Abstract
Environmental and occupational exposure to lead (Pb) remains to be a major public health issue. The purpose of this cross-sectional study was to use non-invasive magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (1H MRS) techniques to investigate whether chronic exposure to Pb in an occupational setting altered brain structure and function of Pb-exposed workers. The Pb-exposed group consisted of 15 workers recruited from either a Pb-smelting factory or a Pb-battery manufacturer. The control group had 19 healthy volunteers who had no history of Pb exposure in working environment or at home. The average airborne Pb concentrations in fume and dust were 0.43 mg/m3 and 0.44 mg/m3, respectively in the smeltery, and 0.10 mg/m3 and 1.06 mg/m3, respectively in the Pb battery workshop. The average blood Pb concentrations (BPb) in Pb-exposed and control workers were 63.5 µg/dL and 8.7 µg/dL, respectively. The MRI examination showed that brain hippocampal volume among Pb-exposed workers was significantly diminished in comparison to age-matched control subjects (p<0.01), although the extent of this reduction was relatively small (5–6% of the control values). Linear regression analyses revealed significant inverse associations between BPb and the decreased hippocampal volume on both sides of brain hemisphere. Among five brain metabolites investigated by MRS, i.e., N-acetyl-aspartate (NAA), creatine (Cr), choline (Cho), inosine (mI), glutamate/glutamine (Glx) and lipids (Lip), a significant decrease in NAA/Cr ratio (7% of controls, p<0.05) and a remarkable increase in Lip/Cr ratio (40%, p<0.01) were observed in the brains of Pb-exposed workers as compared to controls. Furthermore, the increased Lip/Cr ratio was significantly associated with BPb (r = 0.46, p<0.01). Taken together, this study suggests that occupational exposure to Pb may cause subtle structural and functional alteration in human brains. The MRI and MRS brain imaging techniques can be used as the noninvasive means to evaluate Pb-induced neurotoxicity.
Keywords: lead, magnetic resonance imaging, magnetic resonance spectroscopy, occupational exposure, hippocampus, brain metabolites, BPb, N-acetyl-aspartate, lipids
Introduction
By intensive academic research and advocacy, aggressive legislative measures and growing public awareness, the use of lead (Pb) in gasoline and other domestic products has been considerably reduced, if not completely eliminated, in this as well as many other countries. However, even with an indicative trend toward a declining environmental Pb during the past decades (Jacobs et al., 2006; Ona et al., 2006; Parekh et al., 2002; Singh et al., 2006), Pb toxicity remains to be a public health issue, both environmentally and occupationally, for its residual levels in inner cities in the U.S. and for its continuous use as an important industrial ingredient essential to manufacture of many materials in modern society. Thus, investigation of Pb-induced neurotoxicity, particularly among high risk populations such as workers with long-term, low-dose exposure in Pb production or utilization, remains to be a daunting and indispensable task in years to come.
Chronic Pb exposure is known to cause neurological dysfunctions, mainly by damaging the cognitive function. As a metal, Pb ions readily pass across the blood-brain barrier and accumulate in particular brain regions such as hippocampus, resulting in highly selective injury in particular brain regions (Zheng, 2001). Pb also accumulates in the choroid plexus, a brain tissue adjacent to the hippocampal formation and actively participating in regulation of brain homeostasis of chemicals, metabolites and endogenous polypeptides such as beta-amyloids (Zheng et al., 1991, 1996, 2001). Recent evidences indicate that the maternal exposure to Pb in early life may lead to an altered expression of amyloid precursor protein (APP) in later life (Wu et al., 2008; Zawia et al., 2005). Since the question as to whether or not Pb exposure is associated with the onset of Alzheimer’s Disease (AD) remains unanswered, there is a need to investigate the impact of long-term, low-dose exposure to Pb on brain structures and functions that are pertinent to the pathogenesis of AD. To this end, the rapidly developed magnetic resonance imaging (MRI) and spectroscopy (MRS) techniques offer a unique non-invasive, real-life diagnostic advantages.
Both MRI and MRS use the same principles of nuclear magnetic resonance (NMR) to create images. The MRS reveals signals in spectra curves within the same space. The peak values of MRS signals, under a well-controlled condition, represent the contents of chemical species investigated). Studies conducted on AD patients have showed that the coronal cross-session MR images of patient’s brain possess a clear evidence of atrophy in the hippocampal formation and gray matter in posterior temporoparietal and occipital cortex. Thus, a reduction in these areas has been suggested to be a valuable subsidiary index in the diagnosis of AD (Prince et al., 2008; Rabinovici et al., 2008).
More recently, the longitudinal change in 1H MRS metabolite markers has been introduced to characterize the disease progression in AD (Clark et al., 2008; Huang et al., 2001; Kantarci et al., 2007). For example, N-acetylaspartate (NAA), a molecule synthesized in neurons from the amino acid aspartate and acetyl-coenzyme A, has been used as a neuronal integrity marker. To reflect precisely the neuronal metabolic activity in a given volume, the total creatine (Cr) including creatine and creatine phosphate has been used as an internal standard for its relatively stable concentration in both physiological and pathological statuses. Thus, the ratio of NAA/Cr in MRS indicates the neuronal metabolic function, and a reduction of this ratio has been associated with damage or degeneration of neuronal and/or axonal structures in AD (Jeffon et al., 2000; Pametti et al., 1997; Schuff et al., 2002; Shinno et al., 1993, 2007; Van et al., 1992). Choline (Cho) is the precursor of acetylcholine and phospholipidoyl choline. Its status as Cho/Cr has been used to indicate the memory and cognitive activity (with acetylcholine) and myelin sheath integrity (with phospholipidoyl choline) (Clark et al., 2008; Mackay et al., 1996). Nucleoside inosine (mI) plays an important role in translating the genetic code, participating in purine metabolism and maintaining the membrane integrity. A declined MRS ratio of mI/Cr has been associated with several degenerative diseases (Brand et al., 1993; Ross et al., 1991; Shonk et al., 1995). Glutamate and glutamine (Glx) are the indispensible molecular for a variety of neuronal activities. A declined Glx/Cr ratio has been seen in AD brains (Antuono et al., 2001; Ross, 1991). Moreover, lipids (Lip) is an essential component of cellular membrane with the phospholipids binding to saturated and unsaturated fatty acids. An injured cell membrane or myelin sheath can release the free lipids. In clinics, an increased MRS Lip/Cr ratio has been found in metastatic brain tumors (Chernov et al., 2006). Since Pb intoxication may alter brain functions by affecting these brain metabolites, it would be interesting to investigate the status of these metabolites in specific brain regions of Pb-exposed active workers by non-invasive MRI and MRS techniques.
The purpose of this study was to determine whether chronic exposure to Pb in occupational settings altered brain structure and function among active workers. We chose a Pb smelting factory and Pb-containing battery manufacturer for this study for their demonstrated high levels of airborne Pb in working environment and historical Pb intoxication cases. A control group was established with subjects who did not involve in Pb-related production and had not history of Pb exposure in their jobs or daily life. Specifically, this cross-sectional study was designed (1) to determine the airborne Pb levels in working environment for purpose of recruitment of Pb-exposed workers and control subjects, (2) to collect blood samples and conduct physical examination on subjects, (3) to use well-established MRI/MRS method (Jiang et al., 2007) to determine the hippocampal volume and brain metabolites including NAA, Cho, mI, Glx and Lip among study participants, and (4) to investigate whether alterations in MRI and MRS outcomes were associated with blood Pb concentration (BPb).
Subjects and Methods
Study Population
This cross-sectional study was conducted among Pb-exposed workers recruited from a smelting factory and a battery manufacturer. The smelting factory has more than 100 workers and has produced mainly lead products since 1992. The battery factory has the size of the more than 200 workers to manufacture Pb-containing batteries for automobiles and has been in operation since 1964. A total of 15 Pb-exposed workers (7 from smelting plant and 8 from battery factory) were recruited to this study as the Pb-exposed group. A group of 19 control workers, matched with age, sex, years of employment and other socioeconomic status (i.e., salary and education, etc.), were recruited from a slaughter house or other factories with no history of exposure to airborne Pb in working environment or at home. The control workers were in good health with no reported diseases.
Collection of Air Samples, Personal Data and Biological Samples
The air samples in workplace were collected during routine annual industrial environmental safety inspection in 2006. Two primary locations in the factories, one near sintering and smelting sites for Pb in fume and the other close to charging and fragmenting sites for Pb in dust, were identified as the monitor sites according to the positions where workers usually worked. The airborne Pb concentrations were determined in the breathing zone of the workers by station air samplers. Air samples were collected by a Model BFC-35 D air pump (No. 2 Factory of Jianhu Electron Instrument, Jiangsu, China) equipped with a micro-porous filter, which has a diameter of 40 mm and the pore size of 0.8 um. Air flow was pumped at a flow rate of 5 L/min for 15 min every hour for 4 hours.
All study participants were invited to the First Affiliated Hospital for interview, physical examination and MRI/MRS study. Prior to interview, a written informed consent form was obtained from all study participants. A scheduled interview lasting approximately 60 min was conducted by trained interviewers to obtain detailed information on occupational history, job description, socioeconomic status, lifestyle, and family and personal medical history. The questionnaire also includes self-reported symptoms such as headache, dizziness, nausea, pain in abdomen area, decreased food appetite, deteriorated memory, insomnia and sleep with abnormally high frequency of dreams, lethargy, acting slowly, emotional labiality, fatigue/weakness and limb numbness.
Blood samples were collected in the morning of the day. A volume (5 mL) of venous blood was drawn from a cubital vein. An aliquot (0.5 mL) of whole blood was added to a heparin-contained test tube, followed by thorough mixing. The remaining blood samples were used to separate serum by standing at room temperature for 1 hour, followed by low speed centrifugation. All samples were stored at −80 °C until analyses. All test tubes used in the study were free of metal contamination, as pre-tested by atomic absorption spectrophotometry (AAS).
Determination of Pb Concentrations in Air and Blood Samples
To determine airborne Pb concentrations, the filters were digested with 5 mL of HClO4-HNO3 mixture (1:9 vol/vol) at 200 °C. The dry residues were dissolved in 10 mL of 1% HCl. The solutions were diluted by 20–50 fold and quantified by graphite furnace atomic absorption spectrophotometer (AAS) with a model AA6800 SHIMADZU AAS (Japan), according to a Chinese National Standard Operation Protocol (GB/T16018-1995) for occupational safety surveillance.
Concentrations of Pb in the whole blood (BPb) were quantified following the same standard protocol (Barbosa et al., 2006; Hertz-Picciotto et al., 2000). The standard curves were established using freshly made Pb standards on the day of analysis. The detection limit for this method was 0.2 ng Pb/mL of assay solution with an intra-day variation <5% and inter-day < 9%.
MRI and 1H MRS Examinations
MRI and 1H MRS studies were performed, blinded to any clinical information, including personal data and group identification, on a Philips Achieva 3.0 T whole body MRI scanner (Philips Healthcare, The Netherlands) with a quadrature transmit-receive coil. Axial T1-weighted, T2-weighted, fluid attended inversion recovery (FLAIR), sagittal T1-weighted and diagonal coronal T1-weighted images, vertical to the long axis of hippocampal formation, as well as 1H-MRS data were obtained from each subject.
To obtain routine MRI data, the following parameters were used: for axial fast spin echo (FSE) T2-weighted images, repetition time (TR) = 4000 ms (millisecond), echo time (TE) = 102 ms, thickness = 8 mm, interslice gap = 2 mm; for axial T1 FLAIR, Tr = 2000 ms, TE = minimum, inversion time (TI) = 750 ms, thickness = 8 mm, interslice gap = 2 mm; for sagittal and coronal T1 FLAIR, TR = 2000 ms, TE = minimum, TI = 750 ms, thickness = 5 mm, interslice gap = 1 mm. The postero-terminal line and anterior border of the scanner field were respectively set at the pone of fornix feet and temporal pole, including the entire hippocampus with a field of vision (FOV) 230~240 mm2.
Upon obtaining brain T1-weighted images, the location of the volume of interest (VOI) for the MRS measurement was decided based on the boundary between the head and body of hippocampus. The VOI was adjusted at 3 mm within the anterior border of cerebral peduncle, the outsiders at 3 mm within the temporal horn of lateral ventricle, and the lower boundary at 1 mm within the inferior border of hippocampal head (Webb et al., 1999).
For preliminary scan in spectra collection, the stimulated echo acquisition method (STEAM) sequence was applied to shimming within VOI according to the selected collected sequences, in order to make the half-height and entire width of water peak minimum to match the requirement of 8 Hz (0.1 ppm, ppm means quality fraction, ×106). According to the water resonance frequency in preliminary scan, the computer automatically adjusted the impulse angle of collected sequences to obtain the best effect of water-inhibition.
To obtain 1H-MRS, the referred graphs of anatomical allocation were collected by using spin echo pulse sequence with the following parameters: Tr = 440 ms and Te = 20 ms. The STEAM with the parameters of Tr = 2000 ms, Te = 28 ms, and stimulated times = 128 was applied to collect 1H-MRS. The peaks of N-acetylaspartate (NAA), creatine (Cr), choline (Cho), Glutamate/Glutamine (Clx), inositol (mI) and lipids (Lip) detected by STEAM sequence were identified at 2.0, 3.0, 3.2, 3.78, 3.56, 0.9/1.3 ppm, respectively.
Analyses of Imaging Data
To determine hippocampal formation volume, the borderline of hippocampus in diagonal coronal T1-weighted image was drawn by a computer mouse from hippocampal head to the tail (the postero-terminal line of hippocampal tail was set at fornix feet). An MASS software was used to calculate the volume, which was standardized by Cende’s method reported in literature (Bernasconi et al., 2003; Jack et al., 1989, 1992; Teipel et al., 2006). The following formula was used to calculate the parameters:
where HFV is hippocampal formation volume, CA represents the coronal plane area, and t is the thickness. The intra-calvarium volume was calculated with the same method in transaction. In order to obtain precise volumes, the procedure for the same formation was repeated for 3 times and the average values were used for calculation.
1H MRS data were analyzed using the ”SpectroView” software that comes with the equipment. The baseline and phases of the obtained spectral line were adjust to the same level and the peak heights of each metabolite were then obtained. The ratios of NAA/Cr, Cho/Cr and so on were calculated by using the peak height of Cr as the reference.
Statistical Analyses
Records of interviews and other reports were reviewed and abstracted for demographic data. All data are expressed as the mean ± SD. Comparison of means in Table 1 and 3 was accomplished by using one-way analysis of variance (ANOVA). For data presented in Table 2, a repeated measure of the generalized linear model (GLM) was used to determine the significant differences between the groups. Linear regression analyses were applied to determine the relationship between BPb and MRI/MRS variables and the Pearson correlation coefficients were obtained accordingly. A statistical software SPSS/PC+ for Windows (V. 13.0) was used in data analysis.
Table 1.
Summary of Demographic information
| Control (n=19) | Cases (n=15) | |
|---|---|---|
| Age (year) (95% CI) | 36.7±9.5 (32.1~41.3) | 35.5±7.9 (31.1~39.7) |
| Sex (male/female) (95% CI) | 15/4 | 13/2 |
| Year of employment (year) (95% CI) | 12.32±9.53 (7.7~16.9) | 16.60±8.54 (11.9~21.3) |
| Education (year) (95% CI) | 14.11±0.737 (13.7~14.5) | 13.67±0.617 (13.3~14.0) |
| Cigarette Smoking | 7 (36.8%) | 6 (40.0%) |
| Alcohol | 14 (73.7%) | 10 (66.7%) |
| Air Pb fume level (mg/m3)# | 0.00 | 0.24±0.32 (0.01~1.25)** |
| Air Pb dust level (mg/m3)# | 0.00 | 0.98±1.29 (0.01~3.97)** |
| BPb (µg/dL) (95% CI) | 8.74±1.91 (6.1~12.2) | 63.5±20.9 (17.4~89.6) ** |
p<0.01 compared with the control group.
Note: for airborne Pb levels in working environment, sample numbers were 15, 17 and 10 for Pb in fume, Pb in dust and airborne Pb in control sites, respectively.
Table 3.
Comparisons of brain metabolite contents by MRS between Pb-exposed and control workers
| Group | n | NAA/Cr (95% CI) |
Cho/Cr (95% CI) |
mI/Cr (95% CI) |
Lip/Cr (95% CI) |
Glx/Cr (95% CI) |
|---|---|---|---|---|---|---|
| Control | 19 | 1.314±0.131 (1.252~1.377) |
0.949±0.106 (0.898~1.000) |
0.615±0.094 (0.570~0.662) |
0.264±0.094 (0.218~0.308) |
0.442±0.103 (0.392~0.491) |
| Pb-E | 15 | 1.222±0.082 (1.177~1.267) * |
0.892±0.107 (0.832~0.950) |
0.547±0.113 (0.484~0.610) |
0.369±0.101 (0.315~0.425) ** |
0.483±0.157 (0.396~0.570) |
Data represent mean +/− SD.
p<0.05
p<0.01 compared with the control group.
NAA: N-acetyl-aspartate, Cr: creatine, Cho: choline, mI: inosine, Lip: lipids, Glx: glutamate/glutamine
Table 2.
Comparison of hippocampal volumes by MRI between Pb-exposed and control workers
| Group | n | Left Hippocampus (cm3) (95% CI) | Right Hippocampus (cm3) (95% CI) | Total Hippocampus (cm3) (95% CI) |
|---|---|---|---|---|
| Control | 19 | 2.935±0.087 (2.89~2.98) | 3.093±0.079 (3.06~3.13) | 6.028±0.153 (5.96~6.10) |
| Pb-exposed | 15 | 2.793±0.137 (2.72~2.87)** | 2.911±0.038 (2.83~2.99)** | 5.704±0.278 (5.55~5.86)** |
Data represent mean +/− SD.
p<0.01 compared with the control group.
Materials
AAS standard for Pb was obtained from Alfa Products (Danvers, MA, USA). All reagents were of analytical grade, HPLC grade or the highest available pharmaceutical grade.
Results
Subjects and Exposure Assessment
All study participants were the Chinese Han origin. Of 15 cases and 19 controls, two and four in each respective group were females. Since both case and control groups were matched in age, sex, years of employment, education level, cigarette smoking, alcohol consuming and social-economical status, there were no significant differences between two groups observed for any of these parameters (Table 1).
The average Pb concentrations in fume and dust were 0.24 and 0.98 mg/m3, respectively, which were 8-fold and 19.6-fold more than the permissible concentration-time weighted average (PC-TWA) for Pb in the fume (0.03 mg/m3) and Pb in the dust (0.05 mg/m3) (Ye et al., 2006). The Pb concentrations in fume and dust in control sites were undetectable. Compared with the control BPb (8.74 µg/dL), BPb in Pb-exposed workers (63.5 µg/dL) was increased more than 7 fold (p< 0.01) (Table 1).
Among more than a dozen of self-reported symptoms, the following symptoms showed significant differences between the case and control (ratio of complaints/total workers): fatigue/weakness, 8/15 in cases vs. 0/19 in control (p<0.01 by χ2 test, same below), whole body aching pain, 6/15 in cases vs. 1/19 in control (p=0.039), and limb numbness, 4/15 in cases vs. 0/19 in control (p=0.029). Pb exposure appeared to cause recognizable neurological symptoms among the study population.
Pb Exposure and Changes in Hippocampal Volumes by MRI
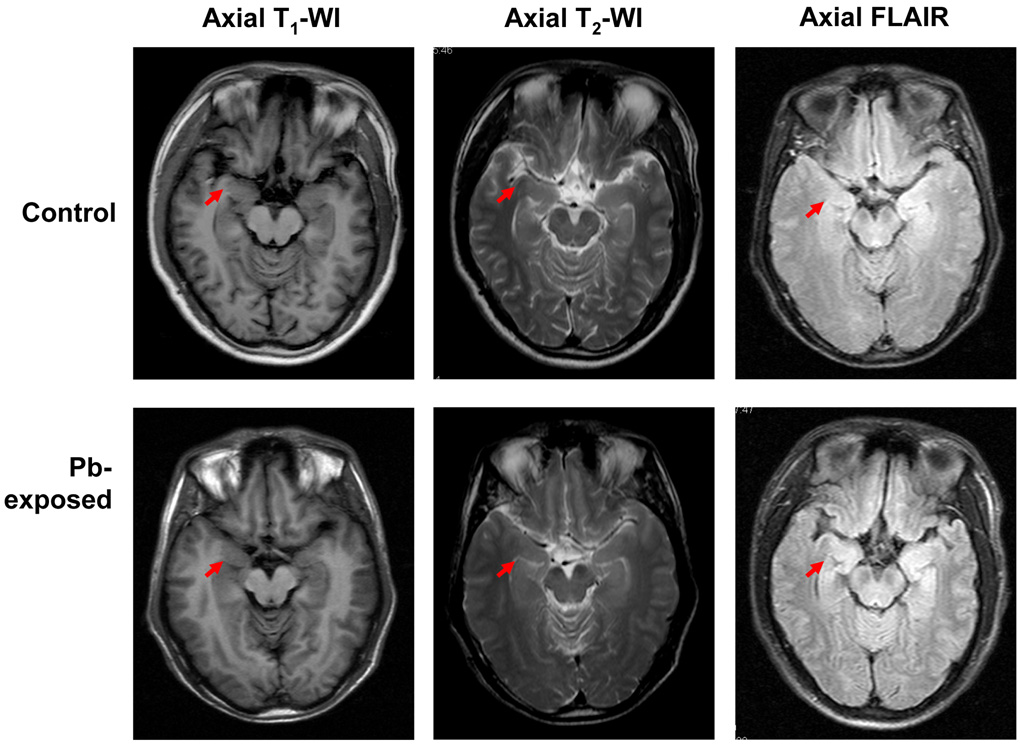
Fig. 1 illustrates the representative images of T1-weighted, T2-weighted or FLAIR MRI among study participants. There were no visible differences in these images between Pb-exposed workers and control subjects. However, when the hippocampal volumes were estimated bilaterally by MRI, the values were decreased by 5% and 6% for left and right hippocampus, respectively, in Pb exposed workers in comparison to those of controls (Table 2). While the percentages of these changes appeared to be relatively small, the statistical analysis indeed revealed that these changes were significantly different in Pb-exposed workers from the control subjects (p<0.01 for both determinants).
Fig. 1. MRI Images.
Representative MRI and fluid attended inversion recovery (FLAIR) of brain imaging of Pb-exposed workers and control subjects. No significant differences in MRI T1-WI, T2-WI and FLAIR were found between two study groups. Arrows indicate the hippocampal head.
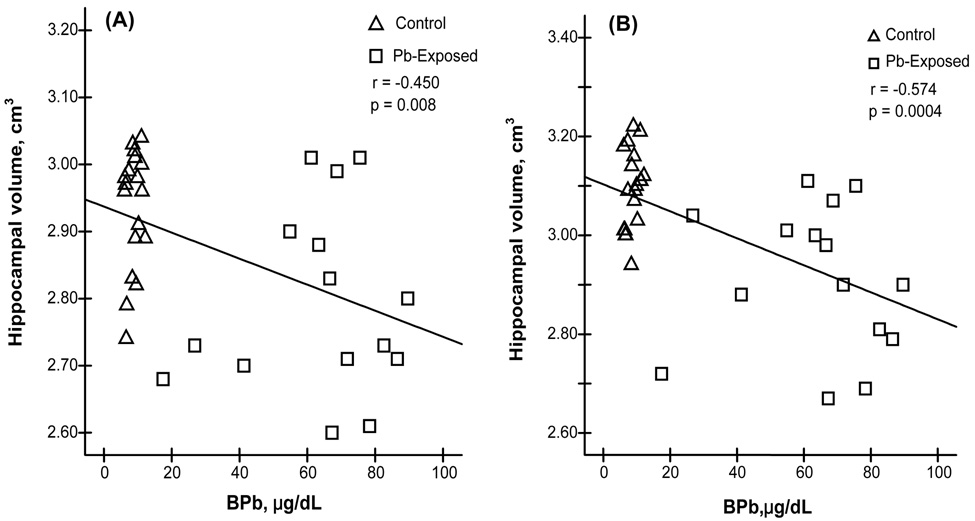
Since a reduced hippocampal volume was apparent in Pb-exposed workers, it became necessary to investigate whether the hippocampal volume was altered as the function of BPb. The linear regression analyses of pooled data from both control and Pb-exposed groups revealed that the decrease in the volumes of the left hippocampal formation was significantly associated with an increase in the BPb among all study participants (r=−0.450, p<0.01) (Fig. 2A), the same was true for changes in the volumes of the right hippocampal formation (r=−0.574, p<0.001) (Fig. 2B). However, when the control and Pb-exposed subjects were independently analyzed within each group, no significant correlations were found between BPb and changes in hippocampal volumes (data not shown).
Fig. 2. Changes of Hippocampal Volume as the Function of BPb.
Reduction of hippocampal volumes as the function of increased BPb. Linear regression analysis revealed significant inverse correlations (A) between BPb and the left hippocampal volume (r = −0.450, p <0.01) and (B) between BPb and the right hippocampal volume (r = −0.574, p < 0.01).
Pb Exposure and Changes of Hippocampal Metabolism by 1H-MRS
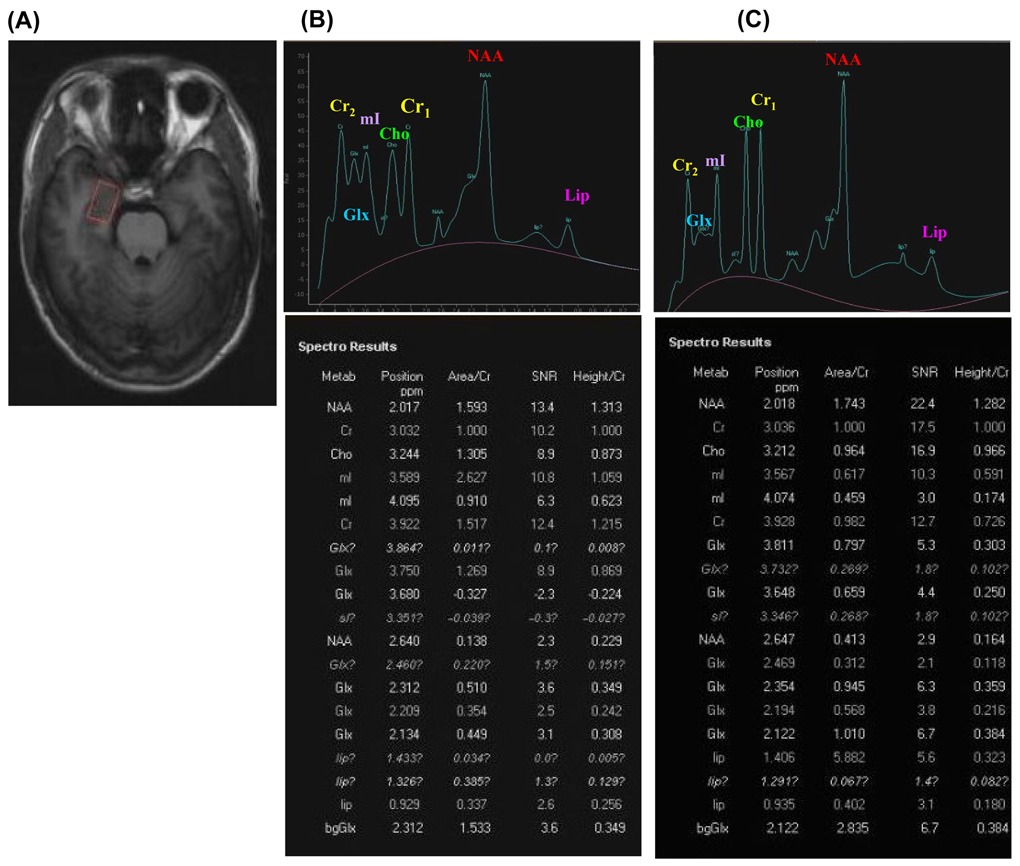
Hippocampal metabolism was evaluated by the typical metabolites such as NAA, Cho, mI, Lip and Glx and normalized by Cr in MRS examination. Fig. 3A demonstrates the area of hippocampus examined; Fig. 3B and C illustrate the typical spectra, along with the original data calculated based on MRS analyses, of control and Pb-exposed brains. By examination of MRS data from all study participants, no significant changes were observed for Cho/Cr, mI/Cr, and Glx/Cr between Pb-exposed and control groups (Table 3).
Fig. 3. 1H-MRS.
Representative hippocampal 1H-MRS spectra (STEAM sequence) of study participants. (A) An MRI indicates the position of MRS sequence. (B) A typical MRS spectra of a healthy volunteer is shown along with the original record of peak position, area/Cr and height/Cr of studied metabolites. (C) A typical MRS spectra of a Pb-exposed worker is shown along with the original record of MRS results.
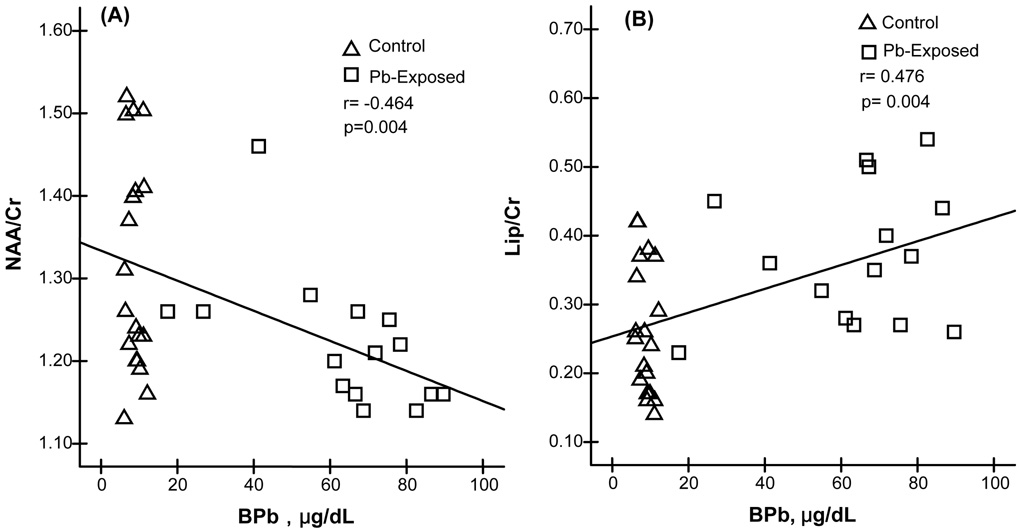
However, when the ratios of NAA/Cr in hippocampus of Pb-exposed workers were compared to those of controls, a small (7%), yet significant decrease (p<0.05) of hippocampal NAA/Cr was observed in Pb-exposed group (Table 3). Surprisingly, a remarkable increase in Lipid/Cr (40%) was detected in Pb-exposed workers as compared to controls (p<0.01) (Table 3). Moreover, linear regression analyses of pooled data from both control and Pb-exposed groups revealed that with the increase of BPb, the ratio of NAA/Cr was significantly decreased (r=−0.464, p<0.01), but the ratio of Lip/Cr was significantly increased (r=0.476, p<0.01) (Fig. 4). When the control and Pb-exposed subjects were independently analyzed within each group, no significant correlations were found between BPb and changes in brain metabolites (data not shown).
Fig. 4. Changes of NAA/Cr and Lip/Cr as the Function of BPb.
Changes of NAA/Cr and Lip/Cr as the function of increased BPb by linear regression analysis. (A) NAA/Cr decreases as the BPb increases (r = −0.464, p<0.01). (B) Lip/Cr increases as the BPb increases (r = 0.476, p<0.01).
Discussion
This cross-sectional case-control study used non-invasive MRI and MRS techniques to compare the hippocampal volumes and five critical brain metabolites between Pb-exposed workers and parameter-matched control subjects. The MRI results revealed that the hippocampal volume was significantly reduced, while small in its magnitude, in Pb-exposed workers as compared to control subjects. The MRS data further revealed a lower NAA and a higher lipid metabolism in Pb-exposed workers than in controls. Significant correlations of BPb to these changes suggest that long-term, chronic exposure to Pb in occupational settings may lead to altered brain structure and function.
Evidences in literature have suggested that Pb ions tend to selectively accumulate in hippocampus after exposure, causing both morphological and functional damages in that particular region (Alfano et al., 1982; Bielarczyk et al., 1996; Costa et al., 1983; Jett et al., 1995; Petit et al., 1983). In newborn rats, chronic Pb exposure induces cell atrophy, cellular nucleus density decrease and reductions in dendrite numbers in hippocampal CA1 and dentate gyrus neurons (Meng et al., 2003). Other studies demonstrate that Pb exposure causes deterioration in hippocampal pyramidal cell thorns (Kiraly et al., 1982), loss in dentate granulosa cells in the dendric range (Alfano et al., 1982), and damage in terminals of moss fibers (Campbell et al., 1982).
Because of cell degeneration, cellular membrane disorganization and interstitial edema following Pb exposure, a diminished brain volume, particularly in hippocampal region, is expected and has indeed been observed in Pb intoxicated subjects as well as in Pb-treated animal models. For example, Stewart et al. (2006) have studied 532 former organolead workers and evaluated 21 brains by MRI. Their results indicate that Pb exposure is significantly associated with reduced total brain volume and, in particular, the smaller volumes in cingulated gyrus and insula. Zhao and Zhou (2005), by using MRI technique to examine patients with sub-acute Pb poisoning, also report an abnormal isometric T1 and T2 signal intensity in ambilateral basal ganglia. In a non-human primate model, Lasky et al. (2005) show that Pb exposure decreases the inter-hemispheric white matter volume in Macaca rhesus monkeys treated with Pb. The current results from Pb-exposed workers were consistent with these previous reports, and further suggested that chronic occupational exposure to Pb may be associated with a reduced bilateral hippocampal volumes. Compared to studies among children, our cohort had a much higher BPb level (64 µg/dL). How the reduced brain mass may relate to clinical manifestation of Pb neurotoxicity and by what mechanism Pb induces the hippocampal volume reduction remain unknown. It is noteworthy that the current study did not determine the total brain volume. How the reduced hippocampal volume is related to the total brain volume among Pb exposed workers is also unknown. These interesting questions deserve further investigation.
Our MRS study revealed a significant alteration in NAA and Lip contents in hippocampal region. NAA, as one of the most concentrated molecules in the brain, functions to maintain brain fluid osmosis and neuronal integrity and to contribute to energy production from the amino acid glutamate in neuronal mitochondria. Previous works by Trope et al. (1998) have used MRS technique to determine NAA status in a 10-year-old boy who has a high BPb. The NAA/Cr ratio in gray and white matter within the frontal region is lower in the Pb-exposed boy than his cousin whose BPb is normal (Trope et al., 1998). This group of investigators has further applied MRS to examine 16 children with high BPb and 5 control children (BPb<10 µg/dL). A lower ratio of NAA/Cr in frontal region gray matter is confirmed among Pb-exposed children in comparison to the control children (Trope et al., 2001). Meng et al. (2005) have also compared the MRS results of 6 severe Pb poisoned children with 6 healthy age-matched children. A significant decrease in NAA in the frontal lobes and hippocampuses is evident in Pb-poisoned children. Furthermore, this group of investigators report that the NAA decrease is associated with IQ scores among these children. The current study based on adult workers confirmed a decreased NAA/Cr in the hippocampus among Pb-exposed workers. Interestingly, a reduction in brain NAA levels has been repeatedly seen among patients who suffer from Alzheimer’s disease. For example, the MRS demonstrates a significant reduction of NAA in the temporal lobe including hippocampus (Jeffon et al., 2000; Pametti et al., 1997; Schuff et al., 2002), parietal lobe (Huang et al., 2001; Schuff et al., 2002), frontal lobe and posterior part of cingulate gyrus (Pametti et al., 1997; Rose et al., 1999). In addition, a diminished NAA has also been found in patients with Huntington disease (Shiino et al., 1993) and Parkinson’s Disease (Jenkins et al., 1993).
In a more recent study, Weisskopf et al. (2007) assessed the association between cumulative Pb exposure in bones and the MRS levels of various brain metabolite ratios in humans. The results indicate that Pb accumulated in patella bone is associated with an increase in the mI/Cr ratio in the hippocampus. The current study did not detect any significant effect of Pb exposure on the ratio of mI/Cr as well as the ratios of Cho/Cr and Glx/Cr, when the values of these parameters were compared to those of control subjects. The difference in mI/Cr between the studies by Weisskopf et al. and ours may be due to the differences in the study populations. However, the lipid level in the hippocampus was found to be markedly increased. In normal brain tissue, lipids are present in cell membrane and myelin sheath. When these structures are injured, the lipids are consequently released to generate free lipid molecules, which can be captured by MRS spectra at the wave crests of 0.9 ppm and 1.3 ppm (Groenendaal et al., 2001). Since Pb is known to generate free radicals and cause oxidative stress in brain (Bennet et al., 2007; Bolin et al., 2006; Chetty et al., 2007), the observed increase in Lip/Cr among Pb-exposed workers may reflect the Pb-induced lipid peroxidation in brain tissues. This hypothesis deserves further experimental verification. As we did not conduct neurobehavioral study including memory test among the study participants, it is not possible to correlate the changes of brain contents of N-acetyl aspartate and lipids to subtle clinical manifestations of Pb poisoning. Nonetheless, the levels of both metabolites measured by MRS may have the potential uses for clinical diagnosis of Pb intoxication.
In summary, this cross-sectional study reveals that brain hippocampal volume among Pb-exposed workers is significantly diminished in comparison to age-matched control subjects as demonstrated by MRI analysis. The decreased hippocampal volumes on both sides of brain hemisphere are significantly associated with an increased BPb. The MRS study further demonstrates a decreased NAA/Cr ratio and an increased Lip/Cr ratio in the brains of Pb-exposed workers as compared to controls. These changes are also significantly associated with BPb, suggesting altered brain metabolic activities and increased oxidative stress. Taken together, this study indicates that chronic occupational exposure to Pb may cause subtle structural and functional alterations in brain. The MRI and MRS brain imaging techniques appear to be a useful noninvasive means to evaluate Pb-induced neurotoxicity.
Acknowledgments
This study was partly supported by PRC Guangxi Science and Technology Commission Grant #0632007-3F (YMJ), USA NIH/National Institute of Environmental Health Sciences Grant #ES-08164 (WZ), and USA Department of Defense Contract #USAMRMC W81XWH-05-1-0239 (WZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfano DP, Petit TL. Neonatal lead exposure alters the dendritic development of hippocampal dentate granule cells. Exp Neurol. 1982;75:275–288. doi: 10.1016/0014-4886(82)90160-1. [DOI] [PubMed] [Google Scholar]
- Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate + glutamine in Alzheimer’s disease detected in vivo with (1) H-MRS at 0.5T. Neurology. 2001;56:737–742. doi: 10.1212/wnl.56.6.737. [DOI] [PubMed] [Google Scholar]
- Barbosa F, Jr, Ramires I, Rodrigues MH, Saint' Pierre TD, Curtius AJ, Buzalaf MR, Gerlach RF, Tanus-Santos JE. Contrasting effects of age on the plasma/whole blood lead ratio in men and women with a history of lead exposure. Environ Res. 2006;102:90–95. doi: 10.1016/j.envres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bennet C, Bettaiya R, Rajanna S, Baker L, Yallapragada PR, Brice JJ, White SL, Bokara KK. Region specific increase in the antioxidant enzymes and lipid peroxidation products in the brain of rats exposed to lead. Free Rad Res. 2007;41:267–273. doi: 10.1080/10715760600889855. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- Bielarczyk H, Tian X, Suszkiw JB. Cholinergic denervation like changes in rat hippocampus following developmental lead exposure. Brain Res. 1996;708:108–115. doi: 10.1016/0006-8993(95)01315-6. [DOI] [PubMed] [Google Scholar]
- Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, Cardozo-Pelaez F. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J. 2006;20:788–790. doi: 10.1096/fj.05-5091fje. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Wooley DE, Vijayan VK, Overmann SR. Morphometric effects of postnatal lead exposure on hippocampal development of the 15-day-old rat. Brain Res. 1982;255:595–612. doi: 10.1016/0165-3806(82)90056-6. [DOI] [PubMed] [Google Scholar]
- Chernov MF, Hayashi M, Izawa M, Ono Y, Hori T. Proton magnetic resonance spectroscopy (MRS) of metastatic brain tumors: variations of metabolic profile. Int J Clin Oncol. 2006;11:375–384. doi: 10.1007/s10147-006-0589-y. [DOI] [PubMed] [Google Scholar]
- Chetty CS, Vemuri MC, Reddy GR, Suresh C. Protective effect of 17-beta-estradiol in human neurocellular models of lead exposure. NeuroToxicology. 2007;28:396–401. doi: 10.1016/j.neuro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Clark CM, Davatzikos C, Borthakur A, Newberg A, Leight S, Lee VM, Trojanowski JQ. Biomarkers for early detection of Alzheimer pathology. NeuroSignals. 2008;16:11–18. doi: 10.1159/000109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Fox DA. A selective decrease of cholinergic muscarinic receptors in the visual cortex of adult rats following developmental lead exposure. Brain Res. 1983;276:259–266. doi: 10.1016/0006-8993(83)90733-3. [DOI] [PubMed] [Google Scholar]
- Groenendaal F, Bianchi MC, Battini R, Tosetti M, Boldrini A, de Vries LS, Cioni G. Proton magnetic resonance spectroscopy (1H-MRS) of the cerebrum in two young infants with Zellweger syndrome. Neuropediatrics. 2001;32:23–27. doi: 10.1055/s-2001-12218. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J. Patterns and determinants of blood lead during pregnancy. Am J Epidemiol. 2000;152:829–837. doi: 10.1093/aje/152.9.829. [DOI] [PubMed] [Google Scholar]
- Huang W, Alexander GE, Chang L, Shetty HU, Krasuski JS, Rapoport SI, Schapiro MB. Brain metabolite concentration and dementia severity in Alzheimer’s disease: A (1)H-MRS study. Neurology. 2001;57:626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jacobs DE, Nevin R. Validation of a 20-year forecast of US childhood lead poisoning: Updated prospects for 2010. Environ Res. 2006;102:352–364. doi: 10.1016/j.envres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Jeffon F, Block W, Trabver F, Keller E, Flacke S, Papassotiropoulos A, Lamerichs R, Heun R, Schild HH. Proton MR spectroscopy detects a relative decrease of N-acetylaspartate in the medial temporal lobe of patients with AD. Neruology. 2000;55:684–688. doi: 10.1212/wnl.55.5.684. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington's disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- Jett DA, Guilarte TR. Developmental lead exposure alters NMDA and muscarinic cholinergic receptors in the rats hippocampus: An autoradiographic study. Neurotoxicology. 1995;16:7–18. [PubMed] [Google Scholar]
- Jiang YM, Zheng W, Long LL, Zhao WJ, Li XG, Mo XA, Lu JP, Fu X, Li WM, Liu SF, Long QY, Huang JL, Pira E. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. NeuroToxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Weigand SD, Petersen RC, Boeve BF, Knopman DS, Gunter J, Reyes D, Shiung M, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007;28:1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly E, Jones DG. Dendtitic spine changes in rat hippocampal pyramidal cells after postnatal lead treatment: A Golgi study. Exp Neurol. 1982;77:236–239. doi: 10.1016/0014-4886(82)90158-3. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Luck ML, Parikh NA. The effects of early lead exposure on the brains of adult rhesus monkeys: A volumetric MRI study. Toxicol Sci. 2005;85:963–975. doi: 10.1093/toxsci/kfi153. [DOI] [PubMed] [Google Scholar]
- MacKay S, Meyerhoff DJ, Constans JM, Norman D, Fein G, Weiner MW. Regional gray and white matter metabolite differences in subjects with AD with subcortical ischemic vascular dementia and elderly controls with 1H magnetic resonance spectroscopic imaging. Arch Neurol. 1996;53:167–174. doi: 10.1001/archneur.1996.00550020079018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Ruan DY, Kang LD, Zhu DM, She JQ, Luo L, Zheng Y, Li X. Age-related morphological impairments in the rat hippocampus following developmental lead exposure: an MRI, LM and EM study. Env Toxicol Pharmacol. 2003;13:187–197. doi: 10.1016/S1382-6689(02)00159-X. [DOI] [PubMed] [Google Scholar]
- Meng XM, Zhu DM, Ruan DY, She JQ, Luo L. Effects of chronic lead exposure on 1H MRS of hippocampus and frontal lobes in children. Neurology. 2005;64:1644–1647. doi: 10.1212/01.WNL.0000160391.58004.D4. [DOI] [PubMed] [Google Scholar]
- Ona LF, Alberto AM, Prudente JA, Sigua GC. Levels of lead in urban soils from selected cities in a central region of the Philippines. Environ Sci Pollut Res Int. 2006;13:177–183. doi: 10.1065/espr2005.08.275. [DOI] [PubMed] [Google Scholar]
- Parekh PP, Khwaja HA, Khan AR, et al. Lead content of petrol and diesel and its assessment in an urban environment. Environ Monit Assess. 2002;74:255–262. doi: 10.1023/a:1014296713553. [DOI] [PubMed] [Google Scholar]
- Pametti L, Tarducci R, Presciutti O, Lowenthal DT, Pippi M, Palumbo B, Gobbi G, Pelliccioli GP, Senin U. Proton magnetic resonance spectroscopy can differentiate Alzheimer’s disease from normal aging. Mech Ageing Dev. 1997;97:9–14. doi: 10.1016/s0047-6374(97)01877-0. [DOI] [PubMed] [Google Scholar]
- Petit TL, Alfano DP, LeBoutillier JC. Early lead exposure and the hippocampus: a review and recent advances. NeuroToxicology. 1983;4:79–94. [PubMed] [Google Scholar]
- Prince SE, Woo S, Doraiswamy PM, Petrella JR. Functional MRI in the early diagnosis of Alzheimer's disease: is it time to refocus? Expert Rev Neurotherapeu. 2008;8:169–175. doi: 10.1586/14737175.8.2.169. [DOI] [PubMed] [Google Scholar]
- Rose SE, Zubicaray CI, Wang D, Galloway GJ, Chalk JB, Eagle SC, Semple J, Doddrell DM. A 1H-MRS study of probable Alzheimer disease and normal aging: implication for longitudinal monitoring of dementia progression. Magn Reson imaging. 1999;17:291–299. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Ross BD. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine: myo-inositol and related metabolites. NMR Biomed. 1991;4:59–63. doi: 10.1002/nbm.1940040205. [DOI] [PubMed] [Google Scholar]
- Schuff N, Capizzano AA, Du AT, Amend DL, O'Neill J, Norman D, Kramer J, Jagust W, Miller B, Wolkowitz OM, Yaffe K, Weiner MW. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58(6):928–935. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino A, Matsuda M, Morikawa S, Inubushi T, Akiguchi I, Handa J. Proton magnetic resonance spectroscopy with dementia. Surg Neurol. 1993;39:143–147. doi: 10.1016/0090-3019(93)90093-g. [DOI] [PubMed] [Google Scholar]
- Shinno H, Inagaki T, Miyaoka T, Okazaki S, Kawamukai T, Utani E, Inami Y, Horiguchi J. A decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulated gyrus are associated with behavioral and psychological symptoms in Alzheimer's disease. J Neurol Sci. 2007;260:132–138. doi: 10.1016/j.jns.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Shonk TK, Moats RA, Gifford P, Michaelis T, Mandigo JC, Izumi J, Ross BD. Probable Alzheimer disease: Diagnosis with proton MR spectroscopy. Radiology. 1995;195:65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- Singh AK, Singh M. Lead decline in the Indian environment resulting from the petrol-lead phase-out programme. Sci Total Environ. 2006;368:686–694. doi: 10.1016/j.scitotenv.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Singh M, Goel P, Singh AK. Biomonitoring of lead in atmospheric environment of an urban center of the Ganga Plain. India Environ Monit Assess. 2005;107:101–114. doi: 10.1007/s10661-005-2146-y. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Shi W, Bassett S, Youssem D. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66:1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Pruessner JC, Faltraco F, Born C, Rocha-Unold M, Evans A, Möller HJ, Hampel H. Comprehensive dissection of the medial temporal lobe in AD: Measurement of hippocampus, amygdala, entorhinal, perirhinal and parahippocampal cortices using MRI. J Neurol. 2006;253:794–800. doi: 10.1007/s00415-006-0120-4. [DOI] [PubMed] [Google Scholar]
- Trope I, Lopez-Villegas D, Lenkinski RE. Magnetic resonance imaging and spectroscopy of regional brain structure in a 10-year-old boy with elevated blood lead levels. Pediatrics. 1998;101(6):1066–1067. doi: 10.1542/peds.101.6.e7. [DOI] [PubMed] [Google Scholar]
- Trope I, Lopez-Villegas D, Cecil KM, Lenkinski RE. Exposure to lead appears to selectively alter metabolism of cortical gray matter. Pediatrics. 2001;107:1437–1442. doi: 10.1542/peds.107.6.1437. [DOI] [PubMed] [Google Scholar]
- Van der Knaap MS, Van der Grond J, Luyten PR, den Hollander JA, Nauta JJ, Valk J. 1H and 31P magnetic resonance spectroscopy of brain in degenerative cerebral disorders. Ann Neurol. 1992;31:202–211. doi: 10.1002/ana.410310211. [DOI] [PubMed] [Google Scholar]
- Webb J, Guimond A, Eldridge P, Chadwick D, Meunier J, Thirion JP, Roberts N. Automatic detection of hippocampal atrophy on magnetic resonance images. Magn Reson Imaging. 1999;17:1149–1161. doi: 10.1016/s0730-725x(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Hu H, Sparrow D, Lenkinski RE, Wright RO. Proton magnetic resonance spectroscopic evidence of glial effects of cumulative lead exposure in the adult human hippocampus. Environ Health Persp. 2007;115:519–523. doi: 10.1289/ehp.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong O. Lead exposure, lead poisoning, and lead regulatory standards in China, 1990–2005. Regul Toxicol Pharmacol. 2006;46:157–162. doi: 10.1016/j.yrtph.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer's disease. Rev Neurosci. 2005;16:325–337. doi: 10.1515/revneuro.2005.16.4.325. [DOI] [PubMed] [Google Scholar]
- Zhao JQ, Zhou SJ. The central nervous system lead poisoning and MRI manifestation due to long-term dyeing. J Clin Res. 2005;22:346–348. In Chinese. [Google Scholar]
- Zheng W. Neurotoxicology of the brain barrier system: New implications. J Toxicol – Clin Toxicol. 2001;39:711–719. doi: 10.1081/clt-100108512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Perry DF, Nelson DL, Aposhian HV. Choroid plexus protects cerebrospinal fluid against toxic metals. FASEB J. 1991;5:2188–2193. doi: 10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]
- Zheng W, Shen H, Blaner WS, Zhao Q, Ren X, Graziano JH. Chronic lead exposure alters transthyretin concentration in rat cerebrospinal fluid: The role of the choroid plexus. Toxicol Appl Pharmacol. 1996;139:445–450. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Lu YM, Lu GY, Zhao Q, Cheung O, Blaner WS. Transthyretin, thyroxin, and retinol-binding protein in human cerebrospinal fluid: Effect of lead exposure. Toxicol Sci. 2001;61:107–114. doi: 10.1093/toxsci/61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]