Abstract
Alterations in genes encoding transforming growth factor-β-signaling components contribute to colon cancer in humans. Similarly, mice deficient in the transforming growth factor-β signaling molecule, Smad3, develop colon cancer, but only after a bacterial trigger occurs, resulting in chronic inflammation. To determine whether Smad3-null lymphocytes contribute to increased cancer susceptibility, we crossed Smad3-null mice with mice deficient in both B and T lymphocytes (Rag2−/− mice). Helicobacter-infected Smad3/Rag2-double knockout (DKO) mice had more diffuse inflammation and increased incidence of adenocarcinoma compared with Helicobacter-infected Smad3−/− or Rag2−/− mice alone. Adoptive transfer of WT CD4+CD25+ T-regulatory cells provided significant protection of Smad3/Rag2-DKO from bacterial-induced typhlocolitis, dysplasia, and tumor development, whereas Smad3−/− T-regulatory cells provided no protection. Immunohistochemistry, real-time reverse transcriptase-polymerase chain reaction, and Western blot analyses of colonic tissues from Smad3/Rag2-DKO mice 1 week after Helicobacter infection revealed an influx of macrophages, enhanced nuclear factor-κB activation, increased BclXL/Bcl-2 expression, increased c-Myc expression, accentuated epithelial cell proliferation, and up-regulated IFN-γ, IL-1α, TNF-α, IL-1β, and IL-6 transcription levels. These results suggest that the loss of Smad3 increases susceptibility to colon cancer by at least two mechanisms: deficient T-regulatory cell function, which leads to excessive inflammation after a bacterial trigger; and increased expression of proinflammatory cytokines, enhanced nuclear factor-κB activation, and increased expression of both pro-oncogenic and anti-apoptotic proteins that result in increased cell proliferation/survival of epithelial cells in colonic tissues.
Patients with chronic inflammatory diseases such as ulcerative colitis and Crohn’s disease are at increased risk for developing colon cancer, indicating that chronic inflammation and cancer are closely linked in the gastrointestinal tract.1,2,3 In addition, bacteria are being increasingly appreciated as etiological agents that contribute to the development of certain cancers, in part by stimulating chronic inflammation.4,5,6 For example, in humans, the induction of gastritis, hepatitis, and colitis by bacterial pathogens has been implicated in the transformation of affected tissues. Host responses to selected intestinal microbial organisms can drive inflammation7 eventually leading to the development of dysplasia and cancer in these diseases. However, it is unclear how individual host factors influence whether particular organisms will induce inflammation followed by cancer, or inflammation followed by bacterial clearance or symbiosis.
Altered responses to transforming growth factor (TGF)-β signaling can contribute to inflammatory bowel disease (IBD) and colon cancer in both animal models and in humans. TGF-β is a multifactorial cytokine that controls many cellular functions including lymphocyte homeostasis, epithelial cell proliferation, apoptosis, and migration.8 TGF-β molecules (TGF-β1, TGF-β2, TGF-β3) bind to heterodimeric receptor complexes of transmembrane serine/threonine kinases called type I and type II receptors (TβR1 and TβR2), which then phosphorylate the Smad family members Smad2 and Smad3. A complex of Smad2 and Smad3 associates with Smad4 and translocates to the nucleus to activate transcription. TGF-β also activates other downstream signaling pathways including Rho GTPases, the extracellular signaling-regulated kinases (ERK), c-Jun NH2-terminals kinase (JNK), and phosphatidylinositol-3 kinase (PI3K).9 Dysregulation of TGF-β signaling pathways are associated with ulcerative colitis, Crohn’s disease, and colorectal cancer. In particular, mutations in the TGFBR2 gene are found in up to 90% of all microsatellite instability-positive cancer patients, whereas inactivating mutations in the Smad2 or Smad4 are associated with 5 to 10% of sporadic colorectal cancers.10 These data underscore the importance of TGF-β signaling molecules in limiting excessive bowel inflammation and the induction of colorectal cancer. However, it is unclear exactly how TGF-β receptors and signaling molecules normally function within different cell populations of inflammatory or colonic epithelial cells to inhibit excessive inflammation and transformation.
We previously used bacterial organisms such as Helicobacter spp. to study how intestinal organisms can induce inflammation, dysplasia, and eventually transformation of colonic epithelial cells.11,12 We found that TGF-β dysregulation in Smad3−/− mice that contain normal, nonpathogenic, commensal bowel flora is insufficient for the development of colon cancer, but requires infection with Helicobacter spp. to initiate inflammation and transformation. To study whether abnormalities in TGF-β signaling in lymphocytes13 contribute to colon cancer susceptibility, we crossed Smad3−/− mice (129SvEv background) with Rag2−/−129SvEv mice. Our results show that Smad3/Rag2-double knockout (DKO) mice, which have defective regulatory adaptive immune responses in addition to defective TGF-β signaling, are acutely sensitive to bacterially induced inflammation and cancer, compared with Smad3−/− or Rag2−/− mice. Mechanisms are suggested by changes in expression levels of inflammatory cytokines, proto-oncogenes, and anti-apoptotic proteins in colonic tissue and epithelial cells from Smad3/Rag2-DKO mice, and offer a murine model to study the early and initial events in inflammation-associated cancer.
Materials and Methods
Mice
Homozygous Smad3−/− (129-Smad3tm/Par/J) mice were generated as described14 with genotypes verified by polymerase chain reaction (PCR). Smad3−/− and Smad3+/− mice were used as donors in adoptive transfer studies and for studies of regulatory T cells (Treg). Smad3/Rag2-DKO mice were generated by intercrossing Smad3−/− and Smad3+/− mice with Rag2−/− (129S6/SvEvTac-Rag2tm1Fwa) mice. Smad3+/−Rag2−/− mice were used in two Helicobacter infection studies and four adoptive transfer experiments. Smad3+/+Rag2+/+ or Smad3+/+ were used as wild-type (WT) controls in all other studies. All mice were specific pathogen-free and annually screened for other rodent pathogens.11 Colon samples were screened annually for Citrobacter rodentium, Salmonella spp., and Clostridium spp. (Phoenix Laboratories, Seattle, WA); some fecal samples were culture- and PCR-positive for Klebsiella oxytoca. Mice were determined to be Helicobacter-free until infected with Helicobacter with infection confirmation by a modified fecal PCR procedure.15 Animals were fed irradiated Picolab Rodent Diet 20 no. 5053 (breeders; PMI Nutrition International, Brentwood, MO) or autoclaved Rodent Chow no. 5013 (study animals; Animal Specialties, Portland, OR), provided autoclaved and acidified water, and monitored as previously described.12 To increase survival of Helicobacter-infected Smad3/Rag2-DKO mice and allow sufficient time for tumor development, mice were intermittently fed medicated rodent chow (metronidazole, amoxicillin, bismuth; BioServe, Frenchtown, NJ). Mice given medicated chow were treated the same across groups within experiments. Differences in medicated chow regimens between experiments that were analyzed together are indicated in the figure legends. Mice were euthanized by CO2 in accordance with the American Veterinary Medical Association Panel on Euthanasia, when they developed severe diarrhea, 20% body weight loss, or rectal prolapse, at which point tissue samples were taken. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Bacterial Infection
H. bilis and H. hepaticus were cultured and mice infected by oral gavage.16 Mice were infected with 2 × 107 H. bilis twice followed by H. hepaticus once, or twice with H. bilis only unless otherwise noted. A cohort of Smad3−/− and DKO mice were infected by oral gavage with Enterococcus faecalis strain OG1RFSS,17 maintained on spectinomycin/streptomycin water (500 μg/ml) to favor E. faecalis colonization, and euthanized and necropsied at 4 to 6 months after infection.
Pathology
Necropsy, tissue sampling, processing, and histological examination were performed as described.15 Tumors were classified according to Boivin and colleagues18 and a tumor burden score assigned based on frankly invasive neoplasms (ie, invasion of neoplastic crypts into and beyond the tunica muscularis). By definition, herniation into the submucosa was not included in this score nor was carcinoma in situ. IBD scores were graded on a 0 to 64 scale,11 which included the cecum, proximal, mid and distal colonic tissues. Histological examination of hematoxylin and eosin (H&E)-stained sections was performed by pathologists (H.B.-O. and P.T.) blinded as to genotype and infection status.
Immunohistochemistry
Immunohistochemistry was done on sections from formalin-fixed tissue from the proximal colon of untreated WT mice, broth-treated DKO mice, and H. bilis-infected DKO mice at 1 week after infection. Three to five samples from each group were evaluated. Briefly, 4-μm sections were cut, deparaffinized, and rehydrated in wash buffer (DAKO, Carpinteria, CA). Antigen retrieval was performed in a steamer (Black and Decker, Towson, MD). Cleaved caspase-3 slides were steamed for 15 minutes in preheated Target Retrieval Solution, High pH (DAKO), and then cooled for 15 minutes. Ki-67 slides were steamed for 40 minutes and F4/80 slides steamed for 20 minutes in preheated Target Retrieval solution (pH 6, DAKO) and cooled for 20 minutes. All subsequent staining steps were performed at room temperature using a DAKO Autostainer. Endogenous peroxide activity was blocked using 3% H2O2 for 8 minutes followed by protein blocking. Sections immunolabeled for cleaved caspase-3 and F4/80 were blocked with biotin block (DAKO) for 10 minutes and then using 15% goat serum and 5% mouse serum (Jackson ImmunoResearch, West Grove, PA) in Tris-buffered saline (TBS) containing 1% bovine serum albumin for 10 minutes. Ki-67 slides were blocked in 15% swine serum and 5% mouse serum in TBS containing 1% bovine serum albumin for 10 minutes. Ki-67 labeling was visualized using a rabbit monoclonal antibody (RM-9106; Lab Vision, Fremont, CA) at a concentration of 0.5 μg/ml for 30 minutes followed by Envision Plus (DAKO) for 30 minutes. Cleaved caspase-3 was detected using a rabbit polyclonal antibody (CP229B; Biocare Medical, Walnut Creek, CA) at a concentration of 0.2 μg/ml for 60 minutes followed by biotinylated goat anti-rabbit at 1:400 for 30 minutes (BA-1000; Vector Laboratories, Burlingame, CA) and Vector Elite ABC. F4/80 (MCA497; Serotec, Oxford, UK) was used at 20 μg/ml followed by biotinylated goat anti-rat (112-065-167, Jackson ImmunoResearch) at 1:200 for 30 minutes followed by Vector Elite ABC. The staining for all slides was visualized with 3,3′-diaminobenzidine (DAKO) for 7 minutes with sections counterstained using hematoxylin (DAKO) for 2 minutes. Concentration-matched isotype control slides were run for each tissue sample (Jackson ImmunoResearch Laboratories).
Flow Cytometric Analysis
Cells were isolated from spleen, thymus, and mesenteric lymph nodes (MLNs), stained for surface markers, and analyzed by flow cytometry as previously described.19 Single cell suspensions were generated from spleen, thymus, and MLN of WT, Smad3+/−, and Smad3−/− mice. Cells were stained with antibodies against the cell surface markers CD25, CD4, CD45RB, and TCR (BD Biosciences, San Diego, CA), followed by intracellular staining for Foxp3 (eBioscience, San Diego, CA). FlowJo (TreeStar Inc., Ashland, OR) was used for data analysis.
Adoptive Transfer Studies
Cells for adoptive transfer studies were isolated from the splenocytes of Smad3−/− or WT mice. CD4+ T cells were isolated by negative selection using a CD4+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA) and an autoMACS (Miltenyi Biotech). Purified WT or Smad3−/− CD4+ T cells were adoptively transferred into recipient mice via intraperitoneal injection. For CD4+CD25+ Treg cell isolation, CD4+ T cells were isolated as above or CD25+ cells were isolated via anti-CD25-PE/anti-PE microbeads (Miltenyi Biotec) and stained with anti-CD4, anti-CD25, and anti-TCR antibodies. CD4+CD25+ T cells were sorted using the FACS Aria cell sorter (BD Biosciences) and adoptively transferred intraperitoneally into recipient mice. After adoptive transfer, recipients were infected with H. bilis (2 or 5 weeks) to initiate infection.
In Vitro Treg Assays
Single cell suspensions were prepared from the spleens of WT, Smad3+/−, or Smad3−/− mice. CD4+ T cells were isolated using the CD4+ T-cell isolation kit (Miltenyi Biotec) followed by magnetic cell isolation on an autoMACS (Miltenyi Biotech). Purified T cells were stained with fluorescent antibodies to anti-CD4, CD25, and TCR. TCR+CD4+CD25+ (Treg) and TCR+CD4+CD25− (T effector) cells were sorted on a FACSARIA cell sorter (BD Biosciences). CD25+ and CD25− T cells were found to be 98% and 99% pure, respectively, after sorting. Separate aliquots of splenocytes were prepared from WT or Smad3−/− mice and used to generate antigen-presenting cells (APCs). Splenocytes were depleted of T cells by labeling with anti-TCR-biotin (BD Biosciences) and binding to streptavidin-conjugated magnetic beads and autoMACS (Miltenyi Biotec). APCs were irradiated before assay setup. Cells were cultured in 96-well round-bottomed plates in 200 μl of RPMI supplemented with 10% fetal bovine serum, nonessential amino acids, l-glutamine, penicillin/streptomycin, sodium pyruvate, HEPES, and 2-mercaptoethanol. Wells contained 2 × 104 T effector cells, 8 × 104 APCs, a titrated number of Tregs starting at a 1:1 Treg:T-effector ratio, and 0.5 μg/ml of either purified anti-CD3 antibody (BD Biosciences) or concavalin A (Sigma, St. Louis, MO). Plates were incubated for 90 hours at 37°C and 5% CO2 in a humidified incubator. Proliferation was determined by measuring [3H] thymidine incorporation during the last 18 hours of culture in triplicate or duplicate wells.
Epithelial Cell Preparations
Colonic epithelial cells were isolated using previously reported methods with minor modification.20 Whole colons were isolated at necropsy, split lengthwise, and flushed with saline to remove fecal material. They were minced into small (3 to 5 mm) pieces and incubated at room temperature for 15 minutes in 1 ml of Hanks’ balanced salt solution containing 5 mmol/L dithiothreitol and 5 mmol/L ethylenediaminetetraacetic acid. They were then transferred to Hanks’ balanced salt solution with 5 mmol/L each of dithiothreitol, CaCl2, and MgCl2, and vortexed gently for 45 to 60 seconds. The resulting cell suspension was separated from tissue pieces and centrifuged at 1200 rpm for 5 minutes to pellet cells. Inflammatory and immune cells were removed by subtractive adsorption to antibodies on magnetic beads, using the mouse hematopoietic progenitor cell enrichment set from BD Bioscience. Total RNA was extracted from cell pellets.
Real-Time Reverse Transcriptase (RT)-PCR
For Helicobacter spp. real-time quantitative PCR (RT-PCR), all of the cecum and 5- to 10-mm tissue samples (and contents) were collected from mid-jejunum, proximal colon, and distal colon. DNA was isolated and real-time RT-PCR performed as previously described.11 For confirmation of altered expression of genes from colonic epithelial cells or whole colonic tissue, total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA). Before use in cDNA synthesis for array or PCR analysis, RNA quality and integrity was assessed by agarose gel electrophoresis and spectrophotometry. Preparations having OD ratios less than 1.9 (260/280) and 1.7 (260/230) were repurified and cDNA was synthesized from RNA using the SuperScript SSIII kit (Invitrogen, Carlsbad, CA) with an oligo dT primer. Real-time RT-PCR was performed in the Mx3005P apparatus (Stratagene, La Jolla, CA) with Brilliant SYBR Green QPCR Master Mix (Stratagene), according to the manufacturer’s instructions. Specific oligonucleotide primers were designed with the MacVector DNA analysis program (Accelrys Corp., San Diego, CA) and purchased from IDT Technologies (Coralville, IA). Relative amounts of PCR products were calculated from threshold (CT) values for each sample after correction for variations in GAPDH content, assuming that a one-cycle difference represents a twofold change in target sequence.
Western Blotting
Cell lysates were prepared by addition of cold lysis buffer (30 mmol/L HEPES, 150 mmol/L NaCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 10% Triton X-100) containing protease and phosphatase inhibitors (Sigma) to epithelial cell preparations. Total protein concentration was determined with the BCA protein assay kit (Pierce Biological, Rockford, IL). Fifty μg of each sample was boiled in 100 ml of sodium dodecyl sulfate sample buffer (0.05 mol/L Tris, pH 6.8, 2% sodium dodecyl sulfate, 0.025% bromphenol blue). Samples were then subjected to electrophoresis in 12% sodium dodecyl sulfate-polyacrylamide gels and transferred electrophoretically to nitrocellulose as described by Hossenlopp and colleagues.21 The transfer buffer contained 15 mmol/L Tris base, 120 mmol/L glycine, and 5% methanol. Membranes were washed in Tris-buffered saline (TBS is 20 mmol/L Tris-HCl, pH 7.5, containing 0.15 mol/L NaCl), and incubated overnight at 4°C with appropriate antibodies in TBS/0.1% Tween 20 containing either 5% nonfat milk (Bio-Rad, Hercules, CA) or 5% bovine serum albumin. They were washed with TBS/0.1% Tween 20, and bands were detected using horseradish peroxidase-linked secondary antibody and enhanced chemiluminescence reagents (ECL system; Amersham Corp., Arlington Heights, IL), according to the manufacturer’s protocol. Films were scanned and densitometry analysis was performed using an AlphaImager 3400 (Alpha Innotech Corp., San Leandro, CA).
Statistical Analysis
Survival curves were generated and compared using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA) software. Statistical significance of differences between curves was determined using the log-rank (Mantel-Cox) test and differences in inflammation and tumor burden scores were tested using one-way analysis of variance followed by a multiple comparisons posttest (Bonferroni). Changes in cell numbers and populations between groups were compared using Student’s t-test assuming unequal variances.
Results
Loss of Rag2 Increases Inflammation and Cancer Severity in Smad3−/− Mice
To examine the roles of B and T lymphocytes in Helicobacter-induced colitis and colon cancer in Smad3−/− mice, we infected Smad3−/−, Rag2−/−, and Smad3/Rag2-DKO mice with Helicobacter and measured subsequent survival, inflammation score, and tumor burden. As shown in Figure 1A, Helicobacter-infected DKO mice showed decreased survival compared with Rag2−/− and Smad3−/− mice. Mean survival times were 24 weeks for Smad3−/−, 19 weeks for Rag2−/−, and 12 weeks for DKO mice (Figure 1A). Decreased survival of Helicobacter-infected DKO mice was attributable to more severe inflammation and greater tumor burden. DKO mice had significantly more inflammation (average score = 57.2) than Rag2−/− mice (average score 40, *P < 0.01) and Smad3−/− mice (average score 40, *P < 0.05) (Figure 1B). Tumor burden scores, which represent only invasive tumors, were significantly higher for DKO mice compared with Rag2−/− and Smad3−/− mice (**P < 0.001, Figure 1C). Helicobacter-infected Smad3+/− and WT mice did not develop colonic inflammation or tumors (data not shown).
Figure 1.
Helicobacter-infected DKO mice have decreased survival and increased inflammation and tumor burden compared with Rag2KO and Smad3KO mice. A: The Kaplan-Meier survival curve for Smad3/Rag2-DKO (n = 9) mice is significantly less than Rag2KO (n = 11, P = 0.037) and Smad3KO (n = 6, P = 0.03) mice. Mice were euthanized when they developed 20% body weight loss or clinical signs of significant IBD. B: Decreased survival of DKO mice was attributable to differences in inflammation and tumor burden. DKO mice had significantly more inflammation than Rag2KO mice (*P < 0.01) and Smad3KO mice (*P < 0.05). C: Tumor burden scores were considerably higher in DKO mice compared with Rag2KO mice and Smad3KO mice (**P < 0.001). Tumor burden scores represent numbers of only invasive tumors in cecum and colon. Rag2KO mice were Smad3+/−Rag2−/−. Helicobacter-infected Smad3+/− (n = 5) and WT (n = 10) mice did not develop inflammation or tumors.
Unlike Helicobacter-infected Smad3−/− mice, which develop focal tumors primarily in the cecum and proximal colon (Figure 2A),12 DKO mice developed multiple tumors throughout the large bowel (Figure 2B). In contrast, Helicobacter-free DKO mice did not develop tumors (Figure 2C). Histologically, lesions in DKO mice were more severe and extensive than those described for Smad3−/− mice.12 Notably, although mucosal inflammation occurred throughout the colon in DKO mice, it was primarily evident in the proximal colon in Smad3−/− mice (Figure 3A and data not shown). Helicobacter-associated hyperplastic colitis in DKO mice was characterized by elongated and irregular glands with loss of goblet cells, increased mitotic figures with interstitial lymphohistiocytic inflammation, crypt abscesses, and glandular ectasia and loss (Figure 3, B–F). Tumors, from early invasive crypts to fully developed mucinous adenocarcinomas, were multicentric in DKO mice, whereas in Smad3−/− mice the neoplasms were primarily focal (data not shown).12 Early in the inflammatory process (1 week after H. bilis inoculation), immunohistochemistry of colonic tissue from DKO mice revealed a moderate to marked influx of F4/80-positive macrophages within the mucosa and submucosa of H. bilis-infected DKO mice, increased epithelial cell proliferation (Ki-67+) within the crypts (extending lumenally and within the expanded lamina propria), and increased numbers of apoptotic cells (cleaved caspase-3+ cells), many of which are superficial and luminal (Figure 4).
Figure 2.
Helicobacter-infected Smad3/Rag2-DKO mice develop tumors throughout the large bowel compared with localized tumors in Helicobacter-infected Smad3KO mice. A: Smad3KO mouse infected with Helicobacter. Tumors are anatomically restricted to the cecocolic junction and proximal colon (arrowheads). The ileum (#) was dissected away and * notes cecum. B: Smad3/Rag2-DKO mouse infected with Helicobacter. The entire colon is markedly thickened with multiple tumors (arrowheads) along the large bowel. Also note the absence of formed feces in the distal colon (arrow). C: Smad3/Rag2-DKO breeder mouse of comparable age maintained Helicobacter-free has no gross or histological evidence of tumors.
Figure 3.
H&E-stained sections of colon from Smad3/Rag2-DKO mice. A: Proximal colon from a Helicobacter-free Smad3/Rag2-DKO mouse. Asterisk = lumen. B: Proximal colon from a Helicobacter-infected Smad3/Rag2-DKO mouse. Note the markedly thickened mucosa with loss of normal architecture compared with A. Asterisk = lumen. C: Proximal colon from the same animal as B with a large serosal focus of mucinous adenocarcinoma (T). Asterisk = lumen. D: A serosal lesion contains abundant mucous and rafts of neoplastic epithelial cells (arrow). E: Early invasive lesion (arrow). Mucus-producing neoplastic cells (arrow) present within the tunica muscularis with extension out toward the serosa (S). Note hyperplastic glands in the overlying mucosa (M). F: Helicobacter-associated hyperplastic colitis in Smad3/Rag2-DKO mice is characterized by elongated and irregular glands with loss of goblet cells, increased mitotic figures with interstitial lymphohistiocytic inflammation, crypt abscesses (asterisk), and glandular ectasia (E), and loss. H&E-stained sections. Original magnifications: ×2 (A, B); ×4 (C); ×10 (D); ×20 (E, F).
Figure 4.
Immunohistochemistry of proximal colonic tissue from WT, DKO-broth, and H. bilis-infected DKO mice at 1 week after infection. H&E-stained section demonstrating the thin regular normal mucosa of the WT and DKO-broth in contrast to the H. bilis-infected DKO where the mucosa is thickened by proliferative epithelium and inflammatory cells. F4/80: Immunohistochemical stain for F4/80 antigen, a macrophage marker. Note the relatively few positive (brown-stained) cells in the WT mouse mucosa. There is a mild increase in positive cells in the DKO-broth colon and a moderate to marked increase in positive signal within the mucosa and submucosa of H. bilis-infected DKO mouse. Ki-67: Immunohistochemical stain for Ki-67, a proliferation antigen, is normally restricted to the base of the crypts (brown staining). Note the increased signal in the DKO-H. bilis colon within the crypts, extending lumenally and within the expanded lamina propria. Cleaved caspase-3: Immunohistochemistry for cleaved caspase-3, a marker of apoptosis. In normal colons, apoptotic cells (brown staining) are present at the top of the crypts as a normal progression of cellular turnover. Note increased positive cells within the mucosa of the H. bilis-infected DKO mouse, likely reflective of increased cell division. Positive cells (brown staining) are present in increased numbers at the top of the crypt, throughout the length of the crypt, and within luminal contents.
Fecal Helicobacter Levels Do Not Correlate with Inflammation or Tumor Burden
Because Helicobacter-infected DKO mice exhibited more diffuse inflammation and cancer compared with Helicobacter-infected Smad3−/− and Rag2−/− mice, we next determined whether Helicobacter load was higher in Helicobacter-infected DKO mice. Using quantitative real-time PCR for Helicobacter spp., we found no differences in number of Helicobacter genomes in fecal samples from mice that develop tumors (Smad3−/−, DKO, and Smad3+/−/Rag2−/−) compared with WT mice that do not (data not shown).
Infection with E. faecalis Does Not Induce Colitis or Colon Cancer in Smad3/Rag2-DKO Mice
To determine whether other bacteria might also trigger inflammation and cancer in Smad3−/− and DKO mice, animals were challenged with E. faecalis, a human commensal that can induce colitis and colon adenocarcinomas in IL-10 knockout mice.22,23 Despite colonization of Smad3−/− mice with E. faecalis for 4 months and DKO mice for 6 months, neither IBD nor subsequent colonic tumors developed in any mouse. Some mice had mild to severe megaesophagus (common in the 129 line), and the only neoplasm noted was a transitional cell carcinoma of the bladder in one DKO mouse colonized with E. faecalis; this tumor was also noted on one occasion in a DKO mouse infected with H. bilis (data not shown).
T-Regulatory Cells from Smad3−/− Mice Function Normally in Vitro
Because the severity of inflammation and multiplicity of tumor development was greater in DKO mice than in Smad3−/− mice, we postulated that Treg cells limit inflammation and confine tumors to the cecum and proximal colon in Helicobacter-infected Smad3−/− mice. Hence, we determined the percentage and total numbers of Treg cells in lymphoid tissues from Smad3−/− and WT mice and assessed their function using an in vitro Treg suppression assay. We found that Smad3−/− mice had comparable percentages and absolute numbers of CD4+CD25+FoxP3+ Treg cells in the thymus, spleen, and MLNs compared with WT and Smad3+/− mice (Figure 5, A and B). In addition, purified Smad3−/− Treg cells (TCR+CD4+CD25+) were as effective as WT Treg cells in suppressing proliferation of purified WT T effector cells (TCR+CD4+CD25−) in response to anti-CD3ε (data not shown) or concanavalin A stimulation (Figure 5C). WT and Smad3−/− Treg cells suppressed proliferation independent of the APC genotype, with similar T-cell suppression results using WT and Smad3−/− Treg cells in assays containing either WT or Smad3−/− T-cell-depleted APCs (data not shown).
Figure 5.
T-regulatory cell numbers and in vitro function in Smad3-null, Smad3-heterozygous, and WT mice. A: Representative flow cytometric profiles showing staining for CD25 and FoxP3 on TCR+CD4+-gated lymphocytes. The profiles indicate similar representation of Treg cells in thymus, spleen, and MLN from WT, Smad3+/−, or Smad3−/− animals. B: The average total numbers of TCR+CD4+CD25+FoxP3+ cells in same tissues in A (n = 3 mice of each genotype). C: Smad−/− Treg cells are able to suppress proliferation of WT effector T cells in the presence of WT APCs. Treg cells were purified from WT or Smad3−/− spleens and set up in assays containing WT effector T cells and APCs (see Materials and Methods for details). Shown is a graph representing the mean cpm of triplicate or duplicate wells at indicated Treg:T-effector ratios. Error bars represent 1 SD of triplicate or duplicate wells using a [3H]thymidine incorporation assay. The assay was repeated twice; once with WT T effectors and APCs and once with Smad3+/− T effectors and APCs. Both assays demonstrated similar results. T cells were stimulated with either concavalin A or anti-CD3ε antibody. Although stimulation with anti-CD3 resulted in less robust proliferative responses, results were similar. An assay using concavalin A stimulation is shown.
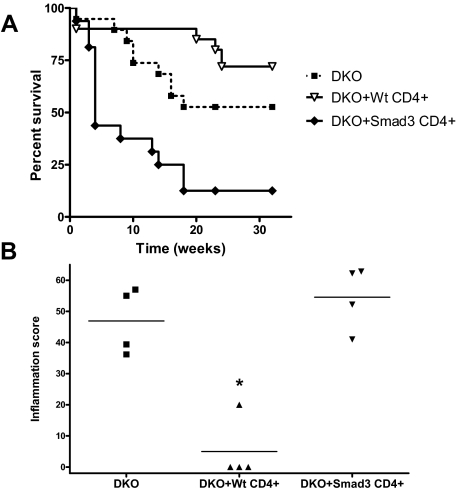
Adoptive Transfer of Total CD4+ or CD4+CD25+ T Cells from WT Mice but Not Smad3−/− Mice Decreased Inflammation and Tumor Burden in Smad3/Rag2-DKO Mice
In vitro assays suggested that T-regulatory cells from Smad3−/− mice function normally. We therefore examined the in vivo ability of total CD4+ cells or CD4+CD25+ Treg cells to protect DKO mice from inflammation and tumors after adoptive transfer. DKO mice were given total CD4+ T cells, or FACS-sorted CD4+CD25+ Treg cells, from WT or Smad3−/− mice, followed by infection with H. bilis 2 to 5 weeks after transplant. Surprisingly, adoptive transfer of total CD4+ T cells from Smad3−/− mice into DKO mice decreased survival compared with DKO mice receiving no cells, whereas adoptive transfer of total WT CD4+ T cells showed a trend toward increased survival (P = 0.089) (Figure 6A). Adoptive transfer of total WT CD4+ T cells also significantly decreased inflammation (P < 0.001), whereas total Smad3−/− T cells had no effect (Figure 6B). Helicobacter-infected DKO mice receiving either WT or Smad3−/− total CD4+ T cells did not develop tumors compared with DKO mice receiving no cells (data not shown); however, DKO mice receiving Smad3−/− cells likely did not survive long enough to develop tumors, because of severe IBD and weight loss.
Figure 6.
Survival and inflammation in Smad3/Rag2-DKO mice receiving adoptively transferred WT or Smad3KO CD4+ cells. A: Kaplan-Meier survival curves for DKO mice adoptively transferred with WT, Smad3KO CD4+ T cells, or no cells (n = 4 per group). DKO mice receiving WT CD4+ cells exhibit a trend of increased survival relative to DKO mice receiving no cells (P = 0.089). Adoptive transfer of Smad3KO CD4+ T cells significantly (P = 0.004) decreased the survival of DKO mice relative to DKO mice receiving no cells. Altered survival curves correlated with differences in inflammation. B: Inflammation was significantly lower (*P < 0.001) in DKO mice receiving WT CD4+ T cells compared with those receiving Smad3KO CD4+ T cells or no cells. Purified WT or Smad3KO CD4+ T cells (4 × 105; 92% pure for both groups) were adoptively transferred into recipient mice by intraperitoneal injection. One month after adoptive transfer, recipients were infected with H. bilis via oral gavage to induce IBD.
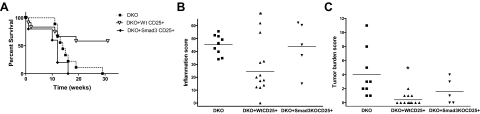
To further evaluate Smad3−/− Treg cell function in vivo, we adoptively transferred sorted CD4+CD25+ cells from WT or Smad3−/− mice into DKO mice and determined the effect of the transferred cells on bowel inflammation and tumor burden. DKO mice receiving WT CD4+CD25+ Treg cells had significantly better survival (P = 0.025) compared with DKO mice receiving Smad3−/− CD4+CD25+ Treg cells or no cells (Figure 7A). Increased survival correlated with significantly lower inflammation scores (Figure 7B) and tumor burden (Figure 7C) in DKO mice receiving WT CD4+CD25+ T cells compared with mice receiving Smad3−/− CD4+CD25+ T cells or no cells (P < 0.01 for both). Variances in survival curves of H. bilis-infected DKO mice across experiments are likely to be related to differences in duration and timing of medicated chow required to maintain animals in studies.
Figure 7.
Survival and inflammation in Smad3/Rag2-DKO mice receiving WT or Smad3KO CD4+CD25+ T cells. A: Kaplan-Meier survival curves for DKO mice adoptively transferred with WT CD4+CD25+ T cells (n = 12), Smad3KO CD4+CD25+ T cells (n = 5), or no cells (n = 9). DKO mice receiving WT CD4+CD25+ T cells had a significantly better survival curve (P = 0.025) than DKO mice receiving Smad3KO CD4+CD25+ T cells, or no cells. Altered survival curves were related to differences in inflammation. Inflammation (B) and tumor burden (C) were significantly lower (*P < 0.01 for both) in DKO mice receiving WT CD4+CD25+ T cells compared with those receiving Smad3KO CD4+CD25+ T cells, or no cells. These data were compiled from two studies. Sorted cells (3.1 × 105, 97.3% CD4+CD25+ for study 1 or 1.3 × 105, > 98% TCR+CD4+CD25+ for study 2) were adoptively transferred into recipient mice by intraperitoneal injection. Recipients were then infected with H. bilis 5 weeks (study 1) or 2 weeks (study 2) after transfer to induce IBD. Medicated chow was given at 7 days after infection for 9 weeks (study 1) and 5.8 weeks (study 2) and was required to maintain animals in studies.
To determine whether the lack of protection by transferred Smad3−/− CD4+CD25+ Treg cells was attributable to insufficient expansion, survival, or trafficking to the gut and associated MLNs, we adoptively transferred sorted Smad3−/− or WT CD4+CD25+ T cells into DKO mice and infected them with H. bilis 2 weeks later. Two and one-half weeks after adoptive transfer, we found comparable numbers of transferred T cells in MLNs (Figure 8, A and B) and spleen (not shown) from DKO mice receiving either Smad3−/− or WT CD4+CD25+ Treg cells. Total splenic cellularity, as well as the percentages and absolute numbers of TCR+CD4+, TCR+CD4+CD25+, and TCR+CD4+CD25− cells, were similar in recipients receiving either Smad3−/− or WT CD4+CD25+ T cells (data not shown). Percentages of these subsets were also similar in the MLN. Interestingly, DKO mice receiving Smad3−/− CD4+CD25+ T cells had increased MLN cellularity and increased absolute numbers of TCR+CD4+ CD25+ T cells (P < 0.05). To determine whether transferred Treg cells trafficked to the colon, we evaluated expression of the Treg-specific gene Foxp3 and the T-cell-specific gene CD3ε using real-time PCR. CD3ε and Foxp3 mRNA expression levels were similar in proximal colons of DKO mice that had received either Smad3−/− or WT CD4+CD25+ T cells, indicating that transferred Tregs of both types trafficked to the colon and survived similarly (Figure 8C). One other consideration is whether loss of Smad3 in DKO recipient mice could result in compensatory increases in TGF-β and/or Smad2, which could affect the function of transferred Treg cells. We thus examined expression of TGFβ and Smad2 in DKO mice and saw no differences in expression of either gene (data not shown).
Figure 8.
Adoptively transferred Smad3KO and WT CD4+CD25+ T cells can be detected at comparable levels in DKO recipients. A: Flow cytometric analysis of MLNs from H. bilis-infected DKO mice that had received 2 × 105 sorted CD4+CD25+TCR+ splenocytes (>98% pure) from Smad3KO or WT mice. Cells were gated on singlets (not shown), live cells (left), CD4+TCR+ (quadrant, middle), and CD25+ or CD25− (right) for analysis. B: Percentage (left) and absolute numbers (right) of indicated cell types. Averages of values from DKO mice receiving sorted Smad3KO cells (n = 4) and DKO mice receiving sorted WT cells (n = 5) are shown (error bars are 1 SD). C: RNA was isolated from proximal colons of DKO adoptive transfer recipients (n = 5 for both groups), converted to cDNA, and analyzed for expression of CD3ε and Foxp3 by real-time RT-PCR. Mean relative expression (expression relative to DKO animal without T-cell transfer) is shown (error bars are 1 SEM). For comparison, values from one unmanipulated WT animal are also shown.
Changes in Cytokine mRNA and Anti-Apoptotic Protein Expression in Colons from Smad3/Rag2-DKO Mice after Helicobacter Infection
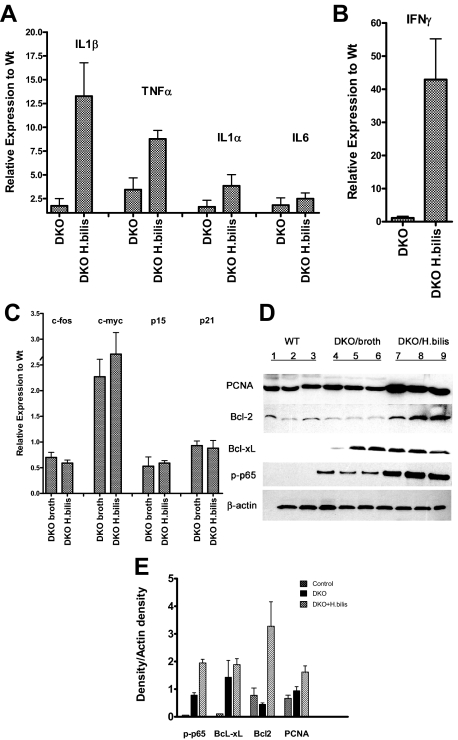
To further evaluate changes in colonic tissue and epithelial cells during the inflammatory phase and before tumor development, colonic tissue at 1 week after infection was analyzed for expression of pro-inflammatory cytokines, cell cycle regulators, and oncogenes by real-time RT-PCR and Western blot analysis. As shown in Figure 9, expression of interleukin (IL)-1β, ΤNF-α (Figure 9A), and interferon (IFN)-γ (Figure 9B) mRNAs was markedly elevated in whole colonic tissue from infected DKO mice relative to WT mice (13-, 9-, and 42-fold, respectively) and uninfected DKO mice. Expression of ΤNF-α was also moderately elevated in uninfected DKO mice relative to uninfected WT mice, suggesting that DKO mice have an intrinsic propensity for an increased inflammatory response, which is greatly exacerbated by the presence of H. bilis. We have previously found similar increases in IL-1β, ΤNF-α, and IFN-γ mRNAs in Helicobacter-infected Smad3−/− colon tissue,12 suggesting that defective TGF-β signaling, rather than loss of Rag2, contributes to enhanced cytokine production in DKO mice. We also evaluated the expression of mRNA for several oncogenes and tumor suppressor genes in purified intestinal epithelial cells. We found c-Myc mRNA levels were increased two to threefold in uninfected and infected DKO mice relative to uninfected WT mice (Figure 9C). In contrast, expression of another oncogene, c-Fos, as well as CDK inhibitors p21 and p15, remained unchanged (Figure 9C). These results are consistent with our previous report for Helicobacter-infected Smad3−/− mice, suggesting that loss of the Smad3 protein increases c-Myc expression in DKO epithelial cells. Further analysis by immunoblot demonstrated increased proliferating cell nuclear antigen (PCNA) in epithelial cells from Helicobacter-infected DKO colons, relative to uninfected DKO and WT colons (Figure 9D). This finding is consistent with increased proliferation of intestinal epithelial cells for Helicobacter-infected DKO mice, as was suggested by immunohistochemical analysis of Ki-67 expression (Figure 4). Increased expression of the anti-apoptotic proteins Bcl-xL and Bcl-2 was observed in Helicobacter-infected DKO mice; Bcl-xL was also increased in uninfected DKO mice, relative to uninfected WT mice (Figure 9E). These data are further evidence for increased survival potential of colonic epithelial cells in DKO mice. Many of the luminal and superficial mucosal apoptotic cells are lost during preparation and cleaning of colons, leaving cells affected by anti-apoptotic survival signals; this may explain apparently conflicting data from immunohistochemical staining and Western blot analysis.
Figure 9.
Relative mRNA and protein expression in colonic tissue from Helicobacter-infected Smad3/Rag2 DKO mice 1 week after infection. RNA was isolated from colonic epithelial cell preparations or whole colonic tissue, and 2 μg were used to generate cDNA for real-time PCR. Data were normalized to GAPDH expression in each sample. mRNA levels in WT, DKO-broth, and H. bilis-infected DKO mice at 1 week after infection are expressed relative to levels in uninfected WT mice. Numbers represent the mean ± SEM, n = 3. There was markedly increased expression of IL-1β and ΤNF-α (13- and 9-fold, respectively) (A), and IFN-γ (42-fold) (B) in whole colonic tissue of infected DKO mice relative to WT mice. ΤNF-α was more moderately increased in uninfected DKO animals relative to uninfected WT-broth mice. C: Levels of c-Myc were constitutively increased two- to threefold in DKO mice and primarily independent of bacterial infection, whereas c-fos, p21, and p15 were not appreciably affected in both uninfected and infected DKO mice. D: For Western analysis, total protein was isolated from epithelial cell preparations or whole colonic tissue, and 30 μg of each sample were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and incubated with appropriate antibodies as described in detail in the text. β-Actin was used as a loading control. For each gel, three samples were included from each group (uninfected WT, DKO-broth, and H. bilis-infected DKO). Analysis blotting of proteins from colonic epithelial cells demonstrated increased levels of PCNA and Bcl-2 in Helicobacter-infected DKO cells relative to WT and uninfected DKO mice. Bcl-xL was also increased in both uninfected and infected DKO mice. Activation of NF-κB, as indicated by p65 phosphorylation, was noted in uninfected DKO mice, which increased further after Helicobacter infection. E: Plot of densitometry scans of data in D. Densities for each sample were divided by corresponding values for β-actin and average values computed for each group of three. Mean ± SEM is shown. There were significant differences noted between WT and H. bilis-infected DKO mice for p65 (P < 0.01), BcL-xL (P < 0.01), BclII (P < 0.05), and PCNA (P < 0.025). When comparing uninfected DKO to H. bilis-infected DKO mice, we noted similar significant differences for all proteins except BcL-xL (ns, P = 0.283).
A key signal transduction pathway that links inflammation with cancer is the canonical nuclear factor (NF)-κB nuclear transcription factor pathway,24 which is triggered by proinflammatory cytokines and several infectious agents.25 The most abundant form of the NF-κB family of transcription factors in nonactivated cells is a complex composed of dimers of p65 (RelA) and p50 or p52, which are retained in the cytoplasm by IκB inhibitor proteins. Phosphorylation of IκB proteins enables release of p65 for translocation to the nucleus, and p65 activity is commonly enabled or modified by phosphorylation as well. Analysis of the NF-κB pathway in colonic tissue by immunoblot revealed that activation of NF-κB (as indicated by p65 phosphorylation) was increased in uninfected DKO colonic tissue relative to WT colonic tissue, and was further elevated after Helicobacter infection (Figure 9D). Collectively, these results suggest that loss of Smad3 increases NF-κB activation in intestinal epithelial cells from DKO mice, which may lead to increased cell survival, proliferation, and enhanced susceptibility to tumorigenesis.
Discussion
We have previously shown that the loss of Smad3 in mice is not sufficient for colon cancer development, but requires a pathogenic bacterial trigger to induce chronic inflammation12 followed by carcinogenesis. In the studies outlined here using the combined null alleles of Smad3 and Rag2, we expand on these findings by showing that the loss of Smad3 predisposes mice to colon cancer by at least two different mechanisms: first, we show that removal of T and B lymphocytes (in Smad3/Rag2-DKO mice) results in more severe inflammation and colon cancer than is observed in Smad3−/− mice alone, and that inflammation and cancer are prevented by the adoptive transfer of WT Treg cells, but not Smad3−/− Treg cells. Second, we show that the combined loss of Smad3 and Rag2 (in Smad3/Rag2-DKO mice) results in more severe inflammation and colon cancer than is observed in Rag2−/− mice alone. Because both in Smad3/Rag2-DKO mice and Rag2−/− mice lack T and B cells, these results suggest that Smad3 also helps limit the development of colon cancer through its actions in other nonlymphoid cell types. When combined with our previous studies, as well as recent studies by other groups, these results reveal important tumor-suppressive functions for Smad3 in T-effector cells,12,26 T-regulatory cells,27 and intestinal epithelial cells, all of which may normally limit the development of colon cancer in response to bacterial inflammation.
Regulatory T cells were originally identified by their ability to suppress autoimmune disease28 and are believed to control inflammation in some models of IBD29 and colon cancer.29,30,31,32 In addition, TGF-β is required for the expression of the forkhead winged-helix transcription factor Foxp3 within Treg cells, and Foxp3 expression is essential for Treg development and function.33,34,35 Because the absence of all lymphocyte populations in Smad3/Rag2-DKO mice resulted in more severe inflammation and colon cancer than in Smad3−/− mice, we hypothesized that Treg cells limit colon cancer in this model, and that Smad3 is required for normal Treg cell development and/or function. Analysis of lymphoid tissues revealed normal numbers of Treg cells in Smad3−/− mice relative to WT controls, which is in contrast to the data of Wang and colleagues27 who found that CD4+CD25+ T cells are increased in Smad3−/− mice. Differences between the latter study and ours may be attributable to differences in genetic background (C57BL/6 versus 129), bacterial colonization of the gut, or methods of data analysis.
With regard to Treg function, Treg cells isolated from Smad3−/− mice were able to suppress the proliferation of WT T-effector cells equally as well as WT Treg cells in vitro. However, although adoptive-transfer of WT Treg cells into DKO mice reduced bacterial-induced inflammation and colon cancer, adoptive-transfer of Smad3−/− Treg cells had no significant effect, despite normal numbers and trafficking to MLNs and colon after transfer. In fact, transfer of total CD4+ T cells (containing both CD4+ T effector and CD4+ Treg cells) from Smad3−/− mice into Smad3/Rag2-DKO mice resulted in increased colonic inflammation and decreased survival, probably because T-effector cells from Smad3−/− mice do not respond normally to TGF-β suppression, as we and others have demonstrated in vitro.12,26 The latter results are consistent with previous studies by Fahlen and colleagues33 in which transgenic mice expressing a dominant-negative TGF-β receptor II in pathogenic CD4+ T cells were refractory to control by Treg cells in a colitis model. However, in contrast to their findings that CD4+CD25+ Treg cells containing dominant-negative TGF-β receptor II retain an ability to suppress colitis in vivo, we find that CD4+CD25+ Treg cells from Smad3−/− mice are not capable of decreasing inflammation and tumor burden or increasing survival in Helicobacter-infected DKO mice, whereas the WT CD4+CD25+ Treg cells are protective. Our results are consistent with the demonstration by Marie and colleagues34 that TGF-β1 was required to maintain normal T-regulatory cell function, and also with an elegant study by Kim and colleagues36 in which T-cell-specific deletion of Smad4 was sufficient for tumorigenesis in multiple tissues. The discrepancy between our in vitro results (suggesting normal Treg function), and our in vivo results (suggesting defective Treg function), are likely attributable to the relatively high concentration of Treg and T-effector cells in microwell plates in vitro, which artificially increase cell contact and local cytokine concentrations, thus potentially skewing the results. Overall, these studies collectively reveal the importance of normal Smad3 signaling in maintaining both T-regulatory and T-effector cell functions and homeostasis, and emphasize the role of inflammation in predisposition to cancer. One unresolved issue, however, is why tumors localize to the cecum and proximal colon in Helicobacter-infected Smad3−/− mice whereas they occur throughout the large colon in Helicobacter-infected DKO mice. In DKO mice, the complete removal of Tregs, and other lymphocyte populations, results in diffuse inflammation and tumor formation throughout the colon and cecum. This could be because total T cells (Tregs and/or T effectors) help limit the amount and extent of inflammation, and/or that Helicobacter organisms are more diffusely spread throughout the colon in DKO mice.
Inflammation within precancerous colonic tissue and colon carcinoma can involve a mix of inflammatory cells, including resident and recruited macrophages, dendritic cells, T and B lymphocytes, and NK cells, all of which can contribute to the cytokine milieu.37 We had previously shown that Helicobacter infection led to increased production of IL-1β, tumor necrosis factor (TNF)-α, IL-6, and IFN-γ in colonic tissue from Smad3−/− mice.12 In this study, we found similar increases in these cytokines in precancerous colonic tissue from Smad3/Rag2-DKO mice after Helicobacter infection, suggesting that these pro-inflammatory cytokines are primarily produced by nonlymphoid cells, such as macrophages, dendritic cells, and potentially colonic epithelial cells. Other studies have shown that pro-inflammatory cytokine production in the pretumor and tumor microenvironment contributes to transformation, tumor growth, progression, and metastasis.37,38 For example, TNF-α may stimulate the production of genotoxic molecules such as reactive oxygen species, leading to DNA damage and tumor initiation.39 In humans, genetic polymorphisms in the TNF-α locus are associated with increased incidence of many cancers including gastric, breast, prostate, colon, and some hematological malignancies.40,41,42,43,44 Mice deficient in the TNF-α receptors TNFR1 and TNFR2 have reduced tumorigenesis,45 and anti-TNF-α neutralizing antibody protects against inflammation-induced liver cancer in MDR2-null mice.46 IL-1β has also been linked to cancer initiation and progression. Polymorphisms in the promoter regions of the gene encoding IL-1β, as well as the gene encoding the IL-1 receptor antagonist IL-1RA, are associated with an increased risk of developing Helicobacter-induced gastric cancer.47 IL-1 increases tumor invasiveness and metastasis by increasing expression of adhesion molecules, cytokines, and pro-angiogenic molecules. Serum levels of IL-6 are increased in patients with colon cancer and correlate with tumor size.48,49 In vitro studies indicate that IL-6 stimulates the growth of colon cancer cells,50 whereas anti-IL-6 antibody inhibits colon cancer in mice.51 Our results collectively suggest that loss of Smad3 alone is sufficient to increase production of proinflammatory cytokines that can contribute to transformation and tumor progression. Helicobacter infection further increases proinflammatory cytokine production in a lymphocyte-independent manner. These results further suggest that Smad3 deficiency may result in hyperactivation of innate immune cells, perhaps in response to TLR signaling.
Activation of toll-like receptors by bacteria or bacterial components such as lipopolysaccharide, as well as the interactions of proinflammatory cytokines such as TNF-α and IL-1 with their cognate receptors, can activate IκB kinase and NF-κB.24,52 Because we found elevated levels of proinflammatory cytokines in DKO mice, we examined NF-κB activation in colonic tissue, both before and after bacterial infection. Notably, elevated levels of phosphorylated p65 (indicative of active NF-κB) were found in colonic tissue from uninfected Smad/Rag2-DKO mice, with further increases in phospho-p65 after Helicobacter infection. Activation of NF-κB paralleled the increases in proinflammatory cytokines we observed in DKO mice. These results suggest that loss of Smad3 is sufficient to result in increased proinflammatory cytokines, which may activate the NF-κB pathway in colonic epithelial cells. Helicobacter infection may drive further increases in proinflammatory cytokine production and NF-κB activation, resulting in recruitment of additional inflammatory cells, increased tissue destruction, and potentially genotoxic damage.
Others have shown that NF-κB may also have distinct roles in intestinal tumorigenesis, independent of its role in inflammatory cells. For example, Greten and colleagues53 previously found that the specific deletion of Ikkβ in intestinal epithelial cells significantly decreased tumor incidence in a mouse colon cancer model, without affecting inflammation. Furthermore, the absence of Ikkβ in enterocytes prevented the induction of the NF-κB target gene and prosurvival protein Bcl-xL, resulting in increased apoptosis of carcinogen-exposed cells. In this study, we found that Bcl-xL was increased in intestinal epithelial cells from both uninfected and Helicobacter-infected DKO mice relative to WT mice, concomitant with increased NF-κB activation, particularly after Helicobacter infection (Figure 9D). In addition, we found that the anti-apoptotic protein Bcl-2 was increased in epithelial cells from Helicobacter-infected DKO mice relative to uninfected DKO and WT mice. These results suggest a model whereby loss of Smad3 is sufficient to induce the production of proinflammatory cytokines from innate immune cells in the intestinal microenvironment, which then activate NF-κB in intestinal cells. Activated NF-κB then stimulates expression of specific target genes including Bcl-xL and c-Myc in intestinal epithelial cells, thereby enhancing their survival and stimulating cell division. Infection with Helicobacter leads to increased expression of the anti-apoptotic protein Bcl-2 and further increases in expression of proinflammatory cytokines. The ability of Helicobacter infection to stimulate increased expression of Bcl-2 protein in intestinal tissue may explain in part why Smad3−/− mice require Helicobacter infection to develop colon cancer. Although loss of Smad3 is sufficient to result in increased expression of the oncogene c-Myc in colonic epithelial cells, c-Myc-induced proliferation is counterbalanced by Myc’s known ability to induce apoptosis.54 The induction of Bcl-2 by Helicobacter may block Myc-induced apoptosis, thus allowing the epithelial cells to proceed toward transformation.
One of the remaining issues to be resolved in this model is the ability of Helicobacter products to directly contribute to the development of colon cancer. Indeed, there is increasing evidence linking certain bacterial products with cancer.6 For example, the Gram-negative facultative intracellular pathogen Bartonella is associated with vasoproliferative tumors in humans55 and utilizes a type IV secretion system to deliver exotoxins that inhibit apoptosis of endothelial cells.56 Similarly, H. pylori utilizes a type IV secretion system to inject the CagA cytotoxin, which has a number of effects including promotion of cell proliferation and invasion and activation of signaling pathways including NF-κB.25 H. hepaticus has also been found to contain components of a type IV secretion system,57 suggesting that H. bilis (predominantly used in these studies) may also mediate its effects via a type IV system. H. bilis could also induce cancer via elaboration of cytolethal distending toxins as described for Escherichia coli and other Gram-negative bacteria.58,59,60 Chronic antigenic stimulation, inflammation, and bacterial cyclomodulins that alter cell cycle and apoptosis have all been proposed as potential mechanisms in bacteria-associated cancers.5 However, not all human cancer-associated bacteria are likely to induce cancer in all animal models. For example, E. faecalis, a Gram-positive human commensal organism that induces colitis and colon cancer in IL-10 knockout mice,22,23 failed to induce inflammation or tumors in Smad3−/− or Smad3/Rag2-DKO mice. However, in the IL-10−/− model, mice were monoassociated with E. faecalis. It is also possible that this organism may have triggered inflammation in the IL-10−/− mice through activation of dysregulated tissue macrophages.61
The Helicobacter-infected Smad3/Rag2-DKO mouse model firmly establishes the importance of bacteria and TGF-β signaling in inflammation and cancer progression. Smad3/Rag2-DKO mice do not develop the colon cancer phenotype when maintained Helicobacter-free; when infected, they develop multiple tumors throughout the colon at sites of inflammation. Our results are consistent with studies by Engle and colleagues62 showing TGFβ−/−Rag2−/− mice only develop colon cancer after infection with H. hepaticus. Similarly, mice expressing dominant-negative TGF-β type I/II receptors and infected with H. pylori develop gastric adenocarcinomas.63 These models, as well as the Smad3/Rag2-DKO mouse model, suggest mechanisms that are relevant to inflammation-associated colon cancer in humans. Mutations in genes involved in TGF-β signaling are found in human colorectal cancer, and TGF-β dysregulation is associated with IBD, which increases risk for colon cancer.64 The fact that inflammation and colon cancer in Smad3/Rag2-DKO mice only occurs in the presence of certain bacteria, notably Helicobacter spp., suggests that the inflammatory response to microorganisms, as controlled by Treg cells, is an early and critical event in the pathogenesis of colon cancer. This model offers an opportunity to examine initial events in carcinogenesis, particularly those associated with inflammation.
Acknowledgments
We thank Andy Chang, Aimee McMillan, and Leno Torres for care and monitoring of mice; and Karen Chase for necropsies of E. faecalis-infected mice.
Footnotes
Address reprint requests to Lillian Maggio-Price, V.M.D., Ph.D., University of Washington, School of Medicine, Dept. of Comparative Medicine, Box 357190, Seattle, WA 98195. E-mail: lmprice@u.washington.edu.
Supported by the Broad Medical Research Program of The Eli and Edythe L. Broad Foundation (to L.M.P.), the Crohn’s and Colitis Foundation of America (grant 1579 to LMP), the National Institutes of Heath (grants 1RO1AI053568 and 2RO1CA68328 to B.M.I.), the College of Veterinary Medicine and Biomedical Sciences (to H.B.-O.), the Department of Veterans Affairs Merit Review Program (to M.M.H.), and the Francis Duffy Endowment (to M.M.H.).
References
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Hahm KB, Im YH, Parks TW, Park SH, Markowitz S, Jung HY, Green J, Kim SJ. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49:190–198. doi: 10.1136/gut.49.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Lax AJ, Thomas W. How bacteria could cause cancer: one step at a time. Trends Microbiol. 2002;10:293–299. doi: 10.1016/s0966-842x(02)02360-0. [DOI] [PubMed] [Google Scholar]
- Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, Tsang M, Shows D, Morrissey P, Viney JL. Dual infection with Helicobacter bilis and Helicobacter hepaticus in P-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793–1806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in smad3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ. TGF-beta signaling in T cells: roles in lymphoid and epithelial neoplasia. Oncogene. 2005;24:5701–5712. doi: 10.1038/sj.onc.1208922. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- Burich A, Hershberg R, Waggie K, Zeng W, Brabb T, Westrich G, Viney JL, Maggio-Price L. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol. 2001;281:G764–G778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- Maggio-Price L, Shows D, Waggie K, Burich A, Zeng W, Escobar S, Morrissey P, Viney JL. Helicobacter bilis infection accelerates and H. hepaticus infection delays the development of colitis in multiple drug resistance-deficient (mdr1a−/−) mice. Am J Pathol. 2002;160:739–751. doi: 10.1016/S0002-9440(10)64894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002;23:529–536. doi: 10.1093/carcin/23.3.529. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci USA. 1999;96:13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Fukushima K, Sasaki I, Matsuno S. Identification of genes involved in mucosal defense and inflammation associated with normal enteric bacteria. Am J Physiol. 2000;279:G492–G499. doi: 10.1152/ajpgi.2000.279.3.G492. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P, Seurin D, Segovia-Quinson B, Hardouin S, Binoux M. Analysis of serum insulin-like growth factor binding proteins using Western blotting: use of the method for titration of the binding proteins and competitive binding studies. Anal Biochem. 1986;154:138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Vogelmann R, Amieva MR. The role of bacterial pathogens in cancer. Curr Opin Microbiol. 2007;10:76–81. doi: 10.1016/j.mib.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Cui YF, Liu YQ, Jin W, Xu H, Jiang ZJ, Lu YX, Zhang Y, Liu XL, Dong B. Increase of CD4(+)CD25(+) T cells in Smad3(−/−) mice. World J Gastroenterol. 2006;12:2455–2458. doi: 10.3748/wjg.v12.i15.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, McHugh RS, Thornton AM, Piccirillo C, Natarajan K, Margulies DH. Control of autoimmunity by regulatory T cells. Adv Exp Med Biol. 2001;490:21–32. doi: 10.1007/978-1-4615-1243-1_3. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim SJ, Fu XY, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- Yan B, Wang H, Rabbani ZN, Zhao Y, Li W, Yuan Y, Li F, Dewhirst MW, Li C-Y. Tumor necrosis factor-α is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- Danforth KN, Rodriguez C, Hayes RB, Sakoda LC, Huang WY, Yu K, Calle EE, Jacobs EJ, Chen BE, Andriole GL, Figueroa JD, Yeager M, Platz EA, Michaud DS, Chanock SJ, Thun MJ, Hsing AW. TNF polymorphisms and prostate cancer risk. Prostate. 2008;68:400–407. doi: 10.1002/pros.20694. [DOI] [PubMed] [Google Scholar]
- Garrity-Park MM, Loftus EV, Jr, Bryant SC, Sandborn WJ, Smyrk TC. Tumor necrosis factor-alpha polymorphisms in ulcerative colitis-associated colorectal cancer. Am J Gastroenterol. 2008;103:407–415. doi: 10.1111/j.1572-0241.2007.01572.x. [DOI] [PubMed] [Google Scholar]
- Hohaus S, Giachelia M, Di Febo A, Martini M, Massini G, Vannata B, D'Alo F, Guidi F, Greco M, Pierconti F, Larocca LM, Voso MT, Leone G. Polymorphism in cytokine genes as prognostic markers in Hodgkin’s lymphoma. Ann Oncol. 2007;18:1376–1381. doi: 10.1093/annonc/mdm132. [DOI] [PubMed] [Google Scholar]
- Malivanova TF, Ostashkin AS, Iurchenko VA, Mazurenko NN. Polymorphisms of tumor necrosis factor genes in sporadic and hereditary breast cancer. Vopr Onkol. 2007;53:664–667. [PubMed] [Google Scholar]
- Sugimoto M, Furuta T, Shirai N, Nakamura A, Xiao F, Kajimura M, Sugimura H, Hishida A. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J Gastroenterol Hepatol. 2007;22:51–59. doi: 10.1111/j.1440-1746.2006.04442.x. [DOI] [PubMed] [Google Scholar]
- Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23:1902–1910. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169–178. doi: 10.1006/clim.2001.5163. [DOI] [PubMed] [Google Scholar]
- Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–226. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett. 2000;151:31–38. doi: 10.1016/s0304-3835(99)00401-2. [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–220. [PubMed] [Google Scholar]
- Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Evan GI. Traps to catch unwary oncogenes. Trends Genet. 1998;14:364–367. doi: 10.1016/s0168-9525(98)01520-0. [DOI] [PubMed] [Google Scholar]
- Dehio C. Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol. 2005;3:621–631. doi: 10.1038/nrmicro1209. [DOI] [PubMed] [Google Scholar]
- Schmid MC, Scheidegger F, Dehio M, Balmelle-Devaux N, Schulein R, Guye P, Chennakesava CS, Biedermann B, Dehio C. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog. 2006;2:e115. doi: 10.1371/journal.ppat.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerbaum S, Josenhans C, Sterzenbach T, Drescher B, Brandt P, Bell M, Droge M, Fartmann B, Fischer HP, Ge Z, Horster A, Holland R, Klein K, Konig J, Macko L, Mendz GL, Nyakatura G, Schauer DB, Shen Z, Weber J, Frosch M, Fox JG. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc Natl Acad Sci USA. 2003;100:7901–7906. doi: 10.1073/pnas.1332093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax AJ. Opinion: bacterial toxins and cancer—a case to answer? Nat Rev Microbiol. 2005;3:343–349. doi: 10.1038/nrmicro1130. [DOI] [PubMed] [Google Scholar]
- Oswald E, Nougayrede JP, Taieb F, Sugai M. Bacterial toxins that modulate host cell-cycle progression. Curr Opin Microbiol. 2005;8:83–91. doi: 10.1016/j.mib.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Chien CC, Taylor NS, Ge Z, Schauer DB, Young VB, Fox JG. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 2000;49:525–534. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ikuta K, Okazaki K, Nakase H, Tabata Y, Matsuura M, Tamaki H, Kawanami C, Honjo T, Chiba T. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig Dis Sci. 2003;48:408–414. doi: 10.1023/a:1021960401290. [DOI] [PubMed] [Google Scholar]
- Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6366. [PubMed] [Google Scholar]
- Hahm KB, Lee KM, Kim YB, Hong WS, Lee WH, Han SU, Kim MW, Ahn BO, Oh TY, Lee MH, Green J, Kim SJ. Conditional loss of TGF-beta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharmacol Ther. 2002;16(Suppl 2):115–127. doi: 10.1046/j.1365-2036.16.s2.3.x. [DOI] [PubMed] [Google Scholar]
- García-González MA, Crusius JB, Strunk MH, Bouma G, Perez-Centeno CM, Pals G, Meuwissen SG, Pena AS. TGFB1 gene polymorphisms and inflammatory bowel disease. Immunogenetics. 2000;51:869–872. doi: 10.1007/s002510000211. [DOI] [PubMed] [Google Scholar]