Summary
Advances in cytokine biology have helped us understand the complex communication that takes place between antigen-presenting cells and cells of the adaptive immune system, such as T cells, which collectively mediate an appropriate immune response to a plethora of pathogens while maintaining tolerance to self-antigens. The interleukin-12 (IL-12) cytokine family remains one of the most important and includes IL-12, IL-23, IL-27, and the recently identified IL-35. All four are heterodimeric cytokines, composed of an α chain (p19, p28, or p35) and a β chain (p40 or Ebi3), and signal through unique pairings of five receptor chains (IL-12Rβ1, IL-12Rβ2, IL-23R, gp130, and WSX-1). Despite the interrelationship between the cytokines themselves and their receptors, their source, activity, and kinetics of expression are quite different. Studies using genetically deficient mice have greatly enhanced our understanding of the biology of these cytokines. However, interpretation of these data has been complicated by the recent realization that p40−/−, p35−/−, and Ebi3−/− mice all lack more than one cytokine (IL-12/IL-23, IL-12/IL-35, IL-27/IL-35, respectively). In this review, we compare and contrast the biology of this expanded IL-12 family and re-evaluate data derived from the analysis of these dual cytokine-deficient mice. We also discuss how the opposing characteristics of the IL-12 family siblings may help to promote a balanced immune response.
Keywords: IL-35, IL-12, IL-27, IL-23, Regulatory T cells
Getting to know the interleukin-12 family
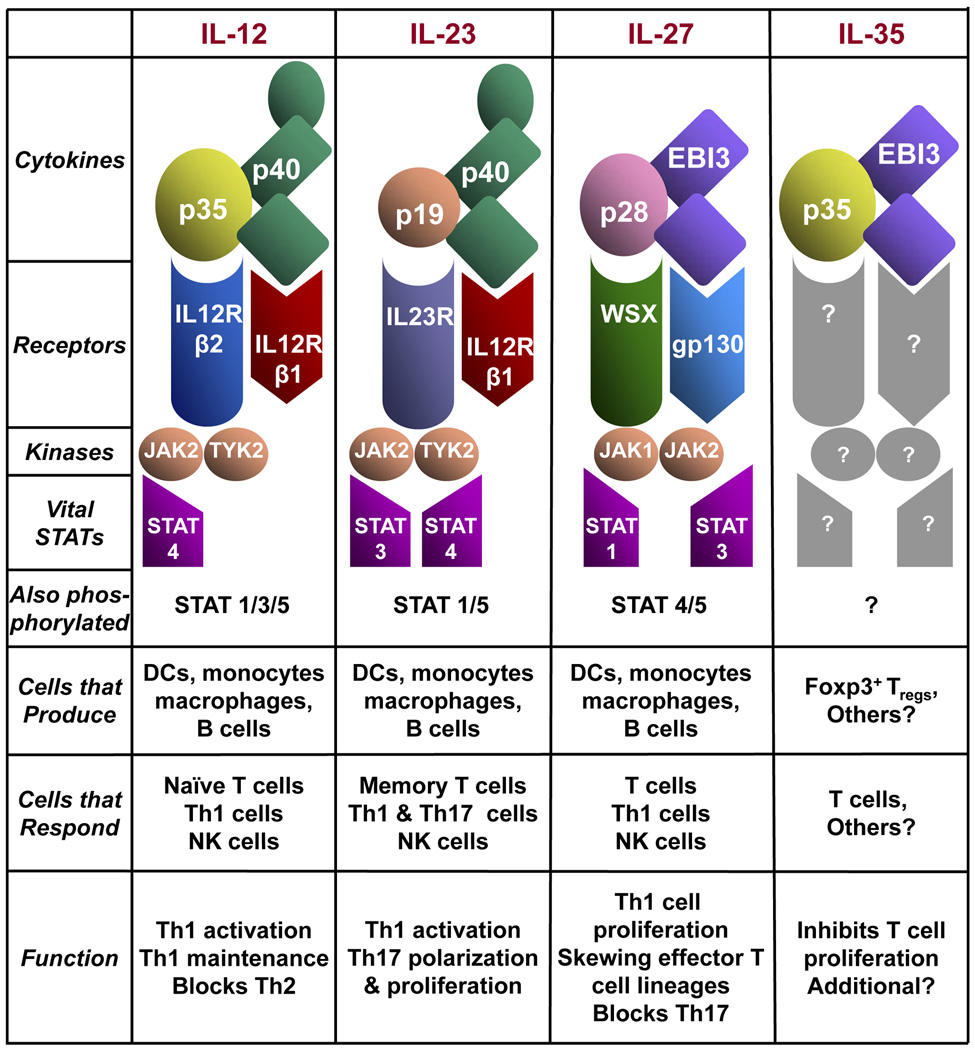
The interleukin-12 (IL-12) family, which is evolutionarily linked to the IL-6 cytokine superfamily, is composed of IL-12, IL-23, IL-27, and the newly identified IL-35(1). The IL-12- related cytokines are heterodimeric proteins comprised of an α chain (p19, p28 or p35) and a β chain (p40 or Ebi3) (Fig. 1). The α chain has a four α helix bundle structure typical of cytokines such as IL-6, granulocyte/macrophage colony-stimulating factor (GM-CSF), while the β chain shares homology with soluble cytokine receptor chains. The patriarch of the family, IL-12, is a disulfide-linked 70 kDa protein composed of p40 and p35 (2–4): the p40 chain can also heterodimerize with p19 to form IL-23 (5), providing the first insight into the extensive chain sharing that has become a characteristic of this cytokine family. Over 10 years ago, a second β chain, Ebi3 was identified in Epstein-Barr virus-infected B cells (6). The α chain partner for Ebi3 remained elusive until the discovery of the p35- and p19-related α chain p28, which pairs with Ebi3 to form IL-27 (7). The newest member of the IL-12 family, IL-35, is composed of the IL-27 β chain Ebi3 and the IL-12 α chain p35 (1). Given the multiple α and β chains and their unique pairings, it is fitting that their expression patterns are quite distinct as well.
Fig. 1. The IL-12 cytokine family; structural and biological characteristics.
Differential expression of the IL-12 family siblings
While these cytokines share binding partners and structural features, there are notable differences in their pattern of expression and secretion (Fig. 1). IL-12, IL-23, and IL-27 are all primarily produced by antigen-presenting cells (APCs), such as macrophages, monocytes, and dendritic cells (DCs), following their activation by recognition of pathogen-specific patterns (7–9). In contrast, secretion of bioactive IL-35 has been described only in forkhead box protein 3 (Foxp3)+ regulatory T cells (Tregs). Analysis of other hematopoietic populations also suggests that Ebi3 and p35 are co-expressed in peripheral γδ T cells, CD8+ T cells, and placental trophoblasts (1, 10). However, secretion of a bioactive heterodimer from these cell types has yet to be demonstrated. Moreover, given the fact that APCs produce both IL-12 and IL-27, it is possible that IL-35 is produced by a subset, or under certain circumstances, by APCs.
Production of each of the four heterodimeric proteins is limited by expression of the α chain. IL-12 and IL-35 assembly is limited because p35 is expressed at lower levels than that of Ebi3 and p40, and co-expression of the α and β chains is required for secretion of the bioactive cytokine (11). Similarly, secretion of IL-23 is limited primarily by the tissue and cell-restricted expression of p19 (5, 11). In cells that secrete IL-12, IL-23, and IL-35, the Ebi3 and p40 subunits are expressed in greater quantities than that required for heterodimer formation. Reports indicate that p40 homodimers, independent of IL-12, signal in DCs and that treatment of p40−/− DCs with a p40 homodimer restores their ability to activate naive T cells (reviewed by Cooper and colleagues in this issue). In addition, free p40 can be secreted from cells alone or as a homodimer, and the homodimer has been reported to function as an antagonist of IL-12 but not IL-23 activity(12) Therefore, it is possible that free Ebi3 or Ebi3 homodimers may antagonize IL-35 or IL-27 activity, although this has not been well studied.
A common feature of all α and β chains of the family is their expression in APCs in response to bacteria or purified bacterial products. However, not all IL-12 family chains are induced to the same degree or by the same types of pathogens. Stimulation of macrophages or DCs with TLR ligands such as lipopolysaccharide (LPS), peptidoglycan, and polyinosinic-polycytidylic acid (Poly I:C) induces significant production of IL-12 (13–15). Although intact Gram-negative bacteria modestly induce p19 expression, purified LPS, a cell-wall component of Gram-negative bacteria, fails to induce expression(16). In fact, the strongest induction of p19 expression is seen in response to Gram-positive bacteria. Moreover, LPS-activated macrophages express the highest level of p28 and Ebi3 in any tissue or cell type described (7, 8, 17). Another interesting difference between the IL-12 family member protein subunits is the kinetics of their expression. Using LPS-activated monocytes, p28, p35, and p40 expression occurs first, with p28 peaking at 4–6 h, p35/p40 peaking at approximately 10–12 h, and all levels restored to background after 24 h (7). However, transcription of EBI3 peaks between 12 and 24 h post-LPS activation and was maintained above background even after 72 h. p19 induction was not measured in these studies. However, based on the differential induction and duration of expression within the same cell type under identical conditions, this observation indicates that these proteins are tightly regulated. Therefore, it is likely that the relative amounts of each chain produced, and thus which cytokine is secreted, may be determined by subtle differences in stimuli, kinetics of stimulation, and relative affinities of each chain for one another.
Divergent function of the IL-12 cytokine family
Synonymous with differential expression, the IL-12 cytokine family also exhibits distinct functional differences (Fig. 1). While IL-12, IL-23, and IL-27 share the common feature of inducing interferon-γ (IFNγ) production and promoting T-helper 1 (Th1) cell differentiation and proliferation, they act differentially on subsets of T cells and with different kinetics. In contrast, IL-35 appears to function solely in an anti-inflammatory fashion by inhibiting T-cell proliferation and perhaps other parameters (1). Therefore, understanding the unique features of IL-12, IL-23, IL-27, and IL-35 and potential synergy and/or competition between the members of the IL-12 cytokine family is of great importance.
IL-12 is a pro-inflammatory cytokine, necessary for cell-mediated immunity and Th1 differentiation (18). The production of IL-12 initiates a complex positive feedback loop involving both APCs and naive T cells. APCs recognize microbial pathogens, which in turn results in the activation and secretion of IL-12. IL-12 secretion by APCs induces IFNγ production, which primes additional APCs for IL-12 production while at the same time initiates the cascade needed to differentiate naive T cells into Th1 cells (9, 19) While T cells do not generate IL-12 themselves, activated T cells induce IL-12 production by monocytes and DCs via CD40-CD40 ligand interaction (20, 21). In combination with IL-27, the Th1 polarizing effect of IL-12 is enhanced. Interestingly, one effect of IL-12 on APCs is to increase p19 (IL-23α) expression (19, 22), suggesting that IL-12, IL-23, and IL-27 may synergize to provide an optimal response to microbial pathogens. IL-12 also promotes IFNγ secretion and cytolytic activity of natural killer (NK) cells, a process which is augmented by costimulation with IL-18 or IL-2 (11, 23, 24).
IL-12 expression and function is regulated by cell-intrinsic and cell-extrinsic factors. IL-12 is positively regulated by IFNγ (9) and negatively regulated by IL-4, IL-10, IL-11, IL-13, and type 1 IFNs (13, 25).The use of various murine models of infection has highlighted the importance of IL-12 in the immune system. The indispensible role of IL-12 in Th1 development and maintenance is evident in infectious models that are dependent upon a robust Th1 response. In this regard, clearance of intracellular bacteria through production of IFNγ, direct lysis of infected cells by NK cells, and induction of anti-bacterial macrophage responses are entirely dependent upon IL-12 (26–28). However, the activity of IL-12 can be antagonized. For example, in patients with systemic lupus erythematosus (SLE), a Th2-driven pathology, ex vivo-stimulated mononuclear cells secrete low levels of IL-2 and IFNγ and high levels of IL-4 and IL-10, consistent with the skewing of cells towards a Th2 response (29). However, despite their predisposition to a Th2-like response, SLE patients possess increased levels of serum IL-12 and IFNγ (30). This highlights two important issues. First, SLE is a complex disease where the Th1/Th2 balance may shift depending upon the disease state or severity. Second, it indicates that the ability of IL-12 to induce a Th1 response can be abrogated by excessive Th2-like cytokines. Similarly, based upon the ability of IL-12 to directly antagonize IL-4 secretion and block Th2 cell responses, the use of IL-12 to inhibit Th2-mediated diseases such as allergy and asthma may have therapeutic potential (31, 32).
Additional support for the importance of IL-12 in the Th1 response also initially came from the observation that anti-IL-12 p40 monoclonal antibody treatment significantly reduces disease in experimental autoimmune encephalomyelitis (EAE), which is induced by pathogenic Th1 cells. In fact, IL-12 p40 neutralization blocks both superantigen-induced and subsequent relapses of EAE (33). The original conclusion was that IL-12 blockade could prevent or reverse disease; however, in light of the discovery of IL-23 and consequent studies, it appears that antip40 antibodies in fact block the IL-23-driven pathogenic Th17 cells, which are instrumental in EAE progression. In support of these observations, macrophages in mice that are genetically susceptible to EAE (SJL mice) and type 1 diabetes [nonobese diabetic (NOD) mice] express aberrantly elevated levels of IL-12 p40, whereas expression of IL-12 p35 remains that same as in non-disease prone strains of mice. This further supports the importance of IL-23 and not IL-12 in susceptibility to disease.
IL-23, like IL-12, is a pro-inflammatory cytokine, which induces IFNγ production and promotes the Th1 response. While a great deal is known about the regulation of IL-12, much less is known about IL-23. One known immunomodulator that appears to regulate expression of IL-23 is prostaglandin E2, which enhances IL-23 expression (34). IL-23 participates in a positive feedback loop promoting IL-12 production by DCs to enhance p19 transcription and further IL-23 production. However, in the absence of IL-23, IFNγ production and Th1 differentiation are normal (35–37), indicating that while IL-12 and IL-23 can synergize for maximal Th1 induction, IL-23 is not absolutely required for Th1 differentiation. In addition, the cell type that is responsive to IL-23 is not conventional naive (CD4+CD45RBhi) T cells, which proliferate predominantly in response to IL-12, but rather memory (CD4+CD45RBlo) T cells (22). Moreover, IL-23 has a role in promoting NK cell activity, in that IL-23 synergizes with IL-12, IL-18, and IL-2 to promotes IFNγ production by NK cells (38). These results suggest responsiveness to the IL-12 cytokines is not static but rather is modified during the course of an inflammatory response. Recently another novel Th1-independent function of IL-23 has been elucidated. Unlike the other IL-12 family cytokines, IL-23 promotes the survival of Th17 cells induced by TGFβ and IL-6 (22). In fact, exposure to IL-23 and IL-1 is necessary for full effector differentiation and function of Th17 cells (35, 39). IL-23 is preferentially expressed by intestinal APCs and appears to drive intestinal inflammation (16). In support of this observation, stimulation via CD40 induces a dramatic increase in IL-23 expression by colonic DCs but only a modest increase by monocyte-derived DCs (16). This elevated IL-23 occurs in DCs of mice with colitis but not in control mice. Moreover, expression of IL-23 was also recently described in psoriatic lesions, where p40 and p19 are preferentially expressed, in the absence of p35 (40). Taken together, these data suggest that in addition to its role in Th1 cell function, IL-23 plays a significant role in Th17 cell development and may exacerbate Th17-driven pathologies.
All three of the original family members, IL-12, IL-23, and IL-27, were initially described as pro-inflammatory/stimulatory cytokines, promoting T-cell proliferation and cytokine production. Interestingly, CD40L-CD40 interactions between T cells and DCs augment production of IL-12, IL-23, and IL-27 (41). Following the discovery of IL-27, initial reports indicated that IL-27, in combination with IL-12, enhanced the production of IFNγ by naive T cells and NK cells by promoting expression of T-bet and IL-12Rβ2 (8, 42). This observation suggests that IL-27 sensitizes T cells to the effects of IL-12 and is critical for the early events leading to Th1 cell polarization. However more recently, a host of immunomodulatory functions have been ascribed to IL-27 in addition to its role in Th1 development (reviewed in 43). Similar to results seen with anti-IL-12 p40 monoclonal antibodies, it has been suggested that anti-IL-27 p28 antibodies are also capable of reducing the severity of EAE, a Th1-driven disease (44, 45). This function of IL-27 appears to be dependent, in part, on IL-10 (43). New reports indicate that IL-27 can inhibit Th1-driven infections (46, 47), limit Th2 activity towards Leishmania major (48), and block the development of Th17 and transforming growth factor-β (TGFβ)-driven induced Treg formation (49–51). Like IL-12, IL-27 can directly inhibit secretion of IL-4 and antagonize IL-2 production, hence limiting Th2 cell differentiation and effector cell function (48). In addition, compared to normal adult blood, umbilical cord blood has significantly higher mRNA expression levels of both Ebi3 and p28 (52), which suggests enhanced IL-27 expression during fetal development and implicates a role for IL-27 in feto-maternal tolerance. In this regard, IL-27 was the first member of the IL-12 family to be shown to have both inflammatory and immunomodulatory activity by skewing development of effector cell lineages.
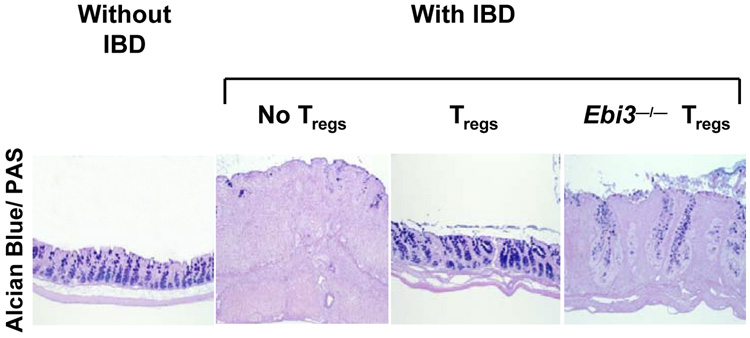
IL-35, the newest member of the IL-12 family, is distinct from its siblings in several ways. Within the CD4+ T-cell population, IL-35 is expressed by resting and activated Tregs but not effector T (Teff) cells (1). Maximal expression and secretion of IL-35 by Tregs requires contact with Teff cells, which potentiates the suppressive capacity of Tregs (LWC and DAAV, unpublished observations). In addition, previous work indicated that Ebi3 and p35 are highly expressed in placental trophoblasts, which suggests that, like IL-27, IL-35 may be an immunomodulator at the feto-maternal border (10). Whether this effect is due to Treg-mediated suppression of maternal responses to the fetus or due to the suppressive capacity of other cell types that might secrete IL-35 remains to be determined. Although many of the biological effects of IL-35 are still being elucidated, it is clear that IL-35 directly suppresses Teff cell proliferation in vitro, in an APC-free culture. Moreover, loss of IL-35 expression results in reduced in vivo suppressive capacity of Tregs (1). IL-35-deficient Tregs fail to control the homeostatic expansion of effector T cells and are less able to cure established colitis when compared to wildtype Tregs (Fig. 2). In addition, it has been suggested that IL-35 can suppress in vivo Th17 development and ameliorate collagen-induced arthritis (53); however, the mechanism by which this occurs requires further study.
Fig. 2. IL-35-deficient Tregs fail to cure inflammatory bowel disease.
Rag1−/− mice received naive effector T cells via the tail vein. After 3–4 weeks, mice developed clinical symptoms of inflammatory bowel disease and were either untreated or given wildtype or Ebi3−/− (IL-35 deficient) Tregs. Histological sections of representative colons were stained with Alcian blue/PAS (Periodic acid Schiff) (1).
Transducing the signal: receptors and intracellular signaling
Chain sharing has become an established hallmark of the IL-12 cytokine family, a feature that may also extend to their receptors (Fig. 1). IL-12Rβ1 and IL-12Rβ2 form the functional IL-12 signaling receptor (54, 55). IL-23 also signals through IL-12Rβ1 which paired with the novel IL-23R (38). In contrast, IL-27 utilizes gp130 and WSX-1 (56) The receptor for IL-35 is currently unknown. However, in light of this propensity for chain sharing, it seems reasonable to propose that IL-35 signals through a combination of receptor chains that are utilized by other family members. Alternatively, a unique receptor chain may be used.
The biological effects of IL-12, IL-23, IL-27, and presumably IL-35 are mediated through their interaction with their receptor chains which bind to and induce phosphorylation of the Janus kinase (Jak) family proteins (38, 57) (Fig. 1). Jak proteins then phosphorylate tyrosine residues on the intracellular domains of the receptor chains, which act as docking sites for various members of the signal transducer and activator of transcription (STAT) family. Phosphorylated STAT proteins form intramolecular complexes that translocate into the nucleus and bind DNA to initiate the gene expression profile and cellular response that typifies the given cytokine.
For IL-12 signaling, the IL-12 p40 subunit interacts primarily with IL-12Rβ1, while p35 binds to IL-12Rβ2 (58–61). Both receptor chains are required to mediate maximal signaling; however, the two chains have independent roles. IL-12Rβ1 is required for high affinity binding to IL-12, and IL-12Rβ2 is required for signal transduction through three tyrosine residues (59). Not surprisingly, both receptor chains are expressed on IL-12-responsive cell types; T cells, NK cells and DCs (62). The IL-12 receptor chains are expressed at very low levels on resting T cells but are induced upon cell activation (63, 64). Consistent with the role of IL-12 in promoting the Th1 response, IFNγ increases T-bet activity that leads to the upregulation of IL-12Rβ2 surface expression and allows for Th1 cell responsiveness to IL-12 (63, 64). On the contrary, the Th2 cytokine IL-4 reduces IL-12Rβ2 expression and thus renders Th2 cells non-responsive (65). This control of receptor expression helps maintain the fidelity of the Th1-Th2 axis and underlies the sensitivity of polarized cells to various cytokines. Binding of IL-12 to the IL-12 receptor induces phosphorylation of Jak2 and Tyk2 proteins (38, 57). STAT1, STAT3, STAT4, and STAT5 have all been reported to be activated by IL-12 signaling; however, only STAT4 is critical and indispensible (57, 66–68). Consistent with the importance of IL-12 signaling in Th1 polarization, direct STAT4 binding to the IFNγ promoter has been shown (69). Moreover, Lck and mitogen-activated protein kinase (MAPK) p38 signaling appears to be critical, as mutation in S721 on STAT4, the target of p38, blocks Th1 differentiation (70).
The IL-23 receptor is composed of IL-12Rβ1 and the novel receptor chain IL-23R (5, 38). Similar to IL-12 signaling, IL-12Rβ1 is required for high affinity binding, while the IL-23R is the signal transducing component signaling through three intracellular tyrosine residues. While the IL-12Rβ1 chain is expressed on many cell types, the IL-23R is weakly expressed on T cells, NK cells, monocytes, and DCs (38), cells that are primarily responsive to IL-12 and generally unresponsive to IL-23. The IL-23R is highly expressed on memory T cells (CD4+CD45RBlo) but is not expressed on naive T cells (CD4+CD45RBhi). Instead, naive T cells secrete IL-2 and IFNγ, which induce expression of IL-12Rβ2 to allow IL-12 signaling in IFNγ-producing, newly activated T cells (38). Naive cells mature and clonally expand to become memory cells which express the IL-23R chain and thus confer responsiveness to IL-23. In addition to memory T cells, IL-23R expression is highly upregulated on Th17 cells, which is consistent with a central role for IL-23 in the maintenance and homeostasis of Th17 cells. The STAT phosphorylation pattern elicited by IL-23 is similar to that seen with IL-12 (STAT1, STAT3, STAT4, and STAT5)(38). However, STAT4 phosphorylation is much weaker than that seen following IL-12R signaling. Instead, STAT3 appears to be the primary mediator of IL-23 signaling.
IL-27 signals through a heterodimeric pairing of gp130 and the previously orphaned receptor chain, WSX-1 (also known as IL-27R, TCCR)(22, 71, 72). The gp130 chain is ubiquitously expressed and is a component of receptors for many cytokines including IL-6, IL-11, and leukocyte inhibitory factor (LIF) (73). In contrast, WSX-1 is primarily expressed on T cells and increases upon activation (43). Initial reports indicated that WSX-1−/− mice have impaired Th1 development, reduced IFNγ production, and are susceptible to intracellular pathogens, commensurate with its central role in mediating IL-27 signal transduction (47, 74). However, more recent reports suggest that WSX-1−/− mice have enhanced resistance to Listeria monocytogenes and EAE (75), which emphasizes the dichotomy of IL-27 functions. The signaling pathway initiated by IL-27 activates JAK1 and JAK2, resulting in phosphorylation of STAT1, STAT2, STAT3, and STAT5 in naive CD4+ T cells. However, only STAT1 and STAT3 are critical to IL-27 bioactivity, as demonstrated by the loss of IL-27 activity in STAT1 and STAT3-deficient mice. Moreover, IL-27 induces T-bet, IL-12Rβ2, and major histocompatibility complex (MHC) class I expression in a STAT1-dependent fashion. However, STAT1 is not required for IL-27-induced proliferation, which instead appears to be dependent on STAT3 (76). Additionally, small interfering RNA (siRNA) knockdown experiments suggest that the suppressive effects of IL-27 are partially dependent upon STAT3, while they are antagonized by STAT1 (77).
Although the IL-35 receptor and signaling pathway are still unknown, it is reasonable to hypothesize that IL-35, like its siblings, will signal through the pairing of known receptor chains utilized by the IL-12 cytokine family. One might deduce that IL-35, being composed of Ebi3 and p35, might signal through receptors that are known to interact with each of these subunits in IL-27 and IL-12, respectively. Since the p35 subunit of IL-12 binds to IL-12Rβ2 to initiate signaling, this chain may be a part of the IL-35 receptor. IL-27 signaling requires both gp130 and WSX-1, however direct binding studies to determine which subunit of the cytokine interacts with the individual receptor chains have not been performed. Therefore, it is possible that either gp130 or WSX-1 may be members of the IL-35 receptor. Whether these pairings are possible and can transduce the IL-35 signal is still under investigation. In addition, it is interesting to speculate, based on the known signaling pathways involved in IL-12 and IL-27 activity, that STAT4 and perhaps STAT1 and STAT3 might be involved in the IL-35 signaling pathway. Moreover, as STAT1 signaling has been shown to induce expression of IL-12Rβ2, if IL-12Rβ2 is a component of the IL-35 receptor, IL-35 might induce expression of its own receptor in a STAT1-dependent manner. If IL-35 does utilize receptor chains and STATs that are similar to those used by its siblings, this would raise an important question: how do T cells translate potentially similar signals into quite contrasting biological outcomes (i.e. proliferation versus suppression)? Of course, given the opposing activities of IL-35 compared with IL-12, IL-23, and IL-27, it remains possible that a unique receptor chain and signaling pathway is utilized that could mediate the distinct biological consequences of IL-35. Resolving these questions remains an important challenge.
What do knockout mice tell us about the function of the IL-12 family cytokines?
The use of genetically deficient mice to determine functions of specific proteins has been extremely useful in defining in vivo protein function. However for the IL-12 family, this approach is less straightforward due to their heterodimeric structure and the frequency of chain sharing. For example, mice that lack p40 fail to produce both IL-12 and IL-23. Consequently, any observed phenotype could be due to the lack of one or both of these cytokines. This was evident from the analysis of models for EAE and collagen-induced arthritis (CIA), as the p35−/− and p40−/− mice have significantly different susceptibilities to disease. Upon discovery of IL-23, conclusions based upon p40 knockout mice or using p40 neutralizing antibodies had to be re-evaluated. Comparing responses in the newly generated p19−/− mice (lacking only IL-23) to responses generated in p35−/− (lacking only IL-12) and p40−/− (lacking both IL-12 and IL-23) has shed new light on the relative contributions of IL-12 and IL-23 to a variety of immune responses. However, inexplicable differences between p35−/− and p40−/− mice still exist.
The recent discovery of IL-35 has significantly complicated this analysis, as it has become clear that p35−/− mice lack IL-35 and IL-12 while Ebi3−/− lack IL-35 and IL-27. The opposing activities of these cytokine pairs (IL-12 vs. IL35; IL-27 vs. IL-35) serve to further complicate analysis, as the loss of one may mitigate the loss of the other, which may underlie the rather mild phenotype see in these mice. For example, if an Ebi3−/− mouse were challenged with a bacterial pathogen, their Th1 response might be diminished due to the lack of IL-27. However, the suppressive effect of IL-35 would also be lost, therefore the net effect might be that the knockout and wildtype mice are equally susceptible to infection. This is likely to be the case for all T-cell-mediated diseases (protective or pathogenic). Therefore, in light of the advent of IL-35, a careful re-evaluation of studies utilizing these mutant mice is warranted. Indeed, it is interesting to ask whether observations and characteristics derived from the analysis of these mice that were previously attributed to IL-27 and IL-12 may in fact be features of IL-35.
IL-12 family cytokines in autoimmune and inflammatory diseases
Mice deficient in either p35 or p40 exhibit reduced IFNγ secretion, impaired Th1 development, enhanced Th2 development, and increased NK cell lytic activity (70, 78). IL-12 secreted by APCs skews the Teff cell population towards Th1 and away from Th2 development, owing to the need for IL-12 in both pathogenic and protective Th1 responses. However, in the context of many diseases and infectious models, p35−/− and p40−/− do not phenocopy one another; therefore, the goal of this section is to reevaluate these studies to determine what differences cannot be explained by the IL-12/IL-23 axis and thus might be attributed to IL-35. In models of T-cell-mediated chronic inflammation such as EAE, CIA, and autoimmune myocarditis, pathogenic T-cell populations drive the inflammatory response. In each of these disease models, the severity or kinetics of infection vary between p35−/− and p40−/− mice, therefore, a reevaluation of these mice in each disease context is warranted. Unfortunately, the Ebi3−/− mice have been evaluated in only a few disease models limiting their comparison with p35−/− mice. Nevertheless, we compare below the susceptibility and responsiveness of each knockout mouse to provide greater insight into the role of IL-12, IL-23, IL-27, and IL-35 in these autoimmune inflammatory disease models.
EAE
EAE is a murine model for the human disease multiple sclerosis characterized by lymphocyte infiltration into the central nervous system (CNS), demyelination of axons, and progressive paralysis (79). Autoreactive Th1 cells, which aberrantly respond to neuro-autoantigens such as myelin basic protein, are considered to be the initiators of EAE (80). Early reports suggested that IL-12 was essential for the pathogenesis of EAE in myelin oligodendrocyte glycopeptide (MOG)-immunized mice (81, 82). In fact, EAE can be prevented by antibodies against IL-12 p40 (33, 83) or by transfer of MOG-specific T cells retrovirally transduced to express IL-12 p40 (84). However, a recent reevaluation of the role of the IL-12 subunits in EAE indicated that while p35−/− and wildtype mice are equally susceptible, p40−/− mice are completely resistant to EAE development (36, 85, 86). No studies have been performed to determine the susceptibility of Ebi3−/− mice to EAE. To determine the relative importance of IL-12 and IL-23 in the EAE model, the susceptibility of p19−/−, p35−/−, and p40−/− mice was compared. These experiments suggested that only p19−/− and p40−/− mice were resistant to EAE and that exogenous IL-23 could render these mice susceptible to EAE (36). The conclusion that was drawn is that IL-23 and not IL-12 is responsible for the T-cell-mediated autoimmune inflammation seen in EAE. However, in light of the recent identification of IL-35, it is noteworthy that p35−/− mice lack both IL-12, which is important to Th1 development and effector function, and IL-35, which suppresses T-cell proliferation. Importantly, Tregs are involved in modulating EAE pathogenesis. In experimentally induced EAE, Tregs co-cultured at high suppressor to responder ratios were found to inhibit both IFNγ production and proliferation of encephalitogenic cells from MOG (35–55)-immunized mice in vitro and limit disease severity in vivo (87). In contrast, depletion of Tregs with anti-CD25 monoclonal antibody significantly reduces the threshold for EAE development (88) and increases the severity of disease(89, 90). Treg depletion also renders naturally resistant B10.S mice susceptible to actively-induced EAE (90). Thus, one cannot exclude a role for Treg-derived IL-35 when interpreting these results.
The p40−/− mice lack two pro-inflammatory cytokines, IL-12 and IL-23. Therefore, in the absence of IL-12 and IL-23 (p40−/− and p19−/−), T cells may be unable to infiltrate and cause disease, while in the absence of IL-12 and IL-35 (p35−/−) the suppressive effects of IL-35 upon T-cell proliferation are released, and IL-23-driven T cells alone are pathogenic. Although p35/p19−/− (double knockout) mice are susceptible to EAE initiated by adoptive transfer of encephalitogenic cells from MOG-immunized mice, this only indicates that IL-23 is not critical for the effector phase of EAE (91). The contribution of IL-35 could be assessed by comparing EAE in p40−/− mice, which lack IL-12 and IL-23, with p35/p19−/− mice, which lack all three cytokines.
CIA
CIA is a murine model of rheumatoid arthritis that can be induced experimentally by immunization with type II collagen (92). CIA is characterized by destruction of bone and cartilage in joints and swelling due to cellular infiltration and secretion of inflammatory mediators such as IL-17 and tumor necrosis factor α (TNFα) (93, 94). After induction of CIA, mice lacking IL-23 (p19−/−) and p40−/− mice generate collagen-specific T cells that produce IFNγ but no IL-17 and are resistant to all CIA pathology. On the contrary, p35−/− mice lacking both IL-12 and IL-35 have heightened IL-17 production and are highly susceptible to CIA, with 80% of mice showing clinical signs of disease. Histological examination revealed disease indistinguishable from that of wildtype infected mice(37). When initially reported, these results were interpreted as indicating that IL-23 is essential for the promotion of CIA, whereas IL-12 is paradoxically protective(37). However, similar to the observations with EAE, in the light of the identification of IL-35, it is possible that IL-35 protects against the inflammatory response initiated by IL-23, but in its absence mice are susceptible to CIA. In support of this hypothesis, similar to their role in EAE, Tregs can both prevent and treat active CIA (95). Interestingly, effective treatments for CIA including delivery of vasoactive intestinal peptide (96), galectin-9 (97), and urocortin (98) suppress CIA by promoting the induction or expansion of Tregs. Moreover, the heightened susceptibility of IFNγR-deficient mice to CIA is associated with functionally impaired Tregs.
Although no studies have been performed to determine the susceptibility of Ebi3−/− mice to CIA, two more recent reports support this hypothesis. First, it was shown that T cells from Ebi3−/− mice (IL-35 and IL-27 deficient) secrete high levels of IL-17 and that there is a greater frequency of IL17-producing cells when polarized toward a Th17 phenotype (99). In addition, an IL-35 fusion protein was recently suggested to inhibit the differentiation of Th17 cells in vitro and to attenuate CIA and suppress IL17 production in vivo (53). These data suggest that IL-35 may suppress the pathogenic T-cell response and protect mice from disease.
Myocarditis
Experimental myocarditis is another T-cell-mediated autoimmune disease, which is induced experimentally by immunization with cardiac myosin (100). Similar to CIA, IL-17-producing T cells appear to promote autoimmune myocarditis. Utilizing a vaccination approach based on VLPs (virus-like particles) to generate IL-17 neutralizing antibodies, neutralization of IL-17 has been shown to completely protect mice from disease (101). Wildtype Balb/c and p35−/− mice are equally susceptible to CIA, producing substantial amounts of IL-17. However, p40−/− mice are completely resistant to disease, and neutralizing IL-23 (p19 monoclonal antibody) treatment reduces disease severity by approximately 75%. Taken together, these data indicate that in addition to IL-23 involvement, susceptibility of p35−/− mice to CIA and autoimmune myocarditis may be a result of the loss of the protective effect of IL-35 through its inhibition of IL-17 production. In addition, although the number of studies is limited, one report indicates that autoimmune myocarditis can be controlled by Tregs. Transfer of glucocorticoid induced tumor necrosis factor receptor (GITR)hi Treg-depleted T cells or thymocytes into BALB/c nude mice results in the development of severe multiorgan inflammation that includes fatal autoimmune myocarditis, suggesting that Tregs may be involved in protection against myocarditis. Analysis of experimental myocarditis in Ebi3−/− mice might help assess the relative importance of IL-35 in this disease.
Inflammatory bowel diseases
Crohn’s disease and ulcerative colitis are inflammatory disorders of the gastrointestinal tract, collectively referred to as inflammatory bowel disease or colitis. These disorders are caused, in part, by deregulated immune responses to commensal gut bacteria that result in the destruction of gut architecture by infiltrating lymphocytes. IL-12 and IL-23 are highly expressed in the gut of mice and patients with inflammatory bowel diseases (102, 103) and anti-p40 monoclonal antibody treatment can reduce inflammation (104–106). Colitis can be induced acutely by rectal administration of haptens trinitrobenzene sulfonic acid (TNBS) or oxazolone. In hapten-induced colitis, p35−/− mice develop a mild form of the disease, whereas p40−/− mice develop a more severe disease. Treatment of p35−/− mice with anti-p40 monoclonal antibody resulted in a disease severity similar to that seen in p40−/− mice (107). More recently, colitis was induced by dextran sodium sulfate (DSS) or TNBS in p19−/− mice (108). Results indicate that p19−/− mice are highly susceptible to colitis and, compared to wildtype control mice, bone marrow-derived DCs express much higher levels of both p35 and p40, indicating that in the absence of IL-23, IL-12 is overexpressed, yet it is unable to protect mice from disease. In fact, IL-12 appears to drive the pathogenesis of colitis as anti-p40 monoclonal antibody treatment could rescue mice from lethal disease (108). Interestingly, the susceptibility of Ebi3−/− mice to colitis appears to be determined by the cell type that drives disease. For instance, Ebi3−/− mice are resistant to oxazolone-induced colitis, which is driven by invariant NKT cells and Th2 cells (109). However, Ebi3−/− mice are indistinguishable from wildtype control mice in TNBS-induced colitis, a Th1-mediated colitis model. Collectively, data from Ebi3−/− and p35−/− mice indicate that in the absence of IL-35, susceptibility to chemically induced colitis is not enhanced. At first, this result may seem contradictory to the necessity of IL-35 for Treg function; however, Tregs are required only for protection against experimental models of colitis in lymphopenic mice (110). Infection with Helicobacter hepaticus, which causes colitis in lymphopenic mice and is cured by Tregs, does not induce immune pathology in wildtype mice, underlining the ability of non-T cells with regulatory capacity, including innate cells and CD8+ or TCRγδ+ T cells to prevent disease (reviewed in 111). Although hapten-mediated colitis is believed to be mediated primarily by Th1 cells, when intact knockout mice are used to determine the affect of various cytokines, it is difficult to determine which cell type is responsible for the phenotype observed. Until recently, this issue could not have been appreciated as a confounding variable as IL-12, IL-23, and IL-27 are produced solely by APCs, not T cells. Therefore, the deficiencies observed were a direct consequence of dysfunctional antigen presentation to T cells or the environment created by these APCs.
However, the identification of IL-35, which is secreted by Tregs, makes interpretation of data derived from Ebi3−/−, p35−/−, and p40−/− mice less clear. Hence, we recently utilized the recovery model of inflammatory bowel disease, a model which is highly dependent on T-cell function (112), to determine the contribution of Treg-derived IL-35 in the cure of colitis (1). In this model, lymphopenic RAG−/− mice develop colitis when they are reconstituted with naive T cells. Approximately 4 weeks later, clinical signs of sickness are evident and Tregs (wildtype, Ebi3−/−, or p35−/−) are injected into mice in an attempt to cure their colitis. Mice that receive IL-35- deficient Tregs (Ebi3−/− or p35−/−) do not resume weight gain and fail to recover from disease as determined by histological scoring of colons (1). To determine the relative contribution of IL-12 and IL-23 to T-cell-mediated colitis, IL10−/− mice (which spontaneously develop disease) were crossed onto either p19−/− or p35−/− background, and the incidence of colitis was monitored. Interestingly, p35−/−IL10−/− mice had accelerated colitis developing by 7 weeks of age, compared to IL10−/− mice, which developed disease by 3 months of age (113). Conversely, p19−/−IL10−/− were still free of colitis at 12 months of age. It was also shown in this study that recombinant IL-23 accelerates colitis by promoting IL17 secretion. From these results, it was concluded that IL-23 and not IL-12 is essential for T-cell-mediated colitis. Similarly, results indicate that in Helicobacter hepaticus-mediated T-cell-dependent colitis, anti-IL10R monoclonal antibody treatment results in severe colitis in p35−/− but not p40−/− mice, suggesting that IL-23 drives intestinal disease (114). Considering that IL-35-deficent Tregs fail to cure established colitis, an alternate or additional interpretation is that the spontaneous colitis arising in IL10−/− mice is exacerbated in the presence of Tregs that lack IL-35 and thus are unable to control development of disease. In this manner, the lack of p35 expression reduces both the pro-inflammatory T-cell cytokine IL-12 and the suppressive Treg cytokine IL-35, resulting in colitis that can still be driven by IL-23 but cannot be inhibited by IL-35. The role of Tregs in controlling inflammatory bowel disease is well documented and reviewed elsewhere (115). Prevention and cure of colitis by Tregs is not limited to disease induced by T cells. In fact, colitis triggered by H. hepaticus can also be suppressed by Tregs, indicating that they can directly suppress pathogenic activation of the adaptive and the innate immune response. Taken together, these studies suggest that while diseases such as EAE, CIA, myocarditis, and inflammatory bowel disease are enhanced by the pathogenic expansion of T cells, which in some instances may be exacerbated by IL-12, IL-23, and IL-27, Treg-derived IL-35 may play an important suppressive role.
IL-12 family cytokines in infectious diseases
Pro-inflammatory effector T-cell responses are necessary for host survival of infectious agents. IL-12 is known to be required for the Th1 response to host infection by many bacteria [Listeria monocytogenes (116), Mycobacterium tuberculosis (117, 118), Francisella tularensis (119), and Mycobacterium bovis (120)], fungi [Cryptococcus neoformans (121)], and parasites [Toxoplasma gondii (122, 123), Schistosomiasis mansoni (124), and Leishmania major (125, 126)]. Mice lacking either the p35 or the p40 subunit differ in their ability to control various infectious agents; however, regardless of the type of infectious agent, p35−/− mice are less susceptible to infections than p40−/− mice.
Bacterial infections
A number of studies have compared the relative susceptibility of p35−/− and p40−/− mice. In Listeria monocytogenes infection, p35−/− mice resisted up to 1000 colony-forming units (cfu) and were able to eliminate Listeria to an even greater degree than wildtype mice (116). In another bacterial infection, Mycobacterium tuberculosis, clearance is elicited by IFNγ-producing T cells. In aerogenically delivered M. tuberculosis, p35−/− mice exhibit antigen-specific responses far superior to those observed in p40−/− mice; however, neither were as capable of controlling the infection as wildtype mice (117). In addition, vaccination elicits a protective response in wildtype and p35−/− mice, characterized by increased secretion of IFNγ and IL-17. However, p40−/− mice fail to accumulate antigen-specific IFNγ- and IL-17-producing T cells and have elevated bacterial burden (118). Francisella tularensis is an intracellular bacterium to which resistance is dependent upon production of IFNγ- and TNFα-producing Th1 cells (119). As reported with other bacterial infections, p35−/− mice displayed enhanced resistance to infection, both in the primary and secondary challenges, whereas p40−/− mice never cleared the bacterium and established a chronic infection (119). In another bacterial infection, Mycobacterium bovis, both p40−/− and p35−/− mice fail to control infection, with p35−/− mice exhibiting elevated bacterial burdens when compared to p40−/− mice. However, despite their lack of IL-17 production, p19−/− mice control Mycobacterium bovis infection to the same degree as wildtype mice (120). Moreover, treatment of mice with an IL-23 p19 specific neutralizing antibody has no affect on M. bovis clearance indicating that IL-23 is not required for control of M. bovis infection. Unfortunately, there are currently no studies that have addressed the impact of bacterial infections upon Ebi3−/− mice.
Fungal and parasitic infections
In the fungal infection Cryptococcus neoformans, the inflammatory response associated with protection is elicited by T cells and macrophages that produce IFNγ, TNFα, and nitric oxide synthase, respectively. Following infection, both p40−/− and p19−/− mice were more susceptible to disease than p35−/− mice. Additionally, recombinant IL-23 treatment enhanced the response of p40−/− mice such that they were as resistant as and had comparable survival to p35−/− mice (121). These results suggest that IL-23 enhances the inflammatory response to C. neoformans. However, p35−/− and p40−/− mice have opposing resistance to disease in two parasitic infections; Schistosomiasis mansoni (124) and Toxoplasma gondii (122, 123). In S. mansoni-infected mice, p35−/− and wildtype mice exhibited equally enhanced lesion size and accelerated death in comparison to p40−/− mice, which appeared nearly entirely resistant to infection. In contrast, similar to that seen with all other infectious agents, in Toxoplasma gondii infection, p40−/− mice succumb to infection more rapidly than p35−/− mice. Indeed, p19−/− mice controlled parasite replication to the same extent as wildtype mice. Interestingly, treatment of p40−/− mice with recombinant IL-23 reduces the parasite burden and enhances survival in T. gondii-infected mice in an IFNγ-independent manner (123). The conclusion that was drawn from these results is that in the absence of IL-12, IL-23 can compensate, albeit suboptimally, for resistance to infection. Another possible interpretation of the results is that in the absence of IL-12 and IL-23 (p40−/−), T cells are unable to control the infection; however in the absence of IL-12 and IL-35 (p35−/−), IL- 35-deficient Tregs are incapable of suppressing the T-cell proliferation mediated by IL-23. Thus, the mice are modestly resistant to disease. This conclusion is supported by the data indicating that rIL-23-treated p40−/− mice exhibit enhanced survival. Additional support for the role of Treg-derived IL-35 in controlling infectious agents comes from experiments using Ebi3−/− mice. Following Leishmania major infection, Ebi3−/− mice were more susceptible than wildtype mice during the early phase of infection, displaying significantly increased lesion size, greater parasite load, and impaired IFNγ production when compared to wildtype mice (126). However, this susceptibility is transient, and Ebi3−/− mice eventually clear the infection. Ebi3 deficiency resulted in skewed cytokine responses away from the protective Th1 response and toward the Th2 response marked by enhanced IL-4, IL-10, and IL-13. The assumption made following these results was that IL-27 derived from DCs was critical for the parasite clearance, although, if true, this effect is clearly not mediated by IL-10. However, similar to p35−/− mice, which lack both IL-12 and IL-35, Ebi3−/− mice lack both IL-27 and IL-35. Therefore, if IL-27 is important for anti-parasite immunity against L. major and IL-35 suppresses this immunity, the net affect of losing both IL-27 and IL-35 might be susceptibility to the infection, at least during the early phase of infection. In fact, there is a growing body of literature that suggests that Tregs are fundamentally involved in the persistence of L. major (125).
Host survival of many infectious agents requires a robust effector T-cell response. However, the reaction must be controlled to prevent excessive tissue damage during pathogen clearance. One of the mechanisms by which the host limits the damage to self-tissues and restores balance following infection is by generating Tregs, which control the intensity of effector cell responses. The induction of Tregs is critical to the host equilibrium but detrimental to the pathogen’s survival and transmission. For this reason, Tregs may be generated by the host to suppress the inflammatory response or may be induced by the pathogen to promote their survival (127). However, recent studies have also shown that Tregs can facilitate the initial protective responses to local viral infections by allowing the timely entry of NK cells, DCs, and T cells into infected tissues (128). It is currently not possible to discern whether Treg generation and thus secretion of IL-35 is the result of the host’s attempt to restore equilibrium and facilitate the early immune response or the pathogen’s attempt to ensure its own survival and transmission. However, re-evaluation of key studies using existing and new mutant mouse models will provide further insight into the potential role of IL-35 in autoimmune and infectious diseases.
Balance epitomizes the immune system: exemplified by the IL-12 family?
In reconsidering the title of this review, it seems that IL-35 is both part of the family and the odd one out. It shares many structural features with other members of this family, which may well extend to its receptor and the signaling process it mediates, and yet its biological function and the cells that produce IL-35 contrast sharply with the characteristics of IL-12, IL-23, and IL-27. While IL-35 is an exciting addition to the IL-12 family, there is still much that needs to be determined about IL-35 biology and the role of the IL-12 family cytokines in general. There are four sets of questions that need to be addressed.
First, while it is known that IL-35 can suppress naive CD4+ T cells, it remains to be determined if IL-35 can inhibit other T-cell (Th1, Th2, Th17, CD8+, etc) and non-T-cell (DCs, macrophages, NK cells, neutrophils, etc) populations. It is also unclear if IL-35 can inhibit functions other than proliferation, such as cytokine production, cytolysis, migration, and kinesis. Lastly, it remains to be determined if IL-35 synergizes with or antagonizes the function of other cytokines. In this regard, any interactions it has with its siblings (IL-12, IL-23, IL-27) or other inhibitory cytokines (IL-10, TGFβ) would be particularly interesting.
Second, as discussed above, the composition of the IL-35 receptor and the signaling cascade it initiates is still unclear. Obviously the expression pattern of this receptor will also provide insight into target cell types. We anticipate some commonality between the receptor chains and STATs utilized by IL-35 and its siblings. If this is true, it will raise the interesting question of how such dramatically contrasting biological outcomes can be mediated by such similar signaling cascades. Similarly interesting problems have been tackled with other receptors that use the same STAT but mediate quite different responses, such as the use of STAT3 by the IL-10R, IL-6R, and IL-22R (129).
Third, while we have shown that Tregs produce IL-35 but no other IL-12 family member, it is possible that other cell types also make IL-35. Indeed, we have shown that some Ebi3 and p35 mRNA is expressed by CD8+, γδ T-cell, and NK cell populations (1). Whether this represents low level possibly mosaic expression that does not confer regulatory potential or expression that is restricted to a specific sub-population of cells that actually secretes bio-active IL-35 remains to be determined. An IL-35-specific reporter mouse in which only the Ebi3:p35 heterodimer, but not IL-12, IL-27, or Ebi3 or p35 alone, is genetically traceable would certainly be valuable. Given that DCs and macrophages can make IL-12 and IL-27, it is interesting to question whether they might also make IL-35. Indeed, its expression may be characteristic of specific sub-types of APCs with regulatory or tolerogenic potential. Additional work is clearly needed to discern whether DCs and macrophages can secrete IL-35 and whether this contributes to a tolerogenic phenotype. A better understanding of the relative pairing affinities and any mechanisms/chaperones that control pairing between the various IL-12 family chains would obviously be valuable here.
Fourth, given that many of the current knockout mice in fact lack production of two cytokines, new mutant mouse models are clearly required. Specifically, a genetic approach is needed that deletes/abrogates only a single cytokine leaving all others unaffected. Purified Treg transfers (either IL-35 sufficient or deficient) have allowed us to better understand the role of Treg-derived IL-35 in various in vivo models (1). Ebi3 or p35 conditional knockout mice, where IL-35 expression is absent in Tregs but IL-12, IL-23, and IL-27 expression is normal in all APCs, would allow one to assess the relative contribution of Treg-derived IL-35 in most disease contexts. As Ebi3 and p35 mRNA is detectible in other non-Treg populations, such as CD8+, γδ T cells, and NK cells, this approach would also facilitate the analysis of IL-35 function in these cell types. Likewise, such mutant mouse models would also help to determine the contribution of any IL-35 produced by potentially tolerogenic DCs and macrophages.
The potency of the immune system to both protect and destroy is clear. As such, the immune system likely had to co-evolve mechanisms to both initiate and dampen immune responses. In this regard, there are several examples of related genes that perform opposing functions, such as CD4 and LAG-3, and CD28 and CTLA4. It seems that the IL-12 family has not ignored the importance of immune balance and contributes cytokines that can both enhance and suppress immune responses. So while IL-12 and IL-27 can have significant suppressive effects, they do have predominantly proinflammatory/prostimulatory functions and are made by APCs. In contrast, IL-35 is produced by Tregs and perhaps other T-cell populations with regulatory capacity and seems to have an exclusively inhibitory capacity. So by comparison, IL-35 seems the odd one out. However, it may be very much part of the immunological family that helps to maintain balance.
Acknowledgments
L.W.C. is supported by the St, Jude Gephardt Postdoctoral Fellowship and an Individual NRSA. D.A.A.V. is supported by the National Institutes of Health, a Cancer Center Support CORE grant, and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 2.Gately MK, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–882. [PubMed] [Google Scholar]
- 3.Gubler U, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23,with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 6.Devergne O, et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 8.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 9.Ma X, et al. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf SF, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 12.Gillessen S, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 13.Hochrein H, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 15.Grohmann U, et al. Positive regulatory role of IL-12 in macrophages and modulation by IFN-gamma. J Immunol. 2001;167:221–227. doi: 10.4049/jimmunol.167.1.221. [DOI] [PubMed] [Google Scholar]
- 16.Krajina T, Leithauser F, Moller P, Trobonjaca Z, Reimann J. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073–1083. doi: 10.1002/eji.200323518. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Shea JJ, Paul WE. Regulation of T(H)1 differentiation--controlling the controllers. Nat Immunol. 2002;3:506–508. doi: 10.1038/ni0602-506. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 20.Shu U, et al. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 21.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belladonna ML, et al. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 23.Nakahira M, et al. Synergy of IL-12 and IL-18 for IFN-gamma gene expression:IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168:1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 24.Walker W, Aste-Amezaga M, Kastelein RA, Trinchieri G, Hunter CA. IL-18 and CD28 use distinct molecular mechanisms to enhance NK cell production of IL-12-induced IFN-gamma. J Immunol. 1999;162:5894–5901. [PubMed] [Google Scholar]
- 25.Takenaka H, et al. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J Leukoc Biol. 1997;61:80–87. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- 26.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. Pathogen-induced Th1 phenotype development in CD4+ alpha beta-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993;5:371–382. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- 28.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 29.Funauchi M, Ikoma S, Enomoto H, Horiuchi A. Decreased Th1-like and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheumatol. 1998;27:219–224. doi: 10.1080/030097498440859. [DOI] [PubMed] [Google Scholar]
- 30.Tokano Y, et al. Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE)--relation to Th1- and Th2-derived cytokines. Clin Exp Immunol. 1999;116:169–173. doi: 10.1046/j.1365-2249.1999.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sur S, Bouchard P, Holbert D, Van Scott MR. Mucosal IL-12 inhibits airway reactivity to methacholine and respiratory failure in murine asthma. Exp Lung Res. 2000;26:477–489. doi: 10.1080/01902140050130374. [DOI] [PubMed] [Google Scholar]
- 32.Wills-Karp M. IL-12/IL-13 axis in allergic asthma. J Allergy Clin Immunol. 2001;107:9–18. doi: 10.1067/mai.2001.112265. [DOI] [PubMed] [Google Scholar]
- 33.Constantinescu CS, et al. Antibodies against IL-12 prevent superantigen-induced and spontaneous relapses of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:5097–5104. [PubMed] [Google Scholar]
- 34.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 36.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 37.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005;201:169–171. doi: 10.1084/jem.20042279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee E, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai T, Devergne O, van Seventer GA, van Seventer JM. Interferon-beta mediates opposing effects on interferon-gamma-dependent Interleukin-12 p70 secretion by human monocyte-derived dendritic cells. Scand J Immunol. 2007;65:107–117. doi: 10.1111/j.1365-3083.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 42.Takeda A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 43.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–1178. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–6471. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 46.Hamano S, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 47.Villarino A, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 48.Artis D, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 50.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neufert C, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 52.Krumbiegel D, Anthogalidis-Voss C, Markus H, Zepp F, Meyer CU. Enhanced expression of IL-27 mRNA in human newborns. Pediatr Allergy Immunol. 2008 doi: 10.1111/j.1399-3038.2007.00685.x. in press. [DOI] [PubMed] [Google Scholar]
- 53.Niedbala W, et al. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 54.Chua AO, et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- 55.Chua AO, Wilkinson VL, Presky DH, Gubler U. Cloning and characterization of a mouse IL-12 receptor-beta component. J Immunol. 1995;155:4286–4294. [PubMed] [Google Scholar]
- 56.Pflanz S, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 57.Bacon CM, et al. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci USA. 1995;92:7307–7311. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishikomori R, Usui T, Wu CY, Morinobu A, O'Shea JJ, Strober W. Activated STAT4 has an essential role in Th1 differentiation and proliferation that is independent of its role in the maintenance of IL-12R beta 2 chain expression and signaling. J Immunol. 2002;169:4388–4398. doi: 10.4049/jimmunol.169.8.4388. [DOI] [PubMed] [Google Scholar]
- 59.Wu C, Wang X, Gadina M, O'Shea JJ, Presky DH, Magram J. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J Immunol. 2000;165:6221–6228. doi: 10.4049/jimmunol.165.11.6221. [DOI] [PubMed] [Google Scholar]
- 60.Presky DH, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stern AS, Gubler U, Presky DH, Magram J. Structural and functional aspects of the IL-12 receptor complex. Chem Immunol. 1997;68:23–37. doi: 10.1159/000058692. [DOI] [PubMed] [Google Scholar]
- 62.Grohmann U, et al. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 63.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 65.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr, et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 68.Thierfelder WE, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen KB, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 70.Mattner F, et al. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 71.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida H, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 73.Muller-Newen G. The cytokine receptor gp130: faithfully promiscuous. Sci STKE. 2003;2003:PE40. doi: 10.1126/stke.2003.201.pe40. [DOI] [PubMed] [Google Scholar]
- 74.Artis D, et al. Cutting edge: early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004;172:4672–4675. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- 75.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge:IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 76.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 77.Huber M, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 78.Magram J, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 79.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 80.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 81.Adorini L. Interleukin-12, a key cytokine in Th1-mediated autoimmune diseases. Cell Mol Life Sci. 1999;55:1610–1625. doi: 10.1007/s000180050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karp CL, Biron CA, Irani DN. Interferon beta in multiple sclerosis: is IL-12 suppression the key? Immunol Today. 2000;21:24–28. doi: 10.1016/s0167-5699(99)01541-8. [DOI] [PubMed] [Google Scholar]
- 83.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costa GL, et al. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–2387. doi: 10.4049/jimmunol.167.4.2379. [DOI] [PubMed] [Google Scholar]
- 85.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gran B, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 87.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 88.McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses. J Allergy Clin Immunol. 2002;110:693–702. doi: 10.1067/mai.2002.129339. [DOI] [PubMed] [Google Scholar]
- 89.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 90.Reddy J, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- 92.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 93.Lubberts E, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol. 2003;170:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 94.Rohn TA, et al. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 95.Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.086066. in press. [DOI] [PubMed] [Google Scholar]
- 96.Delgado M, et al. In vivo delivery of lentiviral vectors expressing vasoactive intestinal peptide complementary DNA as gene therapy for collagen-induced arthritis. Arthritis Rheum. 2008;58:1026–1037. doi: 10.1002/art.23283. [DOI] [PubMed] [Google Scholar]
- 97.Seki M, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez-Rey E, Chorny A, Varela N, O'Valle F, Delgado M. Therapeutic effect of urocortin on collagen-induced arthritis by down-regulation of inflammatory and Th1 responses and induction of regulatory T cells. Arthritis Rheum. 2007;56:531–543. doi: 10.1002/art.22394. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, et al. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgammat. Eur J Immunol. 2008;38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 101.Sonderegger I, et al. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36:2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 102.Monteleone G, et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 103.Parronchi P, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–832. [PMC free article] [PubMed] [Google Scholar]
- 104.Fuss IJ, et al. Both IL-12p70 and IL-23 are synthesized during active Crohn's disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis. 2006;12:9–15. doi: 10.1097/01.mib.0000194183.92671.b6. [DOI] [PubMed] [Google Scholar]
- 105.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simpson SJ, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Camoglio L, et al. Contrasting roles of IL-12p40 and IL-12p35 in the development of hapten-induced colitis. Eur J Immunol. 2002;32:261–269. doi: 10.1002/1521-4141(200201)32:1<261::AID-IMMU261>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 108.Becker C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–2764. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- 109.Nieuwenhuis EE, et al. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci U S A. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 111.Singh B, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 112.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 113.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 116.Brombacher F, et al. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int Immunol. 1999;11:325–332. doi: 10.1093/intimm/11.3.325. [DOI] [PubMed] [Google Scholar]
- 117.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 118.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 119.Elkins KL, Cooper A, Colombini SM, Cowley SC, Kieffer TL. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect Immun. 2002;70:1936–1948. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chackerian AA, Chen SJ, Brodie SJ, Mattson JD, McClanahan TK, Kastelein RA, et al. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect Immun. 2006;74:6092–6099. doi: 10.1128/IAI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol. 2006;176:1098–1106. doi: 10.4049/jimmunol.176.2.1098. [DOI] [PubMed] [Google Scholar]
- 122.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 123.Lieberman LA, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 124.Rutitzky LI, Stadecker MJ. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz. 2006;101 Suppl:327–330. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 125.Belkaid Y. The role of CD4(+)CD25(+) regulatory T cells in Leishmania infection. Expert Opin Biol Ther. 2003;3:875–885. doi: 10.1517/14712598.3.6.875. [DOI] [PubMed] [Google Scholar]
- 126.Zahn S, Wirtz S, Birkenbach M, Blumberg RS, Neurath MF, von Stebut E. Impaired Th1 responses in mice deficient in Epstein-Barr virus-induced gene 3 and challenged with physiological doses of Leishmania major. Eur J Immunol. 2005;35:1106–1112. doi: 10.1002/eji.200425926. [DOI] [PubMed] [Google Scholar]
- 127.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 128.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El Kasmi KC, et al. General nature of the STAT3-activated anti-inflammatory response. J Immunol. 2006;177:7880–7888. doi: 10.4049/jimmunol.177.11.7880. [DOI] [PubMed] [Google Scholar]