Abstract
Inflammation is pivotal in atherosclerosis. M-CSF regulates macrophage growth and differentiation and plays a role in atherogenesis. C-reactive protein (CRP), a cardiovascular risk marker, may promote atherogenesis. However, the effects of CRP on M-CSF release and subsequent macrophage proliferation have not been examined previously. Human aortic endothelial cells (HAEC) were incubated with boiled CRP or native CRP 12.5, 25, and 50 μg/mL for 12–15 h, and M-CSF release was examined by flow cytometry and ELISA. CRP resulted in a significant and dose-dependent increase in M-CSF mRNA and secretion from HAEC as well as human monocyte-derived macrophages (HMDM; P<0.01). Furthermore, conditioned medium (5%) from HAEC pretreated with CRP, when incubated with HMDM, increased macrophage proliferation significantly. This was blocked with M-CSF antibody but not irrelevant antibody. Inhibition of NF-κB resulted in significant abrogation of CRP-induced M-CSF release and subsequent macrophage proliferation. Antibodies to CD32 and CD64 but not CD16 abrogated CRP-induced M-CSF release. Thus, CRP up-regulates M-CSF release from HMDM and HAEC and increased macrophage proliferation. These effects appear to be mediated via activation of NF-κB via CD32 and CD64. These studies provide further evidence for a proatherogenic role for CRP.
Keywords: mechanistic insights, CRP, inflammation
INTRODUCTION
Increasing evidence supports the involvement of inflammation in the pathogenesis of atherosclerosis [1, 2]. One common feature of the inflammatory response is the presence of abundant monocyte/macrophages in all stages of atherogenesis. The infiltration of monocytes into the vascular wall and their transformation into lipid-laden foam cells characterize early atherogenesis. Macrophages are also present in more advanced human atherosclerotic plaques and can produce many mediators that may contribute to lesion formation and progression. Macrophage survival and proliferation are controlled by a group of glycoprotein molecules known as CSFs, such as M-CSF. Macrophages and endothelial cells (EC) are rich sources of M-CSF [3]. Human and rabbit atherosclerotic lesions contain increased levels of M-CSF protein and mRNA compared with normal artery tissue [4,5,6]. In the dietary and apolipoprotein E (apoE) knockout models of atherosclerosis, M-CSF deficiency resulted in significantly reduced macrophages in the lesion and in decreased atherosclerotic lesions [7,8,9]. Also, M-CSF has been shown to accelerate neointimal formation after wire-mediated vascular injury in the femoral artery of wild-type mice [10].
C-reactive protein (CRP), the prototypic marker of inflammation, has been shown in numerous studies to predict cardiovascular events [11, 12]. In addition to being a risk marker, several lines of evidence indicate that CRP, by enhancing inflammation, oxidative stress, and procoagulant activity, may play a role in atherothrombosis [11,12,13,14]. Previously, CRP has been shown to up-regulate adhesion molecules, chemokines, plasminogen activator inhibitor-1, and cytokines and to down-regulate endothelial NO synthase (eNOS) and prostacyclin release from human aortic EC (HAEC) [11,12,13,14,15,16]. In monocyte–macrophages, CRP augments reactive oxygen species, tissue factor, cytokines, matrix metalloproteinase (MMP), and oxidized low-density lipoprotein uptake [11,12,13,14,15,16,17,18]. However, the effect of CRP on M-CSF release and on cell proliferation has not been examined previously and will be the focus of this study.
In this report, we demonstrate for the first time that CRP induces M-CSF release via up-regulation of NF-κB and results in increased macrophage proliferation.
MATERIALS AND METHODS
Human CRP was purified from human ascitic/pleural fluids as described by Du Clos et al. [19]. We have shown that our in-house-purified, dialyzed CRP mediates its inflammatory effects in TLR4 knocked-down cells, providing further cogent data that CRP-mediated effects are not a result of endotoxin contamination [20]. Ficoll and piceatannol (Syk kinase inhibitor) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
HAEC (Invitrogen, East Rutherford, NJ, USA) were grown in endothelial medium supplemented with growth factor (EGM-MV) in a humidified incubator at 37°C under 5% CO2, 95% air, and used between Passages 3 and 5. For the initial experiments, cells were grown in 12-well plates. Upon reaching 80% confluency, the cells were incubated with CRP (6.25, 12.5, 25, and 50 μg/ml) or boiled CRP for 12–15 h. Monomeric CRP (mCRP) was prepared as described previously [21], and HAEC were incubated with mCRP (12.5, 25, and 50 ug/ml) for 15 h. However, for all subsequent experiments involving mechanistic insights, native CRP at 25 μg/mL concentration was used. Conditioned media (CM) from HAEC treated with CRP (25 μg/mL; EC-CM) were collected after the 12- to 15-h incubation for use in the human monocyte-derived macrophage (HMDM) proliferation assays. Supernatants and cells were stored for M-CSF assays at −20°C. EC-CM without CRP was used as control.
Following Ficoll separation, monocytes were obtained from blood of normal human volunteers by negative magnetic separation as described previously [22]. The Institutional Review Board of University of California Davis (Sacramento, CA, USA) approved the protocol. Using this technique in our laboratory, we have shown that at least 86% of cells are CD14-positive by flow cytometry. HMDM were obtained as described previously and used on Day 7 of culture. HMDM were incubated with CRP (0–50 μg/mL) in the absence or presence of inhibitors. In proliferation assays, HMDM were incubated with CRP-treated EC-CM (2.5–10%) for 24 h. Following treatment, supernatants and cells were stored for M-CSF assays. M-CSF release was quantitated by ELISA using reagents from R&D Systems (Minneapolis, MN, USA). M-CSF expression was also assessed by flow cytometry using fluorochrome-labeled antibodies to M-CSF and appropriate isotype controls. RNA was obtained from cells using Trizol, and RT-PCR for M-CSF mRNA was performed as described previously [15].
BrdU proliferation assays were carried out using reagents from Calbiochem (Gibbstown, NJ, USA). Briefly, BrdU, a thymidine analog, is incorporated into newly synthesized DNA strands of actively proliferating cells. Following partial denaturation of double-stranded DNA, BrdU is detected immunochemically, allowing the assessment of the population of cells, which are actively synthesizing DNA. During the final 12 h of culture, BrdU is added to wells of the plate and is incorporated into the DNA of dividing cells. Cells are fixed and permeabilized, and the DNA is denatured. Anti-BrdU mAb is added for 1 h, unbound antibody is then washed away, and HRP-conjugated goat anti-mouse is added, followed by the substrate tetramethylbenzidine, and absorbance at 450 nm is read.
Transfection with dominant-negative IκB vector or control vector was performed as described previously [17, 18, 24]. Previously, we have shown that this transfection compared with control vector results in decreased IκB kinase (IKK) by Western blotting and decreased CRP-induced NF-κB binding by EMSA [23, 24]. Also, small interfering (si)RNA to CD32 or CD64, obtained from Ambion (Austin, TX, USA), was transfected using siPORTamine transfection reagents as described previously [15, 17, 18].
Statistical analysis
All experiments were performed at least three times in duplicate. The comparisons among group means were analyzed using ANOVA. The experimental results are presented as the means ± sd. Paired t-tests were used to compute differences in the variables, and the level of significance was set at P < 0.05.
RESULTS
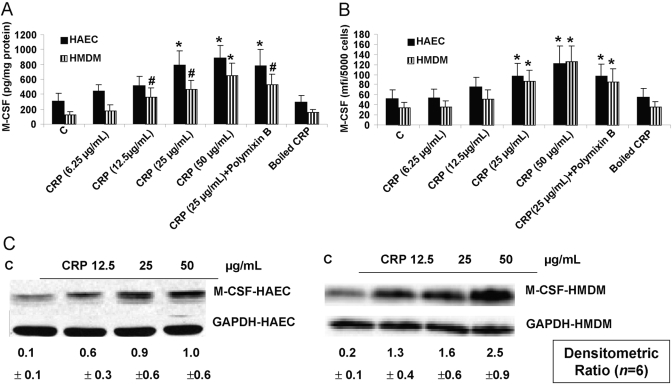
The effect of CRP on M-CSF was tested in HAEC and HMDM. Incubation of HAEC with CRP resulted in a significant dose-dependent induction of M-CSF release (Fig. 1A) and cellular M-CSF by flow cytometry (Fig. 1B). Furthermore, CRP treatment of EC resulted in an increase in M-CSF mRNA (Fig. 1C). Also, CRP had similar effects of inducing M-CSF protein and mRNA from HMDM (Fig. 1, A–C). Boiled CRP failed to have any significant effect. Furthermore, addition of polymixin B failed to suppress CRP-augmented M-CSF. These results indicate CRP-specific induction of M-CSF release from HAEC and HMDM. We also tested the effect of mCRP on M-CSF release. Although mCRP also induced M-CSF release, it was less potent than native, pentameric CRP (control, 212±87 pg/mg protein; CRP 25 μg/mL, 439±104 pg/mg protein*; mCRP 25 μg/mL, 299±101 pg/mg protein; CRP 50 μg/mL, 509±133 pg/mg protein*; mCRP 50 μg/mL, 331±121 pg/mg protein; n=4; *, P<0.05, by ANOVA, native vs. mCRP).
Fig. 1.
CRP augments M-CSF mRNA and protein from HAEC and HMDM. HAEC or HMDM were incubated with CRP (6.25, 12.5, 25, or 50 μg/mL), boiled CRP (25 μg/mL), or polymixin B, and M-CSF release was assessed by ELISA (A) or flow cytometry (B). M-CSF mRNA was assessed by RT-PCR (C) as described in Materials and Methods. n = 6 experiments; *, P < 0.001, compared with control (C); #, P < 0.01, compared with control. mfi, Mean fluorescence intensity.
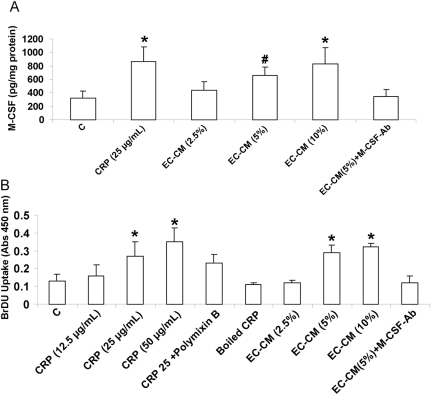
We next treated HMDM with CM from HAEC treated with CRP (25 μg/mL; EC-CM). EC-CM also caused significant up-regulation of M-CSF from HMDM (Fig. 2A). EC-CM without CRP treatment was used as control and failed to up-regulate M-CSF release from HMDM. Also, as an index of the biological activity of M-CSF, macrophage proliferation was assessed. There was significant increase in BrdU uptake, i.e., increased macrophage proliferation following incubation with CRP (25 μg/ml) or with 5% EC-CM (Fig. 2B). To show that the proliferation was indeed a result of M-CSF, HMDM were incubated with EC-CM, pretreated with M-CSF antibody or an irrelevant antibody (IgG). Pretreatment with antibody to M-CSF but not irrelevant antibody resulted in a significant blockade of M-CSF release and HMDM proliferation (Fig. 2B). Of note, treatment with M-CSF antibody did not affect the M-CSF-ELISA results. We also examined cell number as an index of proliferation, and there was a significant increase in the number of macrophages in wells with CRP compared with control (data not shown). These data support a potential paracrine mechanism between HAEC and HMDM in human atheroma.
Fig. 2.
CRP and CRP-CM from HAEC (EC-CM) up-regulate M-CSF release from HMDM and induce HMDM proliferation. HMDM were incubated with CRP or CRP-EC-CM in the absence and presence of M-CSF antibody (10 μg/mL), and M-CSF release (A) and BrdU proliferation (B) were assessed as described in Materials and Methods. n = 4 experiments; *, P < 0.001, compared with control; #, P < 0.01, compared with control.
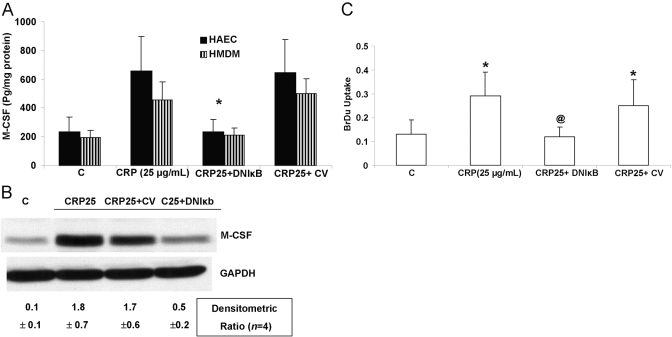
To gain mechanistic insights, as the M-CSF promoter has been shown previously to have NF-κB-binding elements, we transfecting HAEC with a dominant-negative IκB. In addition to abolishing NF-κB activity (as shown previously; refs. [22, 24]), it resulted in a significant decrease in M-CSF protein (Fig. 3A), mRNA (Fig. 3B), and subsequent proliferation (Fig. 3C). Control vector failed to have any effect (Fig. 3).
Fig. 3.
Down-regulation of NF-κB abrogates CRP-induced M-CSF and macrophage proliferation. HAEC and HMDM were incubated with CRP after transfection with vector containing dominant-negative IκB (DNIκB) or control vector (CV), and M-CSF release (A), M-CSF mRNA (B), and macrophage proliferation (C) were assessed as described in Materials and Methods. *, P < 0.001, compared with control; @, P < 0.05, compared with CRP. n = 3 experiments.
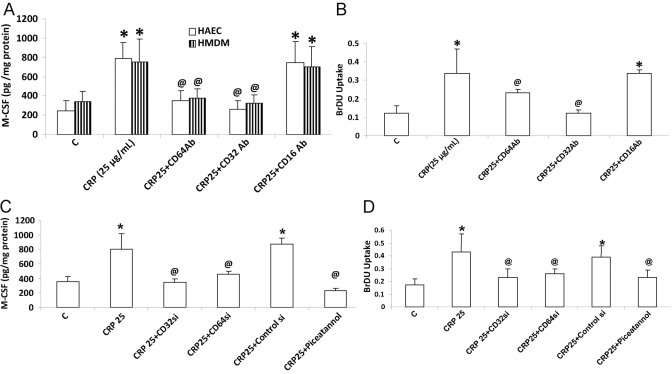
In HAEC and HMDM, CRP binds to the FcγRs CD32 and CD64 and mediated its biological effects. Preincubation of HAEC with antibodies to CD32 and CD64, but not CD16, resulted in a significant decrease in CRP-induced M-CSF release (Fig. 4A) and subsequent HMDM proliferation (Fig. 4B). CD32 antibodies resulted in an 82% reduction, and CD64 antibodies resulted in 53% inhibition of CRP-induced M-CSF (Fig. 4, A and B). Irrelevant IgG antibodies failed to have any effect on CRP-induced M-CSF (data not shown). These experiments were confirmed by transfecting HAEC with siRNA to CD32 and to CD64 (Fig. 4, C and D). CD32 signals through the activation of Syk kinase; thus, we tested the effect of piceatannol, a Syk kinase inhibitor. Piceatannol was able to inhibit CRP-induced M-CSF release from HAEC by 77%. These results indicate that CRP induces M-CSF from HAEC and HMDM transcriptionally via induction of NF-κB, and these effects appear to be mediated largely via activation of CD32 and partly via CD64 activation.
Fig. 4.
Inhibition of CD32 and CD64 decreases CRP-induced M-CSF from HAEC and HMDM and macrophage proliferation. HAEC and HMDM were incubated with CRP after transfection with control siRNA, CD32 siRNA, CD64 siRNA, or antibodies to CD16, CD32, CD64, and M-CSF protein release (A and C), and macrophage proliferation (B and D) was assessed as described in Materials and Methods. *, P <0.001, compared with control; @, P < 0.05, compared with CRP. n = 3 experiments.
DISCUSSION
The infiltration of monocytes into the intima and their transformation into lipid-laden foam cells characterize early atherogenesis [6]. Macrophages are also present in more advanced human atherosclerotic plaques and can produce many mediators that may contribute to lesion formation and progression [25]. M-CSF enhances the proliferation and differentiation of monocyte progenitors and is required for the survival and activation of mature monocytes and macrophages [7,8,9]. M-CSF mRNA has been shown to be several folds higher in human atheromata compared with that found in nonatherosclerotic arteries and veins. Mice deficient in M-CSF and apoE have elevated cholesterol levels but are protected from atherosclerosis. In the dietary and apoE knockout models, M-CSF deficiency resulted in significantly reduced atherogenesis [7,8,9]. M-CSF has emerged as one of the strongest risk factors for adverse outcomes in patients with stable angina [26,27,28,29]. Significantly elevated M-CSF is a harbinger of acute coronary syndrome (ACS) in these patients. Serum M-CSF levels determined 6 weeks after discharge in patients with severe, unstable angina have been demonstrated to be strong predictors of cardiac events during a 2-year follow-up [27].
Various clinical investigations support the concept that high levels of serum CRP are associated with increased risk for cardiovascular disease [10,11,12]. CRP immunoreactivity is also reported to be significantly higher in the plaque of patients with unstable angina pectoris than in those with stable angina pectoris. Importantly, the colocalization of CRP and macrophages has been demonstrated in advanced human atherosclerotic plaque [30]. Also, leukocyte infiltration into plaques has been found to be actively associated with acute coronary events, and raised M-CSF levels in patients with unstable angina are strongly predictive of long-term outcome [26,27,28,29]. CRP levels are also increased in patients with unstable angina and ACS [30,31,32]. In patients with chronic coronary artery disease, the prognostic value of M-CSF is independent and complementary to that of CRP [26,27,28,29]. However, there is a paucity of data examining the direct effect of CRP on M-CSF and on subsequent macrophage proliferation.
In the present study, CRP induced the release of M-CSF from HAEC and HMDM at a CRP concentration of 12.5–50 μg/ml. Importantly, Ridker and Cook [31] have shown that CRP levels >20 μg/mL predict future cardiovascular events. Also, patients with ACS have high levels of circulating CRP (18–90 mg/L) [31, 32]. Therefore, it is likely that local CRP levels in atherosclerotic lesions from circulation or its local synthesis by cells present in arterial lesions, as we and others have shown previously, result in increased M-CSF release and subsequent proliferation and migration of monocytes in the intima. Also, these data support a potential paracrine mechanism between HAEC and HMDM in human atheroma. It is also important to point out that native, unlike mCRP exists in human plasma and atheroma, where the calcium concentration of >2 mM keeps it in the native, pentameric state. The majority of researchers in the CRP area in fact questions the existence of mCRP, as its presence has not been documented in human plasma or atheroma [12, 33, 34]. Furthermore, recent studies in fact indicate that mCRP may be antiatherogenic in vivo [25, 35]. As we have reported previously for other biomediators, in the present report also, we show that native CRP is more potent than mCRP in stimulating M-CSF release [21].
Regarding mechanistic insights, CRP induced the release of M-CSF and subsequent HMDM proliferation via up-regulation of NF-κB. Previously, we and others [17, 18, 23] have shown that CRP up-regulates NF-κB activity directly in HAEC and macrophages using different techniques, EMSA, p65 activity in nuclear extracts, as well as Western blotting for p65 and IKKβ. CRP up-regulates M-CSF at the mRNA level. Previously, modified lipoproteins have been shown to induce M-CSF mRNA and protein via activation of a functional NF-κB-binding site on the M-CSF promoter [36]. Furthermore, human cardiac cells constitutively express M-CSF [37]. This expression of M-CSF in the human heart was down-regulated by dominant-negative IκB transfection and resulted in reduced macrophage survival and differentiation. These observations lend support to our data showing decreased M-CSF and HMDM proliferation with blockade of NF-κB using dominant-negative IκB.
Immunocytochemical detection of FcγRs in human atherosclerotic lesions has been demonstrated [38]. We and others [17, 18, 39,40,41] have shown previously that blocking of CD32/CD64 in HAEC and HMDM abrogates effects of CRP. In addition, in human monocytes and U937 histiocytes, CRP has been shown to induce CCR2 expression via CD64 and MMP-1 expression via CD32. In this paper, we report that CRP mediated M-CSF release, and subsequent macrophage proliferation can be blocked by antibodies to CD32 and CD64 but not CD16; this was confirmed using siRNA. Previously in murine macrophages, CD64 has been shown to regulate M-CSF mRNA transcriptionally [25]. In this study, we show that blockade of CD32 or CD64 abrogated CRP-induced M-CSF; also, the Syk kinase inhibitor, through which CD32 and CD64 signal, also abrogated CRP-induced M-CSF release.
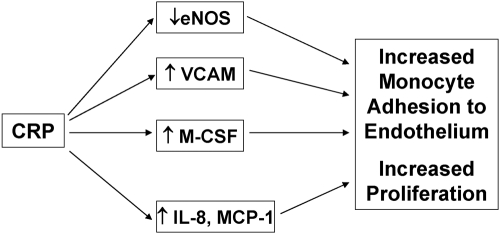
Thus, we show in HAEC and HMDM that CRP augments M-CSF release and biological activity. Release of M-CSF from HAEC results in increased proliferation of HMDM. Furthermore, CRP-induced M-CSF release and macrophage proliferation appear to be mediated via activation of NF-κB and the FcγRs CD32 and CD64. These data, showing that CRP induces M-CSF, combined with the earlier demonstrated effects of CRP on inhibiting eNOS and up-regulating VCAM, IL-8, and MCP-1 and monocyte-EC adhesion under static and shear flow conditions, collectively support a pivotal role for CRP on monocyte recruitment and proliferation (Fig. 5).
Fig. 5.
Schema depicting a pivotal role for CRP in monocyte recruitment and proliferation.
Acknowledgments
This work was supported by National Institutes of Health grant NIHK24 AT00596 as well as RO1 HL074360 to I. J.
References
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65:S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Hansson G K, Robertson A K, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Clinton S K, Underwood R, Hayes L, Sherman M L, Kufe D W, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Inaba T, Shimano H, Gotoda T, Kawamura M, Shiomi M, Yazaki Y, Yamada N. Effect of macrophage colony stimulating factor on the advanced atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Horm Metab Res. 1997;29:507–509. doi: 10.1055/s-2007-979090. [DOI] [PubMed] [Google Scholar]
- Donnelly L H, Bree M P, Hunter S E, Keith J C, Jr, Schaub R G. Immunoreactive macrophage colony-stimulating factor is increased in atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits after recombinant human macrophage colony-stimulating factor therapy. Mol Reprod Dev. 1997;46:92–95. doi: 10.1002/(SICI)1098-2795(199701)46:1<92::AID-MRD14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Inaba T, Gotoda T, Harada K, Shimada M, Ohsuga J, Kawamura M, Yazaki Y, Yamada N. Role of macrophage colony-stimulating factor in the initial process of atherosclerosis. Ann N Y Acad Sci. 1995;748:357–364. doi: 10.1111/j.1749-6632.1994.tb17332.x. [DOI] [PubMed] [Google Scholar]
- Haghighat A, Weiss D, Whalin M K, Cowan D P, Taylor W R. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- Qiao J H, Tripathi J, Mishra N K, Cai Y, Tripathi S, Wang X P, Imes S, Fishbein M C, Clinton S K, Libby P, Lusis A J, Rajavashisth T B. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- Smith J D, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Takahashi M, Yoshioka T, Yajima N, Morimoto H, Izawa A, Ise H, Hatake K, Motoyoshi K, Ikeda U. M-CSF accelerates neointimal proliferation in the early phase after vascular injury in mice. Arterioscler Thromb Vasc Biol. 2007;27:283–289. doi: 10.1161/01.ATV.0000250606.70669.14. [DOI] [PubMed] [Google Scholar]
- Bassuk S S, Rifai N, Ridker P M. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–2150. [PubMed] [Google Scholar]
- Wu J, Stevenson M J, Brown J M, Grunz E A, Strawn T L, Fay W P. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- Bisoendial R J, Kastelein J J, Stroes E S. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195:e10–e18. doi: 10.1016/j.atherosclerosis.2007.04.053. [DOI] [PubMed] [Google Scholar]
- Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43:780–791. doi: 10.1016/j.yjmcc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Xu D Y, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- Singh U, Dasu M R, Yancey P G, Afify A, Devaraj S, Jialal I. Human C-reactive protein promotes oxidized low-density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. J Lipid Res. 2008;49:1015–1023. doi: 10.1194/jlr.M700535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Dasu R, Singh U, Rao L V, Jialal I. CRP stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.05.060. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Clos T W, Zlock L T, Marnell L. Definition of a C-reactive protein binding determinant on histones. J Biol Chem. 1991;266:2167–2171. [PubMed] [Google Scholar]
- Dasu M R, Devaraj S, Du Clos T W, Jialal I. The biological effects of CRP are not attributable to endotoxin contamination: evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48:509–512. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Venugopal S, Jialal I. Native pentameric C-reactive protein displays more potent pro-atherogenic activities in human aortic endothelial cells than modified C-reactive protein. Atherosclerosis. 2006;184:48–52. doi: 10.1016/j.atherosclerosis.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Davis B, Simon S I, Jialal I. CRP promotes monocyte-endothelial cell adhesion via Fc γ receptors in human aortic endothelial cells under static and shear flow conditions. Am J Physiol Heart Circ Physiol. 2006;291:H1170–H1176. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Cheung A T, Jialal I, Griffen S C, Nguyen D, Glaser N, Aoki T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Kumaresan P R, Jialal I. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J Mol Cell Cardiol. 2004;36:405–410. doi: 10.1016/j.yjmcc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Rallidis L S, Zolindaki M G, Pentzeridis P C, Poulopoulos K P, Velissaridou A H, Apostolou T S. Raised concentrations of macrophage colony stimulating factor in severe unstable angina beyond the acute phase are strongly predictive of long term outcome. Heart. 2004;90:25–29. doi: 10.1136/heart.90.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallidis L S, Zolindaki M G, Manioudaki H S, Laoutaris N P, Velissaridou A H, Papasteriadis E G. Prognostic value of C-reactive protein, fibrinogen, interleukin-6, and macrophage colony stimulating factor in severe unstable angina. Clin Cardiol. 2002;25:505–510. doi: 10.1002/clc.4960251106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Kishida H, Tsukada Y, Fukuma Y, Sano J, Yasutake M, Fukuma N, Kusama Y, Hayakawa H. Clinical significance of increased plasma concentration of macrophage colony-stimulating factor in patients with angina pectoris. J Am Coll Cardiol. 2000;35:655–665. doi: 10.1016/s0735-1097(99)00583-5. [DOI] [PubMed] [Google Scholar]
- Ikonomidis I, Lekakis J, Revela I, Andreotti F, Nihoyannopoulos P. Increased circulating C-reactive protein and macrophage-colony stimulating factor are complementary predictors of long-term outcome in patients with chronic coronary artery disease. Eur Heart J. 2005;26:1618–1624. doi: 10.1093/eurheartj/ehi192. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Inoue N, Ohashi N, Terashima M, Matsui Y, Mori T. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of CRP. Arterioscler Thromb Vasc Biol. 2003;23:1398–1404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- Ridker P M, Cook N. Clinical usefulness of very high and very low levels of CRP across the full range of Framingham risk scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- Ridker P M, Cannon C P, Morrow D, Rifai N, Rose L M, McCabe C H, Pfeffer M A, Braunwald E. Pravastatin or atorvastatin evaluation and infection therapy—thrombolysis in myocardial infarction 22 (PROVE IT-TIMI 22) investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Pepys M B, Hirschfield G M. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M B, Hirschfield G M, Tennent G A, Gallimore J R, Kahan M C, Bellotti V, Hawkins P N, Myers R M, Smith M D, Polara A, Cobb A J, Ley S V, Aquilina J A, Robinson C V, Sharif I, Gray G A, Sabin C A, Jenvey M C, Kolstoe S E, Thompson D, Wood S P. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- Schwedler S B, Amann K, Wernicke K, Krebs A, Nauck M, Wanner C, Potempa L A, Galle J. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2005;112:1016–1023. doi: 10.1161/CIRCULATIONAHA.105.556530. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T B, Yamada H, Mishra N K. Transcriptional activation of the macrophage-colony stimulating factor gene by minimally modified LDL. Involvement of nuclear factor-κ B. Arterioscler Thromb Vasc Biol. 1995;15:1591–1598. doi: 10.1161/01.atv.15.10.1591. [DOI] [PubMed] [Google Scholar]
- Frangogiannis N G, Mendoza L H, Ren G, Akrivakis S, Jackson P L, Michael L H, Smith C W, Entman M L. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H483–H492. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- Ratcliffe N R, Kennedy S M, Morganelli P M. Immunocytochemical detection of Fc [INSERT IMAGE]R in human atherosclerotic lesions. Immunol Lett. 2001;77:169–174. doi: 10.1016/s0165-2478(01)00217-6. [DOI] [PubMed] [Google Scholar]
- Ryu J, Lee C W, Shin J A, Park C S, Kim J J, Park S J, Han K H. Fc γ RIIa mediates C-reactive protein-induced inflammatory responses of human vascular smooth muscle cells by activating NADPH oxidase 4. Cardiovasc Res. 2007;75:555–565. doi: 10.1016/j.cardiores.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Williams T N, Zhang C X, Game B A, He L, Huang Y. C-reactive protein stimulates MMP-1 expression in U937 histiocytes through Fc[γ]RII and extracellular signal-regulated kinase pathway: an implication of CRP involvement in plaque destabilization. Arterioscler Thromb Vasc Biol. 2004;24:61–66. doi: 10.1161/01.ATV.0000104014.24367.16. [DOI] [PubMed] [Google Scholar]
- Qin H, Edberg J C, Gibson A W, Page G P, Teng L, Kimberly R P. Differential gene expression modulated by the cytoplasmic domain of Fc γ RIa (CD64) α-chain. J Immunol. 2004;173:6211–6219. doi: 10.4049/jimmunol.173.10.6211. [DOI] [PubMed] [Google Scholar]