Abstract
We previously identified the small molecule harmine as a regulator of peroxisome proliferator activated-receptor γ (PPARγ) and adipocyte differentiation. In an effort to identify signaling pathways mediating harmine’s effects, we performed transcriptional profiling of 3T3-F442A preadipocytes. Inhibitor of DNA biding 2 (Id2) was identified as a gene rapidly induced by harmine but not by PPARγ agonists. Id2 is also induced in 3T3-L1 preadipocytes treated with dexamethasone, 3-isobutyl-1-methylxanthine, and insulin, suggesting that Id2 regulation is a common feature of the adipogenic program. Stable overexpression of Id2 in preadipocytes promotes expression of PPARγ and enhances morphological differentiation and lipid accumulation. Conversely, small interfering RNA-mediated knockdown of Id2 antagonizes adipocyte differentiation. Mice lacking Id2 expression display reduced adiposity, and embryonic fibroblasts derived from these mice exhibit reduced PPARγ expression and a diminished capacity for adipocyte differentiation. Finally, Id2 expression is elevated in adipose tissues of obese mice and humans. These results outline a role for Id2 in the modulation of PPARγ expression and adipogenesis and underscore the utility of adipogenic small molecules as tools to dissect adipocyte biology.
TYPE 2 DIABETES IS a major cause of morbidity and an important risk factor for metabolic diseases. The primary factor underlying this increase is a rapid rise in the prevalence of obesity and obesity-related insulin resistance. Therefore, a better understanding of the molecular mechanisms controlling adipogenesis is needed.
The nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) plays a central role in the adipocyte differentiation program. Downstream targets for PPARγ regulation in fat are well characterized. These include numerous genes involved in lipid accumulation and metabolism, such as aP2, CD36, lipoprotein lipase (LPL), perilipin, and phosphoenol pyruvate carboxykinase (1). By contrast, the transcriptional pathways that control expression of PPARγ itself remain incompletely understood. Early studies in cultured cells showed that induction of CCAAT enhancer binding protein (C/EBP)β and C/EBPδ facilitates expression of PPARγ (2). Mice lacking C/EBPβ and C/EBPδ have a defect in generation of adipose tissues (3). However, PPARγ expression is preserved in fat tissue in these mice, suggesting that additional pathways contribute to PPARγ regulation during adipogenesis.
Consistent with this idea, a number of other transcription factors have also been linked to PPARγ expression. For example, members of the Kruppel-like zinc finger family of transcription factors have been reported to be proadipogenic [KLF5 (4) and KLF15 (5)] or antiadipogenic [KLF2 (6)] modulators of PPAR expression. Other transcription factors linked to the early stages of differentiation include Krox-20 (7), GATA2/3 (8), ternary complex factor (TCF)/Lef (9), E2F (10), and Sma- and Mad-related protein (11). Thus, PPARγ regulation during adipogenesis is complex and requires the integration of multiple transcriptional cascades. It is likely that additional factors contributing to this process remain to be elucidated.
We recently identified the small molecule harmine as an inducer of adipocyte differentiation (12). Remarkably, harmine appears to act by inducing PPARγ mRNA expression in preadipocytes. In the present work, we have used harmine as a tool to probe for transcriptional pathways that trigger PPARγ expression. By profiling the acute effects of harmine on preadipocyte gene expression, we identified Id2 as a mediator of harmine action in adipogenesis.
Id proteins are helix-loop-helix (HLH) domain transcription factors that lack a DNA-binding domain. Ids heterodimerize with other HLH proteins and are involved in a wide range of cellular processes, including development, cell cycle control, differentiation, and tumorigenesis (13). Here we show that ectopic expression of Id2 in preadipocytes promotes PPARγ expression and differentiation, whereas knockdown of Id2 expression is inhibitory. In vivo, Id2 expression is elevated in obese mice and humans. We also show that white adipose tissue (WAT) development is impaired in Id2−/− mice and that Id2−/− mouse embryonic fibroblasts (MEFs) exhibit reduced adipogenic potential. Together, our data demonstrate that Id2 is a transcriptional modifier of PPARγ expression and adipogenesis. These observations shed light on a new factor in adipogenesis and illustrate the utility of adipogenic small molecules as chemical tools to probe fat cell biology.
RESULTS
Identification of Genes Induced by Harmine
We previously identified harmine in a cell-based chemical library screen as a molecule that promotes adipocyte differentiation and PPARγ expression (12). To gain insight into the mechanism of action of this novel adipogenic compound, we performed transcriptional profiling analysis of 3T3-F442A cells. We treated 3T3-F442A cells with either harmine or the PPARγ ligand GW7845 for 24 h. As expected, PPARγ target genes including aP2, adiponectin, and phosphoenol pyruvate carboxykinase were up-regulated in cells treated with either harmine or GW7845 (Fig. 1A). We anticipated that adipogenic genes acting upstream of PPARγ would be regulated by harmine but not by GW7845. A limited number of genes, including Id2, Gremlin, Fseg, Rasl11b, and two uncharacterized genes met our criteria of being up-regulated by harmine (>2.5-fold) and not regulated by GW7845 (<1.3-fold change) (Fig. 1A). Induction of these genes by 24 h harmine treatment was confirmed by quantitative PCR analysis (Fig. 1B). Real-time PCR analysis also confirmed that both Id2 and PPARγ were up-regulated by harmine but not by GW7845, whereas PPARγ target genes such as aP2 and adiponectin were up-regulated by both treatments (Fig. 1B). The increase in Id2 mRNA levels in response to harmine can be attributed to increased transcription, because it was blocked by actinomycin D treatment (Fig. 1E). Because induction of PPARγ expression by harmine is apparent within 4 h (12), we also analyzed the time-dependent regulation of these genes by harmine. Among these genes, Id2 was induced by harmine as rapidly as PPARγ (Fig. 1, B and C). The regulation of Id2 expression by harmine was also observed in 3T3-L1 cells, but not in 293T cell or primary mouse hepatocytes (data not shown), suggesting selectivity for preadipocytes. Interestingly, the standard differentiation cocktail for 3T3-L1 cells [dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), and insulin] (DMI) rapidly induced expression of C/EBPβ, C/EBPδ, KLF5, and Id2, suggesting that Id2 regulation is a common feature of the adipogenic program (Fig. 1D and data not shown).
Figure 1.
Identification of Genes Rapidly Induced by Harmine in 3T3-F442A Preadipocytes
A, Microarray analysis of 3T3-F442A preadipocytes treated with either harmine (10 μm) or PPARγ ligand, GW7845 (30 nm), for 24 h at confluence. PPARγ target genes are shown in the upper panel. Genes that passed the criteria of being up-regulated (>2.5-fold) by harmine and not regulated by GW7845 (<1.3-fold induction or reduction) are shown in the lower panel. B, Comparison of the effects by harmine and GW7845 treatment on Id2, PPARγ, and PPARγ target gene expression. 3T3-F442A preadipocytes were treated with harmine (10 μm) or GW7845 (30 nm) for 48 h at confluence, and mRNA levels were measured by real-time PCR. C, Time course of induction of Id2 and PPARγ by harmine (10 μm) in 3T3-F442A cells as determined by real-time PCR. 3T3-F442A preadipocytes were treated with harmine for the indicated time at confluence. D, Induction of Id2, C/EBPδ, and PPARγ2 expression by adipogenic cocktail (DMI; 1 μm dexamethasone, 0.5 mm IBMX, 5 μg/ml insulin) in 3T3-L1 cells. E, Effect of actinomycin D treatment on induction of Id2 expression by harmine. 3T3-F442A preadipocytes were pretreated with actinomycin D (5 μg/ml) 1 h before harmine treatment. F, Transient overexpression of Id2 induces expression of PPARγ in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were transiently transfected with an Id2 expression vector (pCMX-Id2), PPARγ expression vector (pCMX-PPARγ), or empty vector (pCMX). mRNA was collected 48 h later and gene expression was determined by real-time PCR. Data are representative of three independent experiments. Ctrl, Control; GW, GW7845; Har., harmine; Norm., normalized; PEPCK, phosphoenol pyruvate carboxykinase.
Id2 Stimulates PPARγ Expression and Adipocyte Differentiation
To address the hypothesis that Id2 induction by harmine may contribute to its ability to promote PPARγ expression, we transiently overexpressed either Id2 or PPARγ in 3T3-L1 preadipocytes. Transient overexpression of Id2 increased PPARγ expression (Fig. 1F), whereas overexpression of PPARγ did not alter expression of Id2 (Fig. 1F). Although the magnitude of PPARγ induction in these studies was modest, it was highly reproducible in multiple experiments (data not shown) and was consistent with the 2- to 3-fold up-regulation observed with harmine. This observation suggests that Id2 is upstream of PPARγ in the differentiation cascade (see below).
Based on the ability of Id2 to induce PPARγ expression after short-term transient expression, we asked whether Id2 promotes adipocyte differentiation. To test this possibility, we used a retroviral vector to generate preadipocytes stably expressing Id2. Consistent with the transient transfection results, stable expression of Id2 in 3T3-L1 cells increased expression of PPARγ at confluence (Fig. 2A). After stimulation of differentiation with adipogenic reagents, 3T3-L1 cells stably expressing Id2 displayed increased expression of PPARγ and its target genes aP2, CD36, LPL, adiponectin, and C/EBPα (Fig. 2, A and B). Moreover, Id2-expressing cells showed increased capacity for morphological differentiation and lipid accumulation as determined by Oil red O staining (Fig. 2C). Importantly, promotion of adipocyte differentiation by Id2 was also observed in other preadipocytes cell lines, including C3H10T1/2 (Fig. 2C), 3T3-F442A, and M2–10B cells (data not shown). These data identify Id2 as a novel adipogenic transcription factor that induces PPARγ expression.
Figure 2.
Promotion of Adipocyte Differentiation by Stable Overexpression of Id2 in 3T3-L1 Preadipocytes
3T3-L1 preadipocytes were infected with pBabe-based retrovirus harboring the Id2 gene and large stable pools selected with puromycin (2 μg/ml). Expression of Id2, PPARγ on d 0 and d 6 (A) and PPARγ target genes on d 6 (B) are increased in Id2-overexpressing 3T3-L1 cells. C, Oil red O staining of Id2 overexpressing 3T3-L1 cells and C3H10T1/2 cells. Differentiation of 3T3-L1 and C3H10T1/2 cells into adipocytes was induced by DMI, or GW7845 (GW; 30 nm). DI, DMI and insulin.
Id2 Expression Is Regulated by Wnt Pathway
The Wnt signaling pathway is an important physiological inhibitor of adipocyte differentiation (14). We previously demonstrated that the adipogenic activity of harmine results, at least in part, from its ability to block Wnt signaling in preadipocytes (12). To determine how the Wnt pathway is involved in the regulation of Id2 by harmine, we treated 3T3-L1 and 3T3-F442A cells with Wnt-3a-conditioned media. Consistent with previous work (12,14), Wnt-3a treatment suppressed PPARγ expression and inhibited adipocyte differentiation of 3T3-F442A and 3T3-L1 cells (Fig. 3A and data not shown). Interestingly, Id2 expression was also significantly down-regulated in response to Wnt-3a treatment (Fig. 3B). The suppression of Id2 expression by Wnt-3a occurred at as early as 2 h, a time course consistent with the rapid induction of Id2 and PPARγ expression by harmine (Fig. 3B). In line with previous work showing that harmine antagonizes the ability of Wnt to inhibit adipogenesis (12), treatment of preadipocytes with harmine reversed the inhibitory effect of Wnt-3a on Id2 expression (Fig. 3C).
Figure 3.
Suppression of Id2 Expression by the Wnt Pathway in Preadipocytes
A and B, 3T3-F442A cells were treated with Wnt-3a or control conditioned media for 48 h. mRNA levels were measured by real-time PCR. C, Harmine reverses the inhibitory effect of Wnt-3a on Id2 expression. 3T3-F442A preadipocytes were treated with Wnt-3a conditioned media and/or harmine. D, Effect of harmine derivatives (10 μm) (12) on expression of PPARγ and Id2 in 3T3-F442A preadipocytes. E, dnTCF blunts the induction of Id2 expression by harmine. 3T3-F442A cells stably expressing vector or dnTCF protein were generated by retroviral transduction (12). Id2 expression 48 h after harmine treatment was determined by real-time PCR. Ctrl, Control.
Previous structure-function analysis using six derivatives of harmine, revealed that only 6-methoxyharman shared the ability of harmine to inhibit Wnt and induce PPARγ expression (12). As expected, 6-methoxyharman was also the only derivative besides harmine capable of inducing Id2 (Fig. 3D).
To further establish the role of Wnt signaling in the regulation of Id2 and PPARγ expression by harmine, we generated stable 3T3-F442A cells expressing a dominant-negative form of TCF (dnTCF). This dominant-negative construct was previously shown to reduce Wnt-stimulated gene expression and activity. Induction of Id2 expression by harmine in dnTCF cells was significantly blunted compared with controls (Fig. 3E). Together, these data demonstrate that the regulation of Id2 by harmine results, at least in part, from inhibition of the Wnt signaling pathway.
Id2 Acts Upstream of PPARγ in Adipogenesis
Enforced expression of Id2 in 3T3-L1 cells increased expression of PPARγ, suggesting that Id2 acts upstream of PPARγ in the adipogenic cascade. To further validate this hypothesis, we generated stable 3T3-F442A cells expressing short hairpin RNA (shRNA) against PPARγ. Two independent shRNA constructs targeting different regions of the PPARγ mRNA were used. Both shRNAs effectively reduced PPARγ mRNA expression (Fig. 4A) and protein levels (Fig. 4B). As expected, 3T3-F442A cells expressing PPARγ shRNA showed markedly reduced differentiation and lipid accumulation (data not shown). Expression of downstream target genes of PPARγ, including aP2, adiponectin, and CD36, was markedly reduced in PPARγ shRNA-expressing cells, both in the presence and absence of harmine (Fig. 4D). By contrast, expression of Id2 was not affected by knockdown of PPARγ, demonstrating that Id2 is indeed upstream of PPARγ (Fig. 4C).
Figure 4.
Knockdown of PPARγ Blocks the Adipogenic Effect of Harmine but Does Not Alter Expression of Id2
Stable 3T3-F442A cells expressing two independent shRNAs against PPARγ were generated. A and B, Two independent shRNAs effectively reduce PPARγ mRNA expression (A) and protein levels (B). C, Expression of Id2 is not affected by knockdown of PPARγ, demonstrating that Id2 is upstream of PPARγ. D, Expression of downstream target genes of PPARγ, including aP2, adiponectin, and CD36, is reduced in PPARγ shRNA-expressing cells, both in the presence and absence of harmine. Gene expression was determined by real-time PCR. Ctrl, Control; shPPAR, short hairpin PPAR.
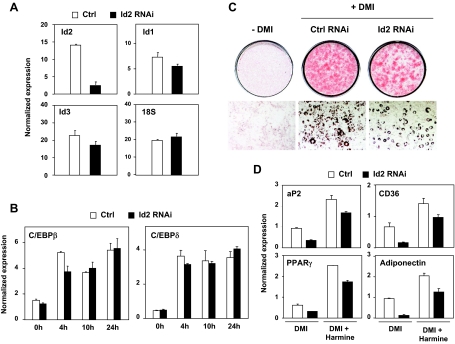
Knockdown of Id2 Inhibits Adipogenesis
To determine the importance of Id2 expression in the regulation of adipocyte differentiation, we conducted Id2 knockdown studies. Transient transfection of 3T3-L1 cells with a pool of four different Id2 siRNA sequences effectively reduced mRNA levels of Id2 but not of the related factors Id1 and Id3 (Fig. 5A). Inhibition of Id2 had no effect on the induction of other early transcription factors, including C/EBPβ and C/EBPδ (Fig. 5B). Id2 knockdown inhibited the ability of 3T3-L1 cells to differentiate (Fig. 5C). In accordance with the effects on morphological differentiation, knockdown of Id2 blunted the induction of PPARγ, aP2, and adiponectin expression by DMI and harmine during adipogenesis (Fig. 5D). Similar results were obtained in parallel knockdown studies using a single independent siRNA sequence (data not shown). Together, these data indicate the transcription factor Id2 as one component of the signaling pathway by which harmine and DMI regulate PPARγ expression and adipocyte differentiation.
Figure 5.
Id2 Knockdown Inhibits 3T3-L1 Adipogenesis
A, Transient transfection of 3T3-L1 cells with a pool of four Id2 siRNA sequences reduces mRNA levels of Id2 but not of the related factors Id1 and Id3 or 18S control. B, Id2 knockdown does not affect induction of the early transcription factors C/EBPβ or C/EBPδ by DMI. C, Oil red O staining of Id2-silenced 3T3-L1 cells (Id2 RNAi) and control (Ctrl RNAi) after 6 d of differentiation. Top, Oil red O staining; bottom, microscopic view. D, Quantitative real-time PCR analysis of expression of the adipogenic markers, adiponectin, aP2, CD36, and PPARγ, in knockdown cells vs. controls. Knockdown of Id2 blunts the induction of PPARγ, aP2, and adiponectin expression by DMI and harmine during adipocyte differentiation. Data are representative of three independent experiments. Ctrl, Control; RNAi, RNA interference.
Id2 Levels Correlate with Adipose Tissue Mass in Mice and Humans
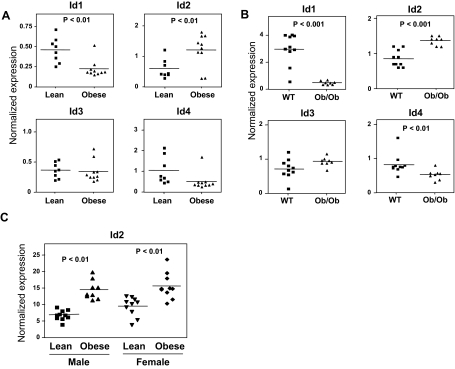
To investigate whether Id2 expression was altered in obesity, we determined the Id2 expression level in the WAT of diet-induced (high-fat diet) and genetic (ob/ob) obese male mice. Id2 expression was significantly elevated in both high-fat-fed and ob/ob mice relative to control mice (Fig. 6, A and B). Interestingly, Id1 and Id4 showed negative correlation with body weight in both obese mice models, whereas Id3 did not show any significant correlation. These data suggest that Id family members may have divergent functions.
Figure 6.
Id2 Is Positively Correlated with Body Mass in Mice and Humans
A and B, Correlation between Ids (Id1–4) expression and body weight in genetic and diet-induced obese mice. Total RNA was collected from epididymal adipose tissues, and Id2 expression was measured by real-time PCR. A, Id2 expression positively correlates with body weight in high-fat-fed mice. C57BL/6 mice were fed with regular chow (n = 10) or high-fat diet for 11 months (n = 10). B, Id2 expression is up-regulated in ob/ob mice. C57BL/6 and ob/ob mice (3 months of age) were used in this study. **, P < 0.05, n = 8–10. C, Expression of Id2 in primary cultured abdominal sc mature adipocytes from nine male and 10 female nondiabetic obese humans. Id2 expression was measured in lean (BMI, 25 kg/m2) and obese (BMI, 55 kg/m2) Pima indians. Id2 expression in humans is also positively correlated with body weight. The microarray data are retrieved in Entrez Gene Expression Omnibus DataSets originally performed by Lee et al. (28). WT, Wild type.
To address the expression of Id2 in human adipose tissue, gene expression data from abdominal adipocytes in nondiabetic lean [body mass index (BMI) 25] and obese (BMI 55) Pima Indians were obtained from National Center for Biotechnology Information Gene Expression Omnibus profiles (www.ncbi.nlm.nih.gov/geo/gds/gds_browse.cgi?gds=1496). In line with our results in mice, we found a very strong correlation of Id2 expression with BMI in human adipose tissue (Fig. 6C). Thus, Id2 expression is correlated with both rodent and human obesity.
Loss of Id2 Expression Affects WAT Development in Mice
Id2 is widely expressed in vivo, including in brown and white adipose tissues (data not shown). To address the potential impact of Id2 deletion on WAT development in vivo, we analyzed Id2 null mice. Both mouse and human models have established that loss of PPARγ causes lipodystrophy (15,16). Thus, it is likely that genes regulating PPARγ expression may also affect adipocyte development in vivo. As previously reported, the gross phenotype of Id2−/− mice at birth was indistinguishable from that of wild-type littermates on the C57BL/6 background (17). However, by d 6, Id2−/− neonates were noticeably runted despite the ability to suckle, and these mice did not survive beyond 2 wk of age. Cross-sections of 6-d-old neonates followed by hematoxylin and eosin staining showed reduced WAT development in Id2−/− pups compared with Id2+/+ controls (Fig. 7A). Dissection at d 4 revealed that neonatal mutant mice exhibited relative deficiencies in interscapular and inguinal fat pads (Fig. 6B and data not shown). Despite this difference in WAT mass, size and morphology of adipocytes and liver were not significantly different (data not shown). These data strongly suggest that Id2 may play a modulatory role in adipocyte development in vivo.
Figure 7.
Reduced WAT Development in Id2−/− Mice
A, Transverse sections at the level of the neck were from Id2+/+ and Id2−/− 6 d after birth. B, Reduced WAT development in Id2−/− pups. Dorsal view of 4-d-old Id2−/− mouse with its littermate control. Note the decreased deposition of fat interscapular regions for the Id2−/− pups. C and D, Reduced adipocyte differentiation in Id2−/− MEFs. Primary MEFs were prepared from E13.5 wild-type and Id2−/− embryos. C, Oil red O staining of Id2+/+ and Id2−/− MEFs. MEFs were differentiated with adipogenic cocktail for 8 d. Top, Oil red O staining; bottom, microscopic view. D, Expression of adipogenic markers, adiponectin, aP2, LPL, and PPARγ, in Id2−/− and Id2+/+ MEFs. Id2 expression is absent in Id2 null cells. The levels of adipogenic markers are reduced whereas C/EBPδ is not changed in Id2−/− cells. At d 6, mRNA was collected and analyzed by real-time PCR. Data are representative of three independent experiments.
To gain additional insight into the importance of Id2 in adipocyte differentiation, and to complement our analysis of Id2−/− pups, primary MEFs were prepared from embryonic d 13.5 (E13.5) wild-type and Id2−/− embryos. MEFs isolated from Id2 null embryos exhibited a modest but highly reproducible reduction in adipogenic potential in multiple independent preparations (Fig. 7C and data not shown). In agreement with changes in morphological differentiation, the levels of PPARγ2, LPL, adiponectin, and aP2 mRNA were reduced in hormone-induced Id2−/− MEFs compared with Id2+/+ MEFs (Fig. 7D). In agreement with our knockdown studies, other early transcription factors such as C/EBPδ were not altered in Id2−/− cells. Together, these data demonstrate that Id2 is a modifier of adipocyte differentiation in vitro and in vivo.
DISCUSSION
We previously identified harmine as a novel adipogenic compound by high throughput screening of preadipocytes (12). Harmine stimulates differentiation through induction of PPARγ expression and inhibition of the Wnt signaling pathway. In the present work, we have used harmine as a chemical tool to identify the transcription factor Id2 as a new player in the adipocyte differentiation program.
By profiling gene expression in preadipocytes treated acutely with harmine, we identified Id2 as a harmine-responsive gene. We showed that expression of Id2 is induced not only by harmine, but also by the classic adipogenic DMI cocktail, suggesting that induction of Id2 is a common feature of the differentiation program. The rapid and transient induction of Id2 by DMI treatment is reminiscent of other early adipogenic PPARγ regulators such as CEBPβ and KLF5. Retroviral expression of Id2 stimulates PPARγ expression and promotes morphological differentiation in multiple preadipocyte cell lines. Furthermore, inhibition of Id2 expression with siRNA approaches inhibited the differentiation of 3T3-L1 cells and blunted the expression of PPARγ and its downstream target genes. Id2 expression is positively correlated with body mass in genetic and diet-induced obese mice. Interestingly, elevated expression of Id2 is also observed in human obese populations. Finally, impaired WAT development in Id2−/− mice and adipocyte differentiation of Id2−/− MEFs are consistent with a role for Id2 in adipose development.
Harmine stimulates differentiation through induction of PPARγ expression and inhibition of the Wnt signaling pathway. Precisely how these effects are accomplished on a molecular level, however, is unknown. In this study, we also explored the connection between harmine, Id2, PPARγ and the Wnt signaling pathway during adipocyte differentiation. First, we determined that Id2 acts downstream of Wnt. Treatment of preadipocytes with Wnt-conditioned medium and purified Wnt-3a inhibited the expression of Id2 and PPARγ (Fig. 3 and data not shown). We also showed that the ability of harmine to induce Id2 and PPARγ was reduced in cells expressing dnTCFs. Second, we established that Id2 acts upstream of PPARγ in the differentiation cascade. Transient and stable expression of Id2 induced PPARγ expression in preadipocytes. Whereas shRNA-mediated knockdown of PPARγ abolished the ability of harmine to stimulate differentiation, Id2 induction by harmine was still detectable. These data suggest that Id2 acts upstream of PPARγ during adipogenesis.
Nevertheless, given the fact that adipogenic effects were not completely blunted in Id2 siRNA transfected cells (Fig. 5D), the possibility that Id2 is not the only mediator of harmine action in adipogenesis must be considered. As we have identified other harmine-responsive genes (Fig. 1), future studies to address the function of these proteins may help to reveal additional adipogenic mechanisms of harmine.
Identification of Id2 as a PPARγ modulator promoted us to test whether Id2 modulates expression and activity of C/EBPs, known PPARγ regulators. Gain and loss of function of Id2 did not alter levels and activity of early adipogenic PPARγ regulators (C/EBPβ and C/EBPδ) (Figs. 5 and 7, and data not shown). Conversely, overexpression of C/EBPδ did not change the Id2 expression whereas it induced PPARγ expression (data not shown). These data suggest that Id2 and C/EBPβ/δ do not act similar in pathways in adipogenic cascades. In fact, the Id proteins lacking DNA-binding domains are believed to function by inhibiting the action of other transcription factors. Previous studies have shown that Ids interact with basic HLH, adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c (18), Ets transcription factor (19), retinoblastoma (20), and E-proteins (21). Several of these factors have been linked to adipogenesis, including adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c and retinoblastoma protein (22,23). Currently, the cis- and trans-acting factors with which Id2 interacts to stimulate PPARγ expression remain to be established. Of note, Id3 has been previously reported to inhibit adipocyte differentiation (18) in contrast to our observations with Id2. We did not observe changes in Id3 expression in response to the adipogenic compound harmine. Accordingly, different Id family members may have different functions in this cell type. Interestingly, recent work from Seidman and colleagues (24) identified Id2 as a key factor in the development of the cardiac conduction system. Thus, our results fit well with the hypothesis that Id2 serves as an important factor in the development of several specialized cell types.
Id2 is ubiquitously expressed, suggesting multiple functions in many types of cells. Id2−/− mice were previously reported to have defects in epithelial proliferation in mammary glands, lacking lymph nodes and Peyer’s patches, and reduced population of natural killer cells and Langerhans cells (25). Additional data further showed that Id2 is critical in CD8+ T cell immunity and cardiac development (17,24). Here we have documented reduced WAT development in Id2−/− mice. Clearly, the reduced adiposity of Id2−/− mice could be secondary to alterations in other tissues and cell types. However, our observation that Id2−/− MEFs show reduced capacity for adipocyte differentiation are consistent with intrinsic defects in adipose tissue in the absence of Id2. Future studies, likely involving tissue-specific knockout mice, will be required to dissect potential roles for Id2 in obesity, insulin resistance, and systemic metabolism.
MATERIALS AND METHODS
Reagents and Plasmids
Harmine hydrochloride was purchased from Sigma Chemical Co. (St. Louis, MO; catalog no. H0625). GW7845 was kindly provided by T. Willson (GlaxoSmithKline, Research Triangle Park, NC). Insulin (no. I0516), dexamethasone (no. D2915), and 3-isobutyl-1-methylxanthine (IBMX, no. I7018) were from Sigma. Actinomycin D was from Calbiochem (La Jolla, CA). Purified Wnt-3a protein was from R&D Systems (Minneapolis, MN; no. 1324-WN). For Wnt-3a-conditioned media, Wnt-3a-expressing L cells or control L cells [American Type Culture Collection (ATCC), Manassas, VA; CRL-2647 and CRL-2648] were cultured in DMEM with 10% fetal bovine serum, and medium was collected as described (12). We used the following primers for cloning of Id2: forward, 5′-gggatccgccgccatgaaagccttcagtccggtgaggtccgtt-3′; and reverse, 5′-gccgtcgacttagccacagagtactttgctatcattcga-3′. Full-length cDNA was cloned into pBabe-puro and pCMX. Oligonucleotides were from Integrated DNA Technologies. siRNAs for Id2 and nonspecific control were purchased from Dharmacon (Lafayette, CO). The target sequences were sense, 5′-gcaaaguacucuguggcuauu-3′; and antisense, 5′-uagccacagaguacuuugcuu-3′ for Id2. shRNAs for PPARγ knockdown were previously described (26).
Cell Culture
3T3-L1 and 3T3-F442A preadipocyte cell lines were maintained and differentiated as previously described (12). M2 mouse mesenchymal stem cells were purchased from American Type Culture Collection. M2 cells were maintained in growth medium consisting of RPMI 1640 with 10% fetal bovine serum and supplemented with 1 mm sodium pyruvate, 100 U/ml penicillin, and 100 U/ml streptomycin. Retroviral overexpression of Id2 was performed using pBabe-puro and the packaging cell line Phoenix E as described elsewhere (27). siRNAs from Dharmacon were resuspended according to the manufacturer’s instructions. Id2 siRNAs were transfected using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA). A concentration of 10 nm siRNA was transfected into 50% confluent 3T3-L1 cells. After 48 h, media were changed and processed further as differentiation protocols. MEFs were isolated from E13.5 Id2+/+ and Id2−/− embryos. MEFs were differentiated in dexamethasone (1 μm), IBMX (0.5 mm), and insulin (5 μg/ml) for 2 d after confluence, followed by insulin alone.
RNA and Protein Analysis
Total RNA was isolated using TRIzol reagent (Invitrogen). Total RNA (0.5 μg) was reverse transcribed using MultiScribe (Applied Biosystems, Foster City, CA) and random hexamers according to the manufacturer’s instructions. Real-time quantitative PCR (SYBR green) analysis was performed on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Expression was normalized to 36B4. The following primers were used for Ids expression:
ID1 forward (ID1F), 5′-gcgagatcagtgccttgg-3′; and ID1R reverse (ID1R), 5′-ctcctgaagggctggagtc-3′; ID2 forward (ID2F), 5′-ggaccacagcttgggcat-3′; ID2 reverse (ID2R), 5′-cgttcatgttgtagagcagactcat-3′; ID3 forward (ID3F), 5′-gaggagcttttgccactgac-3′; ID3 reverse (ID3R), 5′-gagagagggtcccagagtcc-3′; ID4 forward (ID4F); agggtgacagcattctctgc; ID4 reverse (ID4R); ccggtggcttgtttctctta.
Protein preparation, SDS-PAGE, and Western blotting were performed as previously described (12). Antiserum against murine PPARγ (sc-7196) and actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Animals
C57BL/6 wild-type mice, C57BL/6 ob/ob, and C57BLKS/J-Lepr db/db mice were purchased from The Jackson Laboratory (Bar Harbor, ME). For relative gene expression studies in diet-induced obesity, wild-type male (C57BL/6) mice were fed either with regular chow or high-fat diet (60% fat) for 11 months. For genetic induced obesity, 13-wk-old male C57BL/6J ob/ob and wild-type C57BL/6 male mice were used. Id2 knockout mice were generated, maintained as previously described (17). Id2−/− pups were generated by mating heterozygote (+/−), and littermates were used as controls for each experiment. The animal studies were approved by the Institutional Animal Care and Use Committee of University of California, Los Angeles (UCLA) and conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals.
Microarrays
Total RNA from 24 h harmine-treated F442A cells prepared using TRIzol and further purified using RNAeasy columns (QIAGEN, Chatsworth, CA). cDNA preparation and hybridization to Affymetrix Mouse Genome Arrays 430 version 2.0 were performed by UCLA Microarray. Core and data were analyzed using GeneSpring GX 7.3 (Agilent Technologies, Palo Alto, CA).
Acknowledgments
We thank T. Willson for PPAR ligands, and Noam Zelcer and Steve Bensinger for helpful advice. We are grateful to D. Halperin, W. K. Kim, and F. Parhami for providing reagents.
H.W. is a Fellow and P.T. is an Investigator of the Howard Hughes Medical Institute at UCLA.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 18, 2008
Abbreviations: BMI, Body mass index; C/EBP, CCAAT enhancer binding protein; DMI, dexamethasone, IBMX, and insulin; dnTCF, dominant-negative TCF; E13.5, embryonic d 13.5; HLH, helix-loop-helix; IBMX, 3-isobutyl-1-methylxanthine; Id 2, inhibitor of DNA binding; KLF, Kruppel-like factor; LPL, lipoprotein lipase; MEFs, mouse embryonic fibroblasts; PPAR, peroxisome proliferator-activated receptor; shRNA, short hairpin RNA; siRNA, small interfering RNA; TCF, ternary complex factor; WAT, white adipose tissue.
References
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM 2000 Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307 [PubMed] [Google Scholar]
- Wu Z, Xie Y, Bucher NL, Farmer SR 1995 Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev 9:2350–2363 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S 1997 Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J 16:7432–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R 2005 Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab 1:27–39 [DOI] [PubMed] [Google Scholar]
- Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M 2005 Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem 280:12867–12875 [DOI] [PubMed] [Google Scholar]
- Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK 2003 The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-γ expression and adipogenesis. J Biol Chem 278:2581–2584 [DOI] [PubMed] [Google Scholar]
- Chen Z, Torrens JI, Anand A, Spiegelman BM, Friedman JM 2005 Krox20 stimulates adipogenesis via C/EBPβ-dependent and -independent mechanisms. Cell Metab 1:93–106 [DOI] [PubMed] [Google Scholar]
- Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS 2000 Function of GATA transcription factors in preadipocyte-adipocyte transition. Science 290:134–138 [DOI] [PubMed] [Google Scholar]
- Kennell JA, O’Leary EE, Gummow BM, Hammer GD, MacDougald OA 2003 T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with β-catenin to coactivate C/EBPα and steroidogenic factor 1 transcription factors. Mol Cell Biol 23:5366–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Landsberg RL, Huss-Garcia Y, Sardet C, Lees JA, Auwerx J 2002 E2Fs regulate adipocyte differentiation. Dev Cell 3:39–49 [DOI] [PubMed] [Google Scholar]
- Choy L, Derynck R 2003 Transforming growth factor-β inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 278:9609–9619 [DOI] [PubMed] [Google Scholar]
- Waki H, Park KW, Mitro N, Pei L, Damoiseaux R, Wilpitz DC, Reue K, Saez E, Tontonoz P 2007 The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARγ expression. Cell Metab 5:357–370 [DOI] [PubMed] [Google Scholar]
- Ruzinova MB, Benezra R 2003 Id proteins in development, cell cycle and cancer. Trends Cell Biol 13:410–418 [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA 2000 Inhibition of adipogenesis by Wnt signaling. Science 289:950–953 [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S 1999 Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402:880–883 [DOI] [PubMed] [Google Scholar]
- Gurnell M, Wentworth JM, Agostini M, Adams M, Collingwood TN, Provenzano C, Browne PO, Rajanayagam O, Burris TP, Schwabe JW, Lazar MA, Chatterjee VK 2000 A dominant-negative peroxisome proliferator-activated receptor-γ (PPARγ) mutant is a constitutive repressor and inhibits PPARγ-mediated adipogenesis. J Biol Chem 275:5754–5759 [DOI] [PubMed] [Google Scholar]
- Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW 2006 Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol 7:1317–1325 [DOI] [PubMed] [Google Scholar]
- Moldes M, Boizard M, Liepvre XL, Feve B, Dugail I, Pairault J 1999 Functional antagonism between inhibitor of DNA binding (Id) and adipocyte determination and differentiation factor 1/sterol regulatory element-binding protein-1c (ADD1/SREBP-1c) trans-factors for the regulation of fatty acid synthase promoter in adipocytes. Biochem J 344 3:873–880 [PMC free article] [PubMed] [Google Scholar]
- Yates PR, Atherton GT, Deed RW, Norton JD, Sharrocks AD 1999 Id helix-loop-helix proteins inhibit nucleoprotein complex formation by the TCF ETS-domain transcription factors. EMBO J 18:968–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A, Garg P, Lasorella A, Hsu J, Israel MA 1994 The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev 8:1270–1284 [DOI] [PubMed] [Google Scholar]
- Murre C 2005 Helix-loop-helix proteins and lymphocyte development. Nat Immunol 6:1079–1086 [DOI] [PubMed] [Google Scholar]
- Kim JB, Spiegelman BM 1996 ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev 10:1096–1107 [DOI] [PubMed] [Google Scholar]
- Chen PL, Riley DJ, Chen Y, Lee WH 1996 Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev 10:2794–2804 [DOI] [PubMed] [Google Scholar]
- Moskowitz IP, Kim JB, Moore ML, Wolf CM, Peterson MA, Shendure J, Nobrega MA, Yokota Y, Berul C, Izumo S, Seidman JG, Seidman CE 2007 A molecular pathway including Id2, Tbx5, and Nkx2–5 required for cardiac conduction system development. Cell 129:1365–1376 [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P 1999 Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397:702–706 [DOI] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA 2007 Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem 282:14515–14524 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM 1994 Stimulation of adipogenesis in fibroblasts by PPARγ 2, a lipid-activated transcription factor. Cell 79:1147–1156 [DOI] [PubMed] [Google Scholar]
- Lee YH, Nair S, Rousseau E, Allison DB, Page GP, Tataranni PA, Bogardus C, Permana PA 2005 Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia 48:1776–1783;6.6p> [DOI] [PMC free article] [PubMed] [Google Scholar]