Abstract
Cyclooxygenase (COX) encodes a rate-limiting enzyme in the biosynthesis of prostanoids. Although COX-1 is constitutively expressed in many tissues, we found that glucocorticoids cause elevated expression of COX-1 gene in cardiomyocytes. Corticosterone (CT) at physiologically relevant doses (0.05–1 μm) induces transcriptional activation of COX-1 gene as shown by nuclear run-on and promoter reporter assays. An antagonist of glucocorticoid receptor (GR), mifepristone, prevented CT from inducing COX-1. COX-1 gene promoter deletion and mutation studies indicate a role of Sp transcription factors in CT-induced COX-1 gene. EMSAs or chromatin immunoprecipitation assays suggest that GR and Sp3 transcription factor bind to the promoter of COX-1 gene. Coimmunoprecipitation assays found an association of GR with Sp3. Silencing Sp3 protein with small interfering RNA suppressed CT-induced COX-1 promoter activation. Our data suggest that activated GR interacts with Sp3 transcription factor in binding to COX-1 promoter to enhance COX-1 gene expression in cardiomyocytes.
PHYSICAL AND PSYCHOLOGICAL stresses increase the circulating level of corticosteroids. Synthetic corticosteroids are among the most prescribed drugs and are essential for antiinflammatory and immunosuppressive function. Corticosteroids bind to the glucocorticoid receptor (GR), a nuclear receptor protein, to regulate energy metabolism, biochemical homeostasis, and immune response. The binding of corticosteroids to GR triggers a cascade of signaling events, resulting in changes in the expression of genes containing GR response element (GRE). In addition to GR-dependent signaling, corticosteroids also activate intracellular signaling pathways via a GR-independent manner. Despite the wide spread physiological functions and pharmacological applications of corticosteroids, little is known about the biological impact of corticosteroids on cardiac cells. Whereas corticosteroids are well-known inducers of apoptosis in lymphocytes and neuronal cells, cardiomyocytes respond to corticosterone (CT) by eliciting a cytoprotective response (1). Microarray studies first found that CT induces cyclooxygenase-1 (COX-1) gene expression in cardiomyocytes (1).
COX-1 and COX-2 genes encode two distinct isoforms of COX enzyme, which controls the rate-limiting step of prostanoid synthesis. Whereas COX-2 gene is inducible by proinflammatory cytokines, growth factors, carcinogens, and chemical or physical stress (2,3), COX-1 gene usually expresses constitutively and is thought to be a housekeeping gene. However, emerging evidence indicates that COX-1 gene expression can be induced under certain circumstances. For example, female hormones estrogen and progesterone have been reported to induce COX-1 expression in endothelial cells (4,5,6). Additional reported inducers of COX-1 gene include the nerve growth factor, fibroblast growth factor, vascular endothelial growth factor, tobacco carcinogens, phorbol ester, retinoic acid, TNF-α related apoptosis-inducing ligand, and histone deacetylase (HDAC) inhibitors (7,8,9,10,11,12,13,14). Contrary to these inducers, glucocorticoids (GCs) have been shown to down-regulate COX-1 gene expression in endothelial cells (15).
Unlike COX-2, the molecular mechanism underlying the regulation of COX-1 gene has not been fully characterized as evidenced by a limited number of reports. In HDAC inhibitor-induced COX-1 expression in neuronal cells, a Sp1 binding site in COX-1 promoter is critical (16). With estrogen-induced COX-1 expression in endothelial cells, estrogen receptor and AP2 transcription factor appear to be involved, although it is not known whether estrogen receptor and AP2 interact with each other in regulating COX-1 expression (6). In both cases, the GC-rich region of COX-1 promoter immediately upstream of the transcriptional start site is important for targeted regulation of COX-1 gene expression.
Sp proteins, a family of highly conserved zinc-finger transcription factors, bind to GC-rich consensus elements (17). Nine Sp proteins have been discovered, among which Sp1–Sp4 are the best-studied members (18). Sp family members can form homo- or heterodimers for DNA binding. In addition, Sp proteins interact with basal transcription factors, cAMP response element-binding protein-binding protein (CBP), CBP-related p300 protein, and chromatin modulators such as HDACs (18,19). Because a large number of genes contain GC-rich sequences in the promoter region, Sp transcription factors, through regulating these genes, play an important role in physiological or pathophysiological processes (17,18,19,20). In this study, we investigate the mechanism underlying CT-induced COX-1 expression in cardiomyocytes and found a role of Sp3 transcription factor.
RESULTS
CT Causes Transcriptional Activation of COX-1 Gene
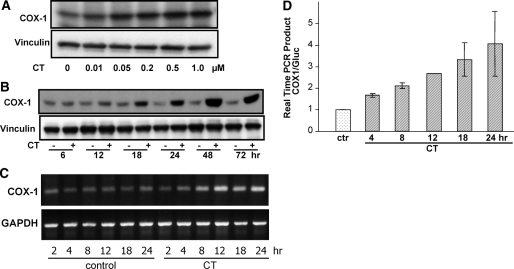
Microarray analyses first detected COX-1 mRNA elevation in rat cardiomyocytes 24 h after 1 μm CT treatment (1). To characterize such induction, we examined the dose response and time course of COX-1 expression after CT treatment. At the protein level, COX-1 was induced by CT at a concentration as low as 50 nm (Fig. 1A). Time course studies showed a clear elevation of COX-1 protein 18 h after CT treatment, and COX-1 protein remained elevated for at least 72 h (Fig. 1B). A time-dependent induction of COX-1 mRNA by CT was observed by RT-PCR (Fig. 1C). Real-time PCR from three independent experiments revealed COX-1 mRNA elevation starting at 4 h and reaching 4-fold induction at 24 h after CT treatment (Fig. 1D).
Figure 1.
GCs Induce Elevations of COX-1 Protein and mRNA in Cardiomyocytes
Cardiomyocytes were treated with various concentrations of CT for 24 h (A) or with 1 μm CT for indicated time (A, C, and D). The cells were harvested for Western blot analyses (A and B), semiquantitative RT-PCR (C), or real-time PCR (D) with vinculin (A and B), GAPDH (C), or glucuronidase (Gluc) (D) as an internal control for equal loading. The control was harvested at 4 h time point for real-time PCR (D). The data are from one experiment representative of three (A–C) or represent means ± sds of three independent experiments (D).
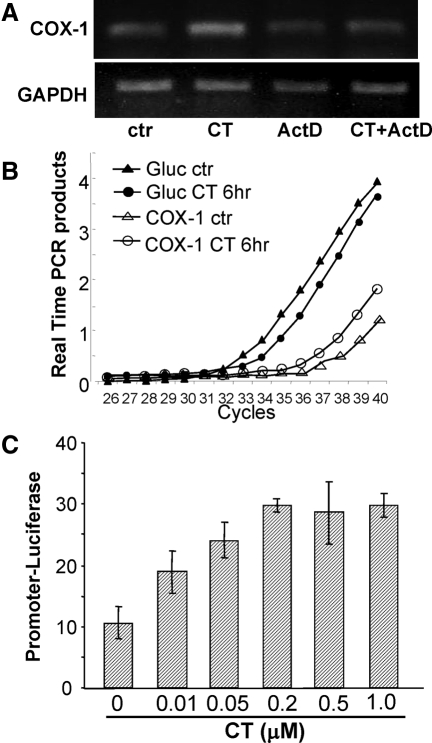
An inhibitor of RNA synthesis, nuclear run-on assay, and promoter activity assay were used to address whether CT caused transcriptional activation of COX-1 gene. Pretreatment of actinomycin D, an inhibitor of RNA synthesis, prevented CT from inducing COX-1 mRNA (Fig. 2A). Nuclear run-on assays show that the nucleus of CT-treated cardiomyocytes exhibited an increased rate of COX-1 transcription (Fig. 2B), about 4.5-fold faster than the control when newly made COX-1 transcripts were corrected by that of β-glucuronidase. A luciferase construct under the control of rat COX-1 promoter was introduced into cardiomyocytes by transient transfection. Treatment with CT caused an increase in the luciferase activity (Fig. 2C). These approaches indicate that CT treatment results in transcriptional activation of COX-1 gene.
Figure 2.
CT Induces COX-1 Gene Transcription
Cardiomyocytes were pretreated with actinomycin D (0.5 μm) for 30 min before 1 μm CT treatment for 6 h (A). Total RNA was used for semiquantitative RT-PCR to detect the mRNA level of COX-1 or the loading control GAPDH (A). The nuclear run-on assay was performed with nuclei harvested 6 h after 1 μm CT treatment for real-time PCR (B). Cardiomyocytes transfected with −172/+8 bp COX-1 promoter luciferase construct were treated with various concentrations of CT for 24 h before harvesting for luciferase activity assay (C). Results are shown from one experiment representative of three. ActD, Actinomycin C; ctr, control; Gluc, glucuronidase.
GR and Sp3 Mediate Transcriptional Activation of COX-1 Gene
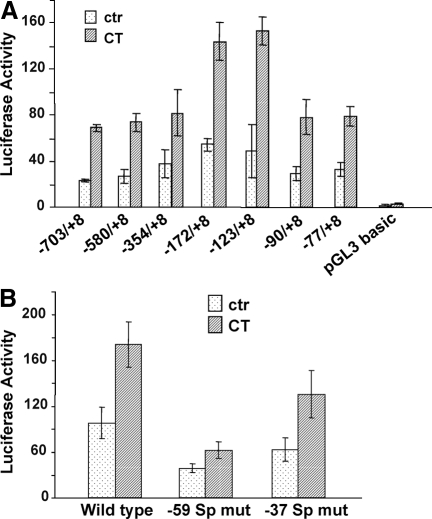
Corticosteroids often bind to GR to turn on transcription of targeted genes through GRE. Based on the sequence information, rat COX-1 gene does not contain a GRE in the promoter. To understand the mechanism of CT-induced COX-1 transcription, we generated seven luciferase reporter constructs, with sequential deletion of rat COX-1 promoter sequence (Fig. 3A). Each of the constructs was transfected into cardiomyocytes for measurements of promoter activation after CT treatment (Fig. 3A). The data show that 5′-deletion of sequences upstream of −77 bp did not significantly affect CT from activating COX-1 promoter (Fig. 3A). These data suggest that the −77/+8-bp region contains key regulatory elements for CT-induced COX-1 transcription.
Figure 3.
−77/+8 GC-Rich Region of COX-1 Promoter Is Critical for CT-Induced Transcriptional Activation
Cardiomyocytes were transfected with COX-1 promoter reporter constructs at various lengths or empty vector (A). GGGGTGG was replaced with GTTGTGG for the −59-bp Sp binding site, whereas GGGAGGGG at the −37-bp Sp binding site was replaced with GTTAGGGG in −172/+8 bp promoter reporter construct (B). Transfected cells were treated with 1 μm CT for 24 h for measurement of luciferase activity. The data represent one of three independent experiments with means ± sds from triplets of one representative experiment. ctr, Control; mut, mutant.
In the promoter region of human COX-1 gene, two GC-rich consensus sequences have been found important for COX-1 gene expression (6,16). The rat COX-1 promoter contains GC-rich putative Sp binding sites located at −59 bp and −37 bp. To test the involvement of these putative Sp binding sites in CT-induced COX-1 gene expression, substitution mutants were generated (Fig. 3B). Mutation of the −59-bp Sp binding site significantly attenuated the capability of CT to activate COX-1 promoter, although a decrease of the basal level promoter activity was also observed (Fig. 3B). Mutation of the −37-bp Sp binding site was less effective in eliminating CT effect (Fig. 3B).
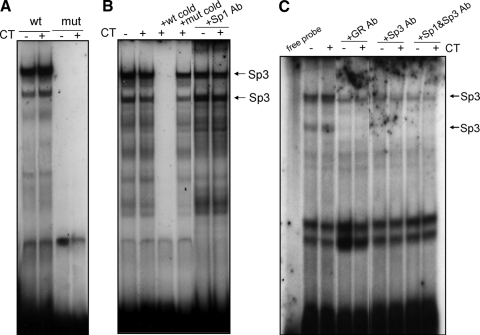
EMSA was performed to demonstrate the interaction of proteins with the Sp binding sequence from COX-1 promoter. The oligonucleotide probes for EMSA were adopted from the −67/−41-bp region of rat COX-1 promoter, containing wild-type or mutant −59-bp Sp binding sequence. With the nuclear extracts from control untreated samples, EMSA with wild-type Sp probe generated two major DNA-protein complexes (Fig. 4A), similar to what have been reported in the literature (21). These complexes were absent with the −59-bp Sp mutant probe (Fig. 4A). Competition analyses using excess wild-type or mutant cold probe further suggest that these two bands are specific complexes of nuclear protein binding to wild-type Sp sequence (Fig. 4B). However, CT treatment did not significantly increase or decrease the binding of nuclear proteins to the Sp cis-element containing oligonucleotide (Fig. 4, A–C).
Figure 4.
GR and Sp3 Bind to −59 Sp Consensus Sequence in Vitro
Cardiomyocytes were treated with vehicle or 1 μm CT for 8 h. Nuclear proteins were extracted from the cardiomyocytes for EMSAs. Wild-type or mutant radiolabeled probes with the sequence from the −67/−41-bp region of rat COX-1 promoter were incubated with nuclear extracts (A). For competition assay, 50× molar excess wild-type or mutant unlabeled probes are included in DNA binding reaction (B). Antibodies against GR (BuGR2), Sp1, and/or Sp3 (1 μg /20 μl) were included during DNA binding reaction (B and C). Ab, Antibody; mut, mutant; wt, wild type.
Sp1 and Sp3 transcription factors are known to bind Sp cis-elements. When Sp1 antibody was added to the nuclear extract, DNA binding was not affected (Fig. 4B). In contrast, Sp3 antibody abolished the bottom band completely and reduced the intensity of the top band (Fig. 4C). Addition of Sp1 antibody did not change the pattern of Sp3 antibody-induced loss of two major bands (Fig. 4C).
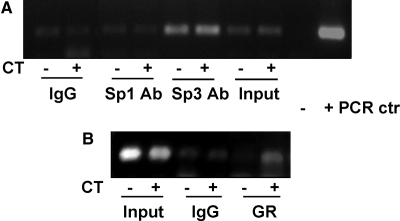
Chromatin immunoprecipitation (ChIP) assays were carried out to examine whether CT treatment induces Sp1 and Sp3 binding to COX-1 promoter in vivo. This assay detected Sp3 transcription factor binding to COX-1 promoter, although the binding was not changed by CT treatment (Fig. 5A). The results from ChIP assay is consistent with that from EMSA, showing Sp3 transcription factor binding to COX-1 promoter and that CT treatment did not alter the binding of Sp3 with COX-1 promoter.
Figure 5.
GR and Sp3 Transcription Factor Bind to COX-1 Promoter in Cardiomyocytes
Cardiomyocytes were treated with vehicle or 1 μm CT for 12 h for ChIP assay. DNA in the Sp1, Sp3, or GR-immunoprecipitated complex was used as a template for PCR with the primers to generate −172/+8 bp fragment of COX-1 promoter. COX-1 luciferase construct or water was used as a template for positive (+) or negative (−) PCR control. Whereas “Input” serves as a loading control, rabbit IgG (top panel) or mouse IgG (bottom panel) immunoprecipitation serves as a negative control for specific Sp1 or Sp3 polyclonal or GR monoclonal antibody binding. Ab, Antibody; ctr, control.
EMSA and ChIP assay were carried out to address whether CT treatment induces GR binding to COX-1 promoter within the region containing Sp binding site. The antibodies against GR reduced the binding of proteins to Sp cis-element in vitro in a manner similar to Sp3 antibody (Fig. 4C). With ChIP assays to determine the interaction of GR with COX-1 promoter in vivo, we found that CT treatment caused GR to bind to COX-1 promoter (Fig. 5B).
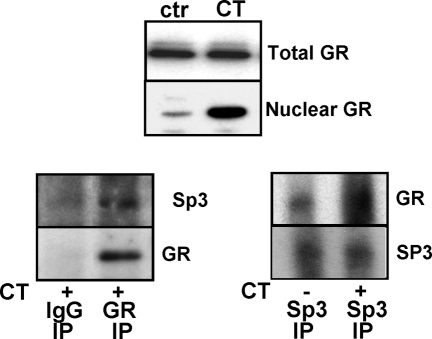
Because GR and Sp3 transcription factor bind to the same region of COX-1 promoter, we tested whether GR interacts with Sp3 transcription factor by coimmunoprecipitation. CT treatment caused nuclear translocation of GR as expected (Fig. 6, top panel). Without CT treatment, the nuclear fraction contains insufficient GR and is therefore not suitable for immunoprecipation. Immunoprecipitation was performed with the nuclear extracts of CT-treated cells using IgG control or an antibody against GR (Fig. 6, left panel). Immunoblotting of GR precipitates detected the presence of Sp3 between 50- and 75-kDa molecular mass markers in the complex (Fig. 6, left panel). A reciprocal immunoprecipitation experiment was performed using nuclear extracts and Sp3 antibody. An increased level of GR protein was found in the immunoprecipitates of Sp3 from the nuclear extract of CT-treated cells (Fig. 6, right panel). These data support the theme that CT caused nuclear translocation of GR, which in turn binds to Sp3 transcription factor in the nuclei.
Figure 6.
GR Interacts with Sp3 Transcription Factor
Cardiomyocytes were treated with vehicle or 1 μm CT for 4 h. Nuclear proteins were harvested for measurements of GR protein level by Western blot (upper panel) or for immunoprecipitation using mouse IgG control, BuGR2 monoclonal antibody, or Sp3 antibody. GR or Sp3 protein in the immunocomplex was determined by Western blot with polyclonal antibodies against GR or Sp3. The same set of membrane was blotted with Sp3 antibody and then GR antibody after stripping (left panel) or vice versa (right panel). The data represent one of three independent experiments. ctr, Control; IP, immunoprecipitation.
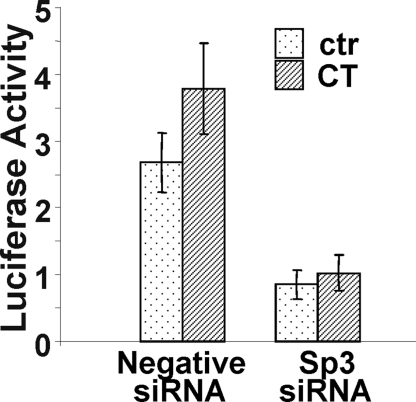
To further verify that Sp3 transcription factor indeed plays a functional role in COX-1 induction, the small interfering RNA (siRNA) technology was applied to block Sp3 gene expression. Technically, transfection of siRNA requires oligofectamine, instead of FuGene6 liposomes, which are typically used for transfecting luciferase reporter DNA constructs. Although the expression level of COX-1 luciferase reporter is relatively low with oligofectamine-mediated transfection, the data show that Sp3 siRNA was capable of abolishing CT-induced COX-1 promoter activation (Fig. 7).
Figure 7.
Inhibiting CT-Induced COX-1 Expression with Sp3 siRNA
Sp3 siRNA or negative control siRNA was introduced into cardiomyocytes by oligofectamine during cotransfection with −123/+8 bp COX-1 promoter reporter construct for 1 μm CT treatment. Cells were harvested for luciferase activity assays at 24 h after CT treatment. The data represent one of three independent experiments with means ± sds of triplet experiments. ctr, Control.
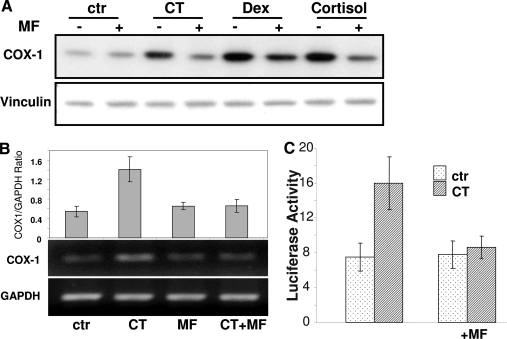
Mifepristone (MF) competes with corticosteroids for GR binding, preventing GR from interacting with DNA and inducing chromatin remodeling (22). Pretreatment with MF abolished COX-1 protein increases induced by three GCs (Fig. 8A). MF also prevented CT from inducing COX-1 mRNA or activating COX-1 promoter (Fig. 8, B and C). These data suggest that CT-induced COX-1 gene expression is GR dependent.
Figure 8.
GR Regulates CT-Induced COX-1 Expression
Cardiomyocytes were treated with 1 μm MF for 1 h before 24 h treatment of 1 μm CT, dexamethasone (Dex), or cortisol (A). Cells were harvested for measurements of COX-1 protein level by Western blot with vinculin as a loading control (A) or for measurements of COX-1 mRNA by RT-PCR with GAPDH as a loading control (B). The intensity of the COX-1 band was quantified by National Institutes of Health Image J software and corrected by that of GAPDH to calculate the ratio (B, top panel). Cardiomyocytes transfected with −172/+8 COX-1 promoter reporter construct were used for luciferase activity assay following the same protocol of MF and CT treatment (C). The data represent one of three independent experiments with means ± sds from three independent experiments (B, top panel). ctr, Control.
DISCUSSION
This study presents a novel finding of corticosteroids increasing the transcription of COX-1 gene. Whereas the promoter of COX-1 gene lacks GRE sequence, our data suggest a GR-dependent mechanism of COX-1 induction. It appears that the Sp3 transcription factor constitutively binds to the rat COX-1 gene promoter. GR nuclear translocation upon ligand binding contributes to its interaction with Sp3 protein on COX-1 promoter, resulting in an enhanced transcription of COX-1 gene.
Our promoter deletion and mutation studies indicate that the Sp cis-element at −59 bp of COX-1 promoter plays a critical role in regulating COX-1 gene expression. The fact that Sp3 constitutively binds to COX-1 promoter, as shown in EMSA and ChIP assays, explains the observed decreases in the basal activity and CT-induced activity of the −59-bp mutant (Fig. 3B) and with Sp3 siRNA cotransfection (Fig. 7). There are several clusters of GC-rich sequence in COX-1 promoter, suggesting a possible involvement of additional transcription factors in CT-induced COX-1 expression. It is known that Sp1 and Sp3 transcription factors target GC-rich sequences. Sp1 and Sp3 transcription factors typically share similar expression pattern, protein structure, and affinity for consensus DNA sequence binding (23). Despite these similarities, our EMSA and ChIP assays reveal that Sp3, instead of Sp1, is the primary transcription factor binding to COX-1 promoter in cardiomyocytes. CT treatment did not change Sp1 or Sp3 binding to COX-1 promoter in vitro by EMSA or in vivo by ChIP assay.
The Sp family of transcription factors has been shown to interact with a wide variety of proteins (19), among which are the nuclear receptor family proteins. Sp1 protein is capable of binding to a number of nuclear receptors, such as estrogen receptor, progesterone receptor, androgen receptor, retinoic acid receptor, retinoic X receptor, peroxisome proliferator-activated receptor γ, and vitamin D receptor (24). The estrogen receptor has been reported to bind to several Sp transcription factors, including Sp3 (25,26). Most of these nuclear receptors interact with a large number of proteins, in particular transcription factors (Sp1, c-Jun, and RelA), chromatin-modulation factors (steroid receptor coactivator 1, CBP/p300, BRG-1, and SNURF), and basal transcription factors (TATA-binding protein and TATA-binding protein-associated factors) (27,28,29). Because GR binds to chromatin modulation factors and basal transcription factors upon activation, the interaction of GR with Sp3 likely results in the formation of a large protein complex on COX-1 promoter containing chromatin modulation factors and basal transcription factors, triggering in transcriptional activation of COX-1 gene. The lack of significant increase of Sp3 protein binding to DNA by CT treatment, as shown in EMSA or ChIP assay, suggests that GR may not form physical contact with DNA in this case but the interaction with Sp3 protein is critical for COX-1 gene transcription.
Although our data suggest that the interaction of GR with Sp3 protein results in an increased rate of COX-1 transcription, we cannot exclude the possibility that GR sequestrates a transcriptional repressor to release the inhibition of COX-1 transcription. Alternatively, GR may induce the expression of a transcriptional activator or suppress the expression of a transcriptional repressor to regulate COX-1 expression. COX-1 promoter deletion studies suggest possible transcriptional repressors associated with the −172-bp upstream and −90-bp downstream regions of COX-1 promoter (Fig. 3A). Because GR is known to interact with a large number of nuclear proteins, the cross talk between GR and other transcription factors may result in either enhancing or repressing the activity of the transcription factors (28,29,30). Therefore, GR interaction with Sp3 may not be the sole mechanism regulating COX-1 gene expression.
A limited number of COX-1 inducers have been reported in the literature. Our study is the first to show that corticosteroids induce COX-1 gene expression. In neuroblastoma cell lines, dexamethasone enhances retinoic acid-induced COX-1 expression, although it alone is not effective for inducing COX-1 expression (12). The only other report studying the effect of GCs on COX-1 shows that GCs decrease COX-1 gene expression in fetal pulmonary artery endothelial cells (15). Both GR and Sp transcription factors are expressed in the endothelial cells (15), suggesting that induction of COX-1 gene by CT is likely a cardiomyocyte cell type-specific phenomenon. Unlike many other cell types in which GCs have been extensively studied, such as lymphocytes, neuronal cells, endothelial cells, smooth muscle cells, and kidney epithelial cells, cardiomyocytes respond to GCs by expressing cytoprotective genes and becoming resistant against apoptosis inducers (1). Because GR translocation is a universal event upon ligand binding, one might postulate that GR-interacting proteins or signaling network may differ among cell types and therefore determine cell type-specific responses. Cardiomyocytes may have a unique set of signaling web or scaffold proteins that orchestrate the interaction between GR and Sp3 transcription factor.
Elevated COX-1 protein was observed with CT at a concentration as low as 50 nm. The plasma level of GCs fluctuates with the diurnal cycle, peaking in the early morning, followed by a decline throughout the day, and reaching the nadir at about midnight for humans. As a result, the basal level of GCs varies from 3–16 μg/dl or 0.09–0.46 μm in the plasma. Physical and psychological stresses elevate the circulating level of GCs, often to a range around or above 25 μg/dl or 0.72 μm. Our data indicate that CT induces COX-1 expression in cardiomyocytes at physiologically relevant doses.
MATERIALS AND METHODS
Cell Culture and Drug Treatment
Cardiomyocytes were prepared from 1- to 2-d-old neonatal Sprague Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) as previously described (1,31). Cardiomyocytes were seeded at a density of 2.5 × 106 cells per 100-mm dish or 0.5 × 106 cells per well of six-well plates in low-glucose DMEM containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Cells were serum starved for 24 h before experiments.
Western Blot Analysis and Coimmunoprecipitation
Whole-cell lysates were prepared using a sodium dodecyl sulfate lysis buffer. After measuring protein concentration by the Warburg-Christian method (32,33), we performed Western blots using primary antibodies against COX-1 (catalog nos. 160109 or 160110; Cayman Chemical, Ann Arbor, MI) or GR (rabbit polyclonal, SC-1004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Vinculin (V9131; Sigma-Aldrich, St. Louis, MO) was used as a loading control.
Coimmunoprecipitation was performed using nuclear proteins with mouse IgG (SC-2025, Santa Cruz Biotechnology) or antibodies against GR (BuGR2 mouse monoclonal, no. 2768; Abcam, Cambridge, MA) or Sp3 (polyclonal, no. 07-107; Upstate Biotechnology) followed by incubation with protein G-agarose beads. The immunocomplexes were separated by SDS-PAGE for Western blot using antibodies against Sp3 (rabbit polyclonal, no. 07-107; Upstate Biotechnology, Lake Placid, NY) or GR (M-20 rabbit polyclonal, SC-1004; Santa Cruz Biotechnology).
Semiquantitative RT-PCR and Real-Time RT-PCR
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) for reverse transcription (RT). COX-1 mRNA was amplified using PCR primer pair of forward 5′-TAAGTACCAGGTGCTGGATGG-3′ and reverse 5′-GGTTTCCCCTATAAGGATGAG-3′. Glyceraldhyde-3-phosphate dehydrogenase (GAPDH) was amplified in parallel as an internal control using primers of forward 5′-AGACAGCCGCATCTTCTTGT-3′ and reverse 5′-CCACAGTCTTCTGAGTGGCA-3′. The real-time PCR was carried out using a rat COX-1-specific primer/probe set (Applied Biosystems, Foster City, CA; COX-1, Rn00566881_m1).
Nuclear Run-On Assay
The nuclei were prepared from 2 × 107 cardiomyocytes for in vitro transcription in a mixture of triphosphate nucleosides containing biotin-16-UTP (34). Isolated nuclei were incubated 1 h at 30 C in a reaction mixture containing 20 mm Tris-HCl (pH 8.0), 5 mm MgCl2, 200 mm KCl, 5 mm dithiothreitol, 4 mm each of ATP, CTP, and GTP, 4 mm biotin-16-UTP (Roche Clinical Laboratories, Indianapolis, IN), 200 mm sucrose, and 20% glycerol. The resulting biotin-labeled RNA was extracted with Trizol (Invitrogen) and isolated by magnetic Dynabeads M-280 covalently linked to streptavidin (Dynal Biotech, Brown Deer, WI). The beads were then resuspended in a reaction mixture for RT and real-time PCR.
Cloning of COX-1 Promoter Reporter Constructs
A series of fragments containing the rat COX-1 promoter sequence were amplified by PCR using rat liver genomic DNA as a template with the forward primers of 5′-GAGGTACCAGTCACCCCTCCCACAATC-3′ for −703 bp, 5′-GAGGTACCCACTGAGGACCAGCAAAGTCT-3′ for −580 bp, 5′-GAGGTACCTTCTCACCTCCTTGCTTCTCA-3′, for −354 bp, 5′-GAGGTACCGAGTGATGGTCTTAGCGGTCA-3′ for −172 bp, 5′-GGTACCTAAGGCTGGAGCAGAGGAAGTTG-3′ for −123 bp, 5′-ATGGTACCCTCTCCGAGGTGACAACTAG-3′ for −90 bp, or 5′-ATGGTACCAACTAGAGGGAGGAGTGG-3′ for −77 bp fragment. The reverse primer was 5′-CTAGCTAGCTGGTGTTACTCCAGCACTGC-3′, spanning −13 to +8 bp of COX-1 gene sequence. The forward primers contain a KpnI restriction site, whereas the reverse primer has a NheI restriction site, allowing restriction digestion and subcloning of the PCR products into a pGL3 Basic vector at 5′ upstream of a firefly luciferase coding sequence (Promega Corp., Madison, WI) to generate −703, −580, −354, −172, −123, −90, and −77 bp COX-1 promoter reporter constructs.
Mutations in −172 bp COX-1 promoter construct were generated by QuikChange Site-Directed Mutagenesis (Stratagene, La Jolla, CA) using the following sequences: forward, 5′-GAGGGAGGAGTGGTTGTGGAGCCGAGGG-3′; and reverse, 5′-CCCTCGGCTCCACAACCACTCCTCCCTC-3′ for −59-bp Sp binding site mutation; forward, 5′-GGATGGGTGGGAGGTTACCCGCCGGTGC-3′; and reverse, 5′-GCACCGGCGGGTAACCTCCCACCCATCC-3′ for −37-bp Sp binding site mutation. The sequences of cloned promoter fragments and mutations were verified by DNA sequencing.
Transient Transfection
COX-1 promoter reporter construct together with pRL-TK-Renilla luciferase construct were incubated with FuGene6 (Roche) in serum-free DMEM before adding to cells. The pRL-TK-Renilla luciferase reporter serves to correct for transfection efficiency. Rat Sp3 siRNA or scrambled siRNA negative control (Ambion, Austin, TX) was cotransfected with a −172-bp COX-1 promoter luciferase construct using oligofectamine (Invitrogen). After 5 h incubation with transfection mixtures, cells were placed in fresh DMEM containing 10% fetal bovine serum for overnight recovery before serum starvation and experimental treatments.
EMSA
Nuclear proteins were extracted by collecting cells in hypotonic buffer and lysis with detergent on ice following manufacturer’s instruction (Active Motif, Carlsbad, CA). Double strands of oligonucleotides corresponding to the −67 to −41-bp region of wild-type rat COX-1 promoter (5′-GAGGAGTGGGTGGAGCCGAGGGATG-3′ and opposite strand) or mutant (5′-GAGGAGTGGTTGTGGAGCCGAGGGATG-3′ and opposite strand) were end labeled with [γ-32P]ATP for incubation with nuclear extracts in a binding buffer containing 0.05 μg/μl polydeoxyinosinic deoxycytidylic acid, 10 mm HEPES (pH 7.9), 0.5 mm EDTA, 1 mm dithiothreitol, and 2.5% glycerol for 30 min. DNA-protein complexes were separated from free DNA probes by electrophoresis through nondenaturing polyacrylamide gels for autoradiography. For competition assays, a 50-fold molar excess of unlabeled wild-type or mutant double-strand oligonucleotides was added to the binding reaction. Nuclear extracts were incubated with antibodies against GR (BuGR2 mouse monoclonal, no. 2768; Abcam), Sp1, or Sp3 (polyclonal, nos. 07-645 or 07-107; Upstate Biotechnology) in the binding buffer for 60 min before the addition of 32P-labeled probes and polydeoxyinosinic deoxycytidylic acid.
ChIP Assay
ChIP assays were carried out using an enzymatic shearing method (Active Motif, Carlsbad, CA). Cell lysates were incubated with normal rabbit IgG or mouse IgG (SC-2027 or SC-2025, Santa Cruz Biotechnology) or antibodies against Sp1, Sp3 (rabbit polyclonal, nos. 07-645 and 07-107; Upstate Biotechnology), or GR (BuGR2 mouse monoclonal, no. 2768; Abcam) for immunoprecipitation. The DNA in the immunocomplex was analyzed by PCR using the following primers: forward, 5′-GAGGTACCGAGTGATGGTCTTAGCGGTCA-3′; and reverse, 5′-CTAGCTAGCTGGTGTTACTCCAGCACTGC-3′, spanning −172 to +8 bp of the COX-1 gene sequence.
Acknowledgments
We thank Ms. Yan Lin for technical assistance.
Footnotes
Current address for H.S.: Department of Anesthesiology, Division of Molecular Medicine, University of California Los Angeles, Los Angeles, California 90095-7115.
This work was supported by National Institutes of Health Grants R01 ES10826, R01 HL 076530, and T32 ES007091 (to Q.M.C.). Real-time PCR was performed at the Genomics Core Facility supported by the Southwest Environmental Health Sciences Center (ES06694).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 3, 2008
Abbreviations: CBP, cAMP response element binding protein-binding protein; ChIP, chromatin immunoprecipitation; COX, cyclooxygenase; CT, corticosterone; GAPDH, glyceraldhyde-3-phosphate dehydrogenase; GC, glucocorticoid; GR, glucocorticoid receptor; GRE, glucocorticoid response element; HDAC, histone deacetylase; MF, mifepristone; siRNA, small interfering RNA.
References
- Chen QM, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S 2005 Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: induction of antiapoptosis, antioxidant, and detoxification genes. Mol Pharmacol 67:1861–1873 [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM 2000 Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182 [DOI] [PubMed] [Google Scholar]
- Warner T, Mitchell J 2004 Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J 18:790–804 [DOI] [PubMed] [Google Scholar]
- Jun SS, Chen Z, Pace MC, Shaul PW 1998 Estrogen upregulates cyclooxygenase-1 gene expression in ovine fetal pulmonary artery endothelium. J Clin Invest 102:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina JA, Krause DN, Duckles SP 2002 17β-Estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke 33:600–605 [DOI] [PubMed] [Google Scholar]
- Gibson LL, Hahner L, Osborne-Lawrence S, German Z, Wu KK, Chambliss KL, Shaul PW 2005 Molecular basis of estrogen-induced cyclooxygenase type 1 upregulation in endothelial cells. Circ Res 96:518–525 [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Pilbeam CC, Gronowicz G, Abreu C, Fletcher BS, Herschman HR, Raisz LG, Hurley MM 1995 Transcriptional induction of prostaglandin G/H synthase-2 by basic fibroblast growth factor. J Clin Invest 96:923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CE, Appleton I, Mitchell JA 1998 Vascular endothelial growth factor upregulates constitutive cyclooxygenase 1 in primary bovine and human endothelial cells. Life Sci 62:2195–2201 [DOI] [PubMed] [Google Scholar]
- Rioux N, Castonguay A 2000 The induction of cyclooxygenase-1 by a tobacco carcinogen in U937 human macrophages is correlated to the activation of NF-κB. Carcinogenesis 21:1745–1751 [DOI] [PubMed] [Google Scholar]
- Smith CJ, Morrow JD, Roberts LJ, Marnett LJ 1993 Differentiation of monocytoid THP-1 cells with phorbol ester induces expression of prostaglandin endoperoxide synthase-1 (COX-1). Biochem Biophys Res Commun 192:787–793 [DOI] [PubMed] [Google Scholar]
- Xu XM, Tang JL, Hajibeigi A, Loose-Mitchell DS, Wu KK 1996 Transcriptional regulation of endothelial constitutive PGHS-1 expression by phorbol ester. Am J Physiol 270:C259–C264 [DOI] [PubMed] [Google Scholar]
- Schneider N, Lanz S, Ramer R, Schaefer D, Goppelt-Struebe M 2001 Up-regulation of cyclooxygenase-1 in neuroblastoma cell lines by retinoic acid and corticosteroids. J Neurochem 77:416–424 [DOI] [PubMed] [Google Scholar]
- Secchiero P, Gonelli A, Ciabattoni G, Melloni E, Grill V, Rocca B, Delbello G, Zauli G 2002 TNF-related apoptosis-inducing ligand (TRAIL) up-regulates cyclooxygenase (COX)-1 activity and PGE(2) production in cells of the myeloid lineage. J Leukoc Biol 72:986–994 [PubMed] [Google Scholar]
- Kaplan MD, Olschowka JA, O’Banion MK 1997 Cyclooxygenase-1 behaves as a delayed response gene in PC12 cells differentiated by nerve growth factor. J Biol Chem 272:18534–18537 [DOI] [PubMed] [Google Scholar]
- Jun SS, Chen Z, Pace MC, Shaul PW 1999 Glucocorticoids downregulate cyclooxygenase-1 gene expression and prostacyclin synthesis in fetal pulmonary artery endothelium. Circ Res 84:193–200 [DOI] [PubMed] [Google Scholar]
- Taniura S, Kamitani H, Watanabe T, Eling TE 2002 Transcriptional regulation of cyclooxygenase-1 by histone deacetylase inhibitors in normal human astrocyte cells. J Biol Chem 277:16823–16830 [DOI] [PubMed] [Google Scholar]
- Philipsen S, Suske G 1999 A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res 27:2991–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Meng A 2005 Sp1-like transcription factors are regulators of embryonic development in vertebrates. Dev Growth Differ 47:201–211 [DOI] [PubMed] [Google Scholar]
- Safe S, Abdelrahim M 2005 Sp transcription factor family and its role in cancer. Eur J Cancer 41:2438–2448 [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R 2003 Sp1- and Kruppel-like transcription factors. Genome Biol 4:206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Ferro TJ 2005 Sp1: regulation of gene expression by phosphorylation. Gene 348:1–11 [DOI] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE 1997 RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 48:129–156 [DOI] [PubMed] [Google Scholar]
- Bouwman P, Philipsen S 2002 Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol 195:27–38 [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K 2004 Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog Nucleic Acid Res Mol Biol 77:1–36 [DOI] [PubMed] [Google Scholar]
- Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, Marme D, Finkenzeller G, Safe S 2000 Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor α and Sp3 proteins. J Biol Chem 275:22769–22779 [DOI] [PubMed] [Google Scholar]
- Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S 2004 Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor α and SP proteins. Oncogene 23:1052–1063 [DOI] [PubMed] [Google Scholar]
- McEwan IJ, Wright AP, Gustafsson JA 1997 Mechanism of gene expression by the glucocorticoid receptor: role of protein-protein interactions. Bioessays 19:153–160 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW 2002 Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O’Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Kassel O, Herrlich P 2007 Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol 275:13–29 [DOI] [PubMed] [Google Scholar]
- Coronella-Wood J, Terrand J, Sun H, Chen QM 2004 c-Fos phosphorylation induced by H2O2 prevents proteasomal degradation of c-Fos in cardiomyocytes. J Biol Chem 279:33567–33574 [DOI] [PubMed] [Google Scholar]
- Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN 1998 Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J 332:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne E 1957 Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol 3:447–454 [Google Scholar]
- Patrone G, Puppo F, Cusano R, Scaranari M, Ceccherini I, Puliti A, Ravazzolo R 2000 Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. Biotechniques 29:1012–1014; 1016–1017 [DOI] [PubMed] [Google Scholar]