Abstract
A hallmark feature of atherosclerosis is that circulating mononuclear cells adhere to the endothelium and migrate into the subendothelial space. This adhesion is mediated by molecules such as selectins that are expressed on the surfaces of both leukocytes and endothelial cells. In this study, we have determined the role of tissue-specific fucosyltransferase VII (FucT-VII), an enzyme necessary for selectin ligand synthesis, in the development of atherosclerosis. We adopted a scheme of transplanting either FucT-VII−/−GFP+ bone marrow into lethally irradiated low-density lipoprotein receptor low density lipoprotein receptor mice or FucT-VII+/+ GFP+ bone marrow into FucT-VII−/−, low density lipoprotein receptor double-mutant mice to evaluate the roles of E- and P-selectin ligands versus L-selectin ligands, respectively, in diet-induced atherosclerosis. GFP was used to track the transplanted cells. Our results indicate that, compared with controls, selective disruption of E- and P-selectin ligand synthesis resulted in a significant reduction in atherosclerosis. Selective disruption of L-selectin ligand production did not reduce atherosclerosis as robustly as disruption of E- and P-selectin ligands. In both groups, however, there was a significant reduction in the accumulation of macrophages in the lesion. These studies indicate that selectin ligands, particularly those for E- and P-selectins, play an important role in the pathogenesis of atherosclerosis by regulating macrophage accumulation in atherosclerotic lesions.
Macrophages play a key role in the development of atherosclerosis. The presence of macrophage foam cells is characteristic in all stages of lesions from early fatty streak to advanced fibrofatty type.1 The importance of the macrophage in atheroma formation has been underlined in studies using osteopetrotic mice. These mice lack the ability to generate macrophage-colony stimulating factor (M-CSF); thus they are deficient in circulating monocytes and tissue macrophages. Consequently, atherosclerotic lesion development is inhibited in these mice when crossed onto the atherosclerosis-prone apolipoprotein E-deficient (ApoE−/−) or low-density lipoprotein receptor deficient (LDLR−/−) background.2,3
Early in the development of atherosclerosis, circulating mononuclear cells adhere to the endothelium and migrate into the intima. Leukocyte adhesion and extravasation is mediated by molecules expressed on the surface membranes of both leukocytes and endothelial cells. These include the selectins and other adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1).4,5,6,7,8
Three selectin adhesion molecules have been identified; E-, P-, and L-selectin. These adhesion molecules are glycoproteins, characterized by an extracellular Ca2+-dependent lectin that binds to its respective ligands.9 E-selectin is primarily expressed on the surface of activated endothelial cells, where it mediates the binding of circulating leukocytes. P-selectin is stored intracellularly in α-granules in platelets and Weibel-Palade bodies in endothelial cells.10,11 After activation, it rapidly translocates to the cell surface membrane. L-selectin is constitutively expressed on many leukocyte types, where it mediates neutrophil, monocyte, and lymphocyte tethering to and rolling on the endothelium.12,13
Selectin ligands that have been characterized include P-selectin glycoprotein ligand-1 (PSGL-1), E-selectin ligand-1 (ESL-1), CD24, CD34, and others.14,15,16 All of these ligands express glycans tipped with a fucosylated and sialylated tetrasaccharide sialyl Lewis x (sLex), which is critical for binding to the lectin domain of the selectins. Addition of fucose to the appropriate underlying glycan to generate the sLex tetrasaccharide in leukocytes and endothelium is mediated by α(1,3)fucosyltransferases (FucT)-IV and -VII.17,18 A recent investigation determined the role of FucT deficiency in atherogenesis using the ApoE−/− mice.19 In this study, FucT-VII−/−/ApoE−/− mice developed less atherosclerosis than ApoE−/− controls, suggesting a role for systemic FucT-VII expression in mediating the progression of this disease. In the current study, we examined the importance of FucT-VII in the development of atherosclerosis using the LDLR−/− mouse model. Using bone marrow transplantation, we generated chimeric mice with FucT-VII deficiency either in the leukocytes or in all cells except the leukocytes to determine the respective contributions of L-selectin or combined E- and P-selectin ligands in atherogenesis.
Materials and Methods
Animals
LDL receptor-deficient mice backcrossed onto the C57BL/6 background (LDLR−/− mice) were purchased from The Jackson Laboratories (Bar Harbor, ME) and bred in-house. The generation and characterization of the FucT-VII−/− mice on the C57BL/6 background is detailed elsewhere.17,18 Double-mutant mice were generated by crossing LDLR−/− mice with FucT-VII−/− mice. In addition, FucT-VII−/− mice were crossed with mice expressing enhanced green fluorescent protein (eGFP) on the C57BL/6 background (The Jackson Laboratories). FucT-VII−/− mice were genotyped by polymerase chain reaction as described elsewhere.17 The animal care committee at the Scripps Research Institute approved all protocols pertaining to experimentation with animals. All procedures used in this study conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Generation of Chimeric Mice and General Study Protocol
To determine the contribution of E- and P-selectin ligand synthesis on atherogenesis, bone marrow transplantation was used to disrupt either E- and P-selectin ligand synthesis (by repopulating LDLR−/− mice with marrow from FucT-VII−/−eGFP+ mice, thereby retaining endothelial L-selectin expression) or L-selectin ligand synthesis (by repopulating FucT-VII−/−LDLR−/− mice with FucT-VII+/+eGFP+ marrow). LDLR−/− mice reconstituted with FucT-VII+/+eGFP+ marrow served as the control group.
Male LDLR−/− mice (8 to 10 weeks old) were lethally irradiated and reconstituted with either FucT-VII−/−eGFP+ (14 mice) or FucT-VII+/+eGFP+ (14 mice) marrow. Twelve FucT-VII−/−LDLR−/− mice were also lethally irradiated and reconstituted with marrow from FucT-VII+/+eGFP+ mice. All mice were allowed 4 weeks to recover, at which time they were fed an atherogenic diet containing 15.8% fat, 1.25% cholesterol, and no cholic acid (no. 94059; Harlan Teklad, Madison, WI).20 Plasma samples were obtained by retro-orbital sinus bleed at 4-week intervals, and plasma cholesterol and triglyceride levels were measured. Cholesterol content of each lipoprotein fraction was analyzed from plasma pooled from all of the mice at each time point using a fast protein liquid chromatography method as previously described.21,22 After 12 weeks of high-fat diet, the mice were sacrificed and perfused with phosphate-buffered saline (PBS) followed by formal-sucrose (4% paraformaldehyde/5% sucrose in PBS, pH 7.4). The hearts were removed and the organ was immersed in formal-sucrose overnight before being embedded in OCT (Sakura Finetek, Torrance, CA) and stored at −80°C until sectioning. The aortas were cleaned of connective tissue and adventitial fat was removed. The isolated aortas were then pinned open en face to allow quantitation of atherosclerotic lesions on the luminal surface.
Analysis of Atherosclerosis and Immunohistochemistry
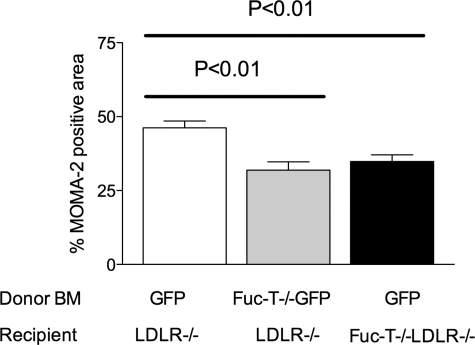
The extent of atherosclerosis in each mouse was assessed by quantitating the lesions in the aortic root as well as on the luminal surface of the aorta, the details of which are described elsewhere.23 Serial (10 μm) cryosections of the aortic root were prepared and every fourth section (for a total of four sections per heart) was stained with Oil Red O, counterstained with Gill’s hematoxylin no. 1 (Fisher Scientific, Pittsburgh, PA), and digitally imaged to quantitate total lesion area. To measure the extent of macrophage infiltration into the lesion in the bone marrow transplantation studies, sections were immunohistochemically stained with a macrophage-specific antibody (MOMA-2, 1:1000; Serotec, Raleigh, NC) and co-localized with eGFP+ regions using a confocal microscope. Areas of the sections stained positive with MOMA-2 antibody were selectively highlighted using computer-assisted morphometry and quantitated as a percentage of MOMA-2-positive area throughout the entire lesion area of the aortic sinus. Trichrome staining was also performed on sections to determine collagen content of the lesion. En face aortas were stained with Sudan IV and digitally imaged for analysis of lesion area, expressed as a percentage of total aortic area, as described elsewhere.23
Statistical Method
Data are presented as mean ± SEM. Statistical significance was determined using either t-test or two-way analysis of variance followed by Bonferroni’s posttest as appropriate. In all cases, significance was determined as P ≤ 0.05.
Results
Before performing the bone marrow transplantation studies, we crossed the FucT-VII−/− mice with the LDLR−/− mice. We then confirmed, as previously reported with ApoE−/− mice, that atherosclerosis was indeed reduced in the LDLR−/− mice with systemic FucT-VII deficiency compared to the control LDLR−/− mice.19 Aortic root lesion area was reduced 36.6% in FucT-VII−/−LDLR−/− mice compared to LDLR−/− controls (FucT-VII −/−LDLR−/− 3.1 × 105 ± 2.9 × 104 μm2 versus LDLR−/− 4.9 × 105 ± 4.7 × 104 μm2, n = 22, P < 0.005). En face aorta lesion area was reduced 57.5% in FucT-VII−/−LDLR−/− mice compared to LDLR−/− controls (FucT-VII−/−LDLR−/− 6.52 ± 0.53% versus LDLR−/− 15.36 ± 1.46%, n = 23, P < 0.0001). However, total cholesterol levels were also reduced in the FucT-VII−/−LDLR−/− mice by 22 to 33% compared to LDLR−/− mice throughout the course of the high-fat diet treatment. We therefore investigated the role of FucT-VII in atherosclerosis using bone marrow transplantation. This technique allowed us to both control for cholesterol level as well as to assess the selective contribution of E- and P-selectin ligands versus L-selectin ligands to atherosclerosis.
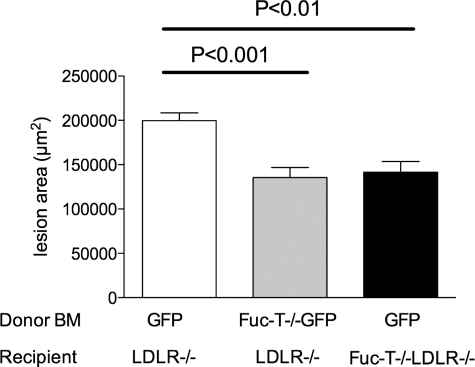
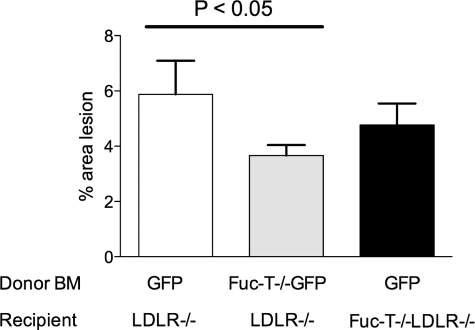
Selective disruption of E- and P-selectin ligand synthesis was achieved by performing bone marrow transplantation into irradiated LDLR−/− mice with marrow cells isolated from FucT-VII−/− mice with one copy of the eGFP gene to track the donor-derived leukocytes. This led to disruption of selectin ligand synthesis on leukocytes that would prevent the leukocytes from interacting with either E- or P-selectin. The extent of atherosclerosis was significantly reduced in these mice compared with controls. Aortic root lesion area was reduced 32.2% in LDLR−/− mice reconstituted with FucT-VII−/−eGFP+ marrow compared with LDLR−/− mice reconstituted with eGFP+ marrow as controls (eGFP+ marrow 2.0 × 105 ± 8.6 × 104 μm2 versus FucT-VII−/−eGFP+ marrow 1.4 × 105 ± 1.1 × 104 μm2, n = 28, P < 0.0005 by unpaired t-test) (Figures 1 and 2). Similarly, there was a 37.8% reduction of the area of lesions on en face aortas in mice reconstituted to lack E- and P-selectin ligand activity compared with controls (eGFP+ marrow 5.88 ± 1.22% versus FucT-VII−/−eGFP+ marrow 3.66 ± 0.38%, n = 28, P < 0.05 by unpaired t-test) (Figure 3). As expected, plasma cholesterol increased throughout time after introduction of the high-fat diet. However, there was no significant difference between plasma cholesterol concentrations in these two groups during the 12-week study (Table 1). Collectively, these data suggest that production of active E- and P-selectin ligands play a role in the natural progression of atherosclerosis.
Figure 1.
Quantification of aortic root lesions stained positive for Oil Red O in LDLR−/− mice receiving GFP+ marrow (white bar), LDLR−/− mice receiving FucT-VII−/−GFP+ marrow (gray bar), and FucT-VII−/−LDLR−/− mice receiving GFP+ marrow (black bar) fed a high-fat diet for 12 weeks. Data are reported as the mean lesion area of four sections taken from the heart of each animal every 40 μm through the aortic valve. The mean ± SEM is shown for each group.
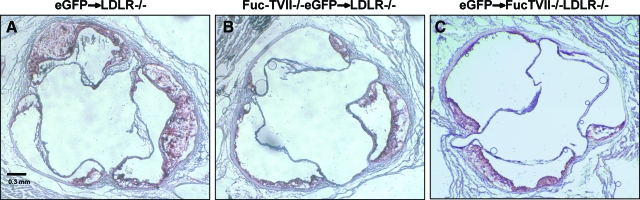
Figure 2.
Representative slides of Oil Red O-stained aortic root sections from LDLR−/− mice receiving GFP+ marrow (A), LDLR−/− mice receiving FucT-VII−/−GFP+ marrow (B), and FucT-VII−/−LDLR−/− mice receiving GFP+ marrow (C) fed a high-fat diet for 12 weeks.
Figure 3.
Quantification of total aortic lesion area stained positive for Sudan IV in LDLR−/− mice receiving GFP+ marrow (white bar), LDLR−/− mice receiving FucT-VII−/−GFP+ marrow (gray bar), and FucT-VII−/−LDLR−/− mice receiving GFP+ marrow (black bar) fed a high-fat diet for 12 weeks. Data are reported as percent lesion area compared to the total aortic surface area for each animal. The mean ± SEM is shown for each group.
Table 1.
Plasma Cholesterol Values at 0, 4, 8, and 12 Weeks
| Chimeric mice | Fuc-T−/−GFP+ → LDLR−/− | GFP+ → LDLR−/− | GFP+ → Fuc-T−/−LDLR−/− |
|---|---|---|---|
| Week 0 | 221 ± 12 mg/dl, n = 14 | 245 ± 8 mg/dl, n = 14 | 260 ± 7 mg/dl, n = 13 |
| Week 4 | 886 ± 44 mg/dl, n = 14 | 1027 ± 70 mg/dl, n = 14 | 1138 ± 110 mg/dl, n = 13 |
| Week 8 | 816 ± 35 mg/dl, n = 14 | 915 ± 47 mg/dl, n = 14 | 1347 ± 78 mg/dl, n = 13* |
| Week 12 | 1115 ± 53 mg/dl, n = 14 | 1196 ± 77 mg/dl, n = 14 | 1268 ± 68 mg/dl, n = 13 |
All data are shown as mean ± SEM. *P < 0.05 as determined by two-way analysis of variance followed by Bonferroni’s post-test.
Selective disruption of active endothelial L-selectin ligand generation also reduced atherosclerotic progression compared to controls, although the reduction was not as robust as with mice in which E- and P-selectin ligand synthesis was disrupted. Aortic root lesion area was reduced 29.7% in FucT-VII−/−LDLR−/− mice reconstituted with eGFP+ marrow compared to LDLR−/− controls (eGFP+ marrow LDLR−/− 2.0 × 105 ± 8.6 × 104 μm2 versus eGFP+ marrow, FucT-VII−/−LDLR−/− 1.4 × 105 ± 1.2 × 104 μm2, n = 27, P < 0.05 by unpaired t-test) (Figures 1 and 2). Similarly, there was an 18.8% reduction in lesioned aortic en face intimal surface in the mice deficient in FucT-VII compared with controls, although this difference was not statistically significant (eGFP+ marrow LDLR−/− 5.88 ± 1.22% versus eGFP+ marrow, FucT-VII−/−LDLR−/− 4.77 ± 0.77%, n = 28, P > 0.05) (Figure 3). Plasma cholesterol was elevated at 8 weeks compared with controls, however, at 4 and 12 weeks no differences were observed (Table 1).
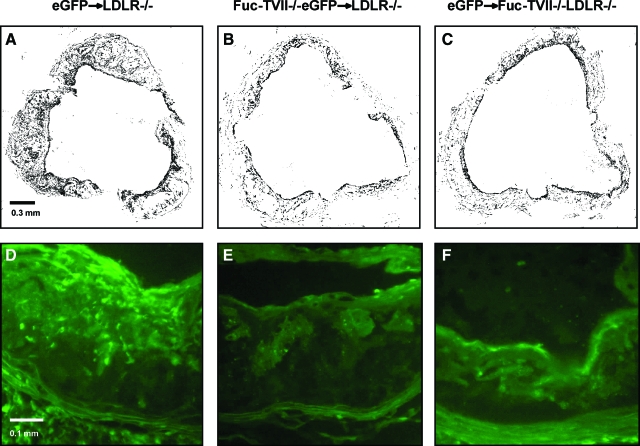
Fluorescence microscopy was used to confirm successful bone marrow reconstitution. The presence of eGFP+ cells in the vessel walls confirmed the presence of donor cells 16 weeks after bone marrow transplant. The presence of macrophages in aortic root lesions was detected by immunohistochemistry, using the anti-MOMA-2 antibody, calculating the percent lesion area that was MOMA-2-positive. MOMA-2-positive staining was reduced in mice lacking active E- and P-selectin ligands on bone marrow-derived cells (eGFP+ marrow 46.18 ± 5.54% versus FucT-VII−/−eGFP+ marrow 31.92 ± 6.91%, n = 12, P < 0.01 by unpaired t-test). To a lesser degree, disruption of active endothelial L-selectin ligands also resulted in reduced area of MOMA-2-positive staining (eGFP+ marrow LDLR−/− 46.18 ± 5.54% versus eGFP+ marrow, FucT-VII−/−LDLR−/− 34.867 ± 5.49%, n = 12, P < 0.01 by unpaired t-test) (Figures 4 and 5). Representative photomicrographs in Figure 4, D–F, also demonstrate that eGFP-positive leukocytes are present in much greater number in the lesions of control mice (Figure 4D) compared with lesions of mice with either E- and P-selectin ligand deficiency (Figure 4E) or L-selectin ligand deficiency (Figure 4F). These results indicate that all three selectin ligands participate in the recruitment of macrophages to the atherosclerotic lesion, although our findings suggest that E- and P-selectin ligands may play a slightly more significant role than L-selectin ligand.
Figure 4.
Representative photomicrographs of aortic heart valve sections from LDLR−/− mice receiving FucT-VII−/−GFP+ marrow (A, D), LDLR−/− mice receiving GFP+ marrow (B, E), and FucT-VII−/−LDLR−/− mice receiving GFP+ marrow (C, F) fed a high-fat diet for 12 weeks. A–C: Black areas representing regions of MOMA-2-positive macrophage staining. The black color represents MOMA-2-positive areas that were selected and highlighted using PhotoShop outfitted with Chroma filter. This was done to demonstrate clearly the macrophages in the lesions. D–F: Representative examples of eGFP-positive cells in the aortic root lesions to demonstrate the accumulation of bone marrow-derived mononuclear cells in atherosclerosis. Original magnifications: ×100 (A–C); ×250 (D–F).
Figure 5.
Quantification of total aortic root lesion area stained positive for MOMA-2 in LDLR−/− mice receiving GFP+ marrow (white bar), LDLR−/− mice receiving FucT-VII−/−GFP+ marrow (gray bar), and FucT-VII−/−LDLR−/− mice receiving GFP+ marrow (black bar) fed a high-fat diet for 12 weeks. Data are reported as percent MOMA-2-positive area compared to the total lesion area in the aortic roots of each animal. The mean ± SEM is shown (n = 6 for each group).
Discussion
Atherosclerosis is a disease mediated in large part by the recruitment of monocytes/macrophages from the circulation into the vessel wall, where they help drive the progression of lesion formation. Fundamental to this process is the capture of the circulating cells by the endothelium, a process in which the selectin family of adhesion molecules are responsible for the initial rolling and tethering. Subsequently, firm attachment followed by migration into the subendothelial space occur mediated by the immunoglobulin adhesion molecules and the integrins.16 Previously, we and others have shown that ablation of the FucT-VII gene results in dramatically reduced capacity of neutrophils and monocytes to adhere to both E- and P-selectins.17,18 These studies also showed that the expression of ligands themselves (such as PSGL-1) are not reduced on the surfaces of leukocytes, indicating that FucT-VII does not participate in the synthesis of the selectin ligands but rather in activating them. Recently, our group has demonstrated that systemic disruption of the selectin pathway through the deletion of FucT-VII, necessary for posttranscriptional modification of the selectin ligands, results in the reduction of atherosclerosis in a mouse model of atherosclerosis,19 and these findings are in concordance with our own findings in the LDLR−/− mouse atherosclerosis model.21,22,23 We have examined the relative contributions of E- and P-selectin ligand and L-selectin ligand production in the development of atherosclerosis. Previous studies have indicated that each of the selectins plays a unique role within the processes of tethering and rolling, with L-selectin capturing leukocytes from the flow in the vessel, E-selectin responsible for rolling, and P-selectin a combination of both.24,25,26,27
The contributions of P- and E-selectin in mediating atherosclerosis have been studied by several groups. Using P-selectin−/− mice crossed onto LDLR−/− mice, Johnson and colleagues28 showed a decrease in rolling leukocytes as determined by intravital microscopy compared with LDLR−/− controls and a twofold reduction in atherosclerosis at 8 weeks in P-selectin-deficient male mice compared with controls. This early effect was attributable to the importance of macrophage recruitment and foam cell formation during the early stages of the disease. In that study, the authors were unable to ascertain whether endothelial- or platelet-derived P-selectin was the more important in contributing to atherosclerosis. In part, this question is answered by our current study, in which the contribution of selectin ligands on hematopoietic cells that bind the selectins expressed by the endothelium appears to be more important than selectin ligand expression on the endothelium. Dong and colleagues29 took this approach a stage further by examining the combined contribution of both endothelially-expressed selectins on atherosclerosis. Simultaneous deficiencies of both E- and P-selectin resulted in a fivefold reduction in the area of heart valve lesions in male mice compared with LDLR deficiency alone at 8 weeks. With the duration of the study being only 8 weeks, however, there were no measurable lesion areas on the en face aortas for comparison.29 Eriksson and colleagues,30 used antibodies against the selectins to examine their relative contributions to leukocyte adhesion and migration, as determined by intravital microscopy. Leukocyte rolling was present in mice with atherosclerotic lesions that were on a western diet but not in control mice on a chow diet. E-selectin inhibition reduced, and P-selectin inhibition abolished, leukocyte rolling in the aortas of ApoE−/−LDLR−/− mice on the western diet. E-selectin inhibition also decreased leukocyte-endothelial contact time and increased leukocyte rolling speed. P-selectin was shown to be highly expressed on regions of the aorta within the atherosclerotic lesion.
In common with these studies, we have shown that inhibition of the endothelially-expressed selectins (via disruption of selectin ligand synthesis in leukocytes) leads to a decrease in the extent of the disease as determined in both the aortic sinus and on the luminal surface of the entire aorta. This is despite a potential limitation of bone marrow transplantation in that not all of the hematopoietic cells would be expected to stem from the donor bone marrow, although in our study successful reconstitution was determined by the presence of eGFP-positive cells within the lesions. Despite this, there was sufficient reduction of E- and P-selectin ligand production by circulating leukocytes to reduce the progression of the disease. Furthermore, it would be expected that secondary tethering would also be disrupted because of a reduction of L-selectin ligands on the leukocytes. Fuc-TVII expression has been shown to be confined to bone marrow and lung tissue, along with low levels in spleen, salivary gland, and skeletal muscle, suggesting that these effects are unlikely to be attributable to a systemic effect in other tissues such as the kidney or the liver.31
By contrast, less work exists in the literature regarding the role of L-selectin and atherosclerosis. Eriksson and colleagues32 described the importance of L-selectin in mediating the secondary capture of leukocytes in atherosclerotic vessels. A possible L-selectin ligand that would be reduced in Fuc-TVII-deficient mice is endoglycan, a molecule related to CD34 endoglycan has been shown to be expressed on endothelial cells.33 Other traditional L-selectin ligands, such as MadCAM-1, GlyCAM-1, and MECA-79 have been shown to be important in smaller vessels, such as high endothelial vessels, rather than the aorta.34 Although deletion of the FucT-VII gene would disrupt synthesis of most selectin ligands, other polysaccharides have been identified as ligands for both P- and L-selectin that do not contain sialic acids or fucose. Although most of these have not been shown to have any activity in vivo,35 these ligands include the heparan sulfate proteoglycans, such as collagen XVIII, which is likely to be present in atherosclerotic plaques. Collagen XVIII has been shown to act as a ligand for L-selectin, and may help the transition between initial rolling and firm adhesion,36,37,38,39 as well as mediate leukocyte-leukocyte adhesion.40 Other proteoglycans have also shown activity as L-selectin ligands, including versican, a chondroitin sulfate proteoglycan,41 although some studies have suggested that this proteoglycan is more important in the human vasculature than the mouse.42 Although sLex-containing L-selectin ligands would have been disrupted in our study, the reduction in atherosclerosis was not significant. The presence of these alternate ligands that are independent of FucT-VII is a possible reason for this finding, although collagen XVIII has not been demonstrated to mediate capture of leukocytes, suggesting that selectins present on the surface of the endothelium play a greater role in macrophage trafficking in the early stages of atherosclerosis than those on the surface of leukocytes. Although these proteoglycan ligands for L-selectin have been shown to be important in the renal vasculature,41,43 little exists in the literature on their role in the development of atherosclerosis with regards to selectins.
There is evidence that platelets can contribute to the development of atherosclerosis. In vivo mouse models have implicated endothelial P-selectin as playing a role in this process, whereby platelets adhere to the endothelium and then recruit circulating leukocytes,44 although the contribution of platelet-mediated leukocyte recruitment to the vasculature is not known. These interactions are likely interrupted via the disruption of selectin ligand synthesis in our study. Thus, it is possible that the reduction in atherosclerosis and in the accumulation of macrophages in the lesions of the LDLR−/− mice reconstituted with FucT-VII−/−eGFP+ marrow compared to LDLR−/− mice reconstituted with eGFP+ marrow was attributable, at least in part, to the disruption of P-selectin and the subsequent adhesion of platelets to the endothelium.
It is not known why plasma cholesterol was lower in the Fuc-TVII−/−LDLR−/− compared to LDLR−/− mice. The fact that plasma cholesterol was not raised in the Fuc-TVII−/−LDLR−/− mice transplanted with wild-type bone marrow cells indicates that it is the systemic expression of FucTVII, rather than the leukocyte source of FucTVII, that is responsible for the reduced plasma cholesterol seen in the Fuc-TVII−/−LDLR−/− mice. Although bone marrow transplantations were performed with donor marrow cells that were LDLR+/+, we have shown in the past that the expression of LDLR by the bone marrow-derived cells does not affect either circulating cholesterol or the extent of atherosclerosis in the LDLR−/− mice.21 Thus, it is unlikely that the LDLR expressed on bone marrow-derived cells influenced the circulating cholesterol levels in our bone marrow chimeric mice.
The observed decrease in cholesterol levels seen in the Fuc-TVII−/−LDLR−/− recipient mice at week 8 is unusual and difficult to explain. It appears from our data that plasma cholesterol levels were raised earlier in these mice than the other two groups. There are reports that α1,6-fucosyltransferase is involved with the LDLR-related protein-1 (LRP-1), a scavenger receptor. Loss of fucosylation after gene deletion impaired function of LRP-1, and it is possible that a similar effect also occurred in the Fuc-TVII−/−LDLR−/− mice that led to an alteration in cholesterol metabolism and increased plasma concentrations.45,46 Additional work would be needed to further explore the relationship between Fuc-TVII and plasma cholesterol.
In summary, our studies suggest that the synthesis of E- and P-selectin ligands, as mediated by the rate-limiting enzyme FucT-VII, plays an important role in the pathogenesis of atherosclerosis. These data suggest that FucT-VII and the E- and P-selectin pathways may prove a fruitful therapeutic target in the prevention or treatment of atherosclerosis.
Footnotes
Address reprint requests to William A. Boisvert, Ph.D., Vascular Medicine Research, Brigham and Women’s Hospital, Harvard Medical School, 65 Landsdowne St., Room 286, Cambridge, MA 02139. E-mail: wboisvert@rics.bwh.harvard.edu.
Supported by the National Institutes of Health (grant HL069474 to W.A.B.) and the American Heart Association (Fellow to Faculty Transition Award 0275023N to J.W.H.).
Current addresses of J.M.G.: College of Pharmacy, University of Kentucky, Lexington, KY; and W.A.B.: Brigham and Women’s Hospital, Harvard Medical School, Cambridge, MA.
References
- Schaffner T, Taylor K, Bartucci EJ, Fischer-Dzoga K, Beeson JH, Glagov S, Wissler RW. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980;100:57–80. [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Printseva OY, Peclo MM, Gown AM. Various cell types in human atherosclerotic lesions express ICAM-1: further immunocytochemical and immunochemical studies employing monoclonal antibody 10F3. Am J Pathol. 1992;140:889–896. [PMC free article] [PubMed] [Google Scholar]
- Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD, Lobb R, Alpers CE. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston RN, Haskard DO, Coucher JR, Gall NP, Johnson-Tidey RR. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol. 1992;140:665–673. [PMC free article] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady HR, Spertini O, Jimenez W, Brenner BM, Marsden PA, Tedder TF. Neutrophils, monocytes, and lymphocytes bind to cytokine-activated kidney glomerular endothelial cells through L-selectin (LAM-1) in vitro. J Immunol. 1992;149:2437–2444. [PubMed] [Google Scholar]
- Hallmann R, Jutila MA, Smith CW, Anderson DC, Kishimoto TK, Butcher EC. The peripheral lymph node homing receptor LECAM-1, is involved in CD18-independent adhesion of human neutrophils to endothelium. Biochem Biophys Res Commun. 1991;174:236–243. doi: 10.1016/0006-291x(91)90511-5. [DOI] [PubMed] [Google Scholar]
- Norman KE, Katopodis AG, Thoma G, Kolbinger F, Hicks AE, Cotter MJ, Pockley AG, Hellewell PG. P-selectin glycoprotein ligand-1 supports rolling on E- and P-selectin in vivo. Blood. 2000;96:3585–3591. [PubMed] [Google Scholar]
- Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, Cumming DA, Larsen GR. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Malý P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferase FucT-VII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- Homeister JW, Daugherty A, Lowe JB. {alpha}(1,3)Fucosyltransferases FucT-IV and FucT-VII control susceptibility to atherosclerosis in Apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol. 2004;24:1897–1903. doi: 10.1161/01.ATV.0000141844.28073.df. [DOI] [PubMed] [Google Scholar]
- Boisvert WA, Black AS, Curtiss LK. ApoA1 reduces free cholesterol accumulation in atherosclerotic lesions of ApoE-deficient mice transplanted with ApoE-expressing macrophages. Arterioscler Thromb Vasc Biol. 1999;19:525–530. doi: 10.1161/01.atv.19.3.525. [DOI] [PubMed] [Google Scholar]
- Boisvert WA, Spangenberg J, Curtiss LK. Role of leukocyte-specific LDL receptors on plasma lipoprotein cholesterol and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17:340–347. doi: 10.1161/01.atv.17.2.340. [DOI] [PubMed] [Google Scholar]
- Boisvert WA, Spangenberg J, Curtiss LK. Treatment of severe hypercholesterolemia in apolipoprotein E-deficient mice by bone marrow transplantation. J Clin Invest. 1995;96:1118–1124. doi: 10.1172/JCI118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MB, Bainton DF, Springer TA. Neutrophil tethering to and rolling on E-selectin are separable by requirement for L-selectin. Immunity. 1994;1:137–145. doi: 10.1016/1074-7613(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Alon R, Fuhlbrigge RC, Finger EB, Springer TA. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol. 1996;135:849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas FW, Ding H, Tan P, Cumming D, Tedder TF, Gerritsen ME. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J Immunol. 1996;157:326–335. [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. Absence of P-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Direct viewing of atherosclerosis in vivo: plaque invasion by leukocytes is initiated by the endothelial selectins. FASEB J. 2001;15:1149–1157. doi: 10.1096/fj.00-0537com. [DOI] [PubMed] [Google Scholar]
- Smith PL, Gersten KM, Petryniak B, Kelly RJ, Rogers C, Natsuka Y, Alford JA, III, Scheidegger EP, Natsuka S, Lowe JB. Expression of the alpha(1,3)fucosyltransferase Fuc-TVII in lymphoid aggregate high endothelial venules correlates with expression of L-selectin ligands. J Biol Chem. 2006;271:8250–8259. doi: 10.1074/jbc.271.14.8250. [DOI] [PubMed] [Google Scholar]
- Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194:205–218. doi: 10.1084/jem.194.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard-Sumnicht K, Varki A. Endothelial heparan sulfate proteoglycans that bind to L-selectin have glucosamine residues with unsubstituted amino groups. J Biol Chem. 1995;270:12012–12024. doi: 10.1074/jbc.270.20.12012. [DOI] [PubMed] [Google Scholar]
- Nelson RM, Cecconi O, Roberts WG, Aruffo A, Linhardt RJ, Bevilacqua MP. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- Kawashima H, Watanabe N, Hirose M, Sun X, Atarashi K, Kimura T, Shikata K, Matsuda M, Ogawa D, Heljasvaara R, Rehn M, Pihlajaniemi T, Miyasaka M. Collagen XVIII, a basement membrane heparan sulfate proteoglycan, interacts with L-selectin and monocyte chemoattractant protein-1. J Biol Chem. 2003;278:13069–13076. doi: 10.1074/jbc.M212244200. [DOI] [PubMed] [Google Scholar]
- Giuffrè L, Cordey AS, Monai N, Tardy Y, Schapira M, Spertini O. Monocyte adhesion to activated aortic endothelium: role of L-selectin and heparan sulfate proteoglycans. J Cell Biol. 1997;136:945–956. doi: 10.1083/jcb.136.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kawashima H, Watanabe N, Miyasaka M. Identification and characterization of ligands for L-selectin in the kidney. II. Expression of chondroitin sulfate and heparan sulfate proteoglycans reactive with L-selectin. FEBS Lett. 1999;444:201–205. doi: 10.1016/s0014-5793(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Wight T, Merrilees M. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Li Y, Watanabe N, Hirose J, Hirose M, Miyasaka M. Identification and characterization of ligands for L-selectin in the kidney. I. Versican, a large chondroitin sulfate proteoglycan, is a ligand for L-selectin. Int Immunol. 1999;11:393–405. doi: 10.1093/intimm/11.3.393. [DOI] [PubMed] [Google Scholar]
- Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li W, Ikeda Y, Miyagawa JI, Taniguchi M, Miyoshi E, Sheng Y, Ekuni A, Ko JH, Yamamoto Y, Sugimoto T, Yamashita S, Matsuzawa Y, Grabowski GA, Honke K, Taniguchi N. Ectopic expression of alpha1,6 fucosyltransferase in mice causes steatosis in the liver and kidney accompanied by a modification of lysosomal acid lipase. Glycobiology. 2001;11:165–174. doi: 10.1093/glycob/11.2.165. [DOI] [PubMed] [Google Scholar]
- Lee SH, Takahashi M, Honke K, Miyoshi E, Osumi D, Sakiyama H, Ekuni A, Wang X, Inoue S, Gu J, Kadomatsu K, Taniguchi N. Loss of core fucosylation of low-density lipoprotein receptor-related protein-1 impairs its function. leading to the upregulation of serum levels of insulin-like growth factor-binding protein 3 in Fut8−/− mice. J Biochem. 2006;139:391–398. doi: 10.1093/jb/mvj039. [DOI] [PubMed] [Google Scholar]