Abstract
The nervous system (both central and peripheral) is anatomically and physiologically differentiated between the sexes, ranging from gender-based differences in the cerebral cortex to motoneuron number in the spinal cord. Although genetic factors may play a role in the development of some sexually differentiated traits, most identified sex differences in the brain and behavior are produced under the influence of perinatal sex steroid signaling. In many species, the ability to display an estrogen-induced luteinizing hormone (LH) surge is sexually differentiated, yet the specific neural population(s) that allows females but not males to display such estrogen-mediated “positive feedback” has remained elusive. Recently, the Kiss1/kisspeptin system has been implicated in generating the sexually-dimorphic circuitry underlying the LH surge. Specifically, Kiss1 gene expression and kisspeptin protein levels in the anteroventral periventricular (AVPV) nucleus of the hypothalamus are sexually differentiated, with females displaying higher levels than males, even under identical hormonal conditions as adults. These findings, in conjunction with accumulating evidence implicating kisspeptins as potent secretagogues of gonadotropin-releasing hormone (GnRH), suggest that the sex-specific display of the LH surge (positive feedback) reflects sexual differentiation of AVPV Kiss1 neurons. In addition, developmental kisspeptin signaling via its receptor GPR54 appears to be critical in males for the proper sexual differentiation of a variety of sexually dimorphic traits, ranging from complex social behavior to specific forebrain and spinal cord neuronal populations. This review discusses the recent data, and their implications, regarding the bidirectional relationship between the Kiss1 system and the process of sexual differentiation.
Keywords: metastin, kisspeptin, Kiss1, GPR54, sexual differentiation, sex differences, AVPV, tyrosine hydroxylase, development
1. Introduction
Vertebrates have evolved numerous physiological and behavioral mechanisms ensuring their reproductive success and survival, and a great many of these adaptations are controlled directly or indirectly by the brain. Importantly, males and females differ in many of these physiological and behavioral adaptations, such as copulatory behavior or neuroendocrine physiology, and these critical sex differences presumably reflect underlying sex differences in brain circuitries and neuronal mechanisms. In this review, I discuss key aspects of sexual differentiation of the brain and behavior as it relates to the recently-identified neuronal Kiss1 system. Several other papers have extensively reviewed the current literature on the Kiss1 system (including kisspeptins and their receptor GPR54), particularly in relation to reproduction and puberty (50, 78, 80, 84) as well as human clinical issues (2, 11, 22, 44, 85). In the present paper, I provide some essential background on Kiss1 biology and then focus on the latest findings connecting the kisspeptins and GPR54 to sexual differentiation. Specifically, I discuss evidence suggesting a bi-directional relationship between sexual differentiation and kisspeptin-GPR54 signaling; that is, kisspeptin-GPR54 signaling during critical periods of development is required for proper sexual differentiation of the brain and behavior, and certain populations of Kiss1 neurons themselves are one aspect of the brain that becomes sexually differentiated.
2. Characterization of the Kiss1 System, including Kisspeptins and their Receptor
In humans, the KiSS1 gene encodes a 145 amino acid precursor protein, which is processed post-translationally to produce a 54 amino acid peptide called kisspeptin-54 (107). Kisspeptin-54 has also been termed “metastin”, based on initial efforts identifying Kiss1 as a cancer metastasis suppressor gene (52, 59). The rodent Kiss1 gene, which is 46% to 52% homologous to the human KiSS1 gene, encodes a 130 amino acid precursor protein which is processed to generate a 52 or 54 amino acid mature kisspeptin peptide. In addition to kisspeptin-54 or -52, several other smaller peptide fragments derived from the precursor protein have been identified (kisppetin-14, -13, -10), all sharing a distinct structural RF-amide motif (Arg-Phe-NH2) and each able to bind and activate GPR54 with similar efficacy (54, 68, 73).
In rodents, sheep, and primates (including humans), Kiss1 mRNA has been detected by either in situ hybridization or RT-PCR in discrete regions of the forebrain, including the hypothalamic anteroventral periventricular nucleus (AVPV; or preoptic area in sheep) and arcuate nucleus (ARC; infundibular nucleus in primates) (1, 26, 37, 51, 69, 81, 88, 97). Kiss1 is also expressed in several peripheral tissues, most notably, the placenta, ovary, testis, pancreas, and liver (54, 58, 68, 73). In 2001, kisspeptins were shown to be natural high-affinity ligands for an orphan G-protein-coupled membrane receptor termed GPR54 (54, 68, 73). Like Kiss1, the GPR54 gene is expressed in several peripheral tissues (placenta, pancreas, kidney, testis, and pituitary) as well as in the brain— most notably the hypothalamus, preoptic area, midbrain, hippocampus, amygdala, and medulla (54, 58, 68).
3. The Relationship Between the Kisspeptin-GPR54 System and Reproduction
Gonadotropin-releasing hormone (GnRH) neurons are the final common conduit through which the brain regulates the secretion of pituitary gonadotropins, and hence, all of reproduction. Converging evidence accumulated in the past 5 years supports the notion that kisspeptin-GPR54 signaling directly regulates GnRH secretion. In 2003, several groups reported that humans and mice with either spontaneous or genetically-targeted mutations in the gene for GPR54 exhibit severe deficits in reproductive function, including delayed sexual maturation, low levels of gonadal sex steroids, absent spermatogenesis and ovulation, and impaired estrous or menstrual cyclicity (19, 29, 83). Mutations and deletions of GPR54 are also associated with diminished luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion, which has been linked to a deficiency in secretion of GnRH (49, 62, 83, 86); similar impairments in reproductive function have also recently been reported for Kiss1 knock-out (KO) mice (18, 55). In rodents, sheep, and primates (including humans), exogenous kisspeptin treatment elicits rapid and robust increases in plasma levels of LH and FSH (21, 37, 49, 61, 70, 71, 88). Although GPR54 is expressed both in the pituitary and GnRH neurons (46, 62), evidence suggests that kisspeptin’s stimulation of gonadotropin secretion reflects direct activation of GnRH neurons rather than pituitary gonadotropes. For example, kisspeptin treatments increase electrical activity and Fos protein induction in GnRH neurons, and fail to stimulate LH secretion in the presence of GnRH receptor antagonists (37, 41, 49, 61, 62, 101). In addition, kisspeptin-containing axonal fibers have been reported to appose GnRH neurons, suggesting direct innervation of GnRH cells by kisspeptin signaling (10).
The secretion of GnRH is regulated by sex steroids (i.e., positive and negative feedback), but the cellular and molecular mechanisms underlying this regulation are not entirely known. GnRH neurons do not express estrogen receptor α (ERα) or the androgen receptor (AR) (43), which are thought to mediate steroidal feedback effects, suggesting that other steroid-sensitive neurons “upstream” of GnRH neurons receive and transmit steroid feedback signals to the reproductive axis. Recent evidence has implicated hypothalamic Kiss1 neurons as these upstream steroid-sensitive neurons. In rodents, virtually all hypothalamic Kiss1 neurons express ERα and AR, and Kiss1 mRNA is robustly regulated by both estradiol and testosterone (1, 95, 96). Likewise, in sheep, most hypothalamic Kiss1 cells express ERα, as well as progesterone receptor (PR) (97). Interestingly, in rodents, the effects of sex steroids on Kiss1 gene expression in the brain are region-specific. In the ARC, estradiol or testosterone treatment inhibits the expression of Kiss1, whereas in the AVPV these same hormones stimulate Kiss1 expression (51, 95, 96, 100) (1). Conversely, in gonadectomized rodents in which sex steroids are low/absent, Kiss1 mRNA levels are increased in the ARC and decreased in the AVPV (51, 95, 96, 100).
The differential effects of estrogen and testosterone on Kiss1 gene expression in the ARC and AVPV suggest that Kiss1 neurons may be involved in the steroid-mediated positive and negative feedback regulation of GnRH neurons. In rodents, sheep, and primates, the ARC has been proposed to contain the neural substrate mediating the negative feedback regulation of reproduction by sex steroids (27, 82, 94); thus, Kiss1 neurons in this region may be involved in the cellular mechanism(s) orchestrating this negative feedback phenomenon. If so, one would predict that the presence of negative feedback stimuli (i.e., sex steroids) would inhibit the ARC Kiss1 system whereas removal of sex steroids would eliminate inhibition of the Kiss1 system in the ARC. Indeed, as mentioned above, both estradiol and testosterone inhibit Kiss1 gene expression in the ARC whereas removal of sex steroids dramatically increases Kiss1 expression in the ARC (but not elsewhere). Specifically, gonadectomized rodents, sheep, and monkeys display substantially increased Kiss1 expression in the ARC (or its primate homologue, the infundibular nucleus) (89, 97), correlating with increased LH secretion owing to removal of steroid negative feedback. Furthermore, the ability of gonadectomy to remove negative feedback signaling to GnRH neurons and thereby stimulate the reproductive axis is not observed in gonadectomized GPR54 KO mice in which kisspeptin signaling to GnRH neurons is impaired. Thus, although Kiss1 gene expression in the ARC of GPR54 KO mice increases after gonadectomy (similar to wildtype mice), GPR54 KO mice do not display a post-castration rise in plasma LH (24), suggesting that GnRH neurons in these animals are not stimulated after gonadectomy. Similarly, treatment of adult castrated wildtype mice with a selective GPR54 antagonist abolishes the increase in LH secretion typically associated with removal of sex steroid negative feedback signals (A.S. Kauffman, R.A. Steiner, and R.P. Millar, unpublished observations). Collectively, these findings suggest that sex steroids inhibit Kiss1 neurons in the ARC, thereby inhibiting ARC-derived kisspeptin stimulation of GnRH secretion (and consequently, achieving negative feedback). However, the validity of this negative feedback model awaits further studies establishing direct connections between ARC Kiss1 neurons and GnRH neurons, either at GnRH cell bodies or at GnRH axon terminals in the median eminence.
Whereas negative feedback effects of estradiol and testosterone on the reproductive axis occur in both sexes, estradiol can also act in females at a specific stage of their estrous/menstrual cycle to exert a “positive feedback” event, resulting in the stimulation of GnRH/LH secretion which triggers ovulation. In rodents, estrogen-responsive neurons in the AVPV provide the anatomical substrate/circuitry for generating the sexually differentiated preovulatory LH surge (34, 56, 57, 103, 108-110). Convincing evidence suggests that Kiss1 neurons in the AVPV drive the estradiol-induced LH surge in rodents. First, kisspeptin is a potent secretagogue for GnRH, and GnRH neurons express GPR54 (37, 41, 46, 61, 71). Second, Kiss1 neurons in the AVPV project their axonal fibers to GnRH cells and directly innervate these cells (10, 111). Third, Kiss1 expression in the AVPV increases at the time of the LH surge, coincident with induction of the transcription factor Fos in these Kiss1 neurons (1, 100). Fourth, ERα is thought to mediate estrogen’s stimulatory effects on the surge mechanism (16), and virtually all Kiss1 neurons in the AVPV express ERα (1, 95, 96). Lastly, in vivo blockade of kisspeptin signaling using central infusions of kisspeptin antibodies prevents the LH surge from occurring in rats (53). Thus, Kiss1 neurons in the AVPV of female rodents likely serve as the cellular conduit for integrating and relaying estrogen signals to GnRH neurons to generate the preovulatory LH surge.
Unlike rodents, sheep have a relatively small population of Kiss1 neurons in the AVPV/preoptic area which is not strongly regulated by sex steroids (97). In contrast, the ovine mediobasal hypothalamus (containing the ventromedial nucleus and ARC) is believed to contain the neuronal substrates mediating estrogen’s positive feedback effects on GnRH neurons (7, 9, 35, 38). The expression of Kiss1 in the ovine ARC is regulated by sex steroids and markedly increased prior to and during the preovulatory LH surge (26, 97). Thus, in the ewe, both positive and negative feedback effects of sex steroids may be mediated by Kiss1 neurons in the medial basal hypothalamus (i.e., the ARC). Whether these positive and negative feedback effects are mediated by the same ARC Kiss1 neurons or by separate sub-populations of Kiss1 cells within the ARC is unclear. Although primates (including humans) have a distribution of Kiss1 neurons also concentrated in the medial basal hypothalamus/infundibular nucleus (81, 88), the role of the infundibular Kiss1 system in generating the preovulatory LH surge in primates has not yet been studied.
Kisspeptin-GPR54 signaling is undoubtedly critical for regulating GnRH secretion, but whether the gonadotrope cells in the pituitary are also targets for kisspeptin action remains unresolved. GPR54 is expressed in the pituitary of humans and rodents (40, 54, 68, 79, 101), and kisspeptin stimulates gonadotropin release in vitro from cultured rat, ovine, and bovine primary pituitary cells (40, 71, 79, 101, 102). However, other studies have reported no significant effect of kisspeptins on in vitro LH or FSH secretion in cultured primary pituitary cells or anterior pituitary fragments of rats (61, 104). The explanation for these contradictory findings is unknown and may reflect differences in experimental design. However, despite the presence of GPR54 in the pituitary and the ability of kisspeptin to stimulate gonadotropes, recent data in hypothalmao-pituitary disconnected sheep indicate that GnRH signaling is required for kisspeptin’s stimulatory effects on in vivo gonadotropin secretion (99); thus, in the absence of GnRH communication to the pituitary, kisspeptin treatment is unable to induce LH secretion. This finding strengthens the argument that GnRH neurons in the brain are the primary targets for the stimulatory action of kisspeptin on the neuroendocrine reproductive axis.
4. Sexual Differentiation of the Kiss1 System
Animals possess numerous physiological and behavioral adaptations ensuring their reproductive success and survival, and a number of these traits are different between the two sexes. Sex-specific adaptations range from complex copulatory and parental behaviors to endocrine secretion patterns to morphological and developmental parameters. Presumably, many of these critical sex differences reflect underlying sex differences in brain circuitries and neural mechanisms. Indeed, in mammals and other vertebrates, the nervous system is anatomically and physiologically differentiated between the sexes (13, 66, 92), including documented sex differences ranging from the cerebral cortex to the hypothalamus to neurons in the spinal cord.
The central tenet of sexual differentiation is that the brain is “bipotential”, and develops to be male-like or female-like under the direction of the sex steroid environment during the critical perinatal period (92). Although genetic factors, such as sex differences in Y or X chromosome gene expression, may also play a role in the development of some sexually dimorphic traits (20, 30), the majority of presently-known sex differences in the brain and behavior are seemingly produced under the influence of developmental sex steroid signaling. Specifically, the acute secretion of testosterone during critical windows of pre- and/or post-natal life in males, but not females, causes the brain to change its structure and function, thereby differentiating to be masculinized and defeminized. Perinatal testosterone of males directs the brain’s sexual differentiation via activation of either the androgen receptor or estrogen receptors (after its aromatization to estradiol in select neural target tissues). The nature of sexual differences in the brain are diverse and region- and trait-specific; thus, sex differences in the brain range from differences in synapse morphology to neuron size or number to specific gene expression or protein levels (reviewed in (13, 92)). Similarly, neuronal differences between males and females do not tend to always favor one of the two sexes over the other, and hence, there are numerous traits in which males display a greater cell number or larger nucleus size than females, and vice versa. For example, the medial preoptic nucleus of rats contains more neurons in males than females, whereas the AVPV is typically larger in females and possesses more tyrosine-hydroxylase expressing neurons than that of males (13, 92). In many cases, the link between defined sexually-dimorphic neural phenotypes and specific overlying physiological processes or behaviors has been difficult to establish, and thus functional significance of many sex-specific neural traits has remained elusive (and mostly speculative) (87).
In rodents and sheep, one important sexually differentiated trait is the ability of adult females, but not adult males, to display an estrogen-induced, circadian-dependent GnRH/LH surge (i.e., “positive feedback”) (3, 14, 31, 48, 92). [Note that this does not appear to be the case in higher primates, wherein males apparently retain the ability to generate an LH surge in response to E, at least under some circumstances (23, 47, 72, 76).] In male rodents, exposure to testosterone or its estrogenic metabolites during perinatal life (or during prenatal life in male sheep) permanently alters the circuitry in the developing forebrain (66), preventing these animals from being able as adults to generate a GnRH/LH surge in response to estrogen (66). Since the brain of a normal female is not exposed to testosterone or estrogen during the perinatal period, it develops the circuitry/mechanism necessary to generate a GnRH/LH surge in adulthood (91, 92). In support of this model, male rodents that are castrated during the critical window of perinatal development can produce a GnRH/LH surge as adults, just like normal females (14, 28, 31, 32, 42, 77); likewise, females that are exposed to testosterone during the perinatal period lose their ability to generate GnRH/LH surges as adults (12, 17, 36, 92).
Although certain brain regions have been speculated to be involved in the sexually differentiated GnRH/LH surge, the specific population(s) of neurons that drives the GnRH/LH surge, which develops in females and regresses in males, is still being elucidated. In particular, the AVPV has received much experimental support as being a critical part of the surge generating mechanism. Estrogen receptors, including ERα, are expressed in some AVPV neurons, and lesions of the AVPV block spontaneous and steroid-induced preovulatory surges (103, 108, 109), indicating this anatomical site is critical for producing the surge. Furthermore, neuronal populations in the AVPV are sexually dimorphic, with females possessing more neurons overall than males, as well as greater numbers of neurons containing tyrosine hydroxylase (TH; i.e., dopaminergic cells) and GABA/glutamate (74, 90, 91). The sexually dimorphic TH population in the AVPV, like the LH surge phenomenon, has been shown to be sexually differentiated in early development under the influence of the perinatal steroid hormone milieu (90, 93). However, it is unclear whether this TH population actually participates in the LH surge mechanism, as there is no consistent evidence indicating that dopamine regulates GnRH neurons at the time of the surge. Thus, despite the fact that there is a correlation between the sexually differentiated TH population and the sexually differentiated surge phenomenon, it remains to be seen whether there is causation in this relationship.
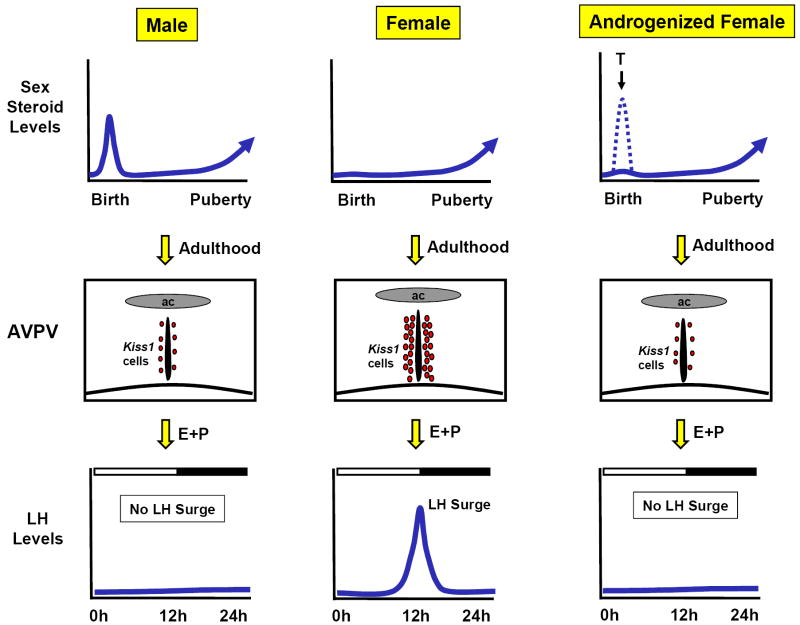
Recent evidence indicates that the Kiss1 system may be a critical component of the sexually differentiated surge. Like TH neurons, Kiss1 neurons in the rodent AVPV are also sexually differentiated, with adult females possessing many more Kiss1 cells than males (Figure 1) (1, 10, 51). In situ hybridization studies have shown that in rats, the number of Kiss1 mRNA-expressing neurons in the AVPV of adult females is as much as 25 times greater than in males (51), and similar sex differences in kisspeptin protein levels in the AVPV (as determined by immunocytochemistry) have also been reported in adult mice (10) and rats (1). Although sex steroids in adulthood stimulate Kiss1 gene expression in the AVPV (98, 100), the sex difference in AVPV Kiss1 neurons is not attributable to sex differences in circulating levels of testosterone or estrogen in adulthood. Gonadectomized male and female rats receiving identical sex steroid treatments (i.e., with or without estradiol implants) as adults still display robust sex differences in Kiss1 expression in the AVPV, regardless of treatment (1, 51); thus, adult females possess more Kiss1 cells in this region than males regardless of sex steroid milieu. In contrast, perinatal sex hormones dramatically affect the sex difference in Kiss1 neurons: female rats treated perinatally with a single injection of androgen (to mimic what a perinatal male normally produces) possess very few Kiss1 neurons in the AVPV as adults, similar to adult males (51).
Figure 1.
Model of the sex steroid-mediated development of the sexually-dimorphic preovulatory LH surge in rodents. The presence of androgens at the time of birth, such as occurs in normal males or in females given an exogenous perinatal treatment of testosterone, results in the development of a male-like AVPV, which includes the absence (or low levels) of Kiss1 neurons. This low level of Kiss1 in the AVPV correlates with the inability of males and androgenized females to display an LH surge in adulthood (in response to exogenous estradiol and progesterone treatment). In contrast, females lack androgen secretion in early perinatal development, and thus have high levels of Kiss1 in the AVPV as adults, correlating with their ability to show an LH surge. It should be noted that this rodent model of sexually-differentiated Kiss1 population in the AVPV may differ in sheep and primates in which positive feedback signaling in females is likely derived from neurons (perhaps kisspeptin cells) in the medial basal hypothalamus (a region including the ARC) rather than the more anterior AVPV/POA region.
The above observations indicate that the Kiss1 system in the AVPV is sexually-differentiated early in development under the influence of perinatal sex steroids, thereby producing significant sex differences in Kiss1 expression in the AVPV in adulthood (and likely accounting for the sex-specific ability of female rodents to produce an LH surge). Thus, the presence of absence of Kiss1 neurons in the AVPV of adult rodents correlates nicely with the ability of inability of these animals to exhibit an LH surge in response to adulthood estradiol and progesterone treatment: adult females have high levels of Kiss1 expression in the AVPV and display an LH surge, whereas adult males and androgenized females (given a single treatment of androgen on the day of birth) have few to no Kiss1 cells in the AVPV and cannot mount an LH surge in response to estradiol and progesterone treatment (Figure 1). This relationship between sexual differentiation and the AVPV Kiss1 system, in conjunction with the compelling line of evidence regarding positive feedback discussed in the previous section, suggests that the sexually-differentiated population of Kiss1 neurons in the AVPV drives the sexually-differentiated E-induced GnRH/LH surge. Since the ability to generate a GnRH/LH surge is sexually differentiated, occurring only in females (3, 14, 31, 45, 92), it is likely that Kiss1 neurons in the AVPV of females serve as the cellular conduit for integrating and relaying circadian and sex steroid signals to GnRH neurons in order to initiate the preovulatory GnRH/LH surge.
The developmental effects of sex steroids on Kiss1 neurons in the AVPV could be mediated by either AR- or ER-dependent pathways. This mechanism has not yet been addressed in rats, but there is some recent evidence reported for mice. Adult female mice containing a mutation in alpha-fetoprotein, which normally binds estradiol during development and prevents it from acting in the brain, show reduced kisspeptin immunoreactivity in the AVPV and decreased Fos induction in kisspeptin neurons after treatment with estradiol and progesterone (33); these same females are incapable of mounting an LH surge in response to sex steroid treatment in adulthood. Thus, development and sexual differentiation of the AVPV kisspeptin system is likely guided by the presence or absence of perinatal actions of estradiol in the brain, and hence, ER-dependent mechanisms; however, this conjecture requires additional support from future studies with ER KO mice, AR KO mice, and aromatase KO mice.
As mentioned previously, the AVPV also contains a sexually dimorphic population of TH-positive (i.e., dopaminergic) neurons (91, 92), which is greater in females than males and which is sexually differentiated in early perinatal development by estradiol. One question that arises is whether the sexually differentiated populations of Kiss1 and TH cells in the AVPV are the same set of neurons or separate sexually dimorphic systems. In situ hybridization experiments indicate that, in female rats, few AVPV neurons coexpress both TH and Kiss1 mRNA, and the few cells that do coexpress both transmitters contain only low levels of TH mRNA (51). Furthermore, given that the expression of both TH and Kiss1 in the AVPV is regulated by sex steroids, but in opposite directions for the two genes, the degree of co-expression was dependent on the sex steroid milieu of the animals: in the presence of estradiol, co-expression between TH and Kiss1 was at a minimum, whereas there was a modest increase in co-expression when sex steroids were absent owing to ovariectomy. However, even in gonadectomized females, only half of the Kiss1 cells co-expressed TH mRNA, and those Kiss1 cells that were double-labeled contained relatively little TH mRNA compared with the more intensely-stained, single-labeled TH cells in the AVPV (51). Thus, although there was a slight overlap in the anatomical distribution of these two cell populations and a low degree of co-labeling for some Kiss1 cells, the sexually-dimorphic Kiss1 neurons and TH-expressing cells in the AVPV appear to represent two separate, sexually-differentiated populations, at least in rats. In contrast, preliminary data suggest that, in mice, many Kiss1 neurons in the AVPV also to coexpress TH mRNA (60), though this preliminary finding is awaiting corroboration and further confirmation. Although the physiological significance of this species difference in Kiss1 and TH coexpression is unknown, it is not the first incidence of differences in reproductive neural circuits/populations between mice and rats and serves to underscore the importance of being cautious when making generalizations about closely related species based on observations in one animal system.
It is interesting that the populations of Kiss1 and TH neurons in the AVPV are regulated in precisely the opposite fashion by sex steroids: the number of Kiss1 cells and the content of Kiss1 mRNA per neuron is highest in the presence of E, whereas the expression of TH in this region is lowest when E is present (and up-regulated in the absence of E) (90). It is unclear whether the differential regulation of these two AVPV systems reflects a cooperative communication between the two populations, with Kiss1 neurons being “activated” at the time of the LH surge (thus, producing enhanced stimulation of GnRH neurons) and TH neurons coincidently being less active (possibly reducing regulatory input to the GnRH system). If so, the decrease in TH at the time of the LH surge may serve to reduce inhibitory signaling to GnRH neurons which, when combined with enhanced kisspeptin release, would produce a larger net stimulatory effect on GnRH release. It is also possible, though as yet untested, that the neighboring Kiss1 and TH systems in the AVPV may communicate with and/or regulate each other. In addition, further studies are required to determine what role the low levels of TH co-expression in some AVPV Kiss1 neurons plays, especially in relation to regulating the LH surge, as well as whether the sexually dimorphic Kiss1 neurons in the AVPV represent the same population as the sexually-dimorphic GABA/glutamate neurons in this region (74).
Although not as well characterized or studied as the sexually dimorphic systems of the AVPV, the ARC also displays several sex differences in neuronal parameters, including the number of both spine and somatic synapses as well as the morphology of astroglia (63-65). However, in contrast to the AVPV, the ARC of adult rodents displays no sex differences in either the number of Kiss1 neurons or the content of Kiss1 mRNA per cell. In adult rats, males and females display similar levels of enhanced Kiss1 expression in the ARC following gonadectomy and similarly reduced Kiss1 expression after testosterone or estrogen treatment (51). Likewise, in adult mice, kisspeptin proteins levels in the ARC, as measured by immunocytochemistry, are similar between gonad-intact males and females (10). Steiner and others have proposed that Kiss1 cells in the ARC of both males and females provide tonic stimulatory input to GnRH neurons and relay negative feedback effects of sex steroids to the GnRH axis (25)(50). Indeed, in both sexes, removal of sex steroid feedback (by gonadectomy) increases the expression of Kiss1 in the ARC and is associated with a concomitant increase in gonadotropin secretion, whereas steroid hormone treatment inhibits the expression of Kiss1 in this region and reduces gonadotropin levels (46, 95, 96). Though still hypothetical at present, the equivalent role of ARC Kiss1 neurons in both sexes certainly matches the lack of sexual differentiation in these neurons, at least in rodents.
Intriguingly, recent analysis of Kiss1 expression in the ARC of other animals has yielded a different picture with regards to sexual differentiation. In particular, emerging evidence suggests that male and female sheep contain different numbers of Kiss1 neurons in the ARC, thus raising the possibility of an important ovine sex difference in the Kiss1 system in this brain region. Specifically, ewes have higher numbers of Kiss1 neurons in the ARC than male sheep. Given the proposed role of the ovine ARC in mediating the sexually dimorphic preovulatory GnRH/LH surge, the presence of a Kiss1 sex difference in this region of sheep is not surprising (8). Additional studies are required to determine if the ARC Kiss1 system of other species (such as monkeys and humans) displays a lack of sex differences, as in rats and mice, or a significant sex difference, as in sheep.
5. Kisspeptin-GPR54 Signaling Promotes Proper Sexual Differentiation
Sexual differentiation of the male brain and the behaviors it controls occurs during critical developmental windows during which testosterone (or its metabolite estradiol) organizes sexually dimorphic neural populations. Recent findings indicate that the process of sexual differentiation is itself dependent upon functional kisspeptin-GPR54 signaling. Specifically, sexual differentiation towards the male phenotype is impaired in the genotypic male GPR54 KO mouse (49), with adult male GPR54 KOs displaying female-like characteristics in several sexually dimorphic traits, ranging from complex partner preference behaviors to neuroanatomical phenotypes in the forebrain to neuron numbers in the peripheral nervous system (49).
In mammals, mate selection and sexual partner preference are sexually dimorphic and influenced by social odor cues processed by the main and accessory olfactory systems (105). Both of these olfactory systems are sexually dimorphic and differentiated in early development under the influence of sex steroids (4, 5, 39, 106). We recently found that adult GPR54 KO males fail to display typical male-like olfactory partner preference behavior, despite chronic testosterone treatment in adulthood. Specifically, adult GPR54 KO males behaved similarly to wildtype female mice when choosing a conspecific partner based on simultaneous access to volatile, non-volatile, and visual cues of stimulus males and females (49). This finding suggests an essential role for kisspeptin-GPR54 signaling in the development and/or display of this sexually dimorphic olfactory preference behavior. Importantly, the female-like preference behavior of GPR54 KO males is not due to anosmia, since GPR54 KO and wildtype males perform similarly in a “hidden cookie test” (49).
Like olfactory partner preference, TH and Kiss1 neurons are sexually-differentiated in response to organizational effects of sex steroids, with females having more TH and Kiss1 neurons in the AVPV than males (51, 90). Similar to olfactory preference behavior, the sexual differentiation of both of these hypothalamic systems was impaired in the absence of kisspeptin-GPR54 signaling. Specifically, the number of TH and Kiss1 neurons in the AVPV of adult GPR54 KO males was greater than that of wildtype males (and similar to that of females) (49), indicating demasculinization and/or feminization of these two neuronal systems. These data support the conjecture that developmental kisspeptin-GPR54 signaling is required for proper male-like sexual differentiation of the brain. Similarly, lack of GPR54 signaling resulted in the impaired development of motoneurons in the spinal nucleus of the bulbocavernosus (SNB), a well-characterized, androgen-dependent sexually dimorphic trait in rodents (5, 6). In adult GPR54 KO males, the number of SNB motoneurons was significantly lower than that of wildtype males and similar to that of wildtype females (49), further suggesting reduced androgen activity in KO animals sometime in early development. Collectively, the findings in preference behavior, TH and Kiss1 neuroanatomy, and spinal cord motoneuron number indicate that kisspeptin-GPR54 signaling during the perinatal period is essential for proper sexual differentiation in normal males, likely through direct or indirect stimulation of testosterone production during the developmental “critical period”.
Although adult GPR54 KO animals of both sexes have markedly diminished reproductive axis (29, 83)(49), including low to absent sex steroid levels, the inability of GPR54 KO males to exhibit normal male-like phenotypes in sexually-dimorphic traits is not due to lack of activational adult testosterone, because all animals were testosterone-replaced in adulthood prior to testing (49). Thus, the female-like phenotypes in the neuroanatomy and olfactory preference behavior of adult GPR54 KO males are likely attributable to impairments in the sexual differentiation process. Despite this conclusion, the exact mechanism of kisspeptin-GPR54 signaling in the perinatal differentiation process is unclear. Given the established role of GPR54 in controlling GnRH secretion in adulthood, one likely possibility is that neural kisspeptin-GPR54 signaling during the developmental critical window is required for GnRH-mediated androgen secretion which occurs in perinatal males; the foregoing conclusion would also suggest that central kisspeptin-GPR54 signaling likely regulates GnRH secretion during early postnatal life, just as it does in adulthood. However, another possibility is that the sex specific perinatal androgen production that occurs in males depends on peripheral kisspeptin-GPR54 signaling occurring in the testis itself, independent of kisspeptin’s “standard” mode of regulation of GnRH secretion in the brain. This intra-testis mechanism is possible, given that both Kiss1 and GPR54 are expressed in the testis, at least in adulthood (54, 73); on the other hand, whether or not these proteins are actually present in the testis during the critical perinatal window of development remains to be determined, as would the mechanism of how GPR54 activation in the testis would result in androgen secretion. Lastly, it is conceivable that perinatal kisspeptin-GPR54 signaling “downstream” of androgen secretion, at the level of developing neurons, is also important for sexual differentiation. However, this mechanism seems less likely as it would require that GPR54 (and kisspeptins) be present during the critical developmental windows at each of the specific sites of sexual differentiation, including the AVPV, the SNB motoneurons, and some component(s) of the sexually dimorphic neuronal circuitry underlying olfactory partner preference. Thus, the most parsimonious explanation is that kisspeptin-GPR54 signaling is necessary for proper gonadal steroid secretion in males during the perinatal critical period, and hence, for proper male-like sexual differentiation.
In rodents, there are two well-established periods of androgen secretion in perinatal males: one occurs during the last few days of gestation, typically ending 1-2 days before birth, and another occurs on the day of birth and lasting for several hours (the total duration of which is species-specific) (15, 67, 75). It is unclear if kisspeptin-GPR54 signaling is involved in regulating the prenatal and/or postnatal surges. Late gestational androgen secretion may be intact in GPR54 KO males, as indicated by the presence of male genitalia and accessory sex organs, along with “normal” male-like anogenital distance at the time of birth (all of which are androgen-dependent) (49). In contrast, significant differences between wildtype and GPR54 KO mice in anogenital distance at 3 weeks of age (49) suggest that postnatal androgen secretion, or its actions, is deficient in GPR54 KO males. Our lab is currently testing this conjecture by measuring postnatal androgen levels in mice of the two genotypes.
Although kisspeptin-GPR54 signaling is involved in puberty onset and reproduction in other non-mouse species, such as sheep or humans, the role of the Kiss1 system in perinatal development and sexual differentiation of the brain/behavior in species other than mice has not been studied. Analysis of kisspeptin signaling effects during sexual differentiation is especially challenging in humans since most male patients with GPR54 mutations are not evaluated until puberty or later (i.e., well after the “critical window’ of sexual differentiation that occurs in early development). Recently, however, one infant with a mutated GPR54 gene was identified at birth and noted to possess a micropenis and cryptorchidism, suggesting reduced androgen exposure during development (86). Whether or not this particular individual, or other humans with similar impairments in kisspeptin-GPR54 signaling, displays alterations in sexually dimorphic phenotypes (brain, anatomy, and/or behavior) in adulthood has not yet been assessed and awaits further clinical investigation.
6. Summary and Perspectives
Recent investigations in many species have yielded a wealth of information detailing the role of kisspeptin-GPR54 signaling in the regulation of reproduction, including, more recently, the bidirectional relationship of the Kiss1 system with sexual differentiation of the brain. Despite being aware for decades of sex differences in the ability of mammals to generate an estradiol-induced GnRH/LH surge, the phenotypic identity and location of the sex steroid-sensitive neurons in the brain that govern the GnRH/LH surge in females, but not males, has remained elusive. Recent evidence in mice and rats now suggest that Kiss1 neurons in the AVPV are sexually-differentiated under the direction of sex steroids early in perinatal development, and that this sexually-differentiated population of Kiss1 cells provides the cellular mechanism for inducing the GnRH/LH surge that occurs in females; likewise, the absence of sufficient Kiss1 expression in the AVPV of adult male may explain their inability to display such a surge. Furthermore, recent evidence suggests that kisspeptin signaling to its receptor, GPR54, is critical for normal sexual differentiation of the central and peripheral nervous systems, as well as certain sexually dimorphic behaviors, perhaps by regulating developmental androgen secretion in perinatal males.
Despite these advances in the fields of kisspeptin biology and sexual differentiation, many questions and challenges remain. Among these are 1) learning how sex steroids influence the development of Kiss1 neurons in the forebrain (apoptosis and programmed cell death or permanent inhibition of Kiss1 gene activation?); 2) determining the degree of interaction, if any, between the two independent sexually dimorphic populations of TH and Kiss1 neurons in the AVPV, and their functional relation to the GnRH/LH surge; 3) elucidating the presence (or absence) of sex differences in Kiss1 neurons in the ARC in species other than mice and sheep, and the functional role of such an ARC Kiss1 sexual differentiation; 4) understanding the role of kisspeptin-GPR54 signaling in timing the perinatal androgen surge that occurs in developing males but not females; and 5.) determining the relationship between the kisspeptin system and sexual differentiation in humans.
Acknowledgments
The author’s research is supported by NICHD grant K99 HD056157
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee I, Clayton P. The genetic basis for the timing of human puberty. J Neuroendocrinol. 2007;19:831–838. doi: 10.1111/j.1365-2826.2007.01598.x. [DOI] [PubMed] [Google Scholar]
- 3.Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- 4.Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- 5.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- 6.Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–181. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- 7.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139:1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Coolen LM, Reale M, Goodman RL, Lee TM, Padmanabhan V, Lehman MN. Sex differences in kisspeptin neurons in the sheep hypothalamus. Society for Neuroscience Annual Meeting #194.14 2007 [Google Scholar]
- 9.Clarke IJ, Pompolo S, Scott CJ, Rawson JA, Caddy D, Jakubowska AE, Pereira AM. Cells of the arcuate nucleus and ventromedial nucleus of the ovariectomized ewe that respond to oestrogen: a study using Fos immunohistochemistry. J Neuroendocrinol. 2001;13:934–941. doi: 10.1046/j.1365-2826.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colledge WH. GPR54 and Kisspeptins. Results Probl Cell Differ. 2008 doi: 10.1007/400_2007_050. [DOI] [PubMed] [Google Scholar]
- 12.Connolly PB, Resko JA. Prenatal testosterone differentiates brain regions controlling gonadotropin release in guinea pigs. Biol Reprod. 1994;51:125–130. doi: 10.1095/biolreprod51.1.125. [DOI] [PubMed] [Google Scholar]
- 13.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 14.Corbier P. Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology. 1985;116:142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- 15.Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- 16.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 17.Crowley WR, Kalra SP. Neonatal exposure to estradiol prevents the expression of ovarian hormone-induced luteinizing hormone and prolactin surges in adulthood but not antecedent changes in neuropeptide Y or adrenergic transmitter activity: implications for sexual differentiation of gonadotropin secretion. Brain Res. 1994;663:257–265. doi: 10.1016/0006-8993(94)91271-8. [DOI] [PubMed] [Google Scholar]
- 18.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 22.Dhillo WS, Murphy KG, Bloom SR. The neuroendocrine physiology of kisspeptin in the human. Rev Endocr Metab Disord. 2007;8:41–46. doi: 10.1007/s11154-007-9029-1. [DOI] [PubMed] [Google Scholar]
- 23.Dorner G, Rohde W, Schnorr D. Evocability of a slight positive oestrogen feedback action on LH secretion in castrated and oestrogen-primed men. Endokrinologie. 1975;66:373–376. [PubMed] [Google Scholar]
- 24.Dungan HM, Gottsch ML, Lawhorn JK, Byquist AC, Kauffman AS, Clifton DK, Steiner RA. Role of kisspeptin-GPR54 signaling in the negative and positive feedback control of GnRH/LH secretion in the mouse. 89th Annual Meeting of the Endocrine Society; #OR8-2 2007 [Google Scholar]
- 25.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 26.Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18:806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferin M, Carmel PW, Zimmerman EA, Warren M, Perez R, Vande Wiele RL. Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female Rhesus monkey. Endocrinology. 1974;95:1059–1068. doi: 10.1210/endo-95-4-1059. [DOI] [PubMed] [Google Scholar]
- 28.Finn PD, McFall TB, Clifton DK, Steiner RA. Sexual differentiation of galanin gene expression in gonadotropin-releasing hormone neurons. Endocrinology. 1996;137:4767–4772. doi: 10.1210/endo.137.11.8895345. [DOI] [PubMed] [Google Scholar]
- 29.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 30.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogan F, Beattie IA, Hery M, Laplante E, Kordon D. Effect of neonatal administration of steroids or gonadectomy upon oestradiol-induced luteinizing hormone release in rats of both sexes. J Endocrinol. 1980;85:69–74. doi: 10.1677/joe.0.0850069. [DOI] [PubMed] [Google Scholar]
- 32.Gogan F, Slama A, Bizzini-Koutznetzova B, Dray F, Kordon C. Importance of perinatal testosterone in sexual differentiation in the male rat. J Endocrinol. 1981;91:75–79. doi: 10.1677/joe.0.0910075. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of GnRH1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory LH surges. Endocrinology. 2008 doi: 10.1210/en.2007-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman RL. The site of the positive feedback action of estradiol in the rat. Endocrinology. 1978;102:151–159. doi: 10.1210/endo-102-1-151. [DOI] [PubMed] [Google Scholar]
- 35.Goodman RL, Inskeep EK. Neuroendocrine control of the ovarian cycle of the sheep. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Elsevier; Boston: 2006. pp. 2389–2448. [Google Scholar]
- 36.Gorski RA. Gonadal hormones and the development of neuroendocrine functions. In: FGW LM, editor. Frontiers in Neuroendocrinology. Oxford University Press; New York: 1971. pp. 273–290. [Google Scholar]
- 37.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 38.Goubillon M, Delaleu B, Tillet Y, Caraty A, Herbison AE. Localization of estrogen-receptive neurons projecting to the GnRH neuron-containing rostral preoptic area of the ewe. Neuroendocrinology. 1999;70:228–236. doi: 10.1159/000054481. [DOI] [PubMed] [Google Scholar]
- 39.Guillamon A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44:377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagon MM, Castano JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 41.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA. Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod. 1985;32:855–864. doi: 10.1095/biolreprod32.4.855. [DOI] [PubMed] [Google Scholar]
- 43.Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Elsevier; Boston: 2006. pp. 1415–1482. [Google Scholar]
- 44.Hiden U, Bilban M, Knofler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord. 2007;8:31–39. doi: 10.1007/s11154-007-9030-8. [DOI] [PubMed] [Google Scholar]
- 45.Hoffman GE, Le WW, Schulterbrandt T, Legan SJ. Estrogen and progesterone do not activate Fos in AVPV or LHRH neurons in male rats. Brain Res. 2005;1054:116–124. doi: 10.1016/j.brainres.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 46.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin Activation of Gonadotropin Releasing Hormone Neurons and Regulation of KiSS-1 mRNA in the Male Rat. Neuroendocrinology. 2005;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 47.Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference bewteen primates and rodents. Science. 1973;179:484–486. doi: 10.1126/science.179.4072.484. [DOI] [PubMed] [Google Scholar]
- 48.Karsch FJ, Foster DL. Sexual differentiation of the mechanism controlling the preovulatory discharge of luteinizing hormone in sheep. Endocrinology. 1975;97:373–379. doi: 10.1210/endo-97-2-373. [DOI] [PubMed] [Google Scholar]
- 49.Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova M, Rhode AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. Journal of Neuroscience. 2007;27:8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 52.Kauffman EC, Robinson VL, Stadler WM, Sokoloff MH, Rinker-Schaeffer CW. Metastasis suppression: the evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J Urol. 2003;169:1122–1133. doi: 10.1097/01.ju.0000051580.89109.4b. [DOI] [PubMed] [Google Scholar]
- 53.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 54.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 55.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Wei Le W, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007 doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 56.Le WW, Berghorn KA, Rassnick S, Hoffman GE. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology. 1999;140:510–519. doi: 10.1210/endo.140.1.6403. [DOI] [PubMed] [Google Scholar]
- 57.Le WW, Attardi B, Berghorn KA, Blaustein J, Hoffman GE. Progesterone blockade of a luteinizing hormone surge blocks luteinizing hormone-releasing hormone Fos activation and activation of its preoptic area afferents. Brain Res. 1997;778:272–280. doi: 10.1016/s0006-8993(97)00971-2. [DOI] [PubMed] [Google Scholar]
- 58.Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O’Dowd BF. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 60.Lee KJ, Maizlin II, Clifton DK, Steiner RA. Coexpression of tyrosine hydroxylase and KiSS-1 mRNA in the anteroventral periventricular nucleus of the female mouse. Society for Neuroscience 35th Annual Meeting Program No. 758.19 2005 [Google Scholar]
- 61.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 62.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 65.Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol. 2001;432:259–267. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- 66.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 67.Motelica-Heino I, Castanier M, Corbier P, Edwards DA, Roffi J. Testosterone levels in plasma and testes of neonatal mice. J Steroid Biochem. 1988;31:283–286. doi: 10.1016/0022-4731(88)90351-2. [DOI] [PubMed] [Google Scholar]
- 68.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 69.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 70.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 71.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- 72.Norman RL, Spies HG. Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology. 1986;118:2608–2610. doi: 10.1210/endo-118-6-2608. [DOI] [PubMed] [Google Scholar]
- 73.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 74.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pang SF, Tang F. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. J Endocrinol. 1984;100:7–11. doi: 10.1677/joe.0.1000007. [DOI] [PubMed] [Google Scholar]
- 76.Pau KY, Gliessman PM, Hess DL, Spies HG. Effects of estrogen on hypothalamic gonadotropin-releasing hormone release in castrated male rhesus macaques. Brain Res. 1988;459:70–75. doi: 10.1016/0006-8993(88)90287-9. [DOI] [PubMed] [Google Scholar]
- 77.Pfeiffer CA. Sexual differences of the hypophyses and their determination by the gonads. Am J Anat. 1936;58:195–226. [Google Scholar]
- 78.Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol. 2006;155(Suppl 1):S11–6. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- 79.Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- 80.Roa J, Tena-Sempere M. KiSS-1 system and reproduction: comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007;153:132–140. doi: 10.1016/j.ygcen.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 81.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and Increased Kisspeptin Gene Expression in the Hypothalamic Infundibular Nucleus of Postmenopausal Women and Ovariectomized Monkeys. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 82.Scott CJ, Kuehl DE, Ferreira SA, Jackson GL. Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology. 1997;138:3686–3694. doi: 10.1210/endo.138.9.5401. [DOI] [PubMed] [Google Scholar]
- 83.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 84.Seminara SB. Mechanisms of Disease: the first kiss-a crucial role for kisspeptin-1 and its receptor, G-protein-coupled receptor 54, in puberty and reproduction. Nat Clin Pract Endocrinol Metab. 2006;2:328–334. doi: 10.1038/ncpendmet0139. [DOI] [PubMed] [Google Scholar]
- 85.Seminara SB. Kisspeptin in reproduction. Semin Reprod Med. 2007;25:337–343. doi: 10.1055/s-2007-984739. [DOI] [PubMed] [Google Scholar]
- 86.Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O’Rahilly S, Aparicio SA. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- 87.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19:432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 90.Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- 91.Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- 92.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 93.Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smith ER, Davidson JM. Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology. 1974;95:1566–1573. doi: 10.1210/endo-95-6-1566. [DOI] [PubMed] [Google Scholar]
- 95.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 96.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 97.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 98.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 99.Smith JT, Pereira A, Rao A, Morgan K, Millar RP. Evidence That Pituitary Gonadotropes Are Not a Major Target of Kisspeptin. 89th Annual Meeting of the Endocrine Society. 2007:P1–393. [Google Scholar]
- 100.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood, but does not increase during the preovulatory luteinizing hormone surge: Evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2007 doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Anim Reprod Sci. 2007 doi: 10.1016/j.anireprosci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Terasawa E, Wiegand SJ, Bridson WE. A role for medial preoptic nucleus on afternoon of proestrus in female rats. Am J Physiol. 1980;238:E533–9. doi: 10.1152/ajpendo.1980.238.6.E533. [DOI] [PubMed] [Google Scholar]
- 104.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 105.Vandenbergh JG. Pheromones and Mammalian Reproduction. In: Neill JD, editor. Knobil and Neill’s Phsyiology of Reproduction. 3. Elsevier; Boston: 2006. pp. 2041–2058. [Google Scholar]
- 106.Weruaga E, Brinon JG, Porteros A, Arevalo R, Aijon J, Alonso JR. A sexually dimorphic group of atypical glomeruli in the mouse olfactory bulb. Chem Senses. 2001;26:7–15. doi: 10.1093/chemse/26.1.7. [DOI] [PubMed] [Google Scholar]
- 107.West A, Vojta PJ, Welch DR, Weissman BE. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1) Genomics. 1998;54:145–148. doi: 10.1006/geno.1998.5566. [DOI] [PubMed] [Google Scholar]
- 108.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 109.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 110.Wiegand SJ, Terasawa E, Bridson WE. Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology. 1978;102:1645–1648. doi: 10.1210/endo-102-5-1645. [DOI] [PubMed] [Google Scholar]
- 111.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]