Abstract
The retinoblastoma protein (RB) regulates cell proliferation and survival by binding to the E2F family of transcription factors. Recent studies suggest that RB also regulates differentiation in a variety of cell types, including myocytes, neurons, adipocytes, and chondrocytes. Rb mutations have been found in ovarian cancer; however, the role of RB in normal and abnormal ovarian function remains unclear. To test the hypothesis that loss of Rb induces ovarian tumorigenesis, we generated an ovarian granulosa cell conditional knockout of Rb (Rb cKO) using the Cre/lox recombination system. Rb cKO females showed 100% survival and no ovarian tumor formation through 9 months of age, but they developed progressive infertility. Prepubertal Rb cKO females showed increased ovulation rates compared with controls, correlating with increased follicle recruitment, higher Fshr and Kitl mRNA levels, and lower anti-Müllerian hormone levels. In contrast, the ovulation rate of 6-wk-old females was similar to that of controls. Morphometric analysis of Rb cKO ovaries from 6-wk-old and older females showed increased follicular atresia and apoptosis. Rb cKO ovaries and preantral follicles had abnormal levels of known direct and indirect target genes of RB, including Rbl2/p130, E2f1, Ccne2, Myc, Fos, and Tgfb2. In addition, preantral follicles showed increased expression of the granulosa cell differentiation marker Inha, decreased levels of Foxl2 and Cyp19a1 aromatase, and abnormal expression of the nuclear receptors Nr5a1, Nr5a2, and Nr0b1. Taken together, our results suggest that RB is required for the temporal-specific pattern of expression of key genes involved in follicular development.
THE RETINOBLASTOMA PROTEIN (RB) and related RB-like (RBL) family members (RBL1/P107 and RBL2/P130) control cell cycle progression by binding and inhibiting E2F transcription factors. The G1-S transition of the cell cycle is dependent on the activity of cyclin D and cyclin E (1), which phosphorylate RB and cause the release of E2F, thereby promoting the transcription of genes required for cell cycle progression (2,3,4). Such progression is under the control of cyclin-dependent kinase inhibitors (CDNs), including CDN2A/P16ink4, CDN1B/P27kip, and CDN1A/P21cip1, which cause cell cycle arrest in cells carrying DNA damage (5). If the DNA damage cannot be repaired, apoptotic pathways will be activated to suppress the propagation of abnormal cells (6).
In addition to its well-known function in cell cycle control, RB plays a role in differentiation of a variety of cell types, including myocytes, adipocytes, neurons, and chondrocytes (7). In the majority of the cases studied to date, the expression of early differentiation markers and the acute proliferation arrest that precedes terminal differentiation are not perturbed in the absence of Rb (8). However, Rb deletion leads to a delay in the onset of late differentiation markers and to ectopic cell proliferation and cell death (8,9). RB control of gene expression during cellular differentiation might be achieved by its activity as a coactivator or corepressor of lineage-specific transcription factors, or by binding and sequestering repressor molecules, depending on the cell type (8,10,11). RB functions in cell differentiation and cell cycle are independently regulated, and the former appears to involve its C-terminal domain, which shares little homology with RBL1/P107 and RBL2/P130 and thus confers RB unique properties (12).
The production of a fertilizable oocyte begins with the recruitment of primordial follicles into a growing follicle pool (activation). Although small follicles are responsive to FSH, early follicular development is under the control of other factors, including Kit ligand (KITL), Forkhead box L2 (FOXL2), and several locally produced TGFβ family members, which either promote [e.g. bone morphogenetic protein 15 (BMP15), growth differentiation factor 9 (GDF9), and FOXL2] or inhibit [e.g. anti-Müllerian hormone (AMH)] follicle growth (13,14,15). A second selection process takes place at later stages of follicular development, in which growing follicles are rescued from atresia by a rise in FSH levels and continue to grow until the preovulatory stage (13,14,16). During folliculogenesis, granulosa cells proliferate until the ovulatory surge, when they exit the cell cycle and undergo luteinization or terminal differentiation (17,18). Studies in knockout mice have shown that cyclin D2 is essential for granulosa cell proliferation, whereas CDN1B/P27kip and CDN1A/P21cip1 are important for terminal differentiation (19,20,21). In terms of hormonal regulation of the cell cycle, in vitro studies showed that FSH and estradiol initiate the G1-S transition by activating cyclin D2 in ovarian granulosa cells (18,22). Activin, a dimeric TGFβ family member, acts synergistically with FSH to promote the G1-S transition of the cell cycle and the inhibitory phosphorylation of RB (22). In contrast, inhibin, another dimeric TGFβ family member, has been proposed to act as a tumor suppressor because female mice lacking the inhibin α-subunit (Inha) develop granulosa cell tumors (23). Loss of the cell cycle regulators Ccnd2 (cyclin D2) and Cdn1b/p27 modify the development of ovarian tumors in Inha−/− females by accelerating (Cdn1b/p27) or delaying (i.e. Ccnd2) tumor formation (24,25). Mutations at the Rb1 and Rbl2/p130 loci also occur in ovarian tumors (26); however, given the fact that Rb loss may trigger apoptosis via increases in E2F transcription factors and downstream targets (4,27), the contribution of RB to the tumorigenic phenotype remains unclear. Because Rb-deficient mice are embryonic lethal (28), the generation of tissue-specific knockout (KO) mouse models have helped our understanding of the cooperation between RB and other tumor suppressor genes in the genesis/progression of brain and lung and ovarian epithelia tumors as well as granulosa cell tumors (9,29). However, the ability of RB to suppress tumorigenesis in the absence of other mutations and its role as a transcriptional regulator of normal ovarian function remain unclear.
To test the hypothesis that loss of Rb induces ovarian tumorigenesis, we generated a granulosa cell conditional KO (cKO) of Rb (Rb cKO) using a mouse line carrying a floxed allele of Rb and anti-müllerian hormone receptor 2-Cre recombinase knock-in transgenic mice. Rb cKO females showed 100% survival and no ovarian tumor formation through 9 months of age, but they developed progressive infertility. Prepubertal Rb cKO females showed increased ovulation rates and follicular recruitment compared with control females, whereas the ovulation rate of 6-wk-old females was similar to that of controls. Morphometric analysis of Rb cKO ovaries from 6-wk-old and older females showed increased follicular atresia and apoptosis. At the molecular level, Rb cKO ovaries and preantral follicles express abnormal levels of known direct and indirect targets of RB as well as the granulosa cell differentiation marker inhibin-α. Taken together, our results suggest that RB is required for the temporal-specific pattern of expression of key genes involved in follicular development.
RESULTS
Conditional Disruption of Rb in the Ovary
To determine the role of Rb in granulosa cells, we generated Rb cKO using the Cre-loxP system (Fig. 1A). We crossed mice carrying the Rb null allele to mice carrying the Amhr2cre knock-in allele (Amhr2cre+) (30). We then crossed Rb+/−/Amhr2cre+ mice and mice carrying the Rb floxed (Rbflox/flox) allele to generate the Rb cKO mice (Rbflox/−, Amhr2Cre+), and control mice (Rbflox/−, Amhr2Cre−, or Rbflox/−) (Fig. 1A). Expression of the Amhr2cre allele in the ovary has been previously described (30), and the allele has been successfully used by our group to delete activin-βA, follistatin (Fst), Smad 1, Smad5, Smad4, and Rb in Inha null mice (29,31,32,33,34).
Figure 1.
Generation of the Rb cKO Mice and Fertility Studies
A, Breeding scheme used to generate Rb cKO mice. B, Efficiency of recombination of the Rb floxed allele by the Amhr2Cre allele in granulosa cells. PCR analysis of genomic DNA from granulosa cells derived from two Rbflox/−Amhr2Cre− (Rb flox/−) and three Rbflox/−Amhr2Cre+ (Rb cKO) mice. Note that the recombined band (Rec) is present only in Amhr2Cre+ granulosa cells. C, Quantitative RT- PCR analysis of exon 19 (floxed) deletion in granulosa cells. D and E, Fertility studies of Rb cKO females. Ten Rbflox/− and Rb cKO females were bred to stud males for 6 months, and the number of pups per litter and number of litters per month were recorded. D, Average number of pups per litter. Rb cKO females had a significantly lower number of pups per litter. E, Total number of pups. The cumulative number of pups produced by 10 Rb cKO females was also lower than that of controls. **, P < 0.01; a, P < 0.05.
We verified the loss of Rb by PCR analysis of cDNA (Fig. 1B) and genomic DNA (Fig. 1C) obtained from granulosa cells of Rb cKO, wild-type (WT), and Rbflox/− females. Analysis of granulosa cell genomic DNA confirmed the presence of the recombined allele in AmhrCre+ but not in Amhr2Cre− samples (Fig. 1B). Real-time quantitative PCR analysis using primers described in our previous studies (29) showed an expected 56% decrease in the levels of Rb transcripts in Rbflox/− granulosa cells (Fig. 1C), whereas an 80% decrease was observed in Rb cKO granulosa cells, indicating recombination of the floxed allele (Fig. 1C).
Rb cKO Female Mice Show Progressive Infertility
To investigate the reproductive performance of Rb cKO mice, females were bred to WT males for 6 months. Rbflox/− females were used as controls because the average number of pups per litter of these females (8.9 ± 0.3) was similar to that of our C57BL/6J;129S5/SvEvBrd mixed hybrid background colony (eight pups per litter). Rb cKO females gave birth to a significantly lower number of pups per litter (Fig. 1D; P < 0.05) and had a significantly lower number of litters per month compared with control females (Table 1; P < 0.05). The observed reduction in the number of pups, which was evident in the first month of mating (controls, 8.0 ± 0.3; Rb cKO, 4.9 ± 0.8; P < 0.05) progressed over time with virtually no pups being produced by the fifth month (controls, 9.5 ± 0.6; Rb cKO, 0.6 ± 0.5; P < 0.05) and the sixth month (controls, 8.4 ± 0.9; Rb cKO, 0.1 ± 0.09; P < 0.05) of breeding (Fig. 1D). The total number of pups produced by Rb cKO females was also significantly lower compared with that of control females (Fig. 1E). A summary of the fertility studies is shown in Table 1.
Table 1.
Fertility Test of Rbflox/− and Rb cKO Females
| Genotype | n | Litters | Total Pups | Pups/Litter | Litters/Month |
|---|---|---|---|---|---|
| Rbflox/− | 10 | 61 | 538 | 8.97 ± 0.29 | 1.02 ± 0.02 |
| Rbflox/−Amhr2Cre+ | 10 | 30 | 133 | 2.23 ± 0.70a | 0.50 ± 0.12a |
Six-week-old Rbflox/−Amhr2Cre− (Rbflox/−) and Rbflox/− Amhr2Cre+ (Rb cKO) females were mated to WT males for 6 months. Results are shown as the mean ± sem. Data were analyzed using the nonparametric Mann-Whitney U test.
P < 0.05.
Rb cKO Females Do Not Develop Ovarian Tumors but Show Increased Follicular Recruitment and Atresia
To understand the decline in fertility observed in Rb cKO females, we analyzed control and Rb cKO mice up to 9 months of age. The latter time-point was chosen because spontaneous loss of heterozygosity in the intermediate lobe of the pituitary gland and in the thyroid gland has been reported to occur around 10 months of age in the Rb+/− strain of mice used in this study (28). Thus, analysis of tumor formation due to deletion of Rb in granulosa cells in older animals might be obscured by the effects of the presence of tumors in extragonadal sites.
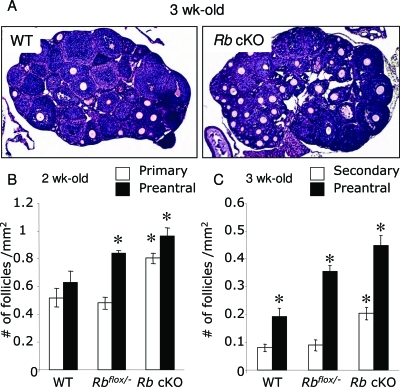
Rb cKO females were viable, showed normal body weights (Rbflox/−, 28.9 ± 1.69 g, and Rb cKO, 29.1 ± 1.13 g; n =10, P > 0.05), and survived up to 9 months of age without obvious anatomical defects. We next analyzed ovaries from 3-, 6-, 12-, and 24-wk-old mice. Grossly, no major differences in size were detected between Rb cKO and control ovaries. Follicular development up to 3 wk of age appeared to be normal in Rb cKO females (Fig. 2A). However, follicle counts revealed a significant increase in the number of primary and preantral follicles in Rb cKO ovaries compared with WT ovaries at 2 wk of age (Fig. 2B; P < 0.05; supplemental Fig. 1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). By 3 wk of age, a significantly higher number of secondary and preantral follicles were observed in Rb cKO ovaries (Fig. 2C; P < 0.05; supplemental Fig. 1B), suggesting increased follicular recruitment. The number of preantral follicles in Rbflox/− ovaries was intermediate between that of controls and Rb cKO ovaries (Fig. 2, B and C; P < 0.05), whereas the number of primary and secondary follicles was the same as that of WT ovaries (Fig. 2, B and C). Ovaries from 6- and 12-wk-old Rb cKO females (Fig. 3, A and C and B and D, respectively) showed a significant increase in the number of atretic follicles and oocyte remnants [Fig. 3, C and D (yellow arrows) and E and F; supplemental Fig. 2, A and B; n = 4–5; P < 0.05]. The number of atretic follicles and oocyte remnants in Rb cKO ovaries at 12 wk of age is twice as many as that of 6-wk-old ovaries. In addition, the number of atretic follicles in 6-wk-old Rbflox/− ovaries was intermediate between that of controls and Rb cKO ovaries (Fig. 3C). The number of primordial follicles was also lower in Rb cKO mouse ovaries, although this difference was not statistically significant (supplemental Fig. 2A). The decrease in the number of primordial follicles was more pronounced at 12 wk in Rb cKO ovaries, although no statistically significant differences were observed between mutant and control ovaries (supplemental Fig. 2B). Interestingly, the number of preantral follicles was significantly higher in Rb cKO ovaries (supplemental Fig. 2B).
Figure 2.
Histological Analysis and Follicle Counts of the Ovaries of Prepubertal Control and Rb cKO Mice
A, Ovaries from 3-wk-old WT and Rbflox/−Amhr2Cre+ (Rb cKO) mice. Note the increased amount of growing follicles present in the Rb cKO ovary. B and C, Follicle counts of 2-wk-old (B) and 3-wk-old (C) WT and Rb cKO mice. Follicles were counted in five histological sections derived from four to five ovarian samples and the number of follicles per square millimeter was calculated as described in Materials and Methods. Two-week-old Rb cKO ovaries show a significantly higher number of primary and preantral (multilayer) follicles compared with WT ovaries, whereas 3-wk-old Rb cKO ovaries show a significantly higher number of secondary and preantral follicles compared with WT. Only follicular stages that displayed significant differences are shown. For counts in all follicle stages, please refer to supplemental Fig. 1, A and B. Magnification, ×50. *, P < 0.05.
Figure 3.
Histological Analysis and Follicle Counts of the Ovaries of Adult Control and Rb cKO Mice
Ovaries from 6-wk-old (A) and 12-wk-old (B) WT and Rb cKO mice. Note the increased amount of oocyte remnants (yellow arrows) present in the Rb cKO ovaries at both 6 wk (C) and 12 wk (D) of age. Follicle counts of 6-wk-old (E) and 12-wk-old (F) Rb cKO mice show a significant increase in the number of atretic follicles and oocyte (zona pellucida) remnants. Only follicular stages that displayed significant differences are shown. For counts in all follicle stages, please refer to supplemental Fig. 2, A and B. Magnification, ×50 (A and B) and ×100 (C and D). *, P < 0.05.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) analysis of 6-wk-old ovaries from WT (Fig. 4A) and Rb cKO (Fig. 4B) females confirmed a significant increase in both the number of apoptotic cells per preantral follicle (Fig. 4C; P < 0.05) and number of apoptotic (atretic) preantral follicles (Fig. 4D; P < 0.05). No significant differences were observed at the antral stage (data not shown). By 9 months of age, a large number of oocyte remnants (Fig. 5, yellow arrows), and only a few growing follicles (Fig. 5) were evident in the ovaries of Rb cKO females, in agreement with the significant decline in fertility observed at this age. No ovarian tumor foci (histological analysis; see Fig. 5 for a representative example) or evidence of metastases (macroscopic analysis, data not shown) were found in any of the samples or animals analyzed (n = 20).
Figure 4.
Confocal Microscopic Analysis of Apoptosis in Six-Week-Old WT and Rb cKO Ovaries
Representative micrographs showing TUNEL staining from WT (A) and Rb cKO (B) 6-wk-old ovaries. TUNEL-positive cells are shown in green; cell nuclei are shown in red (propidium iodine). C, Total and TUNEL-positive follicles and cell numbers were quantified in 10 HPFs (×40) per section, and three individual sections were quantified per specimen (three specimens). D, The number of follicles showing four or more TUNEL-positive cells was also recorded. The significant increase in the number of TUNEL-positive cells and follicles observed in Rb cKO ovaries is limited to the preantral stage. Results are presented as the average of the percent of positive cells per total cell number per HPF (apoptotic index) ± sem and the average number of TUNEL-positive follicles per section ± sem. Magnification, ×250. *, P < 0.05.
Figure 5.
Histological Analysis of Nine-Month-Old Control and Rb cKO Ovaries
A and B, Representative micrographs of WT (A) and Rb cKO (B) mice. Magnification, ×50. C and D, High magnification of ovary from left inset (C) and right inset (D) in B. Note the large amount of periodic acid Schiff-positive zona pellucida remnants (oocyte remnants) (yellow arrowheads). Magnification, ×400.
Serum Hormone and Estrous Cycle Analyses of Rb cKO Females
Analysis of the estrous cycle profiles in Rb cKO and control mice at 2 months of age showed significantly longer diestrus in Rb cKO females (Fig. 6A). The same group of females displayed abnormal cycling patterns at 4 months of age, with significantly shorter proestrus and metestrus and extended diestrus (Fig. 6B). This is consistent with the reduction in the number of pups per litter at this age and the overall decrease in the number of litters per month observed in Rb cKO females (Table 1). Despite estrous cycle abnormalities, serum FSH, estradiol, and LH levels collected at random stages of the estrous cycle showed no significant differences between mutant and control females at any of the ages analyzed (Table 2). However, serum samples from Rb cKO females showed significantly lower levels of FSH at estrus (Fig. 6C).
Figure 6.
Estrous Cycle Profiles of 8-Week-Old and 12-Week-Old Rb cKO Females and Serum FSH Levels at Estrus in Eight-Week-Old Rb cKO Females
Estrous cycle profiles of 8-wk-old (A) and 12-wk-old (C) Rb cKO females. Note that mutant females have significantly longer diestrus even at the earliest time point (n = 5). B, Serum FSH levels at estrus in 8-wk-old females (n = 4). Note the significant decrease in Rb cKO females. Serum levels at random stages of the estrous cycle are presented in Table 2. *, P < 0.05.
Table 2.
Serum Hormone Profiles of Rb cKO Females
| Genotype | FSH (ng/ml) | LH (ng/ml) | E2 (pg/ml) |
|---|---|---|---|
| 3 Months old | |||
| Rbflox/− | 4.42 ± 1.30 | 0.28 ± 0.08 | 24.02 ± 6.72 |
| Rb cKO | 5.95 ± 0.78 | 0.14 ± 0.04 | 25.02 ± 9.94 |
| 6 Months old | |||
| Rbflox/− | 5.15 ± 0.54 | ND | 33.075 ± 13.9 |
| Rb cKO | 6.50 ± 1.30 | ND | 18.37 ± 4.24 |
| 8 Months old | |||
| Rbflox/− | 7.61 ± 1.08 | 0.12 ± 0.02 | 32.63 ± 3.27 |
| Rb cKO | 6.13 ± 1.30 | 0.09 ± 0.04 | 34.95 ± 9.7 |
Results are shown as the mean ± sem. Data were analyzed using the nonparametric Mann-Whitney U test. No statistically significant differences were found between Rbflox/−, Amhr2Cre− (Rbflox/−) and Rbflox/−, Amhr2Cre+ (Rb cKO ). E2, 17β -Estradiol; ND, not determined.
Immature Rb cKO Females Show Increased Ovulation Rates in Response to Gonadotropins
Because of the apparent increase in follicular recruitment observed in Rb cKO prepubertal females, we investigated the response to gonadotropin hormones at 3 wk of age. In these experiments, Rb cKO females ovulated twice as many oocytes as control females (n = 5; P < 0.05; Fig. 7A). Immunostaining and confocal microscopic analysis revealed that 88% of oocytes ovulated by Rb cKO females were normal (Fig. 7, C and D), with the remaining oocytes displaying various defects, including chromosome lagging and misalignment, as well as spindle fiber alterations (Fig. 7C). In contrast, we found no significant differences in the ovulation rates of 6-wk-old Rb cKO and control females (Fig. 7B; n = 4; P > 0.05). These results confirm that prepubertal Rb cKO females display increased ovarian follicular recruitment and show essentially no defects in oocyte maturation. In addition, the low FSH levels observed at estrus suggest that despite the fact that a larger number of follicles enter the growing pool in Rb cKO ovaries, fewer follicles may be rescued by the gonadotropin surge.
Figure 7.
Superovulation and Timed-Mating Experiments in Control and Rb cKO Mice
Four to five 3- or 6-wk-old WT and Rbflox/−Amhr2Cre+ (Rb cKO) were superovulated, and the number of ovulated oocytes per female was recorded. Results are presented as average number of oocytes ± sem (n = 4 and 5). B, Confocal analysis of meiosis II oocytes. Oocytes retrieved from superovulated 3-wk-old WT and Rb cKO females were collected, fixed, and immunostained with β-tubulin to visualize the meiotic spindle (green). Chromatin was stained with propidium Iodide (red). Arrowheads show misaligned chromosomes or unattached spindle fibers. Magnification, ×800. D, Stained oocytes were analyzed by confocal microscopy, and the percentage of oocytes showing abnormal spindles and/or chromosome alignment was recorded (n = 3). Results are shown as the average percentage of normal and abnormal oocytes ± sem. *, P < 0.05.
Ovaries from Rb cKO Females Show Higher Levels of Fshr and FSH Target Genes
Early stages of follicular development are not dependent on FSH signaling, because mice lacking FSH or FSH receptor (FSHR) show a block in folliculogenesis at the multilayer preantral stage (13,35). However, in the mouse ovary, expression of Fshr is observed as early as postnatal d 1 (36). To begin to understand the increase in follicular recruitment observed in Rb cKO ovaries, we evaluated Fshr (Fshr) expression in 2- and 3-wk-old females by quantitative real-time PCR (QPCR) analysis. Because the significantly larger number of preantral follicles present in Rb cKO ovaries may skew gene expression analysis, we also analyzed equal numbers of preantral follicles isolated from 12-d-old WT and Rb cKO females. Our results show that Fshr was significantly increased in both ovaries and preantral follicles (Fig. 8, A and B; n = 3; P < 0.05), which can explain, at least partially, the observed increase in follicular recruitment. However, no significant differences were observed in the expression of Fshr isoforms (data not shown) or LH receptor (Lhcgr, Table 3; P < 0.05). In addition, FSHR downstream targets, cyclin D2 (Ccnd2) (Fig. 8, C and D; n = 3; P < 0.05) and inhibin-α (Inha) (Fig. 8, E and F; n = 3; P < 0.05), were significantly up-regulated in both Rb cKO ovaries and preantral follicles. Immunostaining of ovarian sections from 6-wk-old mice revealed that although inhibin-α protein levels are higher in Rb cKO follicles (supplemental Fig. 3, D–F) compared with controls (supplemental Fig. 3, A–C), its distribution appears to be normal (supplemental Fig. 3, A–F).
Figure 8.
FSHR and FSH Target Gene Expression Changes in Prepubertal Ovaries and Preantral Follicles from Control and Rb cKO Females
Real-time PCR analysis of mRNA from 2- and 3-wk-old control (WT and Rbflox/−) and Rb cKO (Rbflox/−, Amhr2Cre+) ovaries and preantral follicles isolated from 12-d-old females. Ovaries and preantral follicles were collected from three to four independent control and Rb cKO females. Average and sem are shown. A significant increase was seen in the relative quantity (RQ) of Fshr (A and B), Ccnd2 (D and E), and Inha (E and F) in Rb cKO ovaries and preantral follicles. The value of one of the control samples was set to equal 1. *, P < 0.05.
Table 3.
Molecular Changes in Rb cKO Preantral Follicles Compared with WT Follicles
| Gene | WT | Rbflox/− Amhr2Cre+ |
|---|---|---|
| E2f5 | 1.23 ± 0.12 | 5.63 ± 0.52a |
| Sox9 | 1.03 ± 0.25 | 0.88 ± 0.08 |
| Gata4 | 1.12 ± 0.17 | 1.08 ± 0.09 |
| Inhbb | 1.04 ± 0.16 | 3.28 ± 0.51a |
| Inhba | 0.88 ± 0.04 | 1.43 ± 0.26 |
| Lhcrg | 1.25 ± 0.16 | 0.92 ± 0.17 |
Preantral follicles were isolated from 12-d-old WT and Rbflox/− Amhr2Cre+ (Rb cKO) females and subjected to QPCR analysis. Results are shown as the mean ± sem. Data were analyzed using the nonparametric Mann-Whitney U test.
P < 0.05.
Ovaries from Rb cKO Females Show Altered Expression of Two Key Regulators of Early Folliculogenesis: KITL and AMH
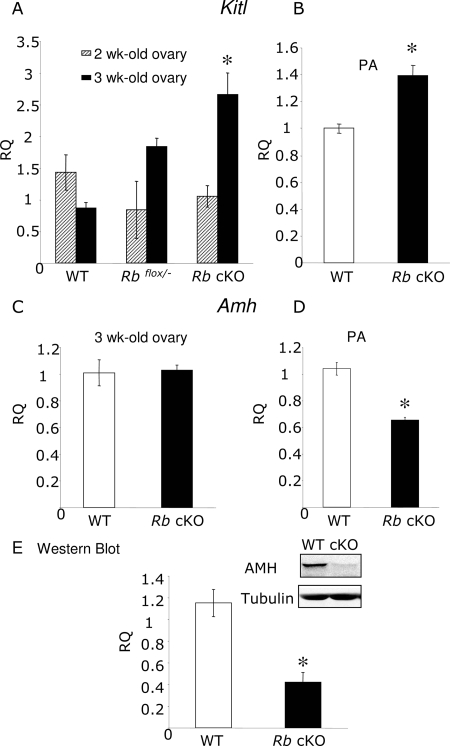
KITL, a growth factor produced by granulosa cells, plays a critical role in the primordial to primary follicle transition by influencing theca cell recruitment and oocyte growth (37,38). Kitl expression has been shown to be under gonadatropin hormone control in rat granulosa cells (39). Because Rb cKO ovaries show a significant increase in primary follicles and higher levels of Fshr, we investigated whether alterations in Kitl levels may contribute to the Rb cKO phenotype. To this end, Kitl mRNA levels were determined in 2- and 3-wk-old ovaries from WT, Rbflox/−, and Rb cKO females as well as isolated preantral follicles from 12-d-old ovaries by QPCR analysis. No significant differences in Kitl levels were observed in 2-wk-old ovaries (Fig. 9A). However, a significant increase in Kitl was found in 3-wk-old Rbflox/− and Rb cKO ovarian samples compared with WT samples (Fig. 9A; n = 3; P < 0.05). The observed increase in Kitl cannot be attributed to the larger number of growing follicles present in Rb cKO ovaries, because similar results were obtained after analysis of samples containing equal numbers of isolated preantral follicles (Fig. 9B; n = 3; P < 0.05).
Figure 9.
KITL and AMH Changes in Prepubertal Ovaries and Preantral Follicles from Control and Rb cKO Females
QPCR of mRNA from 2- and 3-wk-old control (WT and Rbflox/−) and Rb cKO (Rbflox/−, Amhr2Cre+) ovaries and preantral follicles isolated from 12-d-old females. Ovaries and preantral follicles were collected from three to four independent control and Rb cKO females. Average and sem are shown. A significant increase was seen in the relative quantity (RQ) of Kitl in Rb cKO ovaries (A) and preantral follicles (B). Although no changes were observed in 3-wk-old ovaries (C), a significant decrease was seen in the relative quantity (RQ) of Amh was seen in preantral follicles (D). Western blot analysis of preantral follicles confirmed a significant decrease in AMH levels (E). The value of one of the control samples was set to equal 1. *, P < 0.05.
Another granulosa cell-derived factor, AMH, has been shown to be important for folliculogenesis. In the mouse, AMH is expressed in growing follicles from the secondary stage until the small antral stage (38,40). Studies in Amh KO females and in vitro studies show that AMH produced by growing follicles inhibits the initial follicular recruitment and decreases the sensitivity of large preantral and small antral follicles to FSH (16,38,41). Similar to Amh KO mice, Rb cKO females show a significant increase in follicular recruitment and increased follicular atresia and oocyte degeneration during the estrous cycle (42,43). Therefore, we investigated Amh mRNA levels in 3-wk-old ovaries and preantral follicles isolated from 12-d-old WT and Rb cKO ovaries by QPCR analysis. Ovarian samples showed no significant differences in Amh mRNA levels (Fig. 9C). However, a significant decrease in both mRNA and protein levels was observed in isolated Rb cKO preantral follicles as demonstrated by QPCR (Fig. 9D; n = 3; P < 0.05) and Western blot analyses (Fig. 9E; n = 3; P < 0.05), respectively. The apparent discrepancy between Amh levels in ovarian and isolated follicle samples is likely due to the presence of larger numbers of preantral follicles in Rb cKO ovaries. Consistent with these results, immunostaining of ovarian sections from 6-wk-old mice showed lower AMH levels in Rb cKO follicles (supplemental Fig. 3, J–L) compared with controls (supplemental Fig. 3, G–I) and normal AMH distribution (supplemental Fig. 3, G–L).
Taken together, the results show that Rb deletion in ovarian granulosa cells affects the expression of genes that are key regulators of follicular recruitment, thereby causing premature depletion of the follicular pool.
RB and E2F Target Genes Are Deregulated in Rb cKO Preantral Follicles
RB and the related proteins (RBL1/P107 and RBL2/P130) control cell proliferation by binding to members of the E2F family of transcription factors (11). RB can also cooperate with tissue-specific transcription factors to either activate or repress the expression of genes required for cell differentiation (11). E2F1 and cyclin E2 (CCNE2) regulate cell cycle progression and are known to be deregulated in the absence of Rb (4,44). Recent studies show that Rb deletion may lead to compensatory increases in Rbl1/p107 and Rbl2/p130 (9) and that increases in these RB-related proteins can cause cell cycle arrest (45). In addition, RB can directly regulate gene expression by binding to the RB control element. For instance, RB represses the expression of the early and delayed G1 response genes FBJ osteosarcoma oncogene (Fos) and myelocytomatosis (Myc) (46,47), whereas it either stimulates or represses the expression of Tgfb1 depending on the cell type (48).
TGFβ family members play important roles in female reproduction. In mice, TGFβs have been shown to be expressed in the oocyte and in theca and granulosa cells of the immature ovary. Pregnant mare serum gonadotropin or FSH treatments have been shown to increase TGFβ2 in granulosa cells (49,50). Conversely, TGFβs have been shown to increase Fshr mRNA stability and to have an overall positive function in the ovary (51,52). In the rat ovary, Myc and Fos are expressed in granulosa cells and are up-regulated by the gonadotropins (53,54,55,56).
To better understand the impact of Rb deficiency in the ovary, preantral follicles were isolated from 12-d-old WT and Rb cKO ovaries, and mRNA transcripts were determined by QPCR analysis. As expected, E2f1 and cyclin E2 (Ccne2) were significantly increased in Rb cKO preantral follicles compared with WT follicles (Fig. 10, A and B; n = 3; P < 0.05). Rb cKO preantral follicles showed a significant increase in the expression of Rbl2/p130 but not Rbl1/p107 (Fig. 10, C and D; n = 3; P < 0.05). Interestingly, immunostaining of ovarian sections from 6-wk-old mice showed increased cytosolic staining of RBL2 in Rb cKO follicles (supplemental Fig. 4, C and D) compared with controls (supplemental Fig. 3, A and B), in which RBL2 appears to be localized primarily in the nucleus specially in luteal cells (supplemental Fig. 4). Localization of RBL2 in both the nucleus and cytosol has been previously reported, and it has been suggested that RBL2 may play different roles and be subjected to differential regulation in the two compartments (57). We found that, in addition to increased E2f1, Rb cKO follicles expressed significantly higher levels of the transcription factor E2f5, an E2F family member known to interact with RBL2/p130 (Table 3). Finally, RB direct targets Tgfb2, Myc, and Fos were also significantly up-regulated in Rb-deficient preantral follicles (Figs. 10, E and F, and 11F, respectively; n = 3; P < 0.05).
Figure 10.
Gene Expression Changes of RB Target Genes in Preantral Follicles from Control and Rb cKO Females
Real-time PCR analysis of mRNA from WT and Rb cKO (Rbflox/–, Amhr2Cre+) preantral follicles isolated from 12-d-old females. Preantral follicles were collected from three independent control and Rb cKO females. Average and SEM are shown. A significant increase was seen in the relative quantity (RQ) of E2f1 (A), Ccne2 (B), Rbl2/ p130 (D), Tgfb2 (E) and Myc (F) in Rb cKO preantral follicles. No significant changes were observed in Rbl1/p107 levels (C).Other gene expression changes are shown in Fig. 11 and Table 3. The value of one the control samples was set to equal 1. *, P < 0.05.
Other Molecular Changes in Rb cKO Preantral Follicles
Inhibins and activins are TGFβ family members that have been shown to play essential functions in ovarian tumorigenesis and normal ovarian physiology, including the regulation of FSH secretion and granulosa cell steroidogenesis and proliferation (58). Inhibins are heterodimers of α-subunits (encoded by the Inha gene) and β-subunits (encoded by Inhba and Inhbb genes), whereas activins are homodimers/heterodimers of InhBA and InhBB subunits (13). Inhba and Inhbb are predominantly expressed by large preantral and small antral ovarian follicles (59); Inhba is regulated by FSH, whereas Inhbb is regulated by TGFβ1 and TGFβ2 and activins (58). Because young adult female Amh KO mice display high serum levels of inhibins, and we found low AMH levels and high levels of Tgfb2 and Fshr in Rb cKO follicles, we investigated the expression of Inhba and Inhbb in Rb cKO preantral follicles by QPCR analysis. Our results show that Rb cKO follicles have significantly higher levels of Inhbb (Table 3; n = 3; P < 0.05); however, no significant changes in Inhba levels were detected in Rb cKO follicles (Table 3). These results, together with the observation that Inha is also increased, suggest that Rb cKO mice may have increased production of both inhibin B and activin B.
Nuclear receptor subfamily 5, group A member 1 [NR5A1, also known as steroidogenic factor 1 (SF1)] and the structurally related protein NR5A2 [also known as liver receptor homolog 1 (LRH-1) and fetoprotein transcription factor (FTF)] are members of the evolutionarily conserved fushi tarazu F1 family of orphan nuclear hormone receptors. NR5A1/SF1 is essential for normal gonadal development and steroidogenesis and, in the adult ovary, is expressed in thecal and luteal cells as well as in granulosa cells from the primary stage onwards (60). In the postnatal ovary, NR5A2/LRH-1 is expressed in granulosa cells of follicles at all stages of development as well as in newly formed corpora lutea, and it has been shown to play a role in the regulation of steroidogenic enzymes (61,62). Both NR5A1/SF1 and NR5A2/LRH-1 are known to activate the transcription of Inha (63), and FSH increases Nr5a2/Lrh-1 expression (62,64). In addition, Amh expression is stimulated by NR5A1/SF1 (65,66). In this study, we showed that preantral follicles from Rb cKO females express low Amh levels, as well as high Inha and Fshr levels, suggesting that the expression of Nr5a genes might be altered in mutant follicles. Therefore, we evaluated Nr5a1/Sf1 and Nr5a2/Lrh-1 mRNA levels in Rb cKO preantral follicles by QPCR analysis. The results show that although Nr5a1/Sf1 expression is significantly lower in Rb cKO follicles compared with that of controls (Fig. 11A; n = 3; P < 0.05), Nr5a2/Lrh-1 levels were significantly increased in mutant follicles (Fig. 11B; n = 3; P < 0.05).
Figure 11.
Other Gene Expression Changes in Preantral Follicles from Control and Rb cKO Females
Real-time PCR analysis of mRNA from WT and Rb cKO (Rbflox/−, Amhr2Cre+) preantral follicles isolated from 12- d-old females. Preantral follicles were collected from three independent control and Rb cKO females. Average and sem are shown. A significant decrease was seen in the relative quantity (RQ) of Foxl2 (A), Cyp19a1 (B), and Nr5a1/Sf1(C), whereas a significant increase was observed in the levels of Nr5a2/Lrh-1 (D), Nr0b1/Dax-1 (E), and Fos (F) in Rb cKO preantral follicles. The value of one of the control samples was set to equal 1. *, P < 0.05.
FOXL2 is a member of forkhead/hepatocyte nuclear factor 3 family of transcription factors that is expressed in several cell types, including granulosa cells of small and medium size follicles and cumulus cells (67,68). Mutations leading to truncated forms of the FOXL2 protein (humans) or deletion of the Foxl2 gene (mouse) have been shown to cause premature ovarian failure (69,70). Although the phenotype of the Foxl2 KO models differs from that of the Rb cKO described herein (e.g. there is no eyelid defect in the Rb cKO and the squamous to cuboidal granulosa cell transition appears to be largely unaffected) (70,71), we found that various genes controlled either directly or indirectly by FOXL2, namely Amh, Fos, Nr5a1/Sf1, and Nr5a2/Lrh-1 (70,71,72) were also changed in our cKO model (see below). In addition, Cyp19a1 (the gene that encodes the aromatase enzyme responsible for estrogen production) has been reported to be regulated by Foxl2 (73) and Nr5a2/Lrh-1 (64). Therefore, we evaluated by QPCR the levels of Foxl2 and Cyp19a1 and other genes that have been shown to increase in the ovary in response to Foxl2 deletion, including Nr0b1/Dax-1 [nuclear receptor subfamily 0, group B, member 1/dosage-sensitive sex reversal, adrenal hypoplasia congenita critical region on chromosome X, gene 1 (Dax-1)], Fst (follistatin), Gata4 (GATA-binding protein 4) and Sox9 (Sry-box containing gene 9) (69,71). A significant decrease in Foxl2 and Cyp19a1 levels was observed in Rb cKO follicles (Fig. 11, C and D, respectively; n = 3; P < 0.05), whereas a significant increase was observed in the levels of Nr0b1/Dax-1 (Fig. 11E; n = 3; P < 0.05). In contrast, we found no differences in Sox9 (Table 3; P > 0.05), Gata4 (Table 4; P > 0.05), or Fst levels (WT, 1.03 ± 0.18, and Rb cKO, 1.09 ± 0.17; n = 3; P > 0.05) in ovaries from 3-wk-old females.
Table 4.
QPCR Primer Sequences
| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Amh | CGCTATTTGGTGCTAACCGTG | CTTGCAGCTGATCGATGCTCA |
| Tgfb2 | TTCGACGTGACAGACGCTGT | CAAATCTCGCCTCGAGCTCTT |
| Kitl | CGGGATGGATGTTTTGCC | CTTCCAGTATAAGGCTCC |
| E2f5 | TCACTCAGGGCCTATCCATGT | AGCAGCCATGGTGGATTTCT |
| Nr5a1/Sf1 | TCGTACGACGAGGACCTGG | TCTGACTCTCGGTGCACGTG |
| Foxl2 | GCCAAGTTCCCGTTCTACGA | TTCTCGAACATGTCCTCGCA |
| Gata4 | CCCCACAAGGCTATGCATCT | TCCGAGCAGGAATTTGAAGAG |
| Sox9 | ACATCTCTCCTAATGCTATCTTCA | AGCTTGCACGTCGGTTTT |
| Nr0b1/Dax-1 | GTTGAAGACCCTGCGCTTTG | CGGGATCTCCATCATCTCGA |
DISCUSSION
We have recently shown that deletion of Rb in mice carrying a mutation for the Inha gene, which causes granulosa cell tumors, leads to a modest decrease in survival rates and to a significant increase in both cell proliferation and apoptosis (29). However, the role of RB in reproductive tissues in the absence of other mutations remains uncharacterized. Taking advantage of cKO mouse and tissue-specific Cre recombination technologies, we investigated the role of RB in the ovary by generating a granulosa cell-specific KO mouse model. Our results show that Rb deletion in granulosa cells does not promote ovarian tumor development, at least up to 9 months of age; instead, Rb deficiency leads to premature ovarian failure (POF). Pituitary and thyroid gland tumors have been reported to occur in the Rb heterozygous mouse line in 10-month-old and older animals (28). Thus, analysis of tumor formation due to deletion of Rb in granulosa cells in mice older than 9 months of age might be obscured by the presence of tumors in these other two sites. We cannot rule out the possibility that extragonadal tumors detrimentally affect the fertility of older females. However, we think that it is unlikely that it causes POF in Rb cKO mice, because fertility defects are observed in very young (8-wk-old) females, and Rbflox/− females have normal fertility, despite the fact that they are equally susceptible to loss of heterozygosity for Rb. POF in the Rb cKO model appears to arise from a combination of altered hormone/growth factor input and abnormal gene expression, which causes an increase in ovarian follicular recruitment and atresia and leads to early depletion of the follicle pool (Fig. 12A).
Figure 12.
Summary of the Rb cKO Studies and RB Function in Granulosa Cells
A, Summary of gene expression changes in Rb cKO ovaries. RB can regulate transcription in a direct or indirect manner, through its interaction with other transcription factors. Direct targets of RB and E2F1 (left) include Tgfb2, E2f1, Myc and Fos, cyclin E2, Rbl2/p130, and E2f5. E2f1, Rbl2/p130, Myc, and E2f5 are known to cause cell cycle arrest and apoptosis, whereas increases in Fos and Ccne2 (cyclin E2) lead to increased cell proliferation. RB indirect transcriptional effects (right) in the ovary include the regulation of Nr5a1/Sf1, possibly via interaction with SP1, and of Kitl via unidentified mechanisms. Amh expression is also negatively affected, likely due to the decrease in Nr5a1/Sf1. The decrease in Amh and increase in Fos, in turn, appear to impact Fshr expression, which mRNA is also stabilized by TGFβ2. The combined effect of increased Fshr and Kitl expression as well as the decrease in Amh expression, leads to increased follicular recruitment. FSHR target genes Inha and Ccnd2 (cyclin D2) are also up-regulated in Rb cKO ovaries. It is unclear whether the observed decrease in Foxl2 is a direct or indirect effect of Rb deficiency; regardless of this, the decrease is likely to affect the expression of Cyp19a1 (decrease) and Nr0b1/Dax-1 (increase). The increase in Nr0b1 will counteract the increase in Nr5ab2/Lrh-1, which in combination with the decrease in Foxl2 and Nr5a1/Sf1 may help explain the lower levels of Cyp19a1 in mutant follicles. B, Conditional deletion of Rb leads to POF. In WT mice, primordial follicles are recruited into the growing pool. Multilayer preantral follicles are responsive to FSH, but early follicular development is under the control of other factors, which either directly or indirectly promote (e.g. KITL) or inhibit (e.g. AMH) follicle growth. Growing follicles are rescued from atresia by a rise in FSH levels and continue to grow until the preovulatory stage, when a surge of LH induces the ovulation of fertilizable gametes. In the Rb cKO model, an increase in Kitl and decrease in Amh levels increases the number of growing follicles. Other molecular changes may turn the ovarian follicles more susceptible to cell death, which in combination with lower FSH levels at estrus, results in increased follicular atresia, lower number of viable gametes and births, early depletion of the follicle pool, and POF. Increased follicular atresia in this model may arise from increased E2f1 levels, which are known to induce apoptosis, and possibly E2f5 and Rbl2/p130. Based on our results, we propose that RB in granulosa cells is necessary for the correct temporal expression of genes required for follicular development and to prevent apoptosis, whereas the cell cycle functions of RB can be compensated by other RB family members.
AMH is a TGFβ family member that regulates both primordial follicle recruitment and FSH responsiveness of growing follicles (41). Amh KO females show increased follicular recruitment and lower levels of FSH at estrus. Despite the increased sensitivity of AMH null ovaries to FSH, females have normal litter sizes and ovulate normal numbers of oocytes due to an increase in follicular atresia, which occurs primarily at the small to large preantral transition (42,43). The Rb cKO model has striking similarities with the Amh KO model in terms of follicle and oocyte loss; however, and as expected from the pleiotropic functions of RB, the phenotype of Rb cKO mice is more severe, showing a significant decrease in litter size from the first month of mating. Analysis of estrous cycle length and periodicity indicates that Rb cKO females have longer cycles and spend more time in diestrus. Over time, the majority of the females reached estrus only occasionally and became essentially acyclic; this is in agreement with the significant reduction in the number of litters per month observed in the cKO group. Four lines of evidence suggest that despite the findings that more follicles are being recruited into the growing pool in Rb cKO ovaries, fewer are being rescued by the FSH surge. First, we found that although twice as many oocytes were ovulated by 3-wk-old Rb cKO females in response to exogenous gonadotropins, 6-wk-old Rb cKO and control females had a similar response to superovulation; second, a reduced number of pups was produced by Rb cKO females from the first month of breeding; third, an increase in follicular atresia and oocyte (zona pellucida) remnants was observed after 6 wk of age; and fourth, similar to the situation observed in Amh KO mice (43), FSH levels at estrus were significantly reduced in 8-wk-old Rb cKO females. Despite these abnormalities, some follicles successfully escape cell death. Mislocalization of factors (others than AMH or inhibin-α) and/or the degree of chimerism for Cre expression (32) may positively contribute to the survival of those follicles.
Gene expression analysis showed that Fshr and the FSH targets cyclin D2 (Ccnd2) and inhibin-α (Inha), are increased in Rb cKO preantral follicles, indicating that the increase in Fshr mRNA levels correlates with the production of functional FSHR protein (Fig. 12B). Only a few factors have been shown to up-regulate FSHR expression including FSH (species-specific), TGFβ, and activins (74). The rat FSHR promoter contains several regulatory elements, which include binding sites for activation protein-1 (JUN/FOS), NR5A1/SF1, and E2F, although E2F1 does not appear to bind the E2F site (75). RB does not seem to directly regulate the Fshr promoter; instead, our results suggest that the observed increase in Fshr levels is the result of changes in the levels of secreted factors, including activins, AMH, and TGFβ, as well as the levels of the transcriptional regulators FOS and NR5A1/SF1. First, the expression of Inhbb, which encodes one of the two β-subunits of activins and inhibins, is increased in Rb cKO follicles. Activins have been previously reported to regulate both Fshr and Ccnd2 expression, although studies in Inhbb and Inhba double-KO granulosa cells suggest that activins have no direct effect and that they act by enhancing FSH actions (33). Second, Rb cKO follicles show low levels of Amh and increased sensitivity to FSH as has been reported in Amh KO mice, albeit the molecular bases for this phenomenon have not been investigated (41).Third, Tgfb2 expression is increased in Rb cKO follicles, and previous reports show that both TGFβ1 and TGFβ2 increase Fshr mRNA stability and the number of FSH-binding sites in rat granulosa cells (51,52). The increase in Tgfb2 levels may result from the loss of Rb because it has been shown that human Tgfb2 is a direct target of RB (76). However, RB regulation of the mouse Tgfb2 promoter awaits determination. Alternatively, the increase in Tgfb2 expression observed in Rb cKO follicles may be the result of decreased Amh levels, because recent studies show that AMH down-regulates Tgfb2 mRNA in rat follicles (77). The results presented herein show a significant decrease in Nr5a1/Sf1 levels, which appear to contradict the findings that NR5A1/SF1 up-regulates Fshr expression (78,79). This disparity might be explained by the fact that although NR5A1/SF1 increases Fshr expression, it is not required for it (80), and that changes in the levels of other transcription factors known to bind the Fshr promoter (i.e. Fos, see below) may compensate for the decrease in Nr5a1/Sf1. Alternatively, the increase in Fshr mRNA could be primarily due to posttranscriptional events (i.e. increased stability) with a minor contribution from transcriptional activation.
Our analysis also shows changes in Nr5a2/Lrh-1, another member of the NRA5 family of orphan receptors (81). NR5A1/SF1 and NR5A2/LRH-1 share a high degree of homology, particularly in their DNA-binding domains, and can recognize the same DNA sequences (81). The observed up-regulation of Nr5a2/Lrh-1 in Rb cKO follicles is in agreement with studies in rat granulosa cells, in which activation of FSHR downstream signaling pathway has been shown to increase NR5A2/LRH-1 levels (64). The increase in Inha expression observed in the Rb cKO mouse model is also in agreement with the hypothesis that in ovarian granulosa cells, there is a switch in the occupancy of the Inha proximal promoter from NR5A1/SF1 in the basal state to NR5A2/LRH-1 upon FSH stimulation and that this switch stimulates transcription (63).
Two important regulators of early follicular development, namely AMH and KITL, were deregulated in Rb cKO follicles (Fig. 10A). KITL, through its interaction with its receptor KIT, has been shown to be important for primordial follicle activation, granulosa cell proliferation, and oocyte growth (37,82). The increase in Kitl expression in Rb cKO ovaries can be attributed to the increased responsiveness of mutant follicles to FSH because FSH has been shown to up-regulate Kitl in granulosa cells (83). Amh expression has been previously reported to be under the control of various factors including NR5A1/SF1 and the RB-interacting partner, trans-acting transcription factor 1 (SP1) (65,66,84). The decrease in Amh levels observed in the Rb cKO can be explained at least in part by the decrease in Nr5a1/Sf1. The Nr5a1/Sf1 promoter itself appears to be under the control of SP1 (85), a factor capable of binding both SP1 sites and promoter elements regulated by RB (86,87). Interestingly, RB-SP1 interaction results in superactivation of SP1-mediated transcription (88,89), and thus, it is likely that RB deficiency will have a negative impact on the expression of SP1-dependent targets in granulosa cells.
FOXL2 is another factor expressed during early folliculogenesis and is necessary for follicular quiescence (69,71). Some of the changes in gene expression that we found in the Rb cKO model, namely Amh down-regulation and Nr0b1/Dax-1 up-regulation (see below) (71), were similar to those found in the Foxl2 KO, which prompted us to evaluate Foxl2 levels in our cKO model. Although we found a significant decrease in Foxl2 expression, other genes reportedly altered in Foxl2 KO ovaries (71), including Fst, Gata-4, and Sox-9, were unchanged. The decrease in Amh observed in the Foxl2 mutant is likely to arise from the absence of growing, multilayer follicles, which produce AMH, although a direct effect on transcription cannot be discarded. To our knowledge, direct binding of RB or E2F to the Foxl2 promoter has not been reported, and thus it is unclear whether the observed down-regulation is a direct or indirect effect of Rb loss. Fos, Nr5a1/Sf1, and Nr5a2/Lrh-1 have been reported to increase after Foxl2 overexpression in KGN cells (human steroidogenic granulosa-like cell line) (72). Our results are clearly in contrast to these findings; a likely explanation for this is that Rb deficiency may affect the expression/interaction of coactivators and/or repressors required for the expression of such factors. A good example of this is Fos, which has been shown to be up-regulated by FOXL2 (72) but repressed by RB (see below).
Our studies also demonstrate a decrease in Cyp19a1 (P450 aromatase) levels. Aromatase is the enzyme responsible for estrogen production and is present in small ovarian follicles, although larger preovulatory follicles show the highest levels of expression (90). Nr5a2/Lrh-1 has been reported to up-regulate Cyp19a1 levels in the ovary (60,64) and other tissues (91,92); therefore, the decrease in Cyp19a1 expression found in Rb cKO preantral follicles appears to contradict these reports. These disparities might be explained by the concomitant decrease in Foxl2 and increase in Nr0b1/Dax-1 levels observed in our model. Foxl2 has been show to activate P450 aromatase expression in KGN cells and in goat and fish ovaries (72,73,93). Although similar studies have not been carried out in the mouse, it is possible that the decrease of Foxl2 in Rb cKO ovaries may contribute to Cyp19a1 down-regulation.
Nr0b1/Dax-1 is an orphan nuclear receptor that, in the ovary, is expressed in both thecal and granulosa cells. In humans, duplications of chromosome regions containing the DAX-1 gene cause phenotypic sex reversal in XY individuals, whereas DAX-1 gene mutations cause adrenal hypoplasia congenitally associated with hypogonadotropic hypogonadism (94). NR0B1/DAX-1 interacts with a variety of receptors, including NR5a1/SF1, androgen receptor, progesterone receptor, estrogen receptors-α and -β (ESR1 and ESR2), and NR0B2/LRH-1, impairing their transactivation activity (94,95,96,97). A few factors have been reported to regulate Nr0b1/Dax-1 expression, including NR0B1/DAX-1 itself, Wilm’s tumor suppressor gene 1 (Wt1) and β-catenin (94,98). In addition, NR5a1/SF1 has been shown to increase Nr0b1/Dax-1 expression, although it is not essential for it (94). Importantly, NR0B1/DAX-1 inhibits, directly or indirectly, the expression of Amh and Cyp19a1 (94). Thus, our studies suggest that the high levels of Nr5a2/Lrh-1 observed in the Rb cKO model will be counteracted by the simultaneous increase in Nr0b1/Dax-1, which ultimately will have a negative impact in the expression of some NR5A1/LRH-1 target genes, as shown for Cyp19a1 (also regulated by FOXL2). The fact that Inha, another Nr5a2/Lrh-1 target, is not decreased might be explained by the differential regulation of the promoters by other factors, which remain unchanged in the Rb cKO model (e.g. GATA4). Despite the fact that the expression of Cyp19a1 is decreased per ovarian follicle in Rb cKO mice, randomly cycling females show normal levels of serum estrogens. These findings are puzzling because FSH and LH levels in Rb cKO females were also comparable to those of control mice. However, it is possible that evaluation of hormone levels at particular stages of the estrous cycle will unmask differences in the mutant females, as we showed for FSH at estrous.
Finally, RB has been shown to repress the expression of the early-response (Fos) and delayed-response (Myc) genes and in various mammalian cell types. In agreement with this, RB deficiency in ovarian granulosa cells leads to a significant increase in both transcripts. Additionally, E2F1 and MYC have been shown to increase each other’s transcription (99), and MYC has been shown to up-regulate Ccnd2 transcription (100). Thus, factors required for the G1 to S transition of the cell cycle, including E2F1, cyclin D2, cyclin E, FOS, and MYC are increased in Rb cKO follicles as expected (Fig. 10A). However, the fact that increases in Myc and E2f1 can trigger apoptosis (99,101), together with the up-regulation of Rbl2/p130 and E2f5, which can control cell cycle progression, may help explain why Rb cKO females do not develop ovarian tumors and show increased follicular atresia (Fig. 12A). It is interesting to note that some of the cell cycle-related genes that were altered in Rb cKO ovaries were also deregulated in Inha/Rb double-mutant tumors (102) and that, as in Rb cKO, a high rate of apoptosis was observed in those tumors, despite the concomitant increase in mitotic figures, which appears to arise from the combination of both mutations (102). In fact, in the absence of secondary mutations, tumor development has been observed only in a fraction of the mouse models in which Rb has been conditionally deleted (28,103,104,105), whereas defects in cell differentiation are more frequently found (106,107). It is well established that the default path of the majority of growing follicles is death; thus, it is tempting to speculate that Rb deficiency will increase the susceptibility of the follicles to undergo apoptosis as reported for other cell types (108). In the context of the Rb cKO ovary, it is likely that the altered hormonal environment will contribute to such a negative outcome.
In summary, Rb deletion in granulosa cells causes increased follicular recruitment by altering the expression of both positive and negative regulators of follicle activation (Fig. 12B).We hypothesize that Rb cKO follicles display an increased susceptibility to apoptosis due to changes in gene expression, which in combination with lower FSH levels at estrus, suggest that follicles fail to be rescued by the FSH surge, resulting in a reduced number of embryos produced by cKO females in the absence of exogenous gonadotropins (Fig. 12B). Our results also imply that whereas the role of RB in the cell cycle might be partially compensated by RB family members (especially RBL2/P130), the unique functions of RB as a transcriptional regulator are abolished in the Rb cKO granulosa cells (Fig. 12, A and B). Taken together, our results suggest that RB is required for the temporal-specific pattern of expression of key granulosa cell genes involved in follicular development.
MATERIALS AND METHODS
Generation and Genotyping Rb cKO Mice
The Rb null allele (Rbtm1Tyj) (designated throughout Rb+/−), and the Rb conditional allele (Rb1tm2Brn) (designated throughout as Rbflox) have been previously characterized (28,104) and were maintained on a C57BL/6J;129S5/SvEvBrd mixed hybrid background. The Rb null allele was generated by insertion of a PGK-neo cassette and 3-bp changes in exon 3, which introduces two stop codons and renders a truncated RB. The Rb conditional allele creates a truncated protein by deletion of exon 19, which is functionally equivalent to the null allele (104,109). Mice were genotyped from tail genomic DNA using PCR primers as described (104). To generate Rb-deficient granulosa cells, Rb+/− mice were bred to Amhr2cre+ mice. Rb+/−;Amhr2cre+ mice were then bred to Rbflox/flox mice, to create Rbflox/−;Amhr2cre+ (experimental, designated throughout as Rb cKO) and Rbflox/−;Amhr2cre− (designated throughout as Rbflox/−) mice (Fig. 1A). Recombination of the Rb conditional allele in granulosa cells was confirmed in genomic DNA and cDNA from granulosa cells using PCR primers as previously described (29).
Fertility, Weight, and Survival Studies of Rb cKO Female Mice
Mice were weighed once per week for a period of 4–14 wk and were monitored for signs of cachexia or distress, which can be used as indicators of tumor development. Survival of the mice was also recorded. To evaluate reproductive performance, ten 6-wk-old Rb cKO and Rbflox/− individually caged females were bred to WT C57BL/6J;129S5/SvEvBrd hybrid males of known fertility. The number of litters and the number of pups were recorded over a 6-month period.
Serum Analysis and Estrous Cycle Length
To evaluate estrous cycle periodicity and length, daily vaginal smears were taken from Rbflox/− and Rb cKO females at 8 and 12 wk of age (same group of six females was used for the two time points) for a continuous period of 3 wk. Smears were examined under the microscope and staged as previously described (42). To evaluate hormone serum levels, randomly cycling mice of various ages were anesthetized by isoflurane inhalation (Abbott Laboratories, North Chicago, IL), and blood was collected by cardiac puncture. In a separate set of experiments, samples from 8-wk-old females (n = 5) were taken at estrus to determine FSH levels. Serum was prepared using microtainer tubes (Becton and Dickinson, Franklin Lakes, NJ) and stored at −20 C until assayed. FSH, LH, and estradiol levels were assayed by the University of Virginia Ligand Core Facility (Specialized Cooperative Center Program in Reproductive Research National Institute of Child Health and Human Development/National Institutes of Health U54 HD28934) as described. Assay information is available at http://www.healthsystem.virginia.edu/internet/crr/ligand.cfm. Sensitivity and coefficients of variation for these assays have been previously reported (32).
Histological and Morphometric Analyses
For histological and morphometric studies, ovaries from females of various ages were collected. For each mouse, one ovary was snap frozen for RNA or protein extraction, and the other ovary was fixed overnight in 10% neutral buffered formalin and embedded following standard protocols. Five-micrometer sections were stained with the periodic acid Schiff reaction and hematoxylin. Follicle classification was based on Pedersen and Peters (110). Four to five ovaries were analyzed per genotype and per age. Ovaries were serially sectioned, and every 10th section was kept. Follicles were counted from five of the largest sections and normalized to the total area of the section as previously described (32). Measurements were collected using the AxionVision 4.0 software (Carl Zeiss, Jena, Germany) and plotted as average number of follicles per square millimeter.
Superovulation and Oocyte Immunostaining
Superovulation experiments were carried out as described (111). Four to five 21-d-old or 6-wk-old females were given ip injections of 5 IU pregnant mare serum gonadotropin (Calbiochem, La Jolla, CA) for 46 h followed by an injection with 5 IU Novarel (Ferring Pharmaceuticals, Parsippany, NJ). Cumulus-oocyte complexes were isolated from the ampulla 16 h after Novarel and collected in M2 medium (Sigma Chemical Co., St. Louis, MO) containing 1 mg/ml hyaluronidase (Sigma) to dissociate cumulus cells. The number of retrieved oocytes per female was recorded; oocytes were then collected and fixed in 2% formalin for 30 min at room temperature. Immunostaining of oocytes was performed as previously described (112) using a monoclonal antibody against β-tubulin (Sigma; T4026) to visualize the meiotic spindle and propidium iodine (Vectashield with propidium iodine mounting medium; Vector Laboratories, Burlingame, CA) to visualize chromatin. Analysis of meiosis spindle morphology and collection of digital images from uncompressed oocytes was performed using a Zeiss LSM 5 Pascal confocal microscope with a ×40 Plan-Apo objective and LSM 5 software. The percentage of oocytes showing abnormal spindles and/or chromosome alignment was recorded.
TUNEL Staining
Analysis of apoptosis in 6-wk-old ovaries was carried out by TUNEL assay using the ApopTag Plus fluorescein in situ apoptosis detection kit (catalog no. S7111; Chemicon International, Temecula, CA). Ovaries were fixed in 10% formalin and embedded, and three consecutive 5-μm-thin sections were placed onto one slide. At least five different specimens from Rb cKO and WT ovaries were analyzed in parallel. TUNEL assay was performed according to the manufacturer’s instructions, and slides were mounted in Vectashield (Vector) containing propidium iodine to visualize chromatin. Slides were analyzed by confocal microscopy. Total and TUNEL-positive follicles and cell numbers were quantified in 10 high-power fields (HPFs) (×40) per section, and three individual sections were quantified per specimen. The number of follicles showing four or more TUNEL-positive cells was also recorded. Results are presented as the average of the percentage of positive cells per total cell number per HPF (apoptotic index) ± sem and the average number of TUNEL-positive follicles per section ± sem.
Preantral Follicle Isolation
Ovaries from 12- or 13-d-old individual females were isolated in collection medium [DMEM/F12 medium (Invitrogen, Carlsbad, CA) containing 0.3% BSA (Sigma)] and incubated in dissociation medium (DMEM/F12 medium containing 0.3% BSA, 1 mg/ml collagenase-Dispase (Roche, Indianapolis, IN), and deoxyribonuclease (Sigma)] for 1 h at 37 C as previously described (102). Briefly, ovaries were pipetted in dissociation medium 50 times to release the follicles and then filtered through a 40-μm nylon mesh (Nalgene, Rochester, NY). Isolated follicles and debris were recovered from the filter with collection medium. Follicle size was evaluated under a stereomicroscope, and at least 50 preantral follicles devoid of thecal cells were collected per female. Follicles were snap frozen for further use in RNA or protein extractions.
PCR and QPCR Analyses
Total RNA from preantral follicles or ovaries from 12- or 21-d-old females was reverse transcribed in a 50-μl reaction using 250 IU Superscript II reverse transcriptase (Invitrogen) and random primers (Invitrogen). Samples were used undiluted (1 μl) for each PCR or diluted 10-fold, and 10 μl was used for each QPCR. Real-time QPCR was performed on the ABI Prism 7500 Sequence Detection System [Applied Biosystems (ABI), Foster City, CA] using predesigned TaqMan Assays-On-Demand (ABI) PCR primer and probe sets and mouse Gapd as an endogenous control. The following TaqMan assays were used: cyclin E2 (Ccne2), Mm00438077; cyclin D2 (Ccnd2), Mm00438071; inhibin-α (Inha), Mm00439683; inhibin-βa (Inhba), Mm00434338; inhibin-βb (Inhbb), Mm03023992; FSHR (Fshr), Mm00442819; LH receptor (Lhcgr), Mm00442931; nNR5a2/LRH-1 (Nr5a2/Lrh-1), Mm00446088; Cyp19a1, Mm00484049; follistatin (Fst), Mm00514982; and Gapd (4352339E, primer limited). TaqMan PCR was performed using the TaqMan Universal PCR Master Mix (ABI) in a 20-μl reaction. Primers against E2f transcription factor 1 (E2f1), Rb1 exon 19, Rbl1/p107, and Rbl2/p130 were the same as we recently reported (29). Primer sequences against E2f transcription factor 5 (E2f5), KITL (Kitl), NR5a1/SF1 (Nr5a1/Sf1), AMH (Amh), TGFβ2 (Tgfb2), and FOXL2 (Foxl2) are described in Table 4. Ten-fold serial dilutions were used to determine amplification efficiency for each primer set. Reaction conditions were the same reported in Pangas et al. (32). The relative amount of transcript was calculated by the cycle threshold method as described by ABI using the ABI 7500 System Software (version 1.2.3) and normalized to the endogenous reference (Gapd). The calibrator sample was randomly chosen from the WT samples. The relative amount of target gene expression for each sample was calculated and plotted as the average ± sem.
Western Blot Analysis
Lysates from isolated preantral follicles were subjected to electrophoresis through a 15% sodium dodecyl sulfate-polyacrylamide gel at 100 V. Immunoblotting was performed using a polyclonal antibody raised against hormone (AMH, 1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were reprobed with a 1:1000 dilution of mouse anti-β-tubulin (Sigma; T4026) to verify equivalent loading of the samples. Bands were visualized using the Supersignal West Pico chemiluminescence substrate (Pierce, Rockford, IL) and quantified by densitometry using Image J software (National Institutes of Health, Bethesda, MD). After quantification, the concentration of each target protein was normalized against β-tubulin and plotted as the average ratio ± sem. A minimum of three samples per genotype was analyzed in three independent experiments.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded 4-μm-thick sections using the Vectastain ABC kits (Vector) according to the manufacturer’s instructions. Polyclonal antibodies against AMH and RBL2 were purchased from (Santa Cruz Biotechnology). The rabbit polyclonal anti-inhibin-α antibody was a gift from W. Vale (The Salk Institute, La Jolla, CA). Immunoreactivity was visualized by diaminobenzidine and counterstained in hematoxylin. For direct comparisons, control and experimental ovary sections were placed on the same slide and processed together.
Statistical analysis
Statistical analysis was carried out using the JMP version 5.1 statistical package (SAS Software, Cary, NC). Statistical differences were tested using the nonparametric Mann-Whitney U test for single comparisons or Kruskal-Wallis analysis of ranks test for multiple comparisons. P < 0.01 and P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Richard Behringer for the gift of the Amhr2-Cre knockin transgenic mice and Dr. Diego Vieyra for critical reading of the manuscript.
Footnotes
This work has been supported by National Institutes of Health Grant CA60651 (to M.M.M.) and a grant from the Lalor Foundation (to C.A.-V.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 3, 2008
Abbreviations: AMH, Anti-Müllerian hormone; CDN, cyclin-dependent kinase inhibitor; cKO, conditional knockout; FOXL2, Forkhead box L2; FSHR, FSH receptor; HPF, high-power field; KITL, Kit ligand; LRH-1, liver receptor homolog 1; NR5A1, nuclear receptor subfamily 5, group A member 1; POF, premature ovarian failure; QPCR, quantitative real-time PCR; RB, retinoblastoma protein; RBL, RB-like; SF1, steroidogenic factor 1; SP1, trans-acting transcription factor 1; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling.
References
- Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A 1999 The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev 20:501–534 [DOI] [PubMed] [Google Scholar]
- Classon M, Harlow E 2002 The retinoblastoma tumour suppressor in development and cancer. Nat Rev 2:910–917 [DOI] [PubMed] [Google Scholar]
- Nevins JR 2001 The Rb/E2F pathway and cancer. Hum Mol Genet 10:699–703 [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC 2000 The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 14:2393–2409 [DOI] [PubMed] [Google Scholar]
- Elledge SJ, Winston J, Harper JW 1996 A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol 6:388–392 [DOI] [PubMed] [Google Scholar]
- Delston RB, Harbour JW 2006 Rb at the interface between cell cycle and apoptotic decisions. Curr Mol Med 6:713–718 [DOI] [PubMed] [Google Scholar]
- Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T 1996 Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev 10:1633–1644 [DOI] [PubMed] [Google Scholar]
- Nguyen DX, McCance DJ 2005 Role of the retinoblastoma tumor suppressor protein in cellular differentiation. J Cell Biochem 94:870–879 [DOI] [PubMed] [Google Scholar]
- Wikenheiser-Brokamp KA 2006 Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell Mol Life Sci 63:767–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossel MJ, Hinds PW 2006 Beyond the cell cycle: a new role for Cdk6 in differentiation. J Cell Biochem 97:485–493 [DOI] [PubMed] [Google Scholar]
- Korenjak M, Brehm A 2005 E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev 15:520–527 [DOI] [PubMed] [Google Scholar]
- Sellers WR, Novitch BG, Miyake S, Heith A, Otterson GA, Kaye FJ, Lassar AB, Kaelin Jr WG 1998 Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev 12:95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM 2000 Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol 163:61–66 [DOI] [PubMed] [Google Scholar]
- Pangas SA 2007 Growth factors in ovarian development. Semin Reprod Med 25:225–234 [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Treier M 2006 Foxl2 function in ovarian development. Mol Genet Metab 88:225–234 [DOI] [PubMed] [Google Scholar]
- Gruijters MJ, Visser JA, Durlinger AL, Themmen AP 2003 Anti-Mullerian hormone and its role in ovarian function. Mol Cell Endocrinol 211:85–90 [DOI] [PubMed] [Google Scholar]
- Rolaki A, Drakakis P, Millingos S, Loutradis D, Makrigiannakis A 2005 Novel trends in follicular development, atresia and corpus luteum regression: a role for apoptosis. Reprod Biomed Online 11:93–103 [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 12:924–940 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA 1996 Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384:470–474 [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A 1996 Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 85:721–732 [DOI] [PubMed] [Google Scholar]
- Jirawatnotai S, Moons DS, Stocco CO, Franks R, Hales DB, Gibori G, Kiyokawa H 2003 The cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem 278:17021–17027 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Yogo K, Ishida N, Takeya T 2003 Synergistic effects of activin and FSH on hyperphosphorylation of Rb and G1/S transition in rat primary granulosa cells. Mol Cell Endocrinol 210:31–38 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A 1992 α-Inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360:313–319 [DOI] [PubMed] [Google Scholar]
- Burns KH, Agno JE, Sicinski P, Matzuk MM 2003 Cyclin D2 and p27 are tissue-specific regulators of tumorigenesis in inhibin α knockout mice. Mol Endocrinol 17:2053–2069 [DOI] [PubMed] [Google Scholar]
- Cipriano SC, Chen L, Burns KH, Koff A, Matzuk MM 2001 Inhibin and p27 interact to regulate gonadal tumorigenesis. Mol Endocrinol 15:985–996 [DOI] [PubMed] [Google Scholar]
- Scambia G, Lovergine S, Masciullo V 2006 RB family members as predictive and prognostic factors in human cancer. Oncogene 25:5302–5308 [DOI] [PubMed] [Google Scholar]
- Lazzerini Denchi E, Helin K 2005 E2F1 is crucial for E2F-dependent apoptosis. EMBO Rep 6:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA 1992 Effects of an Rb mutation in the mouse. Nature 359:295–300 [DOI] [PubMed] [Google Scholar]
- Andreu-Vieyra C, Chen R, Matzuk MM 2007 Effects of granulosa cell-specific deletion of Rb in Inha-α null female mice. Endocrinology 148:3837–3849 [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR 2003 Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol 211:15–19 [DOI] [PubMed] [Google Scholar]
- Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM 2004 Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol 18:953–967 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM 2006 Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol 20:1406–1422 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, Kumar TR, Matzuk MM 2007 Intraovarian activins are required for female fertility. Mol Endocrinol 21:2458–2471 [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM 2008 Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 28:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM 2003 Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHβ subunit knockout mice. Reproduction 125:165–173 [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, McLelland D, McBride MW 1997 Regulation of luteinizing hormone-receptor and follicle-stimulating hormone-receptor messenger ribonucleic acid levels during development in the neonatal mouse ovary. Biol Reprod 57:602–608 [DOI] [PubMed] [Google Scholar]
- Thomas FH, Vanderhyden BC 2006 Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol 4:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK 2005 Regulation of primordial follicle assembly and development. Human Reprod Update 11:461–471 [DOI] [PubMed] [Google Scholar]
- Ismail RS, Okawara Y, Fryer JN, Vanderhyden BC 1996 Hormonal regulation of the ligand for c-kit in the rat ovary and its effects on spontaneous oocyte meiotic maturation. Mol Reprod Dev 43:458–469 [DOI] [PubMed] [Google Scholar]
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA 1995 Anti-Mullerian hormone and anti-Mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136:4951–4962 [DOI] [PubMed] [Google Scholar]
- Visser JA, Themmen AP 2005 Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol 234:81–86 [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP 1999 Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140:5789–5796 [DOI] [PubMed] [Google Scholar]
- Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, de Jong FH, Themmen AP 2007 Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology 148:2301–2308 [DOI] [PubMed] [Google Scholar]
- Zhu L 2005 Tumour suppressor retinoblastoma protein Rb: a transcriptional regulator. Eur J Cancer 41:2415–2427 [DOI] [PubMed] [Google Scholar]
- Classon M, Dyson N 2001 p107 and p130: versatile proteins with interesting pockets. Exp Cell Res 264:135–147 [DOI] [PubMed] [Google Scholar]
- Robbins PD, Horowitz JM, Mulligan RC 1990 Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature 346:668–671 [DOI] [PubMed] [Google Scholar]
- Moses HL, Yang EY, Pietenpol JA 1990 TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 63:245–247 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee HD, Robbins PD, Busam K, Sporn MB, Roberts AB 1991 Regulation of transforming growth factor β1 gene expression by the product of the retinoblastoma-susceptibility gene. Proc Natl Acad Sci USA 88:3052–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglieri C, Khatchadourian C, Tabone E, Hendrick JC, Benahmed M, Menezo Y 1995 Immunolocalization of transforming growth factor-β1 and transforming growth factor-β2 in the mouse ovary during gonadotrophin-induced follicular maturation. Hum Reprod 10:2115–2119 [DOI] [PubMed] [Google Scholar]
- Gueripel X, Benahmed M, Gougeon A 2004 Sequential gonadotropin treatment of immature mice leads to amplification of transforming growth factor β action, via upregulation of receptor-type 1, Smad 2 and 4, and downregulation of Smad 6. Biol Reprod 70:640–648 [DOI] [PubMed] [Google Scholar]
- Dunkel L, Tilly JL, Shikone T, Nishimori K, Hsueh AJ 1994 Follicle-stimulating hormone receptor expression in the rat ovary: increases during prepubertal development and regulation by the opposing actions of transforming growth factors β and α. Biol Reprod 50:940–948 [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakamura K, Abe K, Hirakawa T, Tsuchiya M, Oomori Y, Matsuda H, Miyamoto K, Minegishi T 2003 Mechanisms of action of transforming growth factor β on the expression of follicle-stimulating hormone receptor messenger ribonucleic acid levels in rat granulosa cells. Biol Reprod 69:1238–1244 [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Sundfeldt K, Hedin L 1997 The expression of c-myc during follicular growth and luteal formation in the rat ovary in vivo. J Endocrinol 152:395–406 [DOI] [PubMed] [Google Scholar]
- Delidow BC, White BA, Peluso JJ 1990 Gonadotropin induction of c-fos and c-myc expression and deoxyribonucleic acid synthesis in rat granulosa cells. Endocrinology 126:2302–2306 [DOI] [PubMed] [Google Scholar]
- Ness JM, Kasson BG 1992 Gonadotropin regulation of c-fos and c-jun messenger ribonucleic acids in cultured rat granulosa cells. Mol Cell Endocrinol 90:17–25 [DOI] [PubMed] [Google Scholar]
- Sharma SC, Richards JS 2000 Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. Relation of JunD and Fra2 to terminal differentiation. J Biol Chem 275:33718–33728 [DOI] [PubMed] [Google Scholar]
- Popov B, Chang LS, Serikov V 2005 Cell cycle-related transformation of the E2F4-p130 repressor complex. Biochem Biophys Res Commun 336:762–769 [DOI] [PubMed] [Google Scholar]
- Thompson TB, Cook RW, Chapman SC, Jardetzky TS, Woodruff TK 2004 βA versus βB: is it merely a matter of expression? Mol Cell Endocrinol 225:9–17 [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM 1999 Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol 13:1018–1034 [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Kamat A 2007 Mechanisms in the regulation of aromatase in developing ovary and placenta. J Steroid Biochem Mol Biol 106:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerboom D, Pilon N, Behdjani R, Silversides DW, Sirois J 2000 Expression and regulation of transcripts encoding two members of the NR5A nuclear receptor subfamily of orphan nuclear receptors, steroidogenic factor-1 and NR5A2, in equine ovarian cells during the ovulatory process. Endocrinology 141:4647–4656 [DOI] [PubMed] [Google Scholar]
- Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS 2003 Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144:3598–3610 [DOI] [PubMed] [Google Scholar]
- Weck J, Mayo KE 2006 Switching of NR5A proteins associated with the inhibin α-subunit gene promoter after activation of the gene in granulosa cells. Mol Endocrinol 20:1090–1103 [DOI] [PubMed] [Google Scholar]
- Yu FQ, Han CS, Yang W, Jin X, Hu ZY, Liu YX 2005 Activation of the p38 MAPK pathway by follicle-stimulating hormone regulates steroidogenesis in granulosa cells differentially. J Endocrinol 186:85–96 [DOI] [PubMed] [Google Scholar]
- Rey R, Lukas-Croisier C, Lasala C, Bedecarras P 2003 AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 211:21–31 [DOI] [PubMed] [Google Scholar]
- Arango NA, Lovell-Badge R, Behringer RR 1999 Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99:409–419 [DOI] [PubMed] [Google Scholar]
- Moumne L, Batista F, Benayoun BA, Nallathambi J, Fellous M, Sundaresan P, Veitia RA 2008 The mutations and potential targets of the forkhead transcription factor FOXL2. Mol Cell Endocrinol 282:2–11 [DOI] [PubMed] [Google Scholar]
- Pisarska MD, Bae J, Klein C, Hsueh AJ 2004 Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology 145:3424–3433 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M 2004 The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933–942 [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G 2004 Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13:1171–1181 [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D 2005 Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 14:2053–2062 [DOI] [PubMed] [Google Scholar]
- Batista F, Vaiman D, Dausset J, Fellous M, Veitia RA 2007 Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. Proc Natl Acad Sci USA 104:3330–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannetier M, Fabre S, Batista F, Kocer A, Renault L, Jolivet G, Mandon-Pepin B, Cotinot C, Veitia R, Pailhoux E 2006 FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development. J Mol Endocrinol 36:399–413 [DOI] [PubMed] [Google Scholar]
- Findlay JK, Drummond AE 1999 Regulation of the FSH receptor in the ovary. Trends Endocrinol Metab 10:183–188 [DOI] [PubMed] [Google Scholar]
- Heckert LL, Griswold MD 2002 The expression of the follicle-stimulating hormone receptor in spermatogenesis. Recent Prog Horm Res 57:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Wagner S, Liu F, O’Reilly MA, Robbins PD, Green MR 1992 Retinoblastoma gene product activates expression of the human TGF-β2 gene through transcription factor ATF-2. Nature 358:331–334 [DOI] [PubMed] [Google Scholar]
- Nilsson E, Rogers N, Skinner MK 2007 Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction 134:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL 2001 Activation of the rat follicle-stimulating hormone receptor promoter by steroidogenic factor 1 is blocked by protein kinase A and requires upstream stimulatory factor binding to a proximal E box element. Mol Endocrinol 15:704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Heckert LL 2007 Transcriptional regulation of the FSH receptor: new perspectives. Mol Cell Endocrinol 260–262:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levallet J, Koskimies P, Rahman N, Huhtaniemi I 2001 The promoter of murine follicle-stimulating hormone receptor: functional characterization and regulation by transcription factor steroidogenic factor 1. Mol Endocrinol 15:80–92 [DOI] [PubMed] [Google Scholar]
- Fayard E, Auwerx J, Schoonjans K 2004 LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol 14:250–260 [DOI] [PubMed] [Google Scholar]
- Hutt KJ, McLaughlin EA, Holland MK 2006 Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Human Reprod 12:61–69 [DOI] [PubMed] [Google Scholar]
- Driancourt MA, Reynaud K, Cortvrindt R, Smitz J 2000 Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod 5:143–152 [DOI] [PubMed] [Google Scholar]
- Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA 1994 Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651–661 [DOI] [PubMed] [Google Scholar]
- Woodson KG, Crawford PA, Sadovsky Y, Milbrandt J 1997 Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol Endocrinol 11:117–126 [DOI] [PubMed] [Google Scholar]
- Udvadia AJ, Rogers KT, Higgins PD, Murata Y, Martin KH, Humphrey PA, Horowitz JM 1993 Sp-1 binds promoter elements regulated by the RB protein and Sp-1-mediated transcription is stimulated by RB coexpression. Proc Natl Acad Sci USA 90:3265–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchio C, Lingrell S, Vance DE 2007 Sp-1 binds promoter elements that are regulated by retinoblastoma and regulate CTP:phosphocholine cytidylyltransferase-α transcription. J Biol Chem 282:14827–14835 [DOI] [PubMed] [Google Scholar]
- Udvadia AJ, Templeton DJ, Horowitz JM 1995 Functional interactions between the retinoblastoma (Rb) protein and Sp-family members: superactivation by Rb requires amino acids necessary for growth suppression. Proc Natl Acad Sci USA 92:3953–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe V, Alemany C, Chasin LA, Ciudad CJ 1998 Retinoblastoma protein associates with SP1 and activates the hamster dihydrofolate reductase promoter. Oncogene 16:1931–1938 [DOI] [PubMed] [Google Scholar]
- Gray SA, Mannan MA, O’Shaughnessy PJ 1995 Development of cytochrome P450 aromatase mRNA levels and enzyme activity in ovaries of normal and hypogonadal (hpg) mice. J Mol Endocrinol 14:295–301 [DOI] [PubMed] [Google Scholar]
- Carreau S, Bourguiba S, Lambard S, Silandre D, Delalande C 2004 The promoter (s) of the aromatase gene in male testicular cells. Reprod Biol 4:23–34 [PubMed] [Google Scholar]
- Pezzi V, Sirianni R, Chimento A, Maggiolini M, Bourguiba S, Delalande C, Carreau S, Ando S, Simpson ER, Clyne CD 2004 Differential expression of steroidogenic factor-1/adrenal 4 binding protein and liver receptor homolog-1 (LRH-1)/fetoprotein transcription factor in the rat testis: LRH-1 as a potential regulator of testicular aromatase expression. Endocrinology 145:2186–2196 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Yamaguchi S, Hirai T, Kitano T 2007 Follicle-stimulating hormone signaling and Foxl2 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem Biophys Res Commun 359:935–940 [DOI] [PubMed] [Google Scholar]
- Lalli E, Sassone-Corsi P 2003 DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol 17:1445–1453 [DOI] [PubMed] [Google Scholar]
- Iyer AK, McCabe ER 2004 Molecular mechanisms of DAX1 action. Mol Genet Metab 83:60–73 [DOI] [PubMed] [Google Scholar]
- Saxena D, Escamilla-Hernandez R, Little-Ihrig L, Zeleznik AJ 2007 Liver receptor homolog-1 and steroidogenic factor-1 have similar actions on rat granulosa cell steroidogenesis. Endocrinology 148:726–734 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kasahara M, Yoshioka H, Umesono K, Morohashi K 2002 LXXLL motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Endocr Res 28:537 [DOI] [PubMed] [Google Scholar]
- Kim J, Prawitt D, Bardeesy N, Torban E, Vicaner C, Goodyer P, Zabel B, Pelletier J 1999 The Wilms’ tumor suppressor gene (wt1) product regulates Dax-1 gene expression during gonadal differentiation. Mol Cell Biol 19:2289–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura I, Tanaka H, Kanakura Y 2003 E2F1 and c-Myc in cell growth and death. Cell Cycle 2:333–338 [PubMed] [Google Scholar]
- Nasi S, Ciarapica R, Jucker R, Rosati J, Soucek L 2001 Making decisions through Myc. FEBS Lett 490:153–162 [DOI] [PubMed] [Google Scholar]
- Almasan A, Yin Y, Kelly RE, Lee EY, Bradley A, Li W, Bertino JR, Wahl GM 1995 Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc Natl Acad Sci USA 92:5436–5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Downs SM 1984 Chemical signals that regulate mammalian oocyte maturation. Biol Reprod 30:1–11 [DOI] [PubMed] [Google Scholar]
- Maddison LA, Sutherland BW, Barrios RJ, Greenberg NM 2004 Conditional deletion of Rb causes early stage prostate cancer. Cancer Res 64:6018–6025 [DOI] [PubMed] [Google Scholar]
- Vooijs M, van der Valk M, te Riele H, Berns A 1998 Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene 17:1–12 [DOI] [PubMed] [Google Scholar]
- Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U 2005 The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development 132:2377–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks ID, Hacker E, Irwin N, Muller HK, Keith P, Mould A, Zournazi A, Pavey S, Hayward NK, Walker G, Kay GF 2005 Melanocytes in conditional Rb−/− mice are normal in vivo but exhibit proliferation and pigmentation defects in vitro. Pigment Cell Res 18:252–264 [DOI] [PubMed] [Google Scholar]
- Skapek SX, Pan YR, Lee EY 2006 Regulation of cell lineage specification by the retinoblastoma tumor suppressor. Oncogene 25:5268–5276 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q 2005 Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci USA 102:16090–16095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A 2000 Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 14:994–1004 [PMC free article] [PubMed] [Google Scholar]
- Pedersen T, Peters H 1968 Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557 [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM 2002 Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 16:1154–1167 [DOI] [PubMed] [Google Scholar]
- Cowell IG, Aucott R, Mahadevaiah SK, Burgoyne PS, Huskisson N, Bongiorni S, Prantera G, Fanti L, Pimpinelli S, Wu R, Gilbert DM, Shi W, Fundele R, Morrison H, Jeppesen P, Singh PB 2002 Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma 111:22–36;6.6p> [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.