Abstract
The individual and synergistic effects of extracellular matrix interactions on isolated islet function in culture were investigated within a three-dimensional poly(ethylene glycol) (PEG) hydrogel encapsulation environment. First, we observed similar glucose-stimulated insulin secretion from unencapsulated murine islets and islets photoencapsulated in PEG gels. Then islets were encapsulated in gels containing the basement membrane proteins collagen type IV and laminin, individually and in combination, at a total protein concentration of 100 μg/ml, and islet insulin secretion in response to high glucose was measured over time. Specific laminin interactions were investigated via islet encapsulation with adhesive peptide sequences found in laminin as well as via functional blocking of cell surface receptors known to bind laminin. Over 32 days, islet interactions with collagen type IV and laminin localized within the three-dimensional extracellular environment contributed to two-fold and four-fold increases in insulin secretion, respectively, relative to islets encapsulated without matrix proteins. Hydrogel compositions containing both matrix proteins and > 75% laminin further increased islet insulin secretion to approximately six-fold that of islets encapsulated in the absence of matrix proteins. Encapsulation with the peptide sequence IKVAV resulted in increased islet insulin secretion, but not to the extent observed in the presence of whole laminin. Increased insulin secretion in the presence of laminin was eliminated when islets were exposed to functionally blocking anti-α6 integrin antibody prior to islet encapsulation with laminin. Our results demonstrate the potential of specific matrix interactions within an islet encapsulation microenvironment to promote encapsulated islet function.
Keywords: islet encapsulation, PEG hydrogel, cell-matrix interactions, collagen type IV, laminin
Introduction
To date, the design of an islet encapsulation barrier has largely focused on optimizing material biocompatibility and tailoring material properties to exclude the transport of antibodies while allowing adequate diffusion of low molecular weight nutrients and metabolites. Immunoisolation materials have been designed to be bioinert for the purposes of minimizing host response and eliminating material toxicity to encapsulated islets. The resulting inert islet encapsulation environments are extremely different from the native islet microenvironment, which is rich in vasculature and extracellular matrix interactions. The loss of islet-matrix interactions during isolation has been associated with reduced islet survival and function (Wang and Rosenberg., 1999a; Nagata et al., 2002; Ris et al., 2002; Hammar et al., 2004; Pinkse et al., 2006), and even implicated in transplanted islet failure (Thomas et al., 1999). Taking this into consideration, bulk-photopolymerized poly(ethylene gycol) (PEG) hydrogels were applied to islet encapsulation not only as another potentially immunoprotective barrier material, but also to address an area that has been largely overlooked, the controlled introduction of matrix interactions that promote islet survival and function within the islet encapsulation microenvironment.
The native extracellular islet environment includes a surrounding islet capsule comprised of the basement membrane-associated proteins, collagen type IV, laminin, and fibronectin (Meda and Bosco, 2001), as well as basement membrane secreted by the dense islet microvasculature, also rich in collagen type IV and laminin (Nikolova et al., 2006). Improved survival and function of isolated islets following the re-establishment of cell-matrix interactions in vitro indicate that at least partial restoration of the islet extracellular environment is possible outside of the native pancreas tissue (Beattie et al., 1991; Perfetti et al., 1996; Wang and Rosenberg, 1999a; Bosco et al., 2000; Ris et al., 2002; Nagata et al., 2002; Edamura et al., 2003; Kaido et al., 2004; Woods et al., 2004; Nikolova et al., 2006; Pinkse et al., 2006; Parnaud et al., 2006;Labriola et al., 2006). In reports that studied whole islet culture on matrix substrates, as opposed to individual β-cell culture, the basement membrane proteins collagen type IV and laminin were repeatedly identified as matrix contacts that improved not only isolated islet cell survival but also increased glucose stimulated insulin secretion (Nagata et al., 2002; Pinkse et al., 2006; Nikolova et al., 2006). Nagata et al. further examined matrix interactions in three-dimensional culture using collagen based hydrogels and found that the addition of collagen type IV and laminin within collagen type I hydrogels improved three-dimensionally cultured islet function (Nagata et al., 2002). However, the complexities of serum protein interactions with collagen type I gels may introduce confounding influences on islet function.
To test the influence of matrix interactions on islet function within a three-dimensional microenvironment, islets were encapsulated in PEG hydrogels presenting cell-matrix interactions found in the native islet environment, specifically laminin and collagen type IV. Because cells do not interact directly with the hydrophilic PEG network, we were able to observe the isolated effects of extracellular interactions with individual and combined matrix components. To better understand the specific cell-matrix interactions influencing islet function, specific laminin interactions were investigated via islet encapsulation with adhesive peptide sequences found in laminin, as well as functional blocking of cell surface receptors known to bind laminin.
Results
Insulin secretion from islets encapsulated in PEG hydrogels
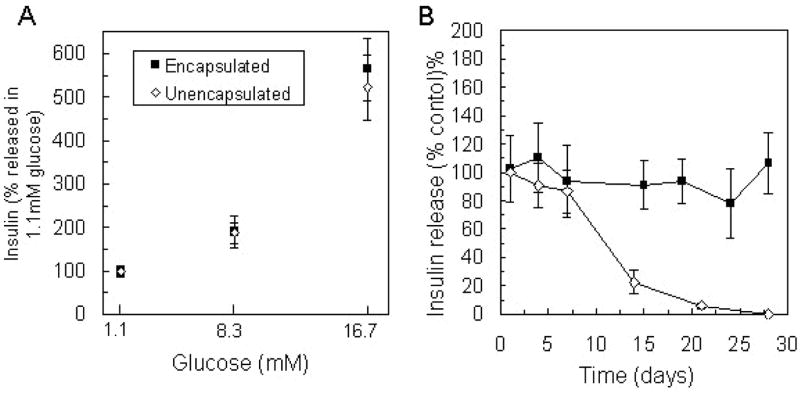
Following isolation, islets were encapsulated in PEG hydrogels and exposed to 1.1mM, 8.3mM, and 16.7mM glucose challenge during the first week in culture. The amount of insulin released from each sample in response to 8.3mM and 16.7mM glucose was normalized to that released in 1.1mM glucose from the respective sample, and the percent increase in insulin secretion over basal conditions was compared to insulin release values from unencapsulated islets exposed to the same stimulation conditions within 3 days of isolation (Figure 1A). Insulin secretion in response to high glucose (16.7) was approximately five-fold greater than basal secretion (1.1mM) from both encapsulated and unencapsulated islets. Insulin release in 8.3mM glucose was approximately twice that in 1.1 mM glucose and also similar between encapsulated and unencapsulated islets. No significant differences were observed in insulin secretion from islets in normal culture (unencapsulated) and from islets within the three-dimensional PEG environment.
Figure 1.
Insulin release from unencapsulated and encapsulated islets (n = 6) in 1 hour static incubation with A) varying glucose concentrations, and B) 16.7mM glucose measured over 28 days in culture represented as a percentage of that released from unencapsulated islets within 24 hours of isolation. No statistical differences in insulin secretion were observed in response to varying glucose concentration or within the first week in culture.
The maintenance of islet function in culture was investigated by stimulation with high glucose (16.7mM) repeatedly over 28 days in culture. The amount of insulin released per encapsulation sample was normalized by the respective ATP content of the sample to eliminate variance between samples due to differing cell number. Insulin secretion from encapsulated islets at specified time points is presented as a percentage of insulin release values for unencapsulated islets within 24 hours of isolation, which was also normalized by ATP content (Figure 1B). The amount of insulin released by encapsulated islets during 1 hour in high glucose solution was sustained over one month in culture, while insulin released from unencapsulated islets diminished between week one and week two and was undetectable after 28 days. In addition to these functional results, islet survival over 28 days within PEG gels was observed via staining with a fluorescent membrane-integrity assay (LIVE/DEAD®, Invitrogen) and previously reported (Weber et al., 2006).
Islets encapsulated with individual basement membrane proteins
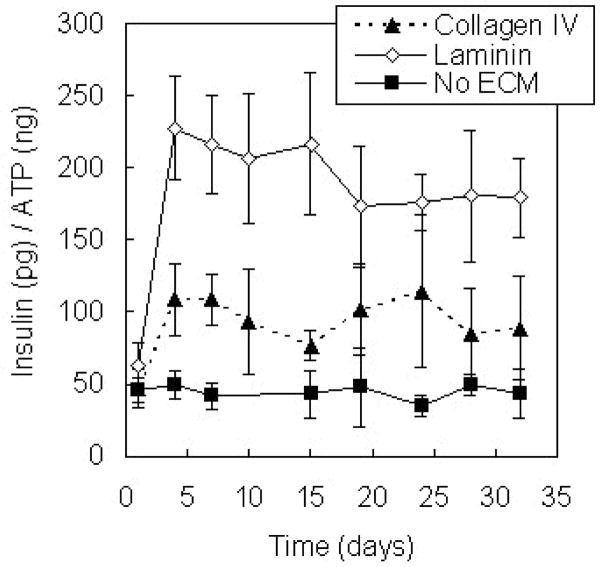
Collagen type IV and laminin are the most abundant matrix proteins found in the vascular basement membrane identified within islets in situ (Nikolova et al., 2006), and the effects of islet-matrix interactions with these proteins on insulin secretion were investigated within PEG hydrogel encapsulation environments. Isolated murine islets were encapsulated in PEG hydrogels containing collagen type IV and laminin, individually, at a concentration of 100 μg/ml, and insulin secretion from islets in response to static glucose stimulation was measured over one month in culture. An ECM protein concentration of 100μg/ml was selected based on the range of ECM concentrations used in previous investigations of islet-matrix interactions in two-dimensional culture (Wang and Rosenberg, 1999; Bosco et al., 2000; Ris et al., 2002; Edamura et al., 2003; Kaido et al., 2004) and in collagen gels (Nagata et al., 2002) as well as results of a preliminary investigation within the three-dimensional PEG encapsulation environment using MIN6 β-cells (Weber et al., accepted). Varying ECM protein concentration from 10 to 250 μg/ml did not affect MIN6 insulin secretion. Islet insulin secretion was normalized by sample ATP content and compared to insulin released from islets encapsulated in the absence of matrix interactions (Figure 2). The presence of collagen type IV in the extracellular environment contributed to an approximately two-fold increase in insulin secretion (p < 0.01), and insulin secretion from islets encapsulated in laminin-containing gels was almost four-fold greater than from islets encapsulated without matrix proteins (p < 0.01).
Figure 2.
Glucose-stimulated insulin release from islets encapsulated in PEG hydrogels (n = 6) containing collagen type IV and laminin compared to that from islets in unmodified PEG environments with culture time. Proteins were encapsulated in the gels at a concentration of 100 μg/ml.
Islets encapsulated with ECM combinations
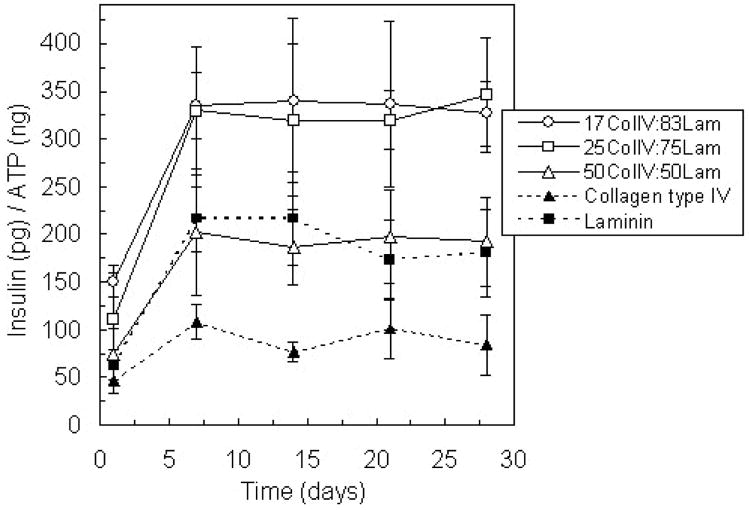
Because the basement membrane is comprised of both collagen type IV and laminin, PEG hydrogel environments were modified with three combinations of these proteins: 17% collagen type IV and 83% laminin, 25% collagen type IV and 75% laminin, and 50% collagen type IV and 50% laminin. For each condition, the total concentration of matrix protein was 100 μg/ml, and therefore, the notations for each combination represent not only the percentage of each protein by weight, but also the matrix protein concentration in μg/ml. The 17% collagen type IV and 83% laminin matrix composition corresponds to a 1:1 molar ratio of the matrix proteins, a similar relative composition to that found in cell-secreted basement membranes (Kleinman et al., 1986). Insulin secretion from islets encapsulated with 50% of each protein was not statistically different from the amount of insulin released from samples containing only laminin (Figure 3). However, islets encapsulated with combinations that contained more laminin than collagen type IV released greater than 50% more insulin compared to laminin alone (p < 0.01). These results suggest that collagen type IV and laminin synergistically influence islet insulin secretion.
Figure 3.
Glucose-stimulated insulin release from islets encapsulated in PEG hydrogels containing varying ratios of collagen type IV and laminin compared to that from islets in PEG environments presenting matrix protein interactions individually over 28 days in culture. Total protein concentration in each gel was 100 μg/ml (n = 4).
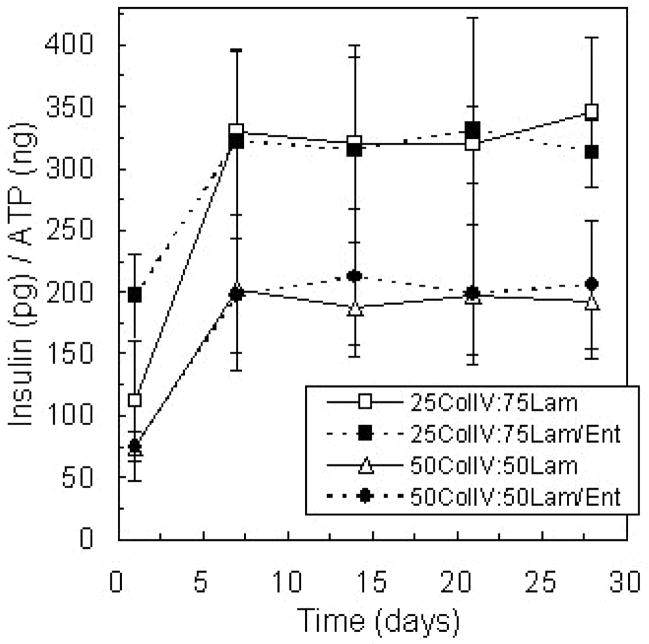
To further explore this intriguing result, the interactions between collagen type IV and laminin were considered. Entactin, also known as nidogen, is a basement membrane protein that facilitates the connection of collagen type IV and laminin networks within basement membrane. Entactin has a high affinity to laminin, and a 1:1 molar laminin/entactin complex can be isolated from mouse EHS tumor (Timpl, 1999). Islets were encapsulated in gels containing entactin in addition to collagen type IV and laminin, to determine if the ability of entactin to direct collagen type IV and laminin binding would further potentiate the synergistic effects of these matrix proteins on encapsulated islet insulin secretion. However, the presence of entactin within PEG gel environments resulted in insulin release values similar to those from islets encapsulated with collagen type IV and purified laminin (Figure 4) in the absence of entactin.
Figure 4.
Glucose-stimulated insulin secretion from islets encapsulated in PEG gel compositions with two relative amounts of collagen type IV and laminin and with and without entactin, a matrix protein known to facilitate binding between collagen type IV and laminin, with culture time. Total protein concentration in each gel was 100 μg/ml (n = 4).
Islets encapsulated with peptide recognition sequences found in laminin
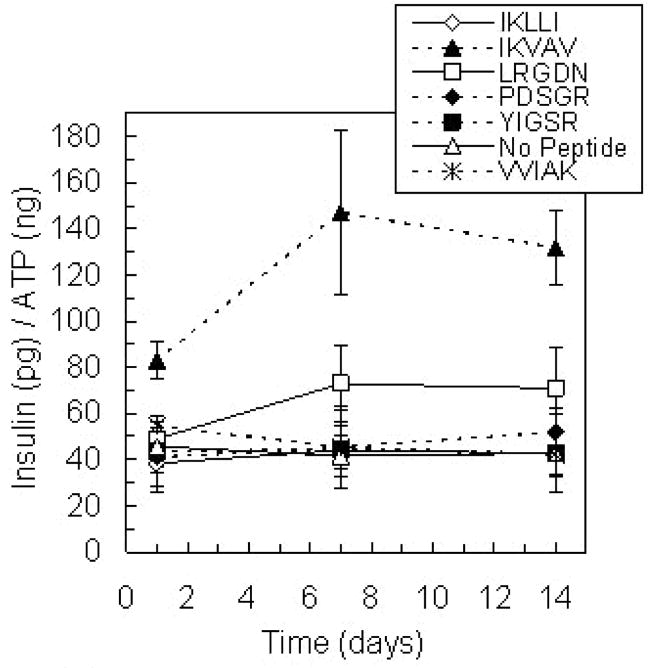
Given the prominent effect of laminin on islet insulin secretion, islets were encapsulated with five adhesive peptide sequences found in laminin: IKLLI, IKVAV, LRGDN, PDSGR, and YIGSR (Yamada, 1991; Masters and Anseth, 2004) to better understand specific cell-matrix interactions. The presentation of IKVAV within the encapsulation environment resulted in statistically higher islet insulin secretion relative to islets with no matrix interactions or any of the other peptide sequences studied (Figure 5, p < 0.01). However, insulin secretion in the presence of IKVAV was significantly less than that secreted by islets in PEG gels containing whole laminin (p < 0.01). Insulin secretion in the presence of a scrambled version of IKVAV, the peptide sequence VVIAK, was not statistically different from control levels secreted in the absence of matrix interactions, indicating that the specific sequence order of IKVAV is critical to the influence of this peptide on islet function. Average insulin secretion values for islets in gels containing LRGDN were greater than control levels, but this increase was not statistically significant (p = 0.06).
Figure 5.
Glucose-stimulated insulin release from islets encapsulated in PEG gels modified with individual adhesive peptide sequences found in laminin over 14 days in culture (n = 4). Each peptide was incorporated in the gels at a concentration of 5 mM. VVIAK served as a scrambled peptide control for IKVAV. Only gel environments containing IKVAV resulted in increased insulin secretion (p < 0.01).
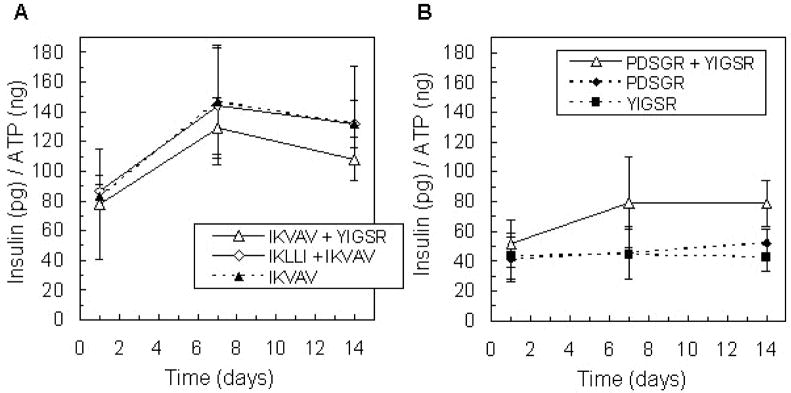
Additionally, the influence of laminin-derived peptide pairs on islet function was investigated. IKVAV and IKLLI were shown to independently promote encapsulated MIN6 β-cell insulin secretion (Weber et al., 2007), and additional synergistic peptide pairings include IKVAV and YIGSR (Tong et al., 2001) and PDSGR and YIGSR (Kleinman et al., 1989; Aucoin et al., 2002). Insulin release from islets encapsulated with peptide pairs containing IKVAV was comparable to that from islets encapsulated with IKVAV alone (Figure 6A). Interestingly, in gel environments modified with both PDSGR and YIGSR, islets secreted more insulin than with either peptide individually (Figure 6B, p < 0.01), but insulin release from islets in these gels was less than that with IKVAV. Clearly, small laminin peptide fragments can enhance islet function by promoting specific cell-matrix interactions, but the nature of the enhancement is less significant than that of the entire laminin protein.
Figure 6.
Glucose-stimulated insulin release from islets encapsulated in PEG gels modified with peptide combinations IKVAV + YIGSR and IKLLI + IKVAV (A), and PDSGR + YIGSR (B), compared to insulin released in response to individual peptides over 14 days in culture (n = 4). Total peptide concentration in each gel was 5 mM.
ECM receptor blocking and islet insulin secretion
The influence of two known laminin receptors, integrin α6β1 and the 67-kDa laminin receptor, on islet-laminin interactions also was explored in a competitive binding experiment. Islets were incubated with blocking antibodies for the integrin subunit α6 and the 67-kDa laminin receptor, and the effects on insulin secretion from islet encapsulated with laminin were observed. Islets exposed to the laminin receptor antibody released insulin at a level similar to those encapsulated with laminin but not exposed to antibody. In contrast, exposure to α6 antibody prior to encapsulation with laminin, completely obliterated the enhanced insulin secretion observed in the presence of laminin and resulted in insulin secretion levels similar to that from islets encapsulated without matrix protein. The values presented in Figure 7 were measured after 7 days in culture and are representative of those observed throughout two weeks in culture.
Figure 7.
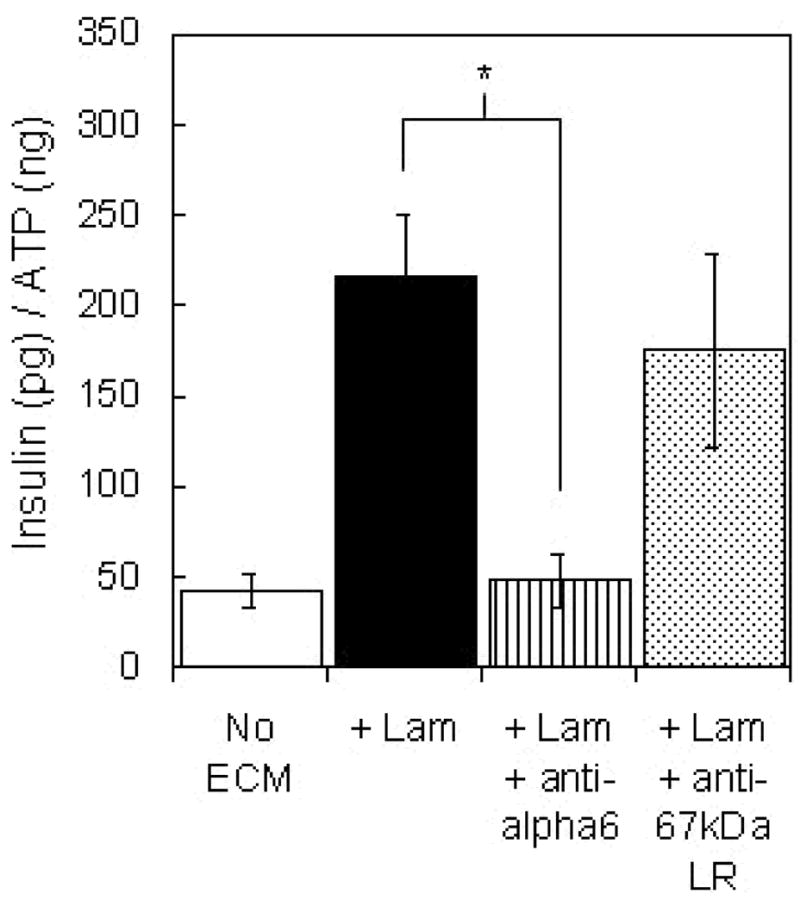
Glucose stimulated insulin release from islets exposed to functional blocking antibodies to integrin α6 and the 67-kDa laminin receptor prior to encapsulation in PEG gels containing laminin compared to that from untreated islets encapsulated with and without laminin after 7 days in culture (n = 6). Laminin concentration in the gels was 100 μg/ml. * denotes statistically significant experimental result (p < 0.01).
Discussion
Glucose-stimulated insulin secretion from islets encapsulated in PEG hydrogels was similar to that of freshly isolated, unencapsulated islets. Furthermore, encapsulated islet insulin secretion in response to glucose challenge (16.7mM) was maintained at levels similar to that of controls over one month in culture. Functional preservation may be due to the three-dimensional support of isolated islet morphology within the hydrogel environment. These results support the cytocompatibility of the photopolymerization conditions used for hydrogel formation and of the subsequent hydrogel environment with respect to maintaining murine islet function in three-dimensional culture. Hydrogel formation within an aqueous solution affords the opportunity to dissolve matrix protein in the precursor solution for physical entrapment upon network photopolymerization and to study the individual and combined effects of cell-matrix interactions on encapsulated islet function.
Islet interactions with laminin and collagen type IV present in the extracellular environment of PEG hydrogels resulted in increased glucose-stimulated insulin secretion. The influence of these individual matrix proteins in a three-dimensional encapsulation environment is in agreement with previous investigations of islets cultured on collagen type IV and laminin substrates (Bosco et al., 2000; Nagata et al., 2002; Edamura et al., 2003; Kaido et al., 2004; Nikolova et al., 2006). Beyond these single component gel systems, hydrogel environments containing both collagen type IV and laminin were synthesized and found to further promote islet insulin secretion, specifically in gels containing a higher ratio of laminin relative to collagen type IV. Interestingly, the synergistic matrix compositions are similar to that found in native basement membrane (Kleinman et al., 1986), the primary ECM component within islets in situ (Nikolova et al., 2006). The ability of matrix interactions within a hydrogel encapsulation environment to positively influence islet insulin secretion in vitro indicates the potential of matrix-functionalized islet encapsulation barriers to promote function of transplanted islets. Because islet donor shortage is a major obstacle in islet transplantation therapy, any encapsulation barrier modifications that result in improved islet function will aid in reducing the amount of islets required for effective treatment.
While much of the basement membrane present within the native islet structure is associated with the islet vasculature, islets are also surrounded by an ECM capsule that contains collagen type IV and laminin (Meda and Bosco, 2001; Nikolova et al., 2006), and this capsule is largely disrupted during islet isolation (Wang et al., 1999b). The presence of matrix proteins within the extracellular encapsulation environment may serve to re-establish cell-matrix interactions originally associated with the native islet capsule. For our initial studies, we used intact mouse islets rather than individual β-cells to avoid additional disruption beyond that caused by initial isolation to complex intra-islet cell communication pathways. It is widely accepted that individual β-cells function differently relative to β-cells within the native islet structure (Meda and Bosco, 2001), and additionally, intact islets are used in current transplantation therapies. Due to the spherical structure of islets, only cells located at the islet surface would be in contact with matrix proteins, yet insulin secretion by β-cells known to be localized to the interior islet region was improved in the presence of ECM. In experiments with cultured β-cells, cell-matrix interactions influenced insulin secretion via alterations in β-cell cytoskeletal organization and intracellular signaling events linked to cytoskeletal arrangement (Li et al., 1999;Thurmond et al., 2003; Hammar et al., 2005; Tomas et al., 2006). Direct β-cell-matrix interactions with the vascular basement membrane located throughout islets could provide this signaling in vivo, but the results presented herein suggest that signaling from cells located along the islet surface also are capable of influencing β-cell function. The molecular mechanisms involved in the coordinated response of multiple islet cell types to matrix interactions should be further investigated.
The observed differences in insulin secretion at early time points from islets encapsulated with matrix moieties also are indicative of complex intracellular changes evoked by islet-matrix contacts. After four days, glucose-stimulated insulin secretion from islets encapsulated with laminin and collagen type IV, individually and in combination, reached maximal levels. However, after only one day, average insulin secretion values from islets encapsulated with laminin were greater that those from islets with no matrix interactions, although not statistically different (p > 0.05). This trend was observed on day one for other gel-matrix conditions as well, suggesting that the effects of matrix interactions on islet function are not immediate but rather the result of a series of intracellular events that occur in response to specific extracellular cues. Toward future studies of these complex intracellular changes, we also investigated specific amino acid sequences within laminin that may interact with islet cell surface receptors as well as specific laminin receptors.
Islet encapsulation in PEG gels containing peptide recognition sequences found in laminin demonstrated the ability of interaction with IKVAV at a concentration of 5 mM to influence islet insulin secretion. However, none of the individual and combined peptide sequences tested affected insulin secretion to the same extent as whole laminin protein. Matrix receptor interactions with the tested peptide sequences may play a role in laminin-islet signaling, but no individual peptide or peptide pairing was identified in these experiments to be solely responsible for the effects of laminin on islet function. The inclusion of matrix interactions within islet encapsulation barrier environments via covalently incorporated peptides may have further application though, due to the potential of peptides to be less immunogenic and more stable in comparison to whole matrix proteins.
The treatment of islets with antibodies to block matrix receptor function prior to encapsulation in laminin-containing gels provided evidence for cell-matrix signaling via integrins containing the α6 integrin subunit. Exposure to anti-α6 antibody effectively eliminated the influence of extracellular laminin on encapsulated islet insulin secretion. Two α6–containing integrins, α6β1 and α6β4, have been shown to interact with laminin (Sasaki and Timpl, 1999), but only the α6β1 integrin has been identified on islet-related cells. Increased insulin secretion from individual β-cells cultured on a laminin-rich cell-secreted matrix was found to be mediated by α6β1 integrin (Bosco et al., 2000), and recent reports have identified the importance of α6β1 integrin in islet development (Wang et al., 2005; Yashpal et al., 2005). In contrast, treatment of islets with an antibody against the 67-kDa laminin receptor did not reduce insulin secretion from islets encapsulated with laminin. However, due to experimental complexities such as variable antibody-receptor binding, this result is inconclusive and does not eliminate the potential importance of the 67-kDa laminin receptor in the effects of laminin on encapsulated islets.
In conclusion, islet encapsulation in PEG hydrogels containing specific matrix interactions resulted in improved glucose-responsive insulin secretion over one month in culture. Accordingly, the re-establishment of islet-matrix contacts within the encapsulated cell microenvironment should be considered in the design of future immunoprotective barrier systems, and although PEG hydrogels are promising candidates for clinical application, presentation of the matrix interactions identified in this work to encapsulated islets could be generally incorporated into a wide range of current bioinert encapsulation schemes. This investigation identified matrix components that support increased islet insulin secretion in three-dimensional culture. Future research should investigate, in concert, the clinical application of these interactions in islet replacement therapies as well as a fundamental understanding of the molecular mechanisms responsible for the observed increases in insulin release and the time course over which these intracellular events occur.
Experimental Procedures
Islet isolation and culture
Islets from adult Balb/c mice were obtained from the Diabetes and Endocrinology Research Center at the Barbara Davis Center for Childhood Diabetes (Denver, CO). Briefly, islets were isolated from mouse pancreata by collagenase (type V; Sigma-Aldrich, St. Louis, MO) digestion (Gotoh et al., 1985) followed by purification on a Histopaque (Sigma-Aldrich) density gradient (Kupfer et al., 2005). Isolated islets were cultured in RMPI 1640 (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin-streptomycin (Gibco), and 0.5 μg/mL fungizone (Gibco) at 37°C in humid conditions with 5% CO2.
Synthesis of extracellular matrix presenting hydrogel environments
Poly(ethylene glycol) dimethacrylate (PEGDM) was synthesized by reacting linear PEG (M̄n = 10000 g/mol) (Sigma-Aldrich) with methacrylic anhydride (Sigma-Aldrich) at a molar ratio of 1:10 via microwave irradiation under solvent free conditions (Lin-Gibson et al., 2004). The macromer product was collected by precipitation into chilled (4°C) ethyl ether (Sigma-Aldrich) and vacuum filtration, and macromer purification was achieved by dialysis in deionized water (diH2O) using cellulose ester dialysis tubing with a molecular weight cutoff of 1000 g/mol (Spectrum Laboratories, Rancho Dominguez, CA). Purified PEGDM was collected by lyophilization and stored at 4°C under nitrogen.
Hydrogels were formed from a precursor solution of 10 wt % PEGDM in Hanks Balanced Salt Solution (HBSS, Gibco) and 0.025 wt % of the photoinitiator 2-hydroxy-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Ciba-Geigy, Basel, Switzerland), exposed to 365nm ultraviolet light at an intensity of ~7mW cm−2 for 10 minutes. For encapsulation, islets were suspended in the precursor solution prior to photopolymerization at a density of ~20 islets/30 μl. Islet survival within the resulting PEG hydrogels has been confirmed previously over 28 days in culture (Weber et al., 2006).
Matrix-presenting PEG hydrogel environments were formed by dissolving matrix proteins at specified concentrations, typically 100 μg/ml, in the 10 wt % PEGDM hydrogel precursor solution prior to polymerization. During photopolymerization of the PEG hydrogel, the following dissolved matrix proteins were physically entrapped throughout the gel network structure, individually and in combination: entactin-free laminin, laminin containing entactin, and collagen IV (BD Biosciences, San Jose, CA).
Adhesive peptide recognition sequences were synthesized using an Applied Biosystems peptide synthesizer (model 433A). Purified IKLLI, IKVAV, LRGDN, PDSGR, and YIGSR were conjugated to mono-acrylated PEG for covalent incorporation into PEG hydrogels during photopolymerization as previously described (Weber et al., 2007). Briefly, peptide-PEG-acrylate was synthesized by reacting the N-terminus of each peptide sequence with an N-hydroxysuccinimidyl group on mono-acrylated PEG (M̄n =3,400 Da) (Acr-PEG-NHS, Nektar Therapeutics, Huntsville, AL) in 0.1 M sodium biocarbonate buffer at pH 8.5 for 2 hrs at room temperature with a 20% molar excess of peptide. Acrylated peptides were then dialyzed in deionized water overnight using cellulose ester dialysis tubing to remove low molecular weight contaminants and excess, unreacted peptide and collected by lyophilization. Peptide-presenting hydrogels were formed by the addition of 5.0 mM acrylated peptide to the hydrogel precursor solution prior to photopolymerization.
Glucose stimulated insulin secretion
Insulin secretion was evaluated by exposure of encapsulated islets to static glucose stimulation for 1 hour at specified time points. Encapsulated and unencapsulated samples were first placed in a low glucose concentration solution (1.1mM) for 45 minutes, followed by incubation in a high glucose concentration buffer (16.7mM) for 1 hour. The insulin concentration in the high glucose buffer solutions after 1 hour was measured by mouse/rat insulin ELISA (Mercodia, Winston Salem, NC). Encapsulated islet insulin release was measured repeatedly over one week in response to varying glucose concentrations (1.1, 8.3, and 16.7mM) and compared to that released from unencapsulated islets within 72 hours of isolation in response to the same glucose concentrations. Samples were exposed to each glucose concentration, and the ratio of insulin secreted with stimulatory glucose concentrations (8.3mM and 16.7mM) to insulin secreted in basal glucose solution (1.1mM) was calculated for each unencapsulated and encapsulated sample, and averaged per condition. Further, insulin secretion from both unencapsulated and encapsulated islets in response to high glucose concentration (16.7mM) was measured over one month in culture to observe any influence of the PEG environment on islet function.
The CellTiter-Glo® Luminescent Cell Viability Assay (Promega, Madison, WI) was used to measure the ATP content of each encapsulation sample. Islet-containing hydrogel samples were incubated in 0.5 ml of culture media combined with 0.5 ml of CellTiter-Glo reagent for 30 minutes on an orbital shaker (~ 200rpm). The luminescence of each sample solution was measured using a microplate reader (Perkin Elmer Wallac Victor2, 1420 Multilabel Counter), and the insulin released from each sample was normalized by the ATP content of the respective sample to account for insulin secretion disparities between samples due to variations in cell number. The relatively small molecular size of ATP allows for the measurement of the total ATP content of each hydrogel sample by simple extraction of the molecule from each sample, avoiding error introduced by the physical destruction of hydrogel samples required for DNA measurement. In control experiments, the amount of ATP in hydrogel samples correlated appropriately to both sample cell number and sample DNA content when ATP was measured immediately following high glucose stimulation (data not shown).
Antibody receptor blocking
For matrix receptor blocking, islets were suspended in culture media containing 25 μg/ml of blocking antibody to the integrin α6 subunit (Santa Cruz Biotechnology, Santa Cruz, CA) or the 67-kDa laminin receptor (Novus Biologicals, Littleton, CO) for 4 hours, and then immediately encapsulated in PEG hydrogels containing laminin. Glucose stimulated insulin secretion from islets exposed to receptor antibodies was measured over 14 days in culture.
Statistical analysis
All results are presented as mean ± standard deviation. A two-tailed, unpaired Student’s t-Test was used to determine statistical significance between experimental conditions and control conditions (p < 0.05), and multiple comparisons were performed by ANOVA followed by Tukey’s secondary test for significance. p values less than 0.01 are noted as such.
Acknowledgments
The authors gratefully acknowledge research funding from the National Science Foundation (Grant #EEC044771) and the Howard Hughes Medical Institute and funding for LMW from the National Science Foundation Graduate Research Fellowship program and the U.S. Department of Education’s Graduate Assistantships in Areas of National Need program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aucoin L, Griffith CM, Pleizier G, Deslandes Y, Sheardown H. Interactions of corneal epithelial cells and surfaces modified with cell adhesion peptide combinations. J Biomater Sci Polym Ed. 2002;13(4):447–462. doi: 10.1163/156856202320253956. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Lappi DA, Baird A, Hayek A. Functional impact of attachment and purification in the short term culture of pancreatic islets. J of Clin Endocrin and Metab. 1991;73:93–98. doi: 10.1210/jcem-73-1-93. [DOI] [PubMed] [Google Scholar]
- Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet β-cell secretion in vitro: role of α6β1 integrin. Diabetes. 2000;49:233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- Edamura K, Nasu K, Iwami Y, Ogawa H, Sasaki N, Ohgawara H. Effect of adhesion or collagen molecules on cell attachment, insulin secretion, and glucose responsiveness in the cultured adult porcine endocrine pancreas: a preliminary study. Cell Transplant. 2003;12(4):439–446. doi: 10.3727/000000003108746867. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplant. 1985;40:437–438. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA. Extracellular matrix protects pancreatic β-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53:2034–2041. doi: 10.2337/diabetes.53.8.2034. [DOI] [PubMed] [Google Scholar]
- Hammar EB, Irminger JC, Rickenbach K, Parnaud G, Ribaux P, Bosco D, Rouiller D, Halban PA. Activation of NF-κB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J of Biol Chem. 2005;280(34):30630–30637. doi: 10.1074/jbc.M502493200. [DOI] [PubMed] [Google Scholar]
- Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human β-cell adhesion, motility, and insulin secretion by collagen IV and its receptor α1β1. J of Biol Chem. 2004;279(51):53762–53769. doi: 10.1074/jbc.M411202200. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Graf J, Iwamoto Y, Sasaki M, Schasteen CS, Yamada Y, Martin GR, Robey FA. Identification of a second active site in laminin for promotion of cell adhesion and migration and inhibition of in vivo melanoma lung colonization. Arch Biochem Biophys. 1989;272(1):39–45. doi: 10.1016/0003-9861(89)90192-6. [DOI] [PubMed] [Google Scholar]
- Kupfer TM, Crawford ML, Pham K, Gill RG. MHC-mismatched islet allografts are vulnerable to autoimmune rejection in vivo. J of Immuno. 2005;175:2309–2316. doi: 10.4049/jimmunol.175.4.2309. [DOI] [PubMed] [Google Scholar]
- Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC. Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol. 2007;263:120–133. doi: 10.1016/j.mce.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Li G, Rungger-Brandle E, Just I, Jonas JC, Aktories K, Wollheim CB. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol Biol of the Cell. 1994;5:1199–1213. doi: 10.1091/mbc.5.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, Horkay F, Washburn N. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280–1287. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- Masters KS, Anseth KS. Cell-material interactions. In: Peppas N, Sefton M, editors. Molecular and Cellular Foundations of Biomaterials, Advances in Chemical Engineering. Vol. 29. Academic Press; Burlington, MA: 2004. pp. 7–46. [Google Scholar]
- Meda P, Bosco D. Communication of islet cells: molecules and functions. In: Habener JF, Hussain M, editors. Molecular basis of pancreas development and function. Kluwer Academic Publishers; Norwell, MA: 2001. pp. 143–163. [Google Scholar]
- Nagata N, Iwanaga A, Inoue K, Tabata Y. Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polymer Edn. 2002;13(5):579–590. doi: 10.1163/15685620260178418. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber H, Ferrar N, Melton DA, Lammert E. The Vascular Basement Membrane: A Niche for Insulin Gene Expression and β cell proliferation. Develop Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. Blockade of β1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat β-cells attached on extracellular matrix. Diabetes. 2006;55:1413–1420. doi: 10.2337/db05-1388. [DOI] [PubMed] [Google Scholar]
- Perfetti R, Henderson TE, Wang Y, Montrose-Rafizadeh C, Egan JM. Insulin release and insulin mRNA levels in rat islets of Langerhans cultured on extracellular matrix. Pancreas. 1996;13:47–54. doi: 10.1097/00006676-199607000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkse GGM, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E. Integrin signaling via RGD peptides and anti-β1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes. 2006;55:312–317. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- Ris F, Hammar E, Bosco D, Pilloud C, Maedler K, Donath MY, Oberholzer J, Zeender E, Morel P, Rouiller D, Halban PA. Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human beta-cells in vitro. Diabetologia. 2002;45:841–850. doi: 10.1007/s00125-002-0840-7. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Timpl R. Laminins. In: Kreis T, Vale R, editors. Guidebook to the extracellular matrix, anchor, and adhesion proteins. Oxford University Press; New York: 1999. pp. 434–443. [Google Scholar]
- Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126:299–304. [PubMed] [Google Scholar]
- Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol Endocrin. 2003;17(4):732–742. doi: 10.1210/me.2002-0333. [DOI] [PubMed] [Google Scholar]
- Timpl R. Nidogen. In: Kreis T, Vale R, editors. Guidebook to the extracellular matrix, anchor, and adhesion proteins. Oxford University Press; New York: 1999. pp. 445–457. [Google Scholar]
- Tomas A, Yermen B, Min L, Pessin JE, Halban PA. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodeling: role of gelsolin and cooperation with the MAPK signaling pathway. J of Cell Sci. 2006;15(10):2156–2167. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- Tong YW, Shoichet MS. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials. 2001;22:1029–1034. doi: 10.1016/s0142-9612(00)00338-0. [DOI] [PubMed] [Google Scholar]
- Wang RN, Rosenberg L. Maintenance of β-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J of Endocrin. 1999a;163:181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- Wang RN, Paraskevas S, Rosenberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J of Histochem Cytochem. 1999b;47:499–506. doi: 10.1177/002215549904700408. [DOI] [PubMed] [Google Scholar]
- Wang R, Li J, Lyte K, Yashpal NK, Fellows F, Goodyer CG. Role for β1 integrin and its associated α3, α5, and α6 subunits in development of the human fetal pancreas. Diabetes. 2005;54:2080–2089. doi: 10.2337/diabetes.54.7.2080. [DOI] [PubMed] [Google Scholar]
- Weber LM, He J, Bradley B, Haskins K, Anseth KS. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled β-cell microenvironments. Acta Biomater. 2006;2:1–8. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated β-cell function within hydrogels functionalized with matrix-derived peptides. Biomaterials. 2007;28(19):3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve β-cell survival and insulin secretion in three-dimensional culture. Tissue Engineering. doi: 10.1089/ten.tea.2007.0238. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods EJ, Walsh CM, Sidner RA, Zieger MAJ, Lakey JRT, Ricordi C, Critser JK. Improved in vitro function of islets using small intestinal submucosa. Transplant Proc. 2004;36:1175–1177. doi: 10.1016/j.transproceed.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Yamada KM. Adhesive recognition sequences. J Biol Chem. 1991;266:12809–12812. [PubMed] [Google Scholar]
- Yashpal NK, Li J, Wheeler MB, Wang R. Expression of β1 integrin receptors during rat pancreas development—sites and dynamics. Endocrinology. 2005;146:1798–1807. doi: 10.1210/en.2004-1292. [DOI] [PubMed] [Google Scholar]