal, Cell Signaling), Pan-ERK (monoclonal, BD Transduction), α-tubulin (monoclonal, Sigma), and cyclin D1 (monoclonal, DCS-6; Santa Cruz Biotechnology). Antibody to caspase-9 and cleaved caspase-3 were obtained from Cell Signaling.
Colony Formation Assay and Assessment of Apoptosis
Three days after infection FSV or CK2β shRNA-transduced cells were trypsinized and resuspended in 0.3% agar diluted in medium at concentration of 3000 cells/ml. One ml of cell suspension was layered on a 0.6% agar-coated 35 mm culture dish. Dishes were incubated at 37°C with saturating humidity and 5% CO2 for 15 days. Colonies were visualized by staining with MTT. Colonies were counted with Quantity One software (Bio-Rad). Hoechst staining was performed by adding Hoechst dye to a final concentration of 0.5 mg/ml to each M96 well. Cells were counted under epifluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Statistical Analysis
Statistical analysis was performed on a database using SPSS for Windows (version 11.5; SPSS Inc., Chicago, IL). The Hscore results were compared by the Mann-Whitney U-test, Student’s t-test, Kruskal-Wallis test, and analysis of variance test when applicable. Correlations between quantitative variables were established through Pearson and Spearman Rho tests. Statistical significance was set at P ≤ 0.05.
Relative Quantification Analysis of CK2β Gene Expression
For gene expression analysis, predesigned gene-specific primer pairs and probe were selected for one target gene (CK2β) and one endogenous control (18s rRNA) within a list of predesigned assays (Assays-on-Demand; Applied Biosystems, Foster City, CA). All assays were based on TaqMan hydrolysis probes labeled with FAM (green fluorescent fluorophore 6-carboxyfluorescein). Assays were performed on an ABI Prism 7000 HT sequence detection system (Applied Biosystems). RNA was obtained from fresh-frozen tissue obtained in the Pathology Department, Hospital Universitari Arnau de Vilanova, shortly after surgery. They included three samples of NE in the proliferative phase, three samples of secretory endometrium, and paired normal and tumor tissue from six patients with EC. Corresponding formalin-fixed, paraffin-embedded tumor tissue from these patients had been included in the tissue microarray that was subjected to immunohistochemical analysis of CK2β. Once RNA was extracted, subsequent reverse transcription analyses to cDNA were performed in 20-μl reaction volumes. For the assays each reaction was comprised of 4 μl of cDNA, 10 μl of 2× qPCR Master Mix, 1 μl of 20× primer/probe assay mix (Applied Biosystems). Samples were assayed in triplicate for each gene, and the mean expression was used during subsequent analysis. Relative expression was calculated using the comparative ΔΔCT method (Bulletin no.2, Applied Biosystems).
5-Bromodeoxyuridine Incorporation
For the determination of DNA, cells were incubated with 3 ng/ml of 5-bromodeoxyuridine (BrdU) (Sigma) for 20 minutes and then fixed with 4% paraformaldehyde. After DNA denaturing with 2 mol/L HCl for 30 minutes and neutralization with 0.1 mol/L Na2B4O7 (pH 8.5) for 2 minutes, cells were blocked in PBS solution containing 5% horse serum, 5% fetal bovine serum, 0.2% glycine, and 0.1% Triton X-100 for 1 hour. Subsequently, cells were subjected to indirect immunofluorescence with a mouse anti-BrdU monoclonal antibody (DAKO), and fluorescein isothiocyanate-conjugated anti-mouse secondary antibody (Molecular Probes, Eugene, OR). Nuclei were counterstained with 5 μg/ml Hoechst 33258 and cells were visualized under an epifluorescence microscope (Leica Microsystems).
Propidium Iodide Cell Death Analysis
Analysis of cell death distribution was determined by propidium iodide (PI) staining and flow cytometry. After treatment, ∼106 cells were fixed in 70% ethanol for at least 1 hour on ice. Cells were then resuspended in 2 ml of cell cycle buffer (20 μg/ml PI in PBS containing 0.1% Triton X-100 and 50 μg/ml RNase A) for 1 hour at 37°C. PI fluorescence emission was measured using a FACSCalibur (BD Biosciences, San Jose, CA), and cell cycle distribution was analyzed with WinMDI 2.9 software (The Scripps Research Institute, La Jolla, CA).
Results
CK2β Expression in EC and NE Samples
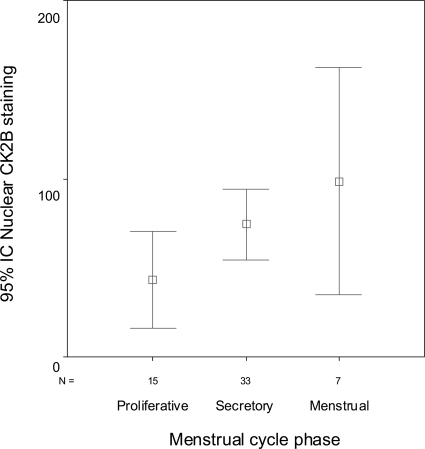
CK2β expression was assessed in NE. There were statistically significant differences in the cytoplasmic (analysis of variance, P = 0.017) and nuclear (analysis of variance, P = 0.001) expression of CK2β among different phases of the menstrual cycle (Figures 1 and 2). Thus, cytoplasmic and nuclear CK2β immunoexpression was low in the proliferative endometrium (mean Hscores, 10.00 and 43.00, respectively) and increased in the secretory (mean Hscores, 32.73 and 74.24, respectively) and menstrual endometrium (mean Hscores, 57.14 and 98.57, respectively). A positive correlation between cytoplasmic and nuclear CK2β immunostaining was detected in NE (Pearson, r = 0.371; P = 0.003). Immunohistochemical results were confirmed at the mRNA level by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). All but one of the samples of secretory endometrium had higher levels of CK2β mRNA than samples of proliferative endometrium.
Figure 1.
Error bar showing that CK2β cytoplasmic expression was significantly higher in the secretory and menstrual endometrium than in proliferative endometrium (analysis of variance, P = 0.017).
Figure 2.
Error bar showing that CK2β nuclear immunoexpression was significantly lower in the proliferative phase of the menstrual cycle, and increases in the secretory and menstrual endometrium (analysis of variance P = 0.001).
CK2β immunoexpression was evaluated in 88 cases, from the initial series of 95 ECs of the TMA; 6 cases were lost during TMA construction, and 1 case did not have representative tumor sample. All of the EC samples showed nuclear CK2β immunostaining (88 of 88; 100%). Cytoplasmic staining was observed in 77 cases (87.5%), but the staining was heterogeneous. The Hscore ranged from 10 to 300 (mean, 100.34); and only 11 cases had negative cytoplasmic staining (Figure 3). There was a strong positive correlation between the cytoplasmic and the nuclear expression in each case (Pearson, r = 0.514; P = 0.000). Thus, EC samples with high cytoplasmic CK2β expression showed also strong nuclear CK2 staining. EC had a very significant higher nuclear CK2β immunostaining compared with NE (mean Hscore in NE, 79.44; mean Hscore in EC, 167.39; Student’s t-test, P = 0.000). Also, the tumor cells showed significantly higher CK2β cytoplasmic expression (mean Hscore, 100.34) than the normal cells (mean Hscore, 29.05; P = 0.000) (Figure 4, A and B). CK2β expression was also checked in full sections of five ECs, to check if the heterogeneity of the staining was attributable to increased staining in the myoinvasive front. However, no statistically significant differences in CK2β expression were seen between the superficial and the deepest area of invasion (Mann-Whitney U-test; P = 0.401 for cytoplasmic staining, and P = 0.599 for nuclear staining). Differences in CK2β expression between EC and corresponding NE were evaluated by quantitative RT-PCR. In all but one of the cases, the CK2β mRNA levels were higher in EC samples than in the corresponding NE samples from the same patients.
Figure 3.
CK2β immunoexpression in ECs. The staining was heterogeneous, cytoplasmic, and nuclear.
Figure 4.
CK2β immunostaining was significantly lower in NE samples (A) compared with ECs (B).
CK2β immunoexpression was slightly higher in the cytoplasm of the endometrioid type of EC (mean Hscore, 107.15) compared with nonendometrioid EC (mean Hscore, 67.00). Also, nonendometrioid EC showed a strong CK2β nuclear staining (mean Hscore, 198.00), but these differences were not statistically significant (Mann-Whitney U-test; P = 0.158, and P = 0.287, respectively). Finally, no significant differences were found in CK2β expression in correlation with the International Federation of Gynecology and Obstetrics grade (Kruskal-Wallis, P = 0.636) or pathological stage (Kruskall-Wallis, P = 0.675).
CK2β and Cellular Proliferation (Ki-67) and Cyclin D1
CK2β nuclear immunostaining demonstrated a trend of correlation with the proliferation index (Ki-67/MIB-1) in EC (Spearman, r = 0.210; P = 0.070). Moreover, CK2β cytoplasmic and nuclear expression increased in cases with high nuclear cyclin D1 expression, but these differences were not significant (Kruskal-Wallis; P = 0.477 for cytoplasmic, and P = 0.494 for nuclear).
CK2β Correlation with AKT, PTEN, and β-Catenin Expression in ECs
We found a positive correlation between CK2β cytoplasmic expression and AKT immunostaining in EC (Spearman, r = 0.243; P = 0.025) and a nearly significant negative correlation between CK2β and PTEN immunoexpression (Spearman, r = −0.194; P = 0.093) (Figure 5, A and B). The correlation between β-catenin and CK2β immunostaining was assessed in 82 ECs. CK2β cytoplasmic and nuclear expression increased in ECs with nuclear β-catenin staining (mean cytoplasmic Hscore,112.50; mean nuclear Hscore, 214.58) (Mann-Whitney U-test, P = 0.054) compared with cases with only β-catenin membranous staining (mean cytoplasmic Hscore, 99.50; mean nuclear Hscore, 158.71).
Figure 5.
High CK2β immunoexpression in ECs (A) was frequently associated with low or negative PTEN expression (B).
CK2β, FLIP Expression, and Apoptotic Index in ECs
Correlation between CK2β and FLIP immunoexpression was assessed in 83 ECs. We demonstrated a significant positive correlation between CK2β and FLIP cytoplasmic immunostaining (Pearson, r = 0.238; P = 0.030) (Figure 6, A and B). However, no correlation was found between CK2β cytoplasmic and nuclear expression and the apoptotic index (Pearson, P = 0.480 and P = 0.282).
Figure 6.
ECs positive for CK2β immunoexpression (A) with high FLIP immunostaining (B).
CK2β Regulates Proliferation and Anchorage-Independent Cell Growth of EC Cells
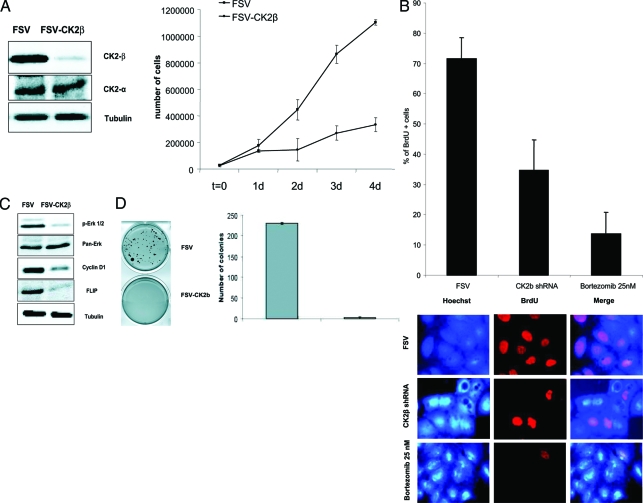
To ascertain whether CK2 regulates proliferation of EC cells, we infected IK cells with lentiviruses carrying either an empty vector (FSV) or a CK2 shRNAs (FSV-CK2β) and 4 days later cell lysates were analyzed by Western blot for the expression of CK2β. As we show in Figure 7A, CK2β shRNA caused a dramatic down-regulation of CK2β protein levels whereas catalytic α CK2 subunit protein levels remained unaffected. To investigate the role of CK2β in proliferation, we infected IK cells with lentiviruses carrying CK2β shRNA, and we assessed cell proliferation by cell counting throughout time. CK2 shRNA (FSV-CK2β)-transduced cells displayed a dramatic decrease in proliferation rate when compared with the empty vector (FSV)-infected cells (Figure 7A). Moreover, to further confirm the role of CK2β in cell proliferation we performed a BrdU analysis. Results indicated that unlike control infected cells, CK2β-silenced cells displayed a marked reduction in cell proliferation. Bortezomib-treated cells were used as positive control (Figure 7B). These data prompted us to determine any putative proliferation marker susceptible to be modulated on CK2β silencing. Western blot analysis suggested a possible role for cyclin D1 and ERK. Figure 7C shows that CK2β shRNA caused a marked decrease in expression of cyclin D1 and phosphorylation of ERK-1/2 without affecting total ERK-1/2 protein levels. Furthermore, consistently with the results described above, CK2β knock-down also resulted in a pronounced decrease in FLIP protein levels. Altogether these results indicated that CK2β is involved in endometrial cancer cell proliferation, and that silencing of CK2β by means of shRNA results in a pronounced blockage of cell growth. To investigate the role of CK2 in growing conditions independent of substrate attachment, we infected IK cells with lentivirus carrying CK2β shRNA or the empty vector. After 3 days, cells were trypsinized and resuspended in soft agar as described in the Materials and Methods section. After 15 days, FSV-infected cells displayed cell growth and colony formation. In contrast, IK cells infected with CK2β shRNA failed to develop colonies (Figure 7D). These results suggest that CK2 regulates both cell proliferation and anchorage-independent cell growth.
Figure 7.
A: Left: IK cells were infected with lentiviruses carrying an empty vector (FSV) or a CK2βshRNA and 4 days later cell lysates were analyzed by Western blot for the expression of CK2β and CK2α. Right: IK cells were infected with lentiviruses carrying the empty vector (FSV) or the CK2βshRNA (FSV-CK2β). Cells were trypsinized and counted at the indicated days after infection. B: Percentage of positive BrdU cells in FSV-infected cells, CK2β-silenced cells, and bortezomib-treated cells. Cell staining shown below. C: Western blot analysis of phospho-ERK-1/2, cyclin D1, and FLIP in CK2β-silenced cells and control cells. Membranes were reprobed with tubulin and total ERK-1/2 antibodies to ensure equal protein loading. D: IK cells were infected with lentiviruses carrying the empty vector (FSV) or the CK2βshRNA (FSV-CK2β). Three days after infection cells were trypsinized and resuspended in 0.3% agar diluted in medium at a concentration of 3000 cells/ml. Fifteen days after plating, colonies were stained with MTT (left) and scored (right).
CK2β shRNA Cytostatic Effects Are Not Caused by an Apoptotic Response of EC Cells
We have previously demonstrated that CK2β is involved in control of TRAIL-induced apoptosis.9 To discard the role of apoptosis in the CK2β-silencing cytostatic effect, FSV/CK2β shRNA-infected cells and bortezomib-treated cells were subjected to propidium iodide staining and apoptotic index was measured by flow cytometry. SubG1 data indicated that CK2β shRNA did not trigger a pronounced apoptotic response compared with those cells treated with bortezomib (Figure 8A). Moreover, Hoechst staining indicated that the decrease in cell proliferation was not attributable to a massive apoptotic cell death in FSV/CK2β-transduced cells because nuclei displaying apoptotic morphology were not primarily appreciated. Consistent with previous results obtained in our laboratory, CK2β-silenced cells treated with TRAIL resulted in an increase in percentage of apoptotic nuclei. This condition was used as a positive control (Figure 8B). Furthermore, we demonstrated by Western blot that caspases were not activated in response to CK2β shRNA because neither inductor caspase-9 nor effector caspase-3 were processed. In contrast, and in agreement with previous available data, we appreciated remarkable caspase activation on treatment of CK2β-silenced cells with TRAIL. Altogether, these data support the notion that CK2β is involved in progression of cell cycle and proliferation and that its decrease is not caused by an apoptotic response.
Figure 8.
A: FSV- and shCK2β-infected IK cells and bortezomib-treated cells were subjected to propidium iodide (PI) staining and monitored by flow cytometry to study the involvement of an apoptotic response. SubG1 phase indicates percentage of apoptotic cells. B: IK cells were subjected to Hoechst staining as described in the Materials and Methods section (right) and apoptotic cells were counted (left). C: Western blot of FSV and CK2β shRNA-infected cells against caspase-9 and caspase-3.
Discussion
Protein kinase CK2 is a serine/threonine kinase that is highly conserved and ubiquitously distributed in eukaryotic organisms. It is typically found in tetrameric complexes consisting of two catalytic (α, and/or α’) subunits, and two regulatory subunits (β). CK2 has been shown to be deregulated in different types of tumors. Several studies in hematopoietic malignancies and solid tumors, such as carcinomas of the prostate, kidney, colon, liver, and lung have demonstrated elevated CK2 activity, and changes in the intracellular location with an enhanced nuclear translocation.3 These studies have demonstrated an increase in the CK2 activity or expression by using biochemical analysis of tissue extracts or immunohistochemical techniques. In some types of tumor, such as those of the head and neck, the level of CK2 is correlated with tumor grade, stage, and clinical outcome. CK2 is involved in protein kinase networks controlling cell growth, proliferation, but also as a potent suppressor of apoptosis. Further evidence about the role of CK2 in tumorigenesis is provided by transgenic mice expressing CK2α in lymphocytes or mammary gland. Both animal models display an increased incidence of lymphomas and breast carcinomas.14,15 More than 300 potential substrates of CK2 (cytoplasmic and nuclear) have been identified, which points to its multiple functional activities. In a recent study we were able to demonstrate for the first time that CK2 played an important role in TRAIL- and Fas-induced apoptosis in EC, by regulating FLIP.9
In the present study we have shown, for the first time, that CK2β is frequently expressed in EC. We have observed a significant increase in cytoplasmic and nuclear CK2β immunostaining in EC in comparison with NE in different phases of the menstrual cycle. Results were confirmed at the mRNA level by RT-PCR. The preferential nuclear distribution of CK2 in tumor cells has also been demonstrated in other tumor types, such as in prostatic carcinoma and squamous carcinoma of the head and neck.16,17 In some types of tumors, CK2 immunoexpression is higher in the infiltrating edge of the tumor and lymphocytes. However, we have not been able to demonstrate such a finding in our series of cases, particularly after having studied full sections from a small selected series of ECs, which allowed us to evaluate the comparative expression of CK2β in the superficial tumor and in areas of deep myometrial invasion.
Interestingly, our results showed a trend of positive correlation between CK2β immunoexpression with the cellular proliferation (Ki-67) in EC, but not in NE. CK2β expression was higher in the secretory and menstrual endometrium than in the proliferative phase, whereas in EC, CK2β expression was associated with increased cell proliferation. As discussed later on, these results obtained in EC samples are in agreement with our results obtained in the Ishikawa cell line. Studies on yeasts and mammalian cells have revealed requirements for CK2 at various stages of the cell cycle, including G1 phase and the G1/S and G2/M transitions.18,19 In other types of tumors, such as prostatic and head and neck carcinomas, the proliferating front of the neoplasm showed higher CK2 immunoexpression.16,20 Recent studies have suggested that overexpression of CK2 in tumor cells may not be simply a reflection of cell proliferation alone but additionally may reflect some pathobiological characteristics of the neoplasm, such as the grade of differentiation of the tumor. In this regard, in our study CK2 immunoexpression had a tendency to increase in nonendometrioid EC and in stage II and III tumors. However, these differences were not statistically significant.
A very interesting result of the present study is the relationship between CK2β and the PI3K and Wnt pathways, two signaling pathways frequently activated in EC. CK2β showed a significant positive correlation with AKT and a nearly significant negative correlation with PTEN immunoexpression. The tumor suppressor gene PTEN is frequently abnormal in EC; loss of heterozygosity at chromosome 10q23 occurs in 40% of cases21,22 and somatic PTEN mutations are also common and occur in 37 to 61% of EC.23,24,25,26 Our results support a role for PTEN, and the PI3K/AKT pathway in the regulation of CK2β expression. It is known that decreased expression of PTEN leads to increased levels of phospho-AKT, which results in activation of antiapoptotic proteins as well as inactivation of proteins involved in cycle progression (p27 and p21). Moreover, Torres and colleagues, 2001, demonstrated that CK2 is able to decrease PTEN stability and increase its proteasome-mediated degradation through the phosphorylation of a cluster of Ser/Thr residues in its C-terminus.27 Moreover, inhibition of PTEN phosphorylation by a CK2 inhibitor diminishes the PTEN protein content. Additional evidence relating CK2 and the PI3K pathway were obtained from the Jurkat cell line, which is PTEN-null. In these cells, down-regulation of CK2 catalytic activity correlated with decreased AKT activity, measured by reduced phosphorylation of typical AKT targets.28
We also found a significant correlation between CK2β immunoexpression and β-catenin nuclear staining. CK2 is a positive regulator of Wnt signaling.29 The Wnt signaling is important in EC, particularly in endometrioid (type I) tumors, because mutations in exon 3 of CTNNB1 are found in 25 to 40% of endometrioid EC. CTNNB1 mutations lead to nuclear accumulation of β-catenin, which has an important impact in transcription of target genes of the Wnt pathway, such as cyclin D1 or MMP-7. CK2 is present in β-catenin complexes and activated in Wnt-signaling cells.30 Recent studies have shown that CK2 enhances β-catenin through phosphorylation of hLEF-1 stimulating the binding and transactivation of β-catenin in vitro.31 In breast cancer cells, CK2 is capable of phosphorylating β-catenin, and regulating its turnover15; in these cells, CK2 activity is essential for maintenance of β-catenin and Drl protein levels.
Also, we found a statistical association between FLIP and CK2β cytoplasmic immunostaining. Such results support the hypothesis that FLIP may be under the regulation of CK2. In a previous study, we demonstrated a role for FLIP in resistance to TRAIL and Fas-induced apoptosis in EC.13 We also demonstrated that FLIP was frequently expressed in EC samples. FLIP is a well-established regulator of TRAIL- and FasL-triggered apoptosis in many cell types, which is constitutively or highly expressed in some tumors such as prostate cancer, Hodgkin’s lymphoma, gastric cancer, bladder carcinoma, and malignant mesothelial cell lines. Moreover, in a very recent study, we have also shown that CK2 is an important regulator of death receptor-induced apoptosis, by regulating the levels of FLIP. We demonstrated that in EC both pharmacological inhibition and CK2 knock-down reduced the levels of FLIP. We also showed that inhibition of CK2 plus addition of either TRAIL- or a Fas-activated caspase-8 as initiator caspase of the extrinsic pathway.9
Several studies have implicated dysregulation of the CK2 activity with enhanced survival of tumor cells. In the rat ventral prostate, CK2 has been implicated in the androgenic regulation of the cells and receptor-mediated apoptosis.32,33,34 In this model loss of CK2 from the cell lead to a cessation of cell growth activity and to induce apoptosis. Other studies have shown that transient overexpression of CK2-α and CK2-αβ in tumor cells resulted in a significant protection against etoposide-mediated apoptosis.7 Recent reports in the carcinoma cell lines PC-3 and ALVA-41, suggest a role for FLIP in regulation of CK2 sensitivity to TRAIL-induced apoptosis.35 Treatment of the cell lines with TRAIL plus a CK2 inhibitor (TBB) lead to a down-regulation of FLIP expression and concomitant caspase-8 activation. Moreover, overexpression of CK2α restored FLIP expression and TRAIL resistance to apoptosis cell death.
Increased CK2 activity is associated with cell growth and proliferation in many types of tumors displaying aberrant or increased CK2 activity.3,36,37 To ascertain whether CK2 regulates proliferation of EC cells in vitro, Ishikawa cells were infected with lentiviruses carrying CK2β shRNA. Knock-down of CK2β protein caused a marked decrease in expression of cyclin D1, phosphorylation of ERK-1/2, decrease of BrdU incorporation, and a marked decrease in proliferation rate that was not caused by a consistent increase in number of apoptotic cells. Interestingly, results from the TMA showed a relationship between CK2 and cyclin D1 that did not reach statistical signification. It is important to keep in mind that cyclin D1 is also a target of other molecular alterations frequently detected in EC (microsatellite instability, mutations in CTNNB1, activation of NF-κB).10,38,39 The presence of these alterations in a certain percentage of tumors might have interfered in the correlation between CK2 and cyclin D1. Finally the Ishikawa EC cell line cells infected with lentivirus carrying CK2β shRNA failed to develop colonies, clearly suggesting that CK2 regulates both cell proliferation and anchorage-independent cell growth. The fact that CK2 has become a highly pleiotropic protein makes the study of the exact mechanism that orchestrates its control over cell growth and proliferation difficult. Many mechanisms have been proposed, as it has been observed that CK2 regulates by phosphorylation many transcription factors such as c-Myc, c-Myb, AP-1, and steroid hormone receptors among others. Moreover, it has also been reported that CK2 can modulate NF-κB activity by phosphorylation of Rel-A (p65) NF-kappa B subunit or by promoting the degradation of its inhibitory subunit IkB.40 Recently, Yde and collaborators41 have suggested that CK2β regulates G2/M transition by controlling stabilization of Wee1 and its subsequent inhibitory phosphorylation of CDK1 on Tyr15 pointing to CK2 as a key regulator of cell cycle progression in lung cancer cells. Our results add important information to the complex network of cell proliferation and cell cycle controlled by CK2 and further research efforts will be required to uncover the precise mechanism.
Footnotes
Address reprint requests to Xavier Matias-Guiu, Department of Pathology and Molecular Genetics, Hospital Universitari Arnau de Vilanova, Av Alcalde Rovira Roure 80, 25198 Lleida, Spain. E-mail: xmatias@arnau.scs.es.
Supported by grants FIS070304, FIS070276, Ministerio de Sanidad y Consumo Marató de TV3 2005-47, 2004XT00090, AGAUR and RTICC (RD06/0020/1034) Ministerio de Sanidad y Consumo; the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo (postdoctoral fellowship CP05/00028 to X.D. and predoctoral fellowship FI05/00191 to D.L.L.); and the Fundación Alicia Cuello de Merigó (fellowship to N.E.).
J.P. and D.L. are first authors and X.D. and X.M.-G. are senior authors.
References
- Evans MP, Podratz K. Endometrial neoplasia: prognostic significance of ploidy status. Clin Obstet Gynecol. 1996;39:696–706. doi: 10.1097/00003081-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu X, Catasus L, Bussaglia E, Lagarda H, Garcia A, Pons C, Munoz J, Arguelles R, Machin P, Prat J. Molecular pathology of endometrial hyperplasia and carcinoma. Hum Pathol. 2001;32:569–577. doi: 10.1053/hupa.2001.25929. [DOI] [PubMed] [Google Scholar]
- Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16:573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- Zhang P, Davis AT, Ahmed K. Mechanism of protein kinase CK2 association with nuclear matrix: role of disulfide bond formation. J Cell Biochem. 1998;69:211–220. [PubMed] [Google Scholar]
- Ahmed K. Nuclear matrix and protein kinase CK2 signaling. Crit Rev Eukaryot Gene Expr. 1999;9:329–336. doi: 10.1615/critreveukargeneexpr.v9.i3-4.170. [DOI] [PubMed] [Google Scholar]
- Tawfic S, Faust RA, Gapany M, Ahmed K. Nuclear matrix as an anchor for protein kinase CK2 nuclear signalling. J Cell Biochem. 1996;62:165–171. doi: 10.1002/(sici)1097-4644(199608)62:2<165::aid-jcb4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Guo C, Yu S, Davis AT, Wang H, Green JE, Ahmed K. A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem. 2001;276:5992–5999. doi: 10.1074/jbc.M004862200. [DOI] [PubMed] [Google Scholar]
- Guo C, Yu S, Davis AT, Wang H, Green JE, Ahmed K. A potential role of nuclear matrix-associated protein kinase CK2 in protection against drug-induced apoptosis in cancer cells. J Biol Chem. 2001;276:5992–5999. doi: 10.1074/jbc.M004862200. [DOI] [PubMed] [Google Scholar]
- Llobet D, Eritja N, Encinas M, Llecha N, Yeramian A, Pallares J, Sorolla A, Gonzalez-Tallada FJ, Matias-Guiu X, Dolcet X. CK2 controls TRAIL and Fas sensitivity by regulating FLIP levels in endometrial carcinoma cells. Oncogene. 2008;27:2513–2524. doi: 10.1038/sj.onc.1210924. [DOI] [PubMed] [Google Scholar]
- Pallares J, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, Palacios J, Prat J, Matias-Guiu X. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol. 2004;204:569–577. doi: 10.1002/path.1666. [DOI] [PubMed] [Google Scholar]
- Pallares J, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, Palacios J, Prat J, Matias-Guiu X. Survivin expression in endometrial carcinoma: a tissue microarray study with correlation with PTEN and STAT-3. Int J Gynecol Pathol. 2005;24:247–253. doi: 10.1097/01.pgp.0000163849.37129.d4. [DOI] [PubMed] [Google Scholar]
- Pallares J, Bussaglia E, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, Sanchez-Verde L, Palacios J, Prat J, Matias-Guiu X. Immunohistochemical analysis of PTEN in endometrial carcinoma: a tissue microarray study with a comparison of four commercial antibodies in correlation with molecular abnormalities. Mod Pathol. 2005;18:719–727. doi: 10.1038/modpathol.3800347. [DOI] [PubMed] [Google Scholar]
- Dolcet X, Llobet D, Pallares J, Rue M, Comella JX, Matias-Guiu X. FLIP is frequently expressed in endometrial carcinoma and has a role in resistance to TRAIL-induced apoptosis. Lab Invest. 2005;85:885–894. doi: 10.1038/labinvest.3700286. [DOI] [PubMed] [Google Scholar]
- Seldin DC, Leder P. Casein kinase II-alpha transgene-induced murine lymphoma—relation to theileriosis in cattle. Science. 1995;267:894–897. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, Seldin DC. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001;227:153–165. [PubMed] [Google Scholar]
- Yenice S, Davis AT, Goueli SA, Akdas A, Limas C, Ahmed K. Nuclear casein kinase-2 (Ck-2) activity in human normal, benign hyperplastic, and cancerous prostate. Prostate. 1994;24:11–16. doi: 10.1002/pros.2990240105. [DOI] [PubMed] [Google Scholar]
- Faust RA, Niehans G, Gapany M, Hoistad D, Knapp D, Cherwit D, Davis A, Adams GL, Ahmed K. Subcellular immunolocalization of protein kinase CK2 in normal and carcinoma cells. Int J Biochem Cell Biol. 1999;31:941–949. doi: 10.1016/s1357-2725(99)00050-3. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Lorenz P, Ansorge W, Pyerin W. Casein kinase-II is required for transition of G0/G1, early G1, and G1/S phases of the cell-cycle. J Biol Chem. 1994;269:6986–6991. [PubMed] [Google Scholar]
- Lorenz P, Pepperkok R, Ansorge W, Pyerin W. Cell biological studies with monoclonal and polyclonal antibodies against human casein kinase-II subunit-beta demonstrate participation of the kinase in mitogenic signaling. J Biol Chem. 1993;268:2733–2739. [PubMed] [Google Scholar]
- Seitz G, Munstermann U, Schneider HR, Issinger OG. Characterization of casein kinase-II in human colonic carcinomas after heterotransplantation into nude-mice. Biochem Biophys Res Commun. 1989;163:635–641. doi: 10.1016/0006-291x(89)92184-0. [DOI] [PubMed] [Google Scholar]
- Nagase S, Sato S, Tezuka F, Wada Y, Yajima A, Horii A. Deletion mapping on chromosome 10q25-q26 in human endometrial cancer. Br J Cancer. 1996;74:1979–1983. doi: 10.1038/bjc.1996.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer SL, Herzog TJ, Tribune DJ, Mutch DG, Gersell DJ, Goodfellow PJ. Allelic loss of sequences from the long arm of chromosome-10 and replication errors in endometrial cancers. Cancer Res. 1995;55:1922–1926. [PubMed] [Google Scholar]
- Bussaglia E, del Rio E, Matias-Guiu X, Prat J. PTEN mutations in endometrial carcinomas: a molecular and clinicopathologic analysis of 38 cases. Hum Pathol. 2000;31:312–317. doi: 10.1016/s0046-8177(00)80244-0. [DOI] [PubMed] [Google Scholar]
- Kong D, Suzuki A, Zou TT, Sakurada A, Kemp LW, Wakatsuki S, Yokoyama T, Yamakawa H, Furukawa T, Sato M, Ohuch N, Sato S, Yin J, Wang S, Abraham JM, Souza RF, Smolinski KN, Meltzer SJ, Horii A. PTEN1 is frequently mutated in primary endometrial carcinomas. Nat Genet. 1997;17:143–144. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- Risinger JI, Hayes AK, Berchuck A, Barrett J. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 1997;57:4736–4738. [PubMed] [Google Scholar]
- Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- Torres J, Pulido R. The tumor suppressor PTBN is phosphorylated by the protein kinase CK 2 at its C-terminus. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- Song DH, Dominguez I, Mizuno J, Kaut M, Mohr SC, Seldin D. CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem. 2003;278:24018–24025. doi: 10.1074/jbc.M212260200. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang HY. Casein kinase 2 is activated and essential for Wnt/beta-catenin signaling. J Biol Chem. 2006;281:18394–18400. doi: 10.1074/jbc.M601112200. [DOI] [PubMed] [Google Scholar]
- Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16:2239–2244. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Ahmed K. Significance of the casein kinase system in cell growth and proliferation with emphasis on studies of the androgenic regulation of the prostate. Cell Mol Biol Res. 1994;40:1–11. [PubMed] [Google Scholar]
- Tawfic S, Goueli SA, Olson MOJ, Ahmed K. Androgenic regulation of phosphorylation and stability of nucleolar protein nucleolin in rat ventral prostate. Prostate. 1994;24:101–106. doi: 10.1002/pros.2990240208. [DOI] [PubMed] [Google Scholar]
- Tawfic S, Olson MOJ, Ahmed K. Role of protein-phosphorylation in posttranslational regulation of protein B23 during programmed cell-death in the prostate-gland. J Biol Chem. 1995;270:21009–21015. doi: 10.1074/jbc.270.36.21009. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Role of protein kinase CK2 in the regulation of tumor necrosis factor-related apoptosis inducing ligand-induced apoptosis in prostate cancer cells. Cancer Res. 2006;66:2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin P, Catasus L, Pons C, Munoz J, Matias-Guiu X, Prat J. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33:206–212. doi: 10.1053/hupa.2002.30723. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Rodriguez-Perales S, Sanchez-Estevez C, Hardisson D, Sarrio D, Prat J, Cigudosa JC, Matias-Guiu X, Palacios J. Cyclin D1 gene (CCND1) mutations in endometrial cancer. Oncogene. 2003;22:6115–6118. doi: 10.1038/sj.onc.1206868. [DOI] [PubMed] [Google Scholar]
- Landesman-Bollag EL, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- Yde CW, Olsen BB, Meek D, Watanabe N, Guerra B. The regulatory β-subunit of protein kinase CK2 regulates cell-cycle progression at the onset of mitosis. Oncogene. 2008;27:4986–4997. doi: 10.1038/onc.2008.146. [DOI] [PubMed] [Google Scholar]