Abstract
GHB is used therapeutically and recreationally, although the precise mechanism of action responsible for its different behavioral effects is not entirely clear. The purpose of this review is to summarize how behavioral procedures, especially drug discrimination procedures, have been used to study the mechanism of action of GHB. More specifically, we will review several different drug discrimination procedures and discuss how they have been used to qualitatively and quantitatively study different components of the complex mechanism of action of GHB. A growing number of studies have provided evidence that the behavioral effects of GHB are mediated predominantly by GABAB receptors. However, there is also evidence that the mechanisms mediating the effects of GHB and the prototypical GABAB receptor agonist baclofen are not identical, and that other mechanisms such as GHB receptors and subtypes of GABAA and GABAB receptors might contribute to the effects of GHB. These findings are consistent with the different behavioral profile, abuse liability, and therapeutic indications of GHB and baclofen. A better understanding of the similarities and differences between GHB and baclofen, as well as the pharmacological mechanisms of action underlying the recreational and therapeutic effects of GHB, could lead to more effective medications with fewer adverse effects.
Keywords: GHB, GABA, drug discrimination, drug abuse, narcolepsy, Xyrem/sodium oxybate
1. Introduction
GHB is an interesting and unique compound in that it is an endogenous molecule (gamma-hydroxybutyric acid), a drug of abuse (illicit GHB), and a marketed therapeutic drug (gamma-hydroxybutyrate, sodium salt; or sodium oxybate).1 The purpose of this review is to summarize how behavioral procedures, especially drug discrimination procedures, have been used to study the mechanism of action of GHB. This review is not intended to serve as a comprehensive summary of the mechanism of action or pharmacological effects of GHB in general, as there are other recent reviews on the biochemistry and neurobiology (e.g., Crunelli et al., 2006; Pardi and Black, 2006), abuse potential (Nicholson and Balster, 2001; Gonzalez and Nutt, 2005; Barker et al., 2007), and therapeutic potential (Mamelak, 2007; Robinson and Keating, 2007) of GHB. Lastly, we will present several conclusions that have been drawn with regard to the pharmacological mechanism of action of GHB, rationale for the continued study of this important endogenous signaling system, and directions for future research.
1.1. GHB as a therapeutic drug
GHB was first developed as a central nervous system depressant (Laborit et al., 1960) and was used as an anesthetic adjuvant for minor surgical procedures in the laboratory (Laborit et al., 1960; Vickers, 1969) and in the clinic (Aldrete and Barnes, 1968; Kleinschmidt et al., 1997; Kleinschmidt et al., 1998). GHB is still approved in Germany (Somsanit®; Kohler) for intravenous anesthesia, although its use as an anesthetic is thought to be decreasing. In the U.S., Canada, the European Union, and Switzerland GHB is approved to treat cataplexy and excessive daytime sleepiness associated with the sleep disorder narcolepsy (Xyrem®, Jazz Pharmaceuticals Inc., Valeant Pharmaceuticals International, and UCB). It is also approved in Italy and Austria to treat alcohol dependence and withdrawal (Alcover®, C.T. Laboratorio Farmaceutico).
Narcolepsy is a sleep disorder that is characterized by fragmented nighttime sleep (i.e., abrupt transitions between wakefulness and REM sleep) and daytime sleepiness, and can also include cataplexy (loss of muscle tone with consciousness intact), hypnogogic (experienced when falling asleep) or hypnopompic (experienced when waking from sleep) hallucinations, and sleep paralysis (for review, see Scammell, 2003). Studies in narcoleptic patients showed that GHB was effective in treating the daytime cataplexy and fragmented sleep/wake cycles of this clinical population (for review, see Mamelak et al., 1986). Nightly doses of GHB were shown to reduce the number of nocturnal awakenings and daytime attacks of cataplexy, and improve the structure of sleep in narcoleptic patients, increasing the delta power and duration of slow wave sleep and reducing the latency to REM sleep at night, and decreasing the frequency of transitions between wakefulness and REM sleep during the day (Broughton and Mamelak, 1979; Broughton and Mamelak, 1980; Scharf et al., 1985; Mamelak et al., 1986; Scrima et al., 1990; Pardi and Black, 2006). In 2002, GHB was approved as sodium oxybate (U.S. Pharmacopeia) under the trade name Xyrem for the treatment of cataplexy associated with narcolepsy, and in 2005, for the treatment of excessive daytime sleepiness associated with narcolepsy (for review, see Fuller and Hornfeldt, 2003).
Around the same time that the effects of GHB on sleep were being examined in the United States, a therapeutic indication for GHB in the treatment of alcoholism was emerging in Italy (for review, see Poldrugo and Addolorato, 1999). Early preclinical studies showed that GHB blocked the convulsant effects of ethanol withdrawal and acetaldehyde administration in rodents (Andronova and Barkov, 1981; Fadda et al., 1989). Clinical trials supported a possible role for GHB in the treatment of ethanol withdrawal, as GHB was found to decrease withdrawal signs and symptoms such as tremor, sweating, nausea, depression, and anxiety (Gallimberti et al., 1989; Addolorato et al., 1999). Additional studies showed that the administration of GHB or the GHB prodrug gamma-butyrolactone (GBL) decreased ethanol consumption in rats (Fadda et al., 1983; Biggio et al., 1992), and in humans, who reported decreased craving for ethanol in parallel with reduced ethanol self-administration (Gallimberti et al., 1992; Addolorato et al., 1996). Thus, the increasing number of reports of the effectiveness of GHB in promoting abstinence in alcoholics led to the approval of GHB under the trade name Alcover in Italy and Austria, for the treatment of alcoholism (Beghe and Carpanini, 2000).
1.2. GHB as a drug of abuse
In the late 1980’s and early 1990’s, GHB was sold over the counter in the United States as a “natural” and “organic” supplement to increase muscle mass (increase growth hormone) and to promote sleep (Dyer, 1991; Sanguineti et al., 1997). As the use of GHB as a natural food supplement increased, the number of reports of GHB intoxication and emergency room visits also increased (e.g., Chin et al., 1998; Li et al., 1998), which resulted in the Centers for Disease Control and the Food and Drug Administration issuing warnings about the potential dangers of GHB (Centers for Disease Control, 1990; United States Federal Register, 2000a). Shortly after the highly-publicized involvement of GHB in cases of drug-facilitated sexual assault, GHB was placed in a bifurcated Federal schedule in the U.S.: nonmedical use of illicit GHB or Xyrem can be prosecuted under Schedule I penalties, whereas legitimate medical use of Xyrem is governed by Schedule III restrictions (United States Federal Register, 2000b).
GHB gained a reputation as a “club drug” due to its reported use by individuals while attending nightclubs, raves, and circuit parties (Miotto et al., 2001; Bellis et al., 2003; Rodgers et al., 2004; Halkitis and Palamar, 2006). The “class” of drugs that is generally referred to as “club drugs” typically also includes methamphetamine, 3,4-methylenedioxy-N-methylamphetamine (MDMA; ecstasy), lysergic acid diethylamide (LSD; acid), and ketamine (special K). Common among these drugs is the setting in which they are most frequently used and the pattern of their use, and not a shared pharmacological mechanism of action. Several studies have shown, however, that some “club drugs”, including GHB, MDMA, and ketamine, are frequently used together, as well as with alcohol, marijuana, and other amphetamines (Degenhardt et al., 2002; Halkitis et al., 2007).
GHB gained a reputation as a “date-rape drug” in the 1990’s as reports of its involvement in drug-facilitated sexual assault began to appear in the media and the scientific literature (e.g., Stillwell, 2002). It has been suggested that the common illicit formulation of GHB as a colorless odorless liquid facilitates the unsuspected addition of GHB to the drinks of individuals in bars and clubs (Smith, 1999; Schwartz et al., 2000; Varela et al., 2004). In addition, some of the reported effects of GHB such as sedation, euphoria, decreased inhibitions, enhanced sex drive, and anterograde amnesia, might lend GHB to being used for drug-facilitated sexual assault.
Although GHB is almost universally referred to as “the date rape drug” in the media, the prevalence of GHB in cases of drug-facilitated sexual assault, when confirmed by toxicological analyses, is relatively low (1–5%; ElSohly and Salamone, 1999; Varela et al., 2004; Association of Chief Police Officers, 2006). Well-validated assays for the detection of exogenous GHB in biological specimens have only recently become available. Thus, many previous case reports and studies have had to rely upon self-reports or anecdotal reports of GHB use, or upon the results of post-mortem toxicological analyses, the results of which have been shown to vary markedly depending on the storage conditions of the samples and the period of time between death and analysis (Kintz et al., 2004; LeBeau et al., 2007). Together, these challenges have made it difficult to identify with confidence that GHB has been involved in cases of suspected drug-facilitated sexual assault.
1.3. The purpose and structure of this review
Despite the fact that GHB is approved for medical use in North America and Europe, the precise pharmacological mechanism of action of GHB that is responsible for its therapeutic and abuse-related effects is not entirely clear. An increasing number of studies over the last several years have focused on examining the mechanism of action of GHB. A GHB receptor has been cloned (Andriamampandry et al., 2007), new receptor binding and functional assays for the GHB receptor have been characterized (e.g., Mehta et al., 2001; Kemmel et al., 2003), new ligands that bind selectively to GHB receptors have been synthesized (e.g., Carter et al., 2005b; Wellendorph et al., 2005), and new behavioral procedures have been developed (e.g., Carter et al., 2004a; Koek et al., 2005). This review focuses on the behavioral, particularly drug discrimination, procedures that have been used to study the mechanism of action of GHB.
2. Pharmacodynamics of GHB
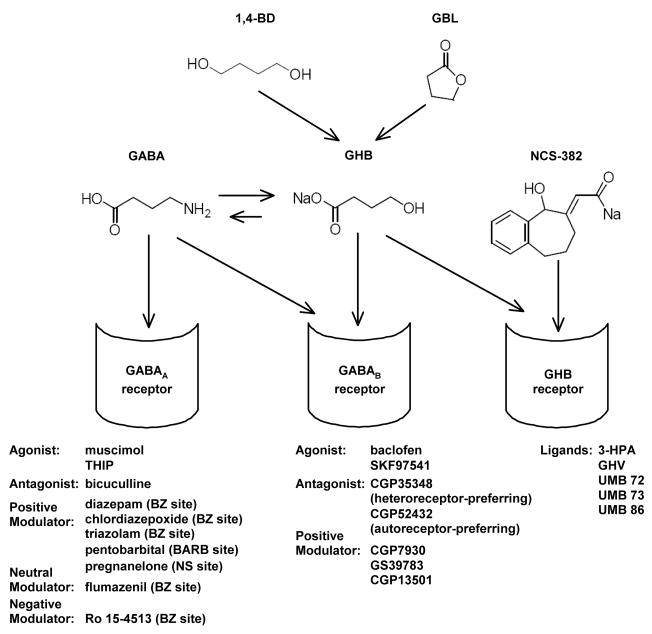
In a review published in 1964, Laborit characterized the in vivo effects of GHB as hypothermic, hypnotic, anesthetic, and anti-convulsant, and without marked respiratory depression or toxicity (Laborit, 1964). At that time, the pharmacological mechanism of action of GHB was unknown; however, the rationale for the synthesis of GHB was to design an analog of gamma aminobutyric acid (GABA) that would cross the blood brain barrier (Laborit et al., 1960; Giarman and Roth, 1964). Thus, a GABAergic mechanism of action for GHB was thought to be likely. Since that time, a number of additional neurotransmitter systems have been implicated in the various effects of GHB, including dopamine, norepinephrine, opioid, orexin/hypocretin, and a specific GHB signaling system. At present, GHB is known to be metabolized to GABA and to bind to GABAB and GHB receptors without exhibiting substantial binding to sites on GABAA receptors (Serra et al., 1991; Xie and Smart, 1992; Fig 1).
Figure 1.
Possible pharmacological mechanisms of GHB. Structures of 1,4-butanediol (1,4-BD) and gamma-butyrolactone (GBL), two GHB prodrugs that are metabolized to GHB in vivo. Structures of GABA, GHB (sodium salt), and NCS-382. GABA and GHB are reversibly metabolized in vivo. Three possible pharmacological mechanisms for GHB. GHB is metabolized to GABA, which binds to GABAA and GABAB receptors, GHB binds to GABAB and GHB receptors, and NCS-382 binds to GHB receptors. Selective ligands for GABAA, GABAB, and GHB receptors are shown below the respective depiction of each receptor.
2.1. GHB prodrugs gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD)
GBL and 1,4-BD are GHB prodrugs that are metabolized to GHB by a calcium-dependent lactonase (Roth and Giarman, 1965; Roth et al., 1967), and by alcohol dehydrogenase and aldehyde dehydrogenase, respectively (Fig 1; Maxwell and Roth, 1972; Poldrugo and Snead, 1984). When first discovered, there was some debate whether each of these compounds (GHB, GBL, 1,4-BD) was biologically active (Bessman and Skolnik, 1964; Sprince et al., 1966); however, the behavioral effects of each compound are correlated with levels of GHB, and not GBL or 1,4-BD, in blood and brain, strongly suggesting that GHB is the pharmacologically active compound (Giarman and Roth, 1964; Roth and Giarman, 1968; Guidotti and Ballotti, 1970).
2.2. GABA receptors
Early electrophysiological evidence and the structural similarity of GHB to GABA suggested that GHB might have GABA-mimetic effects in vivo (Hösli et al., 1983; Snead and Nichols, 1987). Initial studies showed that the inhibitory effects of GHB in the substantia nigra were not blocked by the GABAA receptor antagonist bicuculline (Olpe and Koella, 1979; Osorio and Davidoff, 1979). Later studies showed that the inhibitory effects of GHB on hippocampal and thalamocortical neurons were attenuated by selective GABAB receptor antagonists (Xie and Smart, 1992; Emri et al., 1996). Together, these studies provided the initial evidence that GABAB receptors might play a greater role than GABAA receptors in mediating the effects of GHB. In addition, because GHB is metabolized to GABA, it is possible that GABAC receptors, which were once thought to exist only in retina, but have since been identified in brain (Liu et al., 2004; Schlicker et al., 2004), might also mediate some of the effects of GHB.
Functional evidence for the direct activation of GABAB receptors by GHB was provided by Lingenhoehl and colleagues who showed that GHB stimulated inward current in Xenopus oocytes transfected with functional GABAB receptors (i.e., GABABR1/R2 dimers) and inward-rectifying potassium (Kir3) channels (Lingenhoehl et al., 1999; Fig 1). In this system, the effects of GHB and the GABAB receptor agonist baclofen were completely blocked by three competitive GABAB receptor antagonists (Lingenhoehl et al., 1999).
Activation of GABAB receptors by GHB has also been proposed to explain the apparent effects of GHB on GABAA receptor function (Snead and Liu, 1993). GABAB autoreceptors are known to modulate the firing of GABAergic neurons (Brennan et al., 1981). GABAB receptors are found on GABAergic and non-GABAergic neurons and can modulate neuronal (GABAergic) signaling pre- and post-synaptically by inhibiting neurotransmitter release (Bowery et al., 1980; Bonanno and Raiteri, 1993; Yamada et al., 1999). Activation of GABAB receptors can also increase the synthesis of several neuroactive steroids that positively modulate GABAA receptors (Barbaccia et al., 2002) and greater increases in neurosteroids have been observed in response to GHB administration in rats that have been bred for high sensitivity to GHB compared to rats that have been bred for low sensitivity to GHB (Barbaccia et al., 2005). However, a number of different classes of psychotropic drugs (e.g., antidepressants, selected atypical antipsychotics) increase neurosteroid concentrations, peripheral increases in neurosteroid levels might not correspond to increases in brain, and different neurosteroids have different modulatory effects on GABAA and NMDA receptors (Barbaccia et al., 2004). Thus, further study into the selectivity, specific mechanism, and behavioral relevance of GHB-induced increases in neurosteroid levels via GABAB receptors is warranted.
2.3. GHB receptors
In 1982, specific binding sites for GHB were discovered in rat brain (Benavides et al., 1982; Fig 1). GABA and baclofen did not displace [3H]GHB from these sites, suggesting that they were neither GABAA nor GABAB receptors and might be specific GHB receptors (Benavides et al., 1982). Some argued that these receptors were distinct from GABAB receptors in rat brain (thalamus and cortex) because GHB did not displace [3H]GABA binding and GABAB receptor ligands did not displace [3H]GHB binding more than 40% (Snead, 1996). Developmental data in rats also supported the notion of distinct binding sites; [3H]GABA binding appeared at postnatal day 3, whereas [3H]GHB binding was not observed until postnatal day 17 (Snead, 1994). Furthermore, [3H]GHB binding was not observed in HEK 293 cells transfected with recombinant GABAB receptors (Wu et al., 2004) and [3H]GHB binding was similar in wild type mice and genetically modified mice that lack functional GABAB receptors (Kaupmann et al., 2003).
Evidence showing that [3H]GHB binding in rat and human synaptosomal membranes was specific, competitive, saturable, pH-dependent, protein concentration-dependent, and brain region-dependent suggested that these specific binding sites were receptors (Snead and Liu, 1984). GHB receptors are thought to be metabotropic receptors because application of GHB to rat hippocampal slices stimulates cGMP and inositol phosphate production (Vayer and Maitre, 1989), and pertussis toxin inhibits [3H]GHB binding in rat brain (Snead, 1996). GHB has also been shown to induce 86Rb+ efflux in NCB-20 neurons, an effect that is not mediated by GABAB receptors, suggesting that modulation of K+ channels might be downstream of GHB receptor signaling (Kemmel et al., 2003). Moreover, studies that cloned GHB receptors from rat and human brain have reported that binding of [35S]GTP-γ-S to cloned human GHB receptors is stimulated by GHB and abolished by pertussis toxin (Andriamampandry et al., 2003; Andriamampandry et al., 2007).
There are a variety of pharmacological tools available to study GABAA receptors, including agonists (muscimol, THIP), antagonists (bicuculline), and positive (benzodiazepines, barbiturates), neutral (flumazenil), and negative (Ro 15-4513) allosteric modulators (Fig 1). There is also a growing list of commercially-available pharmacological tools to study GABAB receptors, including agonists (baclofen, SKF97541), antagonists (CGP35348, CGP52432), and most recently, positive allosteric modulators (CGP7930, GS39783, CGP13501; Fig 1). Unlike the selective ligands that are available for studying GABAA and GABAB receptors, there are very few selective and commercially available ligands for studying GHB receptors.
If GHB receptors are indeed functional receptors that are distinguishable from GABAB receptors, then it might be possible to develop selective GHB receptor ligands. In 1988, Maitre and colleagues reported that trans-4-hydroxycrotonic acid (T-HCA), an endogenous GHB analog, displaced [3H]GHB in rat brain (Bourguignon et al., 1988; Hechler et al., 1990; Cash et al., 1996). Further investigation into the structure activity relationship of ligands at the GHB receptor using GHB and T-HCA analogs led to the development of 5-(2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7] annulen-6-ylidene) ethanoic acid (NCS-382), the first putative antagonist at GHB receptors (Maitre et al., 1990; Bourguignon et al., 2000; Fig 1), which was later developed as a radioligand with in vitro selectivity for GHB receptors (Mehta et al., 2001; Gould et al., 2003). Subsequently, the T-HCA and NCS-382 structures have been extended to create a series of ligands that exhibit in vitro selectivity for the GHB receptor, however, the functional effects of these ligands in vitro and in vivo are largely unknown (Wellendorph et al., 2005).
Several other series of GHB-substituted compounds and ethers of 3-hydroxyphenylacetic acid that bind selectively to GHB receptors in vitro have been synthesized and studied in vivo (Wu et al., 2003; Macias et al., 2004; Carter et al., 2005a; Carter et al., 2005b; Chen et al., 2005). These compounds have been used in the behavioral procedures discussed below to examine the possible role of GHB receptors in the effects of GHB. The continued design and synthesis of selective GHB receptor ligands, especially neutral antagonists at GHB receptors, are critical for furthering our understanding of the role that GHB receptors play in the endogenous GHB signaling system and after exogenous administration of GHB and related drugs.
3. Behavioral effects of GHB
The first in vivo effects of GHB were described in 1964 as hypothermic, hypnotic, anesthetic, and anti-convulsant, and without marked respiratory depression or toxicity (Laborit, 1964). Since that time, the mechanisms underlying many of these effects have been further examined and elucidated. For example, as one of the initial and continued therapeutic interests in GHB centered around its potential to induce specific stages of sleep, many early studies focused on examining the electroencephalographic (EEG) effects of GHB. GHB and GBL (at 50–200 mg/kg) produced EEG patterns similar to those observed during slow wave sleep and absence seizures in rats (Winters and Spooner, 1965; Godschalk et al., 1977). The dopaminergic and opioidergic systems were initially implicated in the EEG effects of GHB because the absence seizure-like activity of GHB was blocked or reversed by d-amphetamine and naloxone, respectively (Snead, 1978; Snead and Bearden, 1980). However, later studies showed that selective GABAB receptor antagonists attenuated GHB-induced changes in EEG activity and decreases in firing rate and burst activity of dopaminergic neurons (Snead, 1996; Erhardt et al., 1998). Thus, it is likely that GABAB receptor-mediated inhibition of neurotransmitter release by GHB and baclofen is responsible for the absence seizure-like activity of these two drugs (Banerjee and Snead, 1995). Indeed, 100 mg/kg GBL did not result in absence seizure-like activity in transgenic mice that lack functional GABAB receptors (Kaupmann et al., 2003). Moreover, the GHB antagonist, NCS-382 has not always antagonized the EEG effects of GHB or baclofen, suggesting that GHB binding sites do not play a substantial role in mediating these effects (Snead, 1996; Erhardt et al., 1998; Tremblay et al., 1998).
The hypothermic effects of GHB that were described by Laborit have also been shown to be mediated by GABAB receptors (Quéva et al., 2003). Several other studies have shown that GHB and GHB precursors increase mean arterial pressure, heart rate (560–1,000 mg/kg, iv), and gastric emptying (100–300 mg/kg, ip or po) in rodents, and that these effects are mediated by GABAB receptors (Poggioli et al., 1999; Carai et al., 2002; Gerak et al., 2004; Hicks et al., 2004). Many studies, some of which are described below, have also implicated GABAB receptors as the primary mechanism mediating the hypnotic and anesthetic effects of GHB. The following part of this section on the in vivo effects of GHB will focus specifically on the behavioral effects of GHB and the pharmacological mechanisms of action that are thought to mediate those effects. Although some attention will be given to the acute, chronic, and reinforcing effects of GHB, the primary focus of the following section will be on the discriminative stimulus effects of GHB, and how those effects have been used to examine the mechanism of action of GHB under different conditions.
Consistent with the earliest reports of GHB producing general sedation and hypnosis (Laborit, 1964), GHB has been shown to reduce general locomotor activity and operant behavior in several different species. GHB and the GHB prodrugs, GBL and 1,4-BD, decrease locomotor activity in mice at doses ≥100 mg/kg, ip (Cook et al., 2002; de Fiebre et al., 2004; Carter et al., 2005b) and decrease the rate of operant responding for food in mice (≥200 mg/kg, ip), rats (≥320 mg/kg, ip), and baboons (≥180 mg/kg, ig; Cook et al., 2002; Carter et al., 2004b; Goodwin et al., 2005). At doses larger than those that decrease locomotor activity (doses from 300–1,780 mg/kg, ip), GHB and the GHB prodrugs produce ataxia and loss of righting (Carai et al., 2001; Cook et al., 2002; Carter et al., 2005b;Werawattanachai et al., 2007). Although the behavioral effects described above are not specific to GHB, antagonism studies show that GABAergic mechanisms are involved in these acute behavioral effects of GHB (Kaupmann et al., 2003; Carter et al., 2004b; Carter et al., 2005b).
In addition to sedative and anesthetic effects, GHB and the GHB prodrugs produce catalepsy (maintenance of an abnormal posture) at doses larger than those that decrease locomotion and smaller than those that produce ataxia (Snead and Bearden, 1980; Carter et al., 2005b; Koek et al., 2007b). Although other classes of drugs such as dopaminergic antagonists also produce catalepsy (Navarro et al., 1998; Sevak et al., 2004), the cataleptic effects of GHB and the dopaminergic antagonist haloperidol are differentially modulated by the NMDA receptor antagonist dizocilpine (MK-801), suggesting that different mechanisms are responsible for GHB- and haloperidol-induced catalepsy (Sevak et al., 2004; Sevak et al., 2005). Moreover, GABAB receptor agonists are also known to produce catalepsy (Balsara et al., 1981; Mehta and Ticku, 1987; Wüllner et al., 1987) and the cataleptic effects of GHB and GHB prodrugs are attenuated by GABAB receptor antagonists (Koek et al., 2007b).
There are a few reviews and case reports suggesting that acute doses of GHB produce lasting anterograde amnesia (i.e., memory loss) in humans (e.g., Schwartz et al., 2000; Varela et al., 2004); however, there have been few experimental studies that have examined the effects of GHB on learning and memory. Two studies reported that repeated doses of 100 mg/kg GHB (ip) impaired spatial learning and memory in rats (Sircar and Basak, 2004; García et al., 2006); other studies reported that single doses of GHB did not impair working memory or cognitive (Go/No-go) task performance in rats (up to 500 mg/kg, ip) and rhesus monkeys (up to 250 mg/kg, ig), respectively (Nakamura et al., 1987; Laraway et al., 2008). Data from experimental studies in humans suggest that doses of 10 mg/kg, iv and 4 g/70 kg, po of GHB can affect working memory and the encoding of episodic memory (Grove-White and Kelman, 1971; Carter et al., 2006b); however, the precise mechanism of action underlying these effects of GHB on memory and cognition is not established.
3.1. Tolerance, cross-tolerance, and withdrawal
Tolerance to the behavioral and neurochemical effects of GBL, 1,4-BD, and GHB has been demonstrated in controlled laboratory studies and typically requires large doses and frequent drug administration. Studies from several laboratories have shown that tolerance to the acute of effects of GBL on locomotor activity, catalepsy, and loss of righting develops in rodents treated chronically (i.e., 600–1,920 mg/kg/day, given in drinking water or as repeated injections) with GBL (Gianutsos and Moore, 1978; Giorgi and Rubio, 1981; Van Sassenbroeck et al., 2003). Likewise, tolerance to the acute effects of GHB on locomotor activity, catalepsy, motor coordination, and righting has been observed after repeated administration of large doses of GHB (200–16,000 mg/kg/day for 5–19 days) to rodents (Colombo et al., 1995c; Itzhak and Ali, 2002; Bania et al., 2003; Bhattacharya et al., 2006; Smith et al., 2006; Raybon and Boje, 2007).
In some studies in which tolerance to GHB was observed (after administration of 1,000 mg/kg/day GHB for 9 days), cross tolerance to the (ataxic) effects of ethanol (Colombo et al., 1995c) or the GABAB receptor agonist baclofen (but not the GABAA receptor positive modulator flunitrazepam; Smith et al., 2006) was also observed. Likewise, chronic administration of GBL (480–1,920 mg/kg/day for 13 days) resulted in cross tolerance to the locomotor decreasing effects of baclofen in mice (Gianutsos and Moore, 1978). Chronic treatment with 1,4-BD (640–1,120 mg/kg/day for 42 days) also resulted in cross tolerance to the (operant response) rate decreasing effects of GHB and baclofen in rats (Eckermann et al., 2004). Thus, cross-tolerance to baclofen has been demonstrated after repeated treatment with GHB, GBL, and 1,4-BD, suggesting that GABAB receptors are involved in these effects.
There have been numerous clinical reports of the development of physical dependence following frequent chronic use of illicit GHB as indicated by a withdrawal syndrome upon discontinuation of drug use (Craig et al., 2000; for review, see McDonough et al., 2004); however, many of these reports have not been confirmed by toxicological analysis showing that GHB, and not other substances, was primarily responsible for the reported withdrawal syndrome (Galloway et al., 1997; Hernandez et al., 1998; McDaniel and Miotto, 2001; Miotto et al., 2001). Further, patients did not report tolerance, or symptoms consistent with a GHB withdrawal syndrome in several controlled clinical studies in which therapeutic doses of GHB (3,500–10,000 mg/day or night) were repeatedly administered to humans (Mamelak et al., 1986; Gallimberti et al., 1992; Addolorato et al., 1996).
There have been two studies in which a spontaneous GHB withdrawal syndrome was observed in rats. In one study, doses of GHB (750–2,500 mg/kg) were administered intragastrically (ig) every six hours on the basis of an animal’s current level of intoxication (Bania et al., 2003). This dosing regimen resulted in a relatively mild spontaneous GHB withdrawal syndrome as measured by an observational ethanol intoxication-withdrawal scale (Bania et al., 2003). In a second study, GHB or 1,4-BD was administered intraperitoneally (ip) every three hours in an ascending dose sequence (250–2,000 mg/kg; Quang et al., 2006). With more frequent dosing, a more severe GHB (and 1,4-BD) withdrawal syndrome was observed, which included audiogenic seizures (Quang et al., 2006).
There have been two studies in baboons in which spontaneous and precipitated withdrawal from GHB and GBL has also been observed. In these studies, doses of GHB (350–750 mg/kg/day) or GBL (100–600 mg/kg/day) were administered continuously through an ig catheter (Weerts et al., 2005; Goodwin et al., 2006). In both studies, discontinuation of GHB or GBL treatment resulted in characteristic signs of withdrawal in this species (e.g., abnormal posture, tremor, aggression, vomiting). Moreover, administration of a GABAB receptor antagonist precipitated a similar emergence of withdrawal signs in GHB-treated and in GBL-treated baboons, suggesting that GABAB receptors played a role in mediating these effects (Weerts et al., 2005; Goodwin et al., 2006). If GABAB receptors mediate the dependence producing effects of GHB, then the GHB withdrawal syndrome should be alleviated or reversed by the administration of baclofen. Indeed, a recent clinical case report in which baclofen was administered to a woman undergoing GHB withdrawal suggests that the GHB withdrawal syndrome can be alleviated with baclofen (LeTourneau et al., 2008).
3.2. Reinforcing effects
Epidemiological studies show that the recreational use of GHB differs from many other drugs of abuse. While it is clear that GHB is used recreationally, particularly in combination with other drugs (Liechti et al., 2006; Dresen et al., 2007; Kim et al., 2007), laboratory studies have generally failed to show positive reinforcing effects of GHB under conditions where many other drugs of abuse are positive reinforcers. In procedures such as conditioned place preference that are thought to measure the rewarding effects of drugs, GHB (at doses of 175 and 350 mg/kg, ig in rats and 250 mg/kg, ip in mice) has been shown to produce place preference in rodents (Martellotta et al., 1997; Itzhak and Ali, 2002). Similarly, participants in human abuse liability studies have reported positive subjective effects after administration of doses of 28–114 mg/kg GHB, po (Abanades et al., 2006; Carter et al., 2006b; Abanades et al., 2007). However, in humans and non-humans GHB is typically not self-administered to the same extent as other drugs of abuse such as stimulants and opioids (for review, see Nicholson and Balster, 2001).
In 1995 Colombo et al. reported self-administration of GHB in a study in which rats drank more of a GHB (sodium salt) solution than water in a two-bottle choice procedure (Colombo et al., 1995a). However, using the same procedure, Sardinian alcohol-preferring and Sardinian alcohol-non-preferring rats consumed the same amount of sodium chloride solution as GHB solution, and did so in a (salt) concentration-dependent manner, suggesting that non-pharmacologic factors played a role in maintaining drinking in the previous study (Colombo et al., 1998a). In a subsequent study in which ethanol, GHB, and water were concurrently available to Sardinian alcohol-preferring rats, rats consumed ethanol instead of GHB, further suggesting that any positive reinforcing effects of GHB are modest (Serra et al., 2002).
Studies that have used iv self-administration procedures have also shown the reinforcing effects of GHB to be weak. Intravenous doses of 0.3–7.5 mg/kg/infusion GHB were not self-administered more than saline in rhesus monkeys trained to self-administer phencyclidine (Beardsley et al., 1996). In two out of three rhesus monkeys that were trained to self-administer the barbiturate methohexital, doses of 3.2 and 10 mg/kg/infusion GHB iv was self-administered more than saline, but less than methohexital (Woolverton et al., 1999). Similar results were found for GBL and 1,4-BD, which were not self-administered more than saline by rhesus monkeys trained to self-administer methohexital, with the exception of one monkey at one dose (0.32 mg/kg/infusion) of 1,4-BD (McMahon et al., 2003). Two studies reported iv self-administration of GHB in mice; however, in those studies it is not clear that the behavior was controlled by the drug. In both studies, the doses of GHB available for self-administration were very small (0.01–0.5 mg/kg/infusion) and there was not a clear relationship between the dose self-administered and the behavioral response (Martellotta et al., 1998; Fattore et al., 2001).
Perhaps the strongest evidence for positive reinforcing effects of GHB comes from a recent iv self-administration study in baboons and an abuse liability study in humans. Many iv self-administration procedures (such as the ones mentioned above) allow animals to frequently self-administer drug during sessions lasting an hour or two, and under such conditions fewer injections of GHB are self administered than are other known drugs of abuse (Beardsley et al., 1996; Woolverton et al., 1999). However, some drugs, including sedative/hypnotic drugs, are more readily self administered when the opportunity to receive drug occurs less frequently over an extended (e.g., 24-hr) session (see Griffiths and Weerts, 1997, for review). Under such conditions of extended, infrequent access, GHB (56–130 mg/kg/infusion) and GBL (56–78 mg/kg/infusion) were self-administered by baboons (Weerts et al., 2008). Indirect evidence of positive reinforcing effects of GHB comes from poly-sedative drug abusers who, after taking large doses (50–100 mg/kg) of GHB (under double-blind conditions), report that they would take the drug again and that they would be willing to pay for the drug (i.e., GHB) to the same extent as for other sedative drugs of abuse such as the benzodiazepine triazolam and the barbiturate pentobarbital (Carter et al., 2006b; also see Abanades et al., 2007).
3.3. Discriminative stimulus effects
Discrimination is a particularly useful behavioral procedure for studying the mechanism of action of a novel drug because across many classes, drugs can be distinguished and classified based on their discriminative stimulus effects, and because these classifications often correlate with a specific cellular mechanism of action (Schuster and Johanson, 1988). For example, if a drug with a known mechanism of action is trained as a discriminative stimulus, drugs with the same mechanisms of action at suitable doses typically occasion responding on the same lever as the training drug (i.e., drug-appropriate responding or %DR), consistent with their shared mechanism of action. The same approach can be used to study drugs for which the mechanism of action is not known, by comparing results with compounds with known mechanisms of action. Drug discrimination procedures have been developed in different species (mouse, rat, pigeon, monkey, human) and are often stable over time (e.g., many months without training; see Evola et al., 2007), allowing for the examination of different compounds, antagonism studies, and studies involving chronic drug treatment. As a result, drug discrimination procedures have been useful for studying the mechanism of action of many novel drugs, including GHB.
3.3.1. Effects of GHB in animals trained to discriminate other drugs from vehicle
Some of the earliest studies that examined the effects of GHB in animals trained to discriminate other drugs showed that GHB shared discriminative stimulus effects with the GABAA receptor antagonist pentylenetetrazol and not with the cannabinoid receptor agonist delta-9-tetrahydrocannabinol (Table 1; Shearman and Lal, 1980; Browne and Weissman, 1981). Subsequent studies expanded on these initial investigations by showing that GHB also occasioned drug-appropriate responding in animals discriminating baclofen (77% drug lever responding; Carter et al., 2004a), GBL (>80% drug lever responding; Baker et al., 2005), and in some cases, ethanol (Table 1; Colombo et al., 1995d; Metcalf et al., 2001;). However, GHB did not occasion drug-appropriate responding in all drug discrimination procedures in which it was studied. GHB did not occasion more than 30% drug-appropriate responding in animals discriminating d-amphetamine, phencyclidine, heroin, positive GABAA receptor modulators (e.g., triazolam, diazepam, pentobarbital), and, in some cases, ethanol (Table 1; Colombo et al., 1995d; Shelton, 2004). Likewise, GBL and 1,4-BD did not occasion drug-appropriate responding in animals trained to discriminate pentobarbital, midazolam, or diazepam (McMahon et al., 2003; Carter et al., 2004a). Taken together, these studies provided a basis for the further evaluation of specific GABAergic mechanisms in animals trained to discriminate GHB.
Table 1.
Drug-appropriate responding after administration of GHB in animals trained to discriminate other drugs.
| Training drug (dose, route) | Maximum %DR after administration of GHB* | N (%DR/total)* | Species |
|---|---|---|---|
| Baclofen (3.2 mg/kg, ip) | 77% | 7/11 | Rat (Sprague-Dawley)a |
| D-amphetamine (0.56 or 1 mg/kg, ig) | 29% | 4/4 | Rhesus monkeyb |
| Diazepam (1 mg/kg, ip) | 3% | 10/10 | Rat (Sprague-Dawley)a |
| Ethanol (1 g/kg, ig) | 82% | 6/6 | Rat (Long Evans)c |
| Ethanol (1 g/kg, ig) | 60% | 5/5 | Rat (Long Evans)d |
| Ethanol (2 g/kg, ig) | < 10% | 5/5 | Rat (Long Evans)c |
| Ethanol (1.5 g/kg, ip) | 7% | 5/5 | Mouse (C57BL/6J)e |
| Ethanol (1.5 g/kg, ip) | 2% | 2/4 | Mouse (DBA/2J)e |
| Flumazenil (0.1 mg/kg im) | < 24% | 7/7 | Pigeon (Carneau)f |
| Flumazenil (0.1 or 0.32 mg/kg sc) | 7% | 2/2 | Rhesus monkeyb (5.6 mg/kg diazepam daily) |
| GBL (150 mg/kg, ip) | > 80% | 8/8 | Rat (Sprague-Dawley)g |
| Heroin (0.3 mg/kg, sc) | 20% | 5/5 | Rat (Sprague-Dawley)h |
| PCP (2 mg/kg, ip) | 25% | 4/4 | Rat (Sprague-Dawley)h |
| PCP (2 mg/kg, ip) | 1% | 12/12 | Rat (Sprague-Dawley)i |
| Pentobarbital (10 mg/kg, ig) | 0% | 3/3 | Rhesus monkeyb |
| Pentylenetetrazol (20 mg/kg, ip) | 100% | 4/4 | Rat (Long Evans)j |
| THC (3.2 mg/kg, ip) | NR** | 2/10 | Rat (Sprague-Dawley)k |
| Triazolam (0.1 mg/kg, sc) | 27% | 3/3 | Rhesus monkeyb |
| UMB 86 (100 mg/kg, ip) | 1% | 3/3 | Rat (Sprague-Dawley)l |
The maximum %DR averaged across animals is reported when at least half of the animals responded. N is reported as the number of animals that responded (%DR) over the number of animals tested (total).
NR – not reported, because less than half of the animals responded at the dose(s) studied.
Carter et al., unpublished data.
3.3.2. Discriminative stimulus effects of GHB
In 1981, Winter studied drugs with different mechanisms of action in rats discriminating 200 mg/kg GHB ip from saline (Winter, 1981). As expected, not all drugs that were studied in these animals occasioned GHB-appropriate responding. Consistent with studies in which the effects of GHB were examined in animals discriminating other drugs, GABAergic ligands tended to occasion the most GHB-appropriate responding. Of all the drugs that were studied, baclofen occasioned the greatest amount of GHB-appropriate responding (70%), followed by GBL, the GABAA receptor agonists muscimol and 3-amino-propane sulfonic acid, and the positive GABAA receptor modulator chlordiazepoxide (each about 50% GHB-appropriate responding; Winter, 1981). Serotonergic and dopaminergic agonists did not occasion responding on the GHB-appropriate lever. Thus, even early on, results from animals discriminating GHB suggested a role for GABA receptors, particularly GABAB receptors, in the behavioral effects of GHB.
In a subsequent study in which rats were trained to discriminate ip injections of 200 mg/kg GHB (same dose as the Winter, 1981 study) using procedures that are commonly used for drug discrimination studies (two lever, fixed ratio schedule of responding for food), baclofen occasioned almost 80% GHB-appropriate responding, whereas several different GABAA receptor positive modulators and GBL occasioned less GHB-appropriate responding (Carter et al., 2003). Similar results were found in studies in which rats discriminated 300 mg/kg GHB ig. In these animals, baclofen, and to a lesser extent benzodiazepine positive modulators (e.g., diazepam, alprazolam, flunitrazepam), occasioned GHB-appropriate responding; however, in rats discriminating a larger dose of 700 mg/kg GHB ig, baclofen occasioned GHB-appropriate responding, and diazepam did not (Colombo et al., 1998b; Lobina et al., 1999; Baker et al., 2004; Baker et al., 2005; Baker et al., 2008). As the specificity of a drug’s discriminative stimulus generally increases with training dose (e.g., Koek et al., 2006b), these data suggested that while both GABAA and GABAB receptors appear to play a role in the effects of small doses of GHB, GABAB receptors appear to be predominantly involved in the discriminative stimulus effects of large doses of GHB.
Pigeons, like rats, have also been trained to discriminate GHB, and there have been several drug discrimination studies in pigeons that have also examined the role of GABAA and GABAB receptors in mediating the effects of GHB. As in rats, GHB, GHB prodrugs, and baclofen occasioned substantial GHB-appropriate responding in pigeons discriminating GHB (Koek et al., 2004). However, unlike data from rats discriminating GHB, drugs acting at different sites on the GABAA receptor tend to occasion greater GHB-appropriate responding in pigeons than rats. In these studies, ethanol, pentobarbital, the neutral GABAA receptor modulator flumazenil (benzodiazepine site antagonist), and the negative GABAA receptor modulator Ro 15-4513 (benzodiazepine site inverse agonist), occasioned substantial GHB-appropriate responding, suggesting that GABAA receptors might be more important to the discriminative stimulus effects of GHB in pigeons than in rats (Koek et al., 2004). The finding that flumazenil and Ro 15-4513 occasioned GHB-appropriate responding also suggested that GHB might exert effects at diazepam-insensitive GABAA receptors in the pigeon (Wong et al., 1993; Koek et al., 2004), as these compounds bind to subtypes of the GABAA receptor to which diazepam does not bind. However, when pigeons were trained to discriminate flumazenil (which binds to diazepam-sensitive and diazepam-insensitive GABAA receptors) from vehicle, GHB, GHB prodrugs, and GABAB receptor agonists did not occasion flumazenil-appropriate responding (Koek et al., 2006a). The asymmetric substitution profile of GHB and flumazenil in pigeons (at diazepam-insensitive GABAA receptors) is consistent with the idea that the discriminative stimulus effects of GHB in pigeons involve several components, each mediated by different receptors (i.e., GABA receptors, diazepam-insensitive GABAA receptors). Whether these receptors are involved in the discriminative stimulus effects of GHB in rats awaits further studies (with different training doses).
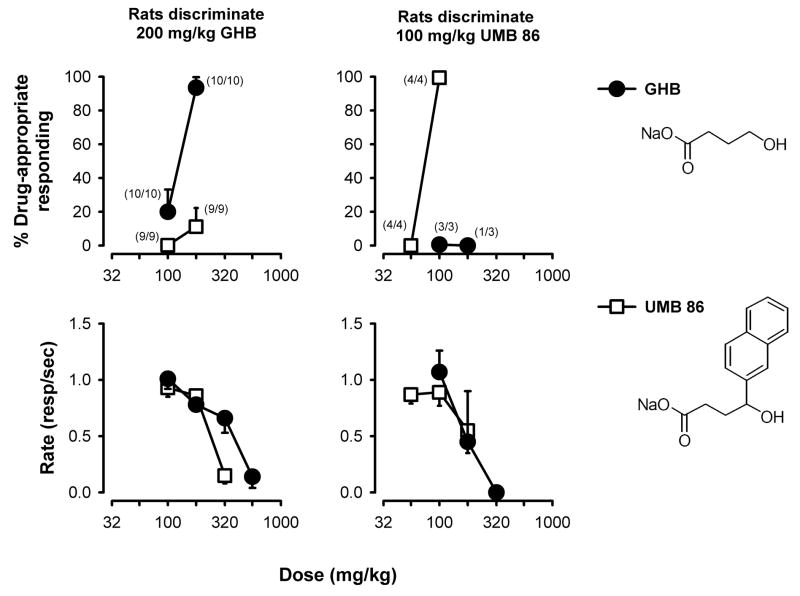
As illustrated in Figure 1, GHB binds to GHB receptors, which also might mediate some of the behavioral effects of GHB. Gamma-hydroxyvalerate (GHV) is a 4-methyl-substituted analog of GHB that is not metabolized to GABA and has lower affinity for GABAA and GABAB receptors as compared to GHB receptors (Carter et al., 2005a). GHV and other selective GHB receptor ligands such as UMB 86 (Fig 2) appear to lack GHB-like discriminative stimulus effects (Wu et al., 2003; Carter et al., 2005a; Carter et al., 2005b). These data, together with those mentioned above, indicate that the discriminative stimulus effects of GHB are not exclusively due to actions at GHB receptors. In addition, the putative GHB receptor antagonist NCS-382 occasions partial GHB-appropriate responding in rats and pigeons discriminating GHB (Carter et al., 2003; Koek et al., 2004). Thus, initial results from a variety of studies using different training and testing drugs indicated that GABAA, GABAB, and GHB receptors played a role in the discriminative stimulus effects of GHB; however, the relative importance of each of these mechanisms to the behavioral effects of GHB was not entirely clear.
Figure 2.
Effects of GHB and the GHB receptor ligand UMB 86 in rats discriminating GHB or UMB 86. The percentage of responses on the drug-appropriate lever (top panels) and the rate of responding (responses/sec; bottom panels) are plotted as a function of dose. Data points and error bars represent the mean ± 1 S.E.M. for 9 or 10 animals discriminating GHB from vehicle (left panels; replotted from Carter et al., 2005b) or for 3 or 4 animals discriminating UMB 86 from vehicle (right panels). The number of animals contributing to each data point in the top panels in given in the numerator adjacent to each point. The structures of the compounds studied are shown on the right-hand side of the figure.
3.3.3. Effects of GHB in animals trained to discriminate a GABAA, GABAB, or GHB receptor ligand from vehicle
Although results obtained in animals discriminating GHB implicated GABAB receptors as being important for the discriminative stimulus effects of GHB, there remained some questions with regard to the additional involvement of GABAA receptors and GHB receptors in these effects. Because drugs acting at GABAA (e.g., diazepam), GABAB (e.g., baclofen), and GHB receptors (e.g., NCS-382) occasioned some GHB-appropriate responding, it was possible that the discriminative stimulus effects of GHB might be a complex stimulus, consisting of multiple components and involving multiple pharmacological mechanisms. Thus, one approach to try to isolate the individual components of the suspected GHB discriminative stimulus was to train different groups of animals to discriminate the individual components of a complex stimulus. This section reviews the effects of GHB and GHB prodrugs in animals trained to discriminate drugs acting at GABAA, GABAB, or GHB receptors.
In studies that have examined the effects of GHB in animals trained to discriminate diazepam (GABAA component) or baclofen (GABAB component), GHB and GHB prodrugs occasioned baclofen-appropriate responding (Carter et al., 2004a), whereas neither GHB nor GHB prodrugs occasioned substantial diazepam-appropriate responding (Carter et al., 2004a). These data are consistent with GABAB receptor agonists occasioning greater GHB-appropriate responding and more GHB-appropriate responding across a larger range of GHB training doses, as compared to results obtained with compounds acting at GABAA receptors (Carter et al., 2003; Koek et al., 2006b). The finding that GHB, GHB precursors, and GABAB receptor agonists occasion similar levels of drug-appropriate responding in animals discriminating GHB and in animals discriminating baclofen, and that these compounds typically do not occasion drug-appropriate responding in animals discriminating diazepam or ethanol, further supports a predominant role for GABAB receptors in the discriminative stimulus effects of GHB in rats (Carter et al., 2004a; Baker et al., 2008).
The recent design and synthesis of selective GHB receptor ligands facilitated studies of GHB receptors in the behavioral effects of GHB. Figure 2 shows data from animals that were trained to discriminate the GHB receptor ligand UMB 86 using procedures identical to those used to train animals to discriminate GHB. UMB 86 is a GHB analog that displaces 50% of [3H]NCS-382 from GHB receptors at a concentration of 13 μM (IC50) and only 30% and 10% of [3H]GABA from GABAA and GABAB receptors, respectively, at a 75-fold higher concentration of 1 mM (Carter et al., 2005b). UMB 86 also shares some behavioral effects with GHB (e.g., decreased locomotion, ataxia, loss of righting), but unlike GHB, GHB precursors, and GABAB receptor agonists, UMB 86 does not produce catalepsy or GHB-like discriminative stimulus effects (Carter et al., 2005b). Data presented in Figure 2 show that GHB did not occasion UMB 86-appropriate responding (Carter et al., unpublished observations). Similarly, baclofen was ineffective as a substitute for the UMB 86 discriminative stimulus (data not shown). These studies further support the view that GHB receptors play a minor role in mediating the discriminative stimulus effects of GHB.
3.3.4. Antagonism of the discriminative stimulus effects of GHB
Although there is a strong predictive relationship between discriminative stimulus effects of drugs and their pharmacological mechanisms of action, antagonism studies are required to confirm that a pharmacodynamic interaction between the ligands and the receptors is responsible for the observed effects. If, as the substitution data suggest, the discriminative stimulus effects of GHB are largely mediated by GABAB receptors, then these effects should be attenuated by selective GABAB receptor antagonists.
Table 2 summarizes discrimination studies in which drugs have been studied for their ability to antagonize the discriminative stimulus effects of GHB. GABAB receptor antagonists reliably attenuate the discriminative stimulus effects of GHB. In rats discriminating 300 or 700 mg/kg GHB ig from vehicle, CGP35348 attenuated the discriminative stimulus effects of GHB (Colombo et al., 1998b; Baker et al., 2005; Baker et al., 2008). In rats discriminating 200 mg/kg GHB, CGP35348 attenuated the discriminative stimulus effects of GHB, baclofen, and another GABAB receptor agonist SKF97541 (Carter et al., 2003; Carter et al., 2006a). In pigeons discriminating GHB, CGP35348 antagonized the discriminative stimulus effects of GHB across several different training doses (Koek et al., 2004; Koek et al., 2006b). Thus, across procedures, species, and training doses, the discriminative stimulus effects of GHB are attenuated by the GABAB receptor antagonist, CGP35348.
Table 2.
Drug discrimination studies in which the putative antagonism of GHB by another drug was studied.
| Training drug (dose, route) | Drug(s) studied with GHB | Species | % Decrease of GHB-appropriate responding |
|---|---|---|---|
| GHB (56 mg/kg, im) | CGP35348 | Pigeon (Carneau)a | 67% |
| Ketamine | 8% | ||
| GHB (100 mg/kg, im) | CGP35348 | 68% | |
| DTT | 0% | ||
| Ketamine | 17% | ||
| GHB (178 mg/kg, im) | CGP35348 | 90% | |
| DTT | 6% | ||
| Flumazenil | 17% | ||
| Haloperidol | 9% | ||
| Ketamine | 0% | ||
| NCS-382 | 0% | ||
|
| |||
| GHB (100 mg/kg, im) | CGP35348 | Pigeon (Carneau)b | 76% |
| Flumazenil | 17% | ||
| Haloperidol | 17% | ||
| Naltrexone | 11% | ||
| NCS-382 | 9% | ||
|
| |||
| GHB (100 mg/kg, im) | GHV | Pigeon (Carneau)c | 13% |
|
| |||
| GHB (100 mg/kg, im) | 3-HPA | Pigeon (Carneau)d | 9% |
| UMB 72 | 25% | ||
| UMB 86 | 27% | ||
|
| |||
| GHB (200 mg/kg, ip) | CGP35348 | Rat (Sprague-Dawley)e | 64% |
|
| |||
| GHB (200 mg/kg, ip) | CGP35348 | Rat (Sprague-Dawley)f | 81% |
| Flumazenil | 16% | ||
| NCS-382 | 54% | ||
|
| |||
| GHB (200 mg/kg GHB, ip) vs. | CGP35348 | Rat (Sprague-Dawley)g | 73% |
| saline or baclofen | CGP52432 | 82% | |
| DTT | 33% | ||
| NCS-382 | 44% | ||
| UMB 86 | 18% | ||
| GHB (200 mg/kg GHB, ip) vs. | CGP35348 | 87% | |
| saline, baclofen, or diazepam | CGP52432 | 93% | |
| DTT | 62% | ||
| NCS-382 | 37% | ||
| UMB 86 | 50% | ||
|
| |||
| GHB (200 mg/kg, ip) | 3-HPA | Rat (Sprague-Dawley)d | 39% |
| UMB 72 | 31% | ||
| UMB 86 | 16% | ||
|
| |||
| GHB (200 mg/kg, ip) | GHV | Rat (Sprague-Dawley)c | 39% |
| Baclofen (3.2 mg/kg, ip) | GHV | 37%h | |
|
| |||
| GHB (300 mg/kg GHB, ig) vs. | CGP35348 | Rat (Sprague-Dawley)i | 94%j |
| 1 g/kg ethanol | NCS-382 | 79% | |
|
| |||
| GHB (300 mg/kg, ig) | CGP35348 | Rat (Sprague-Dawley)k | 80%j |
| NCS-382 | 45% | ||
|
| |||
| GHB (300 mg/kg, ig) | CGP35348 | Rat (Long Evans)l | 65%j |
| GHB (700 mg/kg, ig) | CGP35348 | 85%j | |
|
| |||
| GHB (300 mg/kg, ig) | NCS-382 | Rat (Long Evans)m | 92%j |
| GHB (700 mg/kg, ig) | NCS-382 | 88%j | |
|
| |||
| GHB (600 mg/kg, ig) | DTT | Rats (Long Evans)n | 80%j |
|
| |||
| Baclofen (3.2 mg/kg, ip) | CGP35348 | Rat (Sprague-Dawley)o | 78% |
In these studies;
Values obtained using Graph Digitizer (Datatrend Software);
Carter et al., 2004a; dose of GHB antagonized was 200 mg/kg, ip.
In several of the studies cited above, flumazenil was studied together with GHB to examine a potential antagonism of effects of GHB that might be mediated by the benzodiazepine site on the GABAA receptor. The GHB-like discriminative stimulus effects of diazepam were antagonized by flumazenil, whereas the discriminative stimulus effects of GHB were not (Carter et al., 2003). Likewise, doses of flumazenil that did not occasion GHB-appropriate responding in pigeons did not attenuate the discriminative stimulus effects of GHB in those animals (Koek et al., 2006b). Together, these data suggest that although allosteric modulation of GABAA receptors might play a role in the behavioral effects of GHB at smaller doses, modulation of GABAA receptors at benzodiazepine sites does not appear to play a major role in the effects of large doses of GHB. Studies examining direct or indirect interactions between GHB and the neurosteroid site on GABAA receptors might help further elucidate the potential role of GABAA receptors in the behavioral effects of GHB.
If, as suggested by others, NCS-382 is an antagonist at GHB receptors and if the discriminative stimulus effects of GHB are mediated by GHB receptors, then NCS-382 should block the discriminative stimulus effects of GHB. In fact, the discriminative stimulus effects of GHB in rats trained to discriminate 300 or 700 mg/kg GHB ig were completely attenuated by NCS-382 (Colombo et al., 1995b). However, studies that have further examined the ability of NCS-382 to antagonize the discriminative stimulus effects of GHB or GBL have reported no more than partial antagonism by NCS-382 (Carter et al., 2003; Baker et al., 2005; Koek et al., 2006b). In one study NCS-382 failed to completely attenuate the discriminative stimulus effects of GHB and occasioned some GHB-appropriate responding when given alone (Carter et al., 2003). The partial antagonism of GHB’s discriminative stimulus effects by NCS-382 in that study contrasts with the complete antagonism of the discriminative stimulus effects of GHB and baclofen by CGP35348 in the same animals (Carter et al., 2003). Moreover, in pigeons trained to discriminate GHB from vehicle, NCS-382 occasioned as much GHB-appropriate responding as baclofen and the discriminative stimulus effects of NCS-382 and baclofen were attenuated by CGP35348 (Koek et al., 2004), suggesting that NCS-382 might have agonist effects at GABAB receptors.
Thus, it is not clear whether NCS-382 is a neutral GHB receptor antagonist, largely because it shares discriminative stimulus effects (Koek et al., 2004) with GHB under some conditions, as well as other effects (e.g., decreased intestinal motility, Carai et al., 2002; see Castelli et al., 2004 for review). The apparent shortcomings of NCS-382 as a GHB receptor antagonist highlights the compelling need for the development of better ligands for studying the role of GHB receptors.
Although in large part the discriminative stimulus effects of GHB and baclofen appear to be very similar and are attenuated by selective GABAB receptor antagonists such as CGP35348 and CGP52432, there are some differences between GHB and baclofen and the antagonism of their behavioral effects (e.g., catalepsy; Koek et al., 2007b). For example, in different groups of rats trained to discriminate GHB (200 mg/kg, ip) or baclofen (3.2 mg/kg, ip) from vehicle, CGP35348 attenuated the discriminative stimulus effects of GHB and baclofen; however, approximately 10-fold larger doses of CGP35348 were required to attenuate the effects of GHB and baclofen in rats discriminating baclofen compared to rats discriminating GHB (c.f., Carter et al., 2003; Carter et al., 2004a). Likewise, CGP35348 shifts the baclofen and SKF97541 dose-effect curves further rightward than the GHB dose-effect curve (Carter et al., 2004b; Koek et al., 2005; Carter et al., 2006a).
Differences between baclofen and GHB might involve differential effects on glutamatergic neurons or NMDA receptors. GHB and PCP enhance each others discriminative stimulus effects, whereas baclofen and PCP do not (Koek et al., 2007a). Similarly, the NMDA receptor antagonists ketamine, PCP, and dizocilpine enhance the cataleptic effects of GHB, but not baclofen, a phenomenon that is highly correlated with the potency of the NMDA receptor antagonists to attenuate NMDA-induced convulsions (Koek and France, 2008). Taken together, there is a consistent body of evidence to suggest that the pharmacological mechanisms of GHB and baclofen are not identical, which is consistent with the different therapeutic indications and apparent abuse potential of the two compounds. For example, a recent 3 month clinical trial in narcoleptic patients showed that although baclofen and GHB both increased EEG delta activity during nocturnal sleep, only GHB reduced subjective excessive daytime sleepiness and attacks of cataplexy in these patients (Huang and Guilleminault, 2008).
3.3.5. Effects of GHB in animals trained to discriminate GHB from other drugs
If the discriminative stimulus effects of GHB and baclofen are identical, then animals should not be able to discriminate between the two drugs. However, if the effects of GHB and baclofen are not identical, then animals should be able to discriminate between GHB and baclofen. This premise was evaluated in a study that attempted to train rats to discriminate between GHB (one lever) and vehicle or baclofen (a second lever, with vehicle or baclofen administered on different days) using doses of GHB and baclofen that were most similar in other discrimination studies. A second study attempted to train other rats to discriminate between GHB (one lever) and either vehicle, baclofen, or diazepam (a second lever, with different injections on different days [Koek et al., 2005]). These two discriminations can not be based solely on agonism at GABAB receptors or GABAA receptors, respectively, because a compound that acts at that receptor is paired with each lever. The purpose of this study was to train a discrimination based on stimuli that were not shared by the different training drugs. Thus, any effect shared by, for example, GHB and baclofen would not be the basis for the ability of animals to discriminate between GHB and baclofen. To the extent that GHB and baclofen have common actions at GABAB receptors, those actions would not be the basis of such a discrimination procedure. Rats reliably discriminated GHB, in one study from vehicle or baclofen, and in the second study from vehicle, baclofen, or diazepam. That the discrimination could be established indicates that the discriminative stimulus effects of GHB were not identical to baclofen or to diazepam (Koek et al., 2005). In these drug versus drug(s) discrimination procedures, baclofen and diazepam no longer occasioned GHB-appropriate responding, as they did in rats discriminating GHB from vehicle (Koek et al., 2005).
If GHB receptors play a greater role in the GHB discriminative stimulus under these drug versus drug(s) discrimination procedures, then the effects of GHB receptor ligands (e.g., NCS-382) would be greater and the effects of GABAB receptor ligands would be less, as compared to conditions in which GHB is discriminated from vehicle only. Interestingly, neither NCS-382 nor other GHB receptor ligands occasioned GHB-appropriate responding in animals discriminating GHB from vehicle or baclofen, or in those discriminating GHB from vehicle, baclofen, or diazepam (Koek et al., 2005). In addition, NCS-382 remained ineffective at attenuating the discriminative stimulus effects of GHB in animals discriminating GHB from baclofen, diazepam, or vehicle, suggesting that whatever component of the GHB discriminative stimulus that the animals were trained to discriminate did not involve actions at GHB receptors (Koek et al., 2005).
The effects of different selective GABAB receptor antagonists in animals trained to discriminate GHB from vehicle or other drug(s) suggested that subtypes of the GABAB receptor might be important in mediating the effects of GHB. Although baclofen was paired with the opposite lever in both groups of animals, presumably eliminating any GABAB receptor component of the GHB discriminative stimulus, two selective GABAB receptor antagonists CGP35348 and CGP52432 attenuated the discriminative stimulus effects of GHB in these animals (Koek et al., 2005). Interestingly, the potency of CGP35348 to attenuate the effects of GHB was not changed relative to its potency to attenuate the discriminative stimulus effects of GHB in animals trained to discriminate GHB from vehicle only (Koek et al., 2005); however, the potency of CGP52432 was decreased relative to its potency to attenuate the discriminative stimulus effects of GHB in animals trained to discriminate GHB from vehicle only (Koek et al., 2005). Collectively these results suggest that different GABAB receptors or receptor mechanisms contribute to the discriminative stimulus effects of GHB and of baclofen, perhaps including different subtypes of GABAB receptors or different mechanisms (direct agonism or modulation) at the same receptor subtypes.
One possible explanation for the potency difference of CGP52432 between the groups of animals is that GABAB autoreceptors and GABAB heteroreceptors might play a differential role in the effects of GHB and baclofen. CGP52432 is a GABAB receptor antagonist that, in contrast to CGP35348, exhibits a 100-fold greater Ki at GABAB heteroreceptors (GABAB receptors on glutamatergic neurons) than autoreceptors (GABAB receptors on GABAergic neurons; Lanza et al., 1993). In contrast to the similar potency of CGP35348 across conditions, pairing baclofen with the opposite lever such that the baclofen-like effects of GHB are unlikely to underlie the discrimination, decreased the potency of CGP52423 (autoreceptor-preferring) to antagonize GHB (Koek et al., 2005). That finding suggests that the remaining component of the GHB discriminative stimulus might be more closely related to activity at glutamatergic GABAB heteroreceptors, whereas the baclofen component might be more closely related to activity at (GABAergic) GABAB autoreceptors.
4. Summary and future directions
A growing number of studies, particularly drug discrimination studies, have provided evidence that the behavioral effects of GHB are mediated predominantly by GABAB receptors. However, there are also data that suggest that other mechanisms such as GHB receptors and subtypes of GABAA and GABAB receptors might contribute to the effects of GHB. These findings are consistent with the different behavioral profile, therapeutic indications, and abuse liability of GHB and baclofen. A better understanding of the similarities and differences between GHB and baclofen, as well as the pharmacological mechanism of action underlying the therapeutic and recreational effects of GHB will likely lead to more effective medications with fewer adverse effects.
There is substantial evidence that most, if not all, of the acute behavioral effects of GHB are mediated, at least in part, by GABAB receptors. In addition to discriminative stimulus effects, GABAB receptor antagonists have also been shown to attenuate GHB-induced changes in brain activity, neurotransmitter release (Snead, 1996; Erhardt et al., 1998; Tremblay et al., 1998), mean arterial pressure, heart rate (Gerak et al., 2004; Hicks et al., 2004), gastric emptying (Carai et al., 2002), body temperature, locomotion, (Kaupmann et al., 2003; Quéva et al., 2003), catalepsy (Koek et al., 2007b), operant responding (Cook et al., 2002; Carter et al., 2004b; Goodwin et al., 2005), ataxia, and loss of righting (Carai et al., 2001; Cook et al., 2002; Carter et al., 2005b;Werawattanachai et al., 2007).
Additional evidence that the behavioral effects of GHB are mediated by GABAB receptors comes from studies in which cross-tolerance to baclofen has been demonstrated after repeated treatment with GHB (Smith et al., 2006), GBL (Gianutsos and Moore, 1978), and 1,4-BD (Eckermann et al., 2004), suggesting that chronic treatment with GHB and GHB prodrugs might result in a down regulation or de-coupling of GABAB receptors. Moreover, the administration of a GABAB receptor antagonist can precipitate the emergence of withdrawal signs in baboons that have been chronically treated with GHB or GBL (Weerts et al., 2005; Goodwin et al., 2006).
Consistent with other data, drug discrimination studies implicate GABAB receptors as playing a predominant role in the effects of GHB. Baclofen has been shown to occasion GHB-appropriate responding across different laboratories, in a variety of species, and using a range of different training doses of GHB (Winter, 1981; Colombo et al., 1998b; Carter et al., 2003; Baker et al., 2004; Koek et al., 2004; Baker et al., 2008). The discriminative stimulus effects of baclofen and GHB in animals discriminating GHB have also been shown to be attenuated by selective GABAB receptor antagonists (Colombo et al., 1998b; Carter et al., 2003; Koek et al., 2004; Baker et al., 2005; Baker et al., 2008). Similarly, GHB and GHB prodrugs occasion baclofen-appropriate responding, and the discriminative stimulus effects of baclofen and GHB are attenuated by GABAB receptor antagonists in these animals (Carter et al., 2004a).
For the most part, drug discrimination studies have failed to support a role for GHB receptors in the discriminative stimulus effects of GHB. Selective ligands for GHB receptors do not occasion GHB-appropriate responding in rats or pigeons discriminating GHB (Carter et al., 2005a; Carter et al., 2005b) and, in most cases, the GHB discriminative stimulus is not attenuated by NCS-382 (Carter et al., 2003; Baker et al., 2005; Koek et al., 2006b). In animals discriminating GHB from vehicle, baclofen, or diazepam, the GHB stimulus does not appear to involve a prominent GHB receptor-mediated component. What remains, however, is a stimulus that appears to be mediated by a subtype of GABAB receptor. Thus, although the discriminative stimulus effects of GHB appear to be mediated largely by GABAB receptors, there might be interesting differences in the mechanism of action of GHB and the prototypical GABAB receptor agonist baclofen that involve glutamatergic signaling.
Although most of the research reviewed here provides evidence that the behavioral effects of GHB are predominantly mediated by, and dependent upon, activity at GABAB receptors, there are differences between GHB and baclofen that will likely lead to interesting findings if they are explored further. Several studies that have examined the effects of GHB on glutamate release have shown that the effects of low (nM) concentrations of GHB are antagonized by NCS-382, and not CGP35348, whereas, the effects of higher (mM) concentrations are antagonized by CGP35348, and not NCS-382 (Banerjee and Snead, 1995; Ferraro et al., 2001; Castelli et al., 2003). These data suggest that the effects of low concentrations of GHB on glutamate release might be mediated by GHB receptors and that higher concentrations of GHB on glutamate release might be mediated by GABAB receptors. These data appear to be consistent with NCS-382 occasioning GHB appropriate-responding in pigeons that were trained to lower, but not higher, doses of GHB (Koek et al., 2006b). Thus, the ability to detect GHB receptor mediated effects is likely to be dependent on the dose at which the effect is observed and the relative importance of glutamatergic signaling to that effect.
With regard to the effects of GHB at higher concentrations, an additional layer of complexity is involved when the possibility of GABAB receptor heterogeneity is considered. Previous studies have argued for the existence of GABAB receptor subtypes on the basis of the localization of the receptors and their subsequent ability to inhibit GABA or glutamate release. Animals can discriminate between GHB and baclofen, and might be able to do so on the basis of GHB and baclofen acting at different subtypes of GABAB receptors (Koek et al., 2005). Moreover, recent studies showing that NMDA antagonists enhance the baclofen-like discriminative stimulus and cataleptic effects of GHB, but not baclofen, provide further evidence that the mechanisms mediating the effects of GHB and baclofen are not identical (Koek et al., 2007a; Koek and France, 2008). The possibility that glutamatergic mechanisms might underlie differences between GHB and baclofen is particularly intriguing in light of a growing understanding of the role of glutamatergic signaling in disorders of central pain sensitization such as migraine and fibromyalgia (Sarchielli et al., 2007; Harris et al., 2008). There are ongoing Phase III clinical trials evaluating the clinical efficacy and safety of GHB (sodium oxybate) in fibromyalgia syndrome.
Although clinically effective, the precise mechanism of action of GHB that accounts for its utility in treating the symptoms associated with narcolepsy is still unknown. It is somewhat paradoxical that although most of the behavioral effects of GHB appear to be mediated by GABAB receptors, the prototypical GABAB receptor agonist baclofen is not used as a therapeutic for narcolepsy (Lioresal/baclofen is FDA approved for the treatment of spasticity and muscle rigidity associated with multiple sclerosis). The therapeutic effects of GHB in narcoleptic patients do not appear to be due to a general sedative/hypnotic effect per se (rather, due to a specific increase in delta power and duration of slow wave sleep; see Pardi and Black, 2006). It has been suggested that the effects of GHB on slow wave sleep might be mediated by GHB or GABAB receptors; however, there is little direct evidence to support this idea (Mignot et al., 2002; Pardi and Black, 2006). Moreover, a recent clinical trial showed that although GHB and baclofen increased EEG delta activity during nocturnal sleep, only GHB reduced subjective daytime sleepiness and attacks of cataplexy (Huang and Guilleminault, 2008). A better understanding of the pharmacological mechanism of action of GHB that underlies its clinically relevant effects could lead to the development of drugs that are more effective in treating sleep (and related) disorders and that have fewer undesired effects.
Acknowledgments
Supported in part by USPHS Grants DA15692 (WK) and DA14986 (CPF) and a Senior Scientist Award (DA17918) to CPF. Financial disclosure: Lawrence Carter is an employee of Jazz Pharmaceuticals Inc. and has financial interests in the company.
Abbreviations
- 1,4-BD
1,4-butanediol
- CGP35348
3-aminopropyl(diethoxymethyl) phosphinic acid
- CGP52432
3-[[(3,4-dichlorophenyl)methyl]amino]propyl]diethoxymethyl) phosphinic acid
- GBL
gamma-butyrolactone
- GHB
gamma-hydroxybutyrate
- ig
intragastric
- ip
intraperitoneal
- NCS-382
(5-[3H]-(2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7] annulen-6-ylidene) ethanoic acid)
- SKF97541
3-aminopropyl(methyl) phosphinic acid
Footnotes
Dedication
This review is dedicated to Maharaj (Raj) Ticku, Ph.D., who passed away unexpectedly on November 6th, 2007. Raj was a collaborator on many of the studies presented in this review. He was an extremely accomplished scientist, a talented mentor, and a good friend. Those of us who had the privilege of working with Raj have benefitted immensely from his scientific expertise, keen insight, and good humor.
For the purpose of this review, the chemical name GHB will be used to refer to endogenous gamma-hydroxybutyric acid, chemical grade gamma-hydroxybutyrate, and pharmaceutical grade sodium oxybate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abanades S, Farré M, Barral D, Torrens M, Closas N, Langohr K, et al. Relative abuse liability of gamma-hydroxybutyric acid, flunitrazepam, and ethanol in club drug users. J Clin Psychopharmacol. 2007;27:625–638. doi: 10.1097/jcp.0b013e31815a2542. [DOI] [PubMed] [Google Scholar]
- Abanades S, Farré M, Segura M, Pichini S, Barral D, Pacifici R, et al. Gamma-hydroxybutyrate (GHB) in humans: pharmacodynamics and pharmacokinetics. Ann N Y Acad Sci. 2006;1074:559–576. doi: 10.1196/annals.1369.065. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Castelli E, Stefanini GF, Casella G, Caputo F, Marsigli L, et al. An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects. GHB Study Group. Alcohol Alcohol. 1996;31:341–345. doi: 10.1093/oxfordjournals.alcalc.a008160. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Balducci G, Capristo E, Attilia ML, Taggi F, Gasbarrini G, et al. Gamma-hydroxybutyric acid (GHB) in the treatment of alcohol withdrawal syndrome: a randomized comparative study versus benzodiazepine. Alcohol Clin Exp Res. 1999;23:1596–1604. [PubMed] [Google Scholar]
- Aldrete JA, Barnes DP. 4-Hydroxybutyrate anaesthesia for cardiovascular surgery. A comparison with halothane. Anaesthesia. 1968;23:558–565. doi: 10.1111/j.1365-2044.1968.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Andriamampandry C, Taleb O, Kemmel V, Humbert JP, Aunis D, Maitre M. Cloning and functional characterization of a gamma-hydroxybutyrate receptor identified in the human brain. FASEB J. 2007;21:885–895. doi: 10.1096/fj.06-6509com. [DOI] [PubMed] [Google Scholar]
- Andriamampandry C, Taleb O, Viry S, Muller C, Humbert JP, Gobaille S, et al. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB) FASEB J. 2003;17:1691–1693. doi: 10.1096/fj.02-0846fje. [DOI] [PubMed] [Google Scholar]
- Andronova LM, Barkov NK. The effect of neuroleptics, tranquilizers, narcotics, antidepressants and anticonvulsive drugs on the alterations of mouse behaviour caused by acetaldehyde. Drug Alcohol Depend. 1981;8:85–92. doi: 10.1016/0376-8716(81)90103-4. [DOI] [PubMed] [Google Scholar]
- Association of Chief Police Officers (2006). Report on results of OPERATION MATISSE.
- Baker LE, Pynnonen D, Poling A. Influence of reinforcer type and route of administration on gamma-hydroxybutyrate discrimination in rats. Psychopharmacology. 2004;174:220–227. doi: 10.1007/s00213-003-1744-z. [DOI] [PubMed] [Google Scholar]
- Baker LE, Searcy GD, Pynnonen DM, Poling A. Differentiating the discriminative stimulus effects of gamma-hydroxybutyrate and ethanol in a three-choice drug discrimination procedure in rats. Pharmacol Biochem Behav. 2008;89:598–607. doi: 10.1016/j.pbb.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Baker LE, Van Tilburg TJ, Brandt AE, Poling A. Discriminative stimulus effects of gamma-hydroxybutyrate (GHB) and its metabolic precursor, gamma-butyrolactone (GBL) in rats. Psychopharmacology. 2005;181:458–466. doi: 10.1007/s00213-005-0003-x. [DOI] [PubMed] [Google Scholar]
- Balsara JJ, Muley MP, Vaidya AS, Chandorkar AG. Effects of baclofen on dopamine-dependent behaviors in mice. Psychopharmacology. 1981;75:396–399. doi: 10.1007/BF00435861. [DOI] [PubMed] [Google Scholar]
- Banerjee PK, Snead OC., 3rd Presynaptic gamma-hydroxybutyric acid (GHB) and gamma-aminobutyric acidB (GABAB) receptor-mediated release of GABA and glutamate (GLU) in rat thalamic ventrobasal nucleus (VB): a possible mechanism for the generation of absence-like seizures induced by GHB. J Pharmacol Exp Ther. 1995;273:1534–1543. [PubMed] [Google Scholar]
- Bania TC, Ashar T, Press G, Carey PM. Gamma-hydroxybutyric acid tolerance and withdrawal in a rat model. Acad Emerg Med. 2003;10:697–704. doi: 10.1111/j.1553-2712.2003.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML. Neurosteroidogenesis: relevance to neurosteroid actions in brain and modulation by psychotropic drugs. Crit Rev Neurobiol. 2004;16:67–74. doi: 10.1615/critrevneurobiol.v16.i12.70. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Carai MA, Colombo G, Lobina C, Purdy RH, Gessa GL. Endogenous gamma-aminobutyric acid (GABA)(A) receptor active neurosteroids and the sedative/hypnotic action of gamma-hydroxybutyric acid (GHB): a study in GHB-S (sensitive) and GHB-R (resistant) rat lines. Neuropharmacology. 2005;49:48–58. doi: 10.1016/j.neuropharm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Colombo G, Affricano D, Carai AM, Vacca G, Melis S, et al. GABAB receptor-mediated increase of neurosteroids by γ-hydroxybutyric acid. Neuropharmacology. 2002;42:782–791. doi: 10.1016/s0028-3908(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Barker JC, Harris SL, Dyer JE. Experiences of gamma hydroxybutyrate (GHB) ingestion: a focus group study. J Psychoactive Drugs. 2007;39:115–129. doi: 10.1080/02791072.2007.10399870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Evaluation of the discriminative stimulus and reinforcing effects of gammahydroxybutyrate (GHB) Psychopharmacology. 1996;127:315–322. doi: 10.1007/s002130050092. [DOI] [PubMed] [Google Scholar]
- Beghè F, Carpanini MT. Safey and tolerability of gamma-hydroxybutyrc acid in the treatment of alcohol-dependent patients. Alcohol. 2000;20:223–225. doi: 10.1016/s0741-8329(99)00085-3. [DOI] [PubMed] [Google Scholar]
- Bellis MA, Hughes K, Bennett A, Thomson R. The role of an international nightlife resort in the proliferation of recreational drugs. Addiction. 2003;98:1713–1721. doi: 10.1111/j.1360-0443.2003.00554.x. [DOI] [PubMed] [Google Scholar]
- Benavides J, Rumigny JF, Bourguignon JJ, Cash C, Wermuth CG, Mandel P, et al. High affinity binding sites for gamma-hydroxybutyric acid in rat brain. Life Sci. 1982;30:953–961. doi: 10.1016/0024-3205(82)90624-5. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Skolnik SJ. Gamma hydroxybutyrate and gamma butyrolactone: concentration in rat tissues during anesthesia. Science. 1964;143:1045–1047. doi: 10.1126/science.143.3610.1045. [DOI] [PubMed] [Google Scholar]
- Bhattacharya I, Raybon JJ, Boje KMK. Alterations in neuronal transport but not blood-brain barrier transport are observed during gamma-hydroxybutyrate (GHB) sedative/hypnotic tolerance. Pharm Res. 2006;23:2067–2077. doi: 10.1007/s11095-006-9066-6. [DOI] [PubMed] [Google Scholar]
- Biggio G, Cibin M, Diana M, Fadda F, Ferrara SD, Gallimberti L, et al. Suppression of voluntary ethanol intake in rats and alcoholics by gamma-hydroxybutyric acid: a non-GABAergic mechanism. Adv Biochem Psychopharmacol. 1992;47:281–288. [PubMed] [Google Scholar]
- Bonanno G, Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993;14:259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Bourguignon JJ, Schmitt M, Didier B. Design and structure-activity relationship analysis of ligands of gamma-hydroxybutyric acid receptors. Alcohol. 2000;20:227–236. doi: 10.1016/s0741-8329(99)00086-5. [DOI] [PubMed] [Google Scholar]
- Bourguignon JJ, Schoenfelder A, Schmitt M, Wermuth CG, Hechler V, Charlier B, et al. Analogues of gamma-hydroxybutyric acid. Synthesis and binding studies. J Med Chem. 1988;31:893–897. doi: 10.1021/jm00400a001. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, et al. (−)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brennan MJ, Cantrill RC, Oldfield M, Krogsgaard-Larsen P. Inhibition of gamma-aminobutyric acid release by gamma-aminobutyric acid agonist drugs. Pharmacology of the gamma-aminobutyric acid autoreceptor. Mol Pharmacol. 1981;19:27–30. [PubMed] [Google Scholar]
- Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6:1–6. doi: 10.1017/s0317167100119304. [DOI] [PubMed] [Google Scholar]
- Broughton R, Mamelak M. Effects of nocturnal gamma-hydroxybutyrate on sleep/waking patterns in narcolepsy-cataplexy. Can J Neurol Sci. 1980;7:23–31. [PubMed] [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of Δ9-tetrahydrocannabinol: mechanistic studies. J Clin Pharmacol. 1981;21:227S–234S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Carai MA, Agabio R, Lobina C, Reali R, Vacca G, Colombo G, et al. GABA(B)-receptor mediation of the inhibitory effect of gamma-hydroxybutyric acid on intestinal motility in mice. Life Sci. 2002;70:3059–3067. doi: 10.1016/s0024-3205(02)01553-9. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, et al. Role of GABAB receptors in the sedative/hypnotic effect of γ-hydroxybutyric acid. Eur J Pharmacol. 2001;428:315–321. doi: 10.1016/s0014-2999(01)01334-6. [DOI] [PubMed] [Google Scholar]
- Carai MA, Vacca G, Serra S, Colombo G, Froestl W, Gessa GL. Suppression of GABAB receptor function in vivo by disulfide reducing agent, DL-dithiothreitol (DTT) Psychopharmacology. 2004;174:283–290. doi: 10.1007/s00213-003-1737-y. [DOI] [PubMed] [Google Scholar]
- Carter LP, Chen W, Coop A, Koek W, France CP. Discriminative stimulus effects of GHB and GABA(B) agonists are differentially attenuated by CGP35348. Eur J Pharmacol. 2006a;538:85–93. doi: 10.1016/j.ejphar.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Carter LP, Chen W, Wu H, Mehta AK, Hernandez RJ, Ticku MK, et al. Comparison of the behavioral effects of gamma-hydroxybutyric acid (GHB) and its 4-methyl-substituted analog, gamma-hydroxyvaleric acid (GHV) Drug Alcohol Depend. 2005a;78:91–99. doi: 10.1016/j.drugalcdep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, et al. The role of GABAB receptors in the discriminative stimulus effects of γ-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006b;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Carter LP, Unzeitig AW, Wu H, Chen W, Coop A, Koek W, et al. The discriminative stimulus effects of gamma-hydroxybutyrate (GHB) and related compounds in rats discriminating baclofen or diazepam: the role of GABAB and GABAA receptors. J Pharmacol Exp Ther. 2004a;309:540–547. doi: 10.1124/jpet.103.062950. [DOI] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Cruz CM, Lamb RJ, Koek W, et al. Effects of GHB on schedule-controlled responding in rats: role of GHB and GABAB receptors. J Pharmacol Exp Ther. 2004b;308:182–188. doi: 10.1124/jpet.103.058909. [DOI] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Matthews MM, Mehta AK, Hernandez RJ, et al. Novel gamma-hydroxybutyric acid (GHB) analogs share some, but not all behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther. 2005b;313:1314–1323. doi: 10.1124/jpet.104.077578. [DOI] [PubMed] [Google Scholar]
- Cash CD, Hechler H, Mersel M, Maitre M. Kinetic characterisation and solubilisation of γ-hydroxybutyrate receptors from rat brain. Neurosci Lett. 1996;209:25–28. doi: 10.1016/0304-3940(96)12589-1. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Ferraro L, Mocci I, Carta F, Carai MAM, Antonelli T, et al. Selective γ-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of γ-hydroxybutyric acid. J Neurochem. 2003;87:722–732. doi: 10.1046/j.1471-4159.2003.02037.x. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Pibiri F, Carboni G, Piras AP. A review of pharmacology of NCS-382, a putative antagonist of gamma-hydroxybutyric acid (GHB) receptor. CNS Drug Rev. 2004;10:243–260. doi: 10.1111/j.1527-3458.2004.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Epidemiologic notes and reports multistate outbreak of poisonings associated with illicituse of gamma hydroxy butyrate. MMWR. 1990;39:861–863. [PubMed] [Google Scholar]
- Chen W, Wu H, Hernandez J, Mehta AK, Ticku MK, France CP, et al. Ethers of 3-hydroxyphenylacetic acid as selective gamma-hydroxybutyric acid receptor ligands. Bioorg Med Chem Lett. 2005;15:3201–3202. doi: 10.1016/j.bmcl.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Chin RL, Sporer KA, Cullison B, Dyer JE, Wu TD. Clinical course of gamma-hydroxybutyrate overdose. Ann Emerg Med. 1998;31:716–722. [PubMed] [Google Scholar]
- Colombo G, Agabio R, Balaklievskaia N, Diaz G, Lobina C, Reali R, et al. Oral self-administration of gamma-hydroxybutyric acid in the rat. Eur J Pharmacol. 1995a;285:103–107. doi: 10.1016/0014-2999(95)00493-5. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Bourguignon J, Fadda F, Lobina C, Maitre M, et al. Blockade of the discriminative stimulus effects of γ-hydroxybutyric acid (GHB) by the GHB receptor antagonist NCS-382. Physiol Behav. 1995b;58:587–590. doi: 10.1016/0031-9384(95)00086-x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Fa M, Lobina C, Reali R, et al. Gamma-hydroxybutyric acid intake in ethanol-preferring sP and nonpreferring sNP rats. Physiol Behav. 1998a;64:197–202. doi: 10.1016/s0031-9384(98)00033-x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Fadda F, Gessa GL. Cross-tolerance to ethanol and gamma-hydroxybutyric acid. Eur J Pharmacol. 1995c;273:235–238. doi: 10.1016/0014-2999(94)00687-3. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Fadda F, Gessa GL. Symmetrical generalization between the discriminative stimulus effects of gamma-hydroxybutyric acid and ethanol: occurrence within narrow dose ranges. Physiol Behav. 1995d;57:105–111. doi: 10.1016/0031-9384(94)00215-q. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Gessa GL. Involvement of GABAA and GABAB receptors in the mediation of discriminative stimulus effects of γ-hydroxybutyric acid. Physiol Behav. 1998b;64:293–302. doi: 10.1016/s0031-9384(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Cook CD, Aceto MD, Coop A, Beardsley PM. Effects of the putative antagonist NCS382 on the behavioral pharmacological actions of gammahydroxybutyrate in mice. Psychopharmacology. 2002;160:99–106. doi: 10.1007/s00213-001-0951-8. [DOI] [PubMed] [Google Scholar]
- Craig K, Gomez HF, McManus JL, Bania TC. Severe gamma-hydroxybutyrate withdrawal: a case report and literature review. J Emerg Med. 2000;18:65–70. doi: 10.1016/s0736-4679(99)00163-8. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Emri Z, Leresche N. Unraveling the brain targets of gamma-hydroxybutyric acid. Curr Opin Pharmacol. 2006;6:44–52. doi: 10.1016/j.coph.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, de Fiebre NEC, Coleman SL, Forster MJ. Comparison of the actions of gamma-butyrolactone and 1,4-butanediol in Swiss-Webster mice. Pharmacol Biochem Behav. 2004;77:705–710. doi: 10.1016/j.pbb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Darke S, Dillon P. GHB use among Australians: characteristics, use patterns and associated harms. Drug Alcohol Depend. 2002;67:89–94. doi: 10.1016/s0376-8716(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Dresen S, Kempf J, Weinmann W. Prevalence of gamma-hydroxybutyrate (GHB) in serum samples of amphetamine, methamphetamine and ecstasy impaired drivers. Forensic Sci Int. 2007;173:112–116. doi: 10.1016/j.forsciint.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Dyer JE. γ-Hydroxybutyrate: a health-food product producing coma and seizurelike activity. Am J Emerg Med. 1991;9:321–324. doi: 10.1016/0735-6757(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Eckermann KA, Koek W, France CP. Chronic 1,4-butanediol treatment in rats: cross tolerance to gamma-hydroxybutyrate and (±)-baclofen. Eur J Pharmacol. 2004;484:259–262. doi: 10.1016/j.ejphar.2003.11.016. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Salamone SJ. Prevalence of drugs used in cases of alleged sexual assault. J Anal Toxicol. 1999;23:141–146. doi: 10.1093/jat/23.3.141. [DOI] [PubMed] [Google Scholar]
- Emri Z, Antal K, Crunelli V. Gamma-hydroxybutyric acid decreases thalamic sensory excitatory postsynaptic potentials by an action on presynaptic GABAB receptors. Neurosci Lett. 1996;216:121–124. doi: 10.1016/0304-3940(96)13016-0. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Andersson B, Nissbrandt H, Engberg G. Inhibition of firing rate and changes in the firing pattern of nigral dopamine neurons by gamma-hydroxybutyric acid (GHBA) are specifically induced by activation of GABA(B) receptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:611–619. doi: 10.1007/pl00005215. [DOI] [PubMed] [Google Scholar]
- Evola M, McCorvy JD, Young AM. Retention of drug stimulus control in pigeons trained to a three-key morphine discrimination. Abstract for the CPDD 69th Annual Scientific Meeting; 2007. [accessed Aug 28, 2008]. http://www.cpdd.vcu.edu/Pages/Meetings/2007AbstractBook.html. [Google Scholar]
- Fadda F, Argiolas A, Melis MR, De Montis G, Gessa GL. Suppression of voluntary ethanol consumption in rats by gamma-butyrolactone. Life Sci. 1983;32:1471–1477. doi: 10.1016/0024-3205(83)90913-x. [DOI] [PubMed] [Google Scholar]
- Fadda F, Colombo G, Mosca E, Gessa GL. Suppression by gamma-hydroxybutyric acid of ethanol withdrawal syndrome in rats. Alcohol Alcohol. 1989;24:447–451. [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta C, Deiana S, Fratta W. Baclofen antagonises intravenous self-administration of γ-hydroxybutyric acid in mice. Neuroreport. 2001;12:2243–2246. doi: 10.1097/00001756-200107200-00039. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O’Connor WT, Francesconi W, Loche A, Gessa GL, et al. γ-Hydroxybutyrate modulation of glutamate levels in the hippocampus: an in vivo and in vitro study. J Neurochem. 2001;78:929–939. doi: 10.1046/j.1471-4159.2001.00530.x. [DOI] [PubMed] [Google Scholar]
- Fuller DE, Hornfeldt CS. From club drug to orphan drug: sodium oxybate (Xyrem) for the treatment of cataplexy. Pharmacotherapy. 2003;23:1205–1209. doi: 10.1592/phco.23.10.1205.32756. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Canton G, Gentile N, Ferri M, Cibin M, Ferrara SD, et al. Gamma-hydroxybutyric acid for treatment of alcohol withdrawal syndrome. Lancet. 1989;30:787–789. doi: 10.1016/s0140-6736(89)90842-8. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Ferri M, Ferrara SD, Fadda F, Gessa GL. γ-Hydroxybutyric acid in the treatment of alcohol dependence: a double-blind study. Alcohol Clin Exp Res. 1992;16:673–676. doi: 10.1111/j.1530-0277.1992.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Frederick SL, Staggers FE, Jr, Gonzales M, Stalcup SA, Smith DE. Gamma-hydroxybutyrate: an emerging drig of abuse that causes physical dependence. Addiction. 1997;92:89–96. [PubMed] [Google Scholar]
- García FB, Pedraza C, Arias JL, Navarro JF. Effects of subchronic administration of gamma-hydroxybutyrate (GHB) and spatial working memory in rats. Psicothema. 2006;18:519–524. [PubMed] [Google Scholar]
- Gerak LR, Hicks AR, Winsauer PJ, Varner KJ. Interaction between 1,4-butanediol and ethanol on operant responding and the cardiovascular system. Eur J Pharmacol. 2004;506:75–82. doi: 10.1016/j.ejphar.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Gianutsos G, Moore KE. Tolerance to the effects of baclofen and gamma-butyrolactone on locomotor activity and dopaminergic neurons in the mouse. J Pharmacol Exp Ther. 1978;207:859–869. [PubMed] [Google Scholar]
- Giarman NJ, Roth RH. Differential estimation of gamma-butyrolactone and gamma-hydroxybutyric acid in rat blood and brain. Science. 1964;145:583–584. doi: 10.1126/science.145.3632.583. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Rubio MC. Decreased 3H-L-quinuclidinyl benzilate binding and muscarine receptor subsensitivity after chronic gamma-butyrolactone treatment. Naunyn Schmiedebergs Arch Pharmacol. 1981;318:14–18. doi: 10.1007/BF00503306. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Dzoljic MR, Bonta IL. Slow wave sleep and a state resembling absence epilepsy induced in the rat by gamma-hydroxybutyrate. Eur J Pharmacol. 1977;44:105–111. doi: 10.1016/0014-2999(77)90096-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Nutt DJ. Gamma hydroxy butyrate abuse and dependency. J Psychopharm. 2005;19:195–204. doi: 10.1177/0269881105049041. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology. 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM, et al. Chronic intragastric administration of gamma-butyrolactone produces physical dependence in baboons. Psychopharmacology. 2006;189:71–82. doi: 10.1007/s00213-006-0534-9. [DOI] [PubMed] [Google Scholar]
- Gould GG, Mehta AK, Frazer A, Ticku MK. Quantitative autoradiographic analysis of the new radioligand [3H](2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene) ethanoic acid ([3H]NCS-382) at γ-hydroxybutyric acid (GHB) binding sites in rat brain. Brain Res. 2003;979:51–56. doi: 10.1016/s0006-8993(03)02865-8. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals – implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Grove-White IG, Kelman GR. Effects of methohexitone, diazepam and sodium 4-hydroxybutyrate on short-term memory. Br J Anaesth. 1971;43:113–116. doi: 10.1093/bja/43.2.113. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Ballotti PL. Relationship between pharmacological effects and blood and brain levels of gamma-butyrolactone and gamma-hydroxybutyrate. Biochem Pharmacol. 1970;19:883–894. doi: 10.1016/0006-2952(70)90251-0. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Palamar JJ. GHB use among gay and bisexual men. Addict Behav. 2006;31:2135–2139. doi: 10.1016/j.addbeh.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Palamar JJ, Mukherjee PP. Poly-club-drug use among gay and bisexual men: a longitudinal analysis. Drug Alcohol Depend. 2007;89:153–160. doi: 10.1016/j.drugalcdep.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58:903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- Hechler V, Schmitt M, Bourguignon JJ, Maitre M. Trans-gamma-hydroxycrotonic acid binding sites in brain: evidence for a subpopulation of gamma-hydroxybutyrate sites. Neurosci Lett. 1990;110:204–209. doi: 10.1016/0304-3940(90)90812-n. [DOI] [PubMed] [Google Scholar]
- Hernandez M, McDaniel CH, Costanza CD, Hernandez OJ. GHB-induced delirium: a case report and review of the literature of gamma-hydroxybutyric acid. Am J Drug Alcohol Abuse. 1998;24:179–183. doi: 10.3109/00952999809001706. [DOI] [PubMed] [Google Scholar]
- Hicks AR, Kapusta DR, Varner KJ. Mechanisms underlying the sympathomimetic cardiovascular responses elicited by γ-hydroxybutyrate. J Cardiovasc Pharmacol. 2004;44:631–638. doi: 10.1097/00005344-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Hösli L, Hösli E, Lehmann R, Schneider J, Borner M. Action of γ-hydroxybutyrate and GABA on neurones of cultured rat central nervous system. Neurosci Lett. 1983;37:257–260. doi: 10.1016/0304-3940(83)90440-8. [DOI] [PubMed] [Google Scholar]
- Huang Y, Guilleminault C. Narcolepsy-cataplexy: action of two GABA-2 agonists, baclofen and sodium oxybate on clinical symptoms. Sleep. 2008;31:A213. [Google Scholar]
- Itzhak Y, Ali SF. Repeated administration of gamma-hydroxybutyric acid (GHB) to mice: assessment of the sedative and rewarding effects of GHB. Ann N Y Acad Sci. 2002;965:451–460. doi: 10.1111/j.1749-6632.2002.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, et al. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABAB(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- Kemmel V, Taleb O, Andriamampandry C, Aunis D, Maitre M. Gamma-hydroxybutyrate receptor function determined by stimulation of rubidium and calcium movements from NCB-20 neurons. Neuroscience. 2003;116:1021–1031. doi: 10.1016/s0306-4522(02)00662-0. [DOI] [PubMed] [Google Scholar]
- Kim SY, Anderson IB, Dyer JE, Barker JC, Blanc PD. High-risk behaviors and hospitalizations among gamma hydroxybutyrate (GHB) users. Am J Drug Alcohol Abuse. 2007;33:429–438. doi: 10.1080/00952990701312316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintz P, Villain M, Cirimele V, Ludes B. GHB in postmortem toxicology Discrimination between endogenous production from exposure using multiple specimens. Forensic Sci Int. 2004;143:177–181. doi: 10.1016/j.forsciint.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt S, Grundmann U, Janneck U, Kreienmeyer J, Kulosa R, Larsen R. Total intravenous anaesthesia using propofol, gamma-hydroxybutyrate or midazolam in combination with sufentanil for patients undergoing coronary artery bypass surgery. Eur J Anaesthesiol. 1997;14:590–599. doi: 10.1046/j.1365-2346.1994.00181.x. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt S, Grundmann U, Knocke T, Silomon M, Bach F, Larsen R. Total intravenous anaesthesia with gamma-hydroxybutyrate (GHB) and sufentanil in patients undergoing coronary artery bypass graft surgery: a comparison in patients with unimpaired and impaired left ventricular function. Eur J Anaesthesiol. 1998;15:559–564. doi: 10.1046/j.1365-2346.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- Koek W, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, et al. Discriminative stimulus effects of γ-hydroxybutyrate (GHB) in rats discriminating GHB from baclofen and diazepam. J Pharmacol Exp Ther. 2005;314:170–179. doi: 10.1124/jpet.105.083394. [DOI] [PubMed] [Google Scholar]
- Koek W, Carter LP, Wu H, Coop A, France CP. Discriminative stimulus effects of flumazenil: perceptual masking by baclofen, and lack of substitution with gamma-hydroxybutyrate and its precursors 1,4-butanediol and gamma-butyrolactone. Behav Pharmacol. 2006a;17:239–247. doi: 10.1097/00008877-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Koek W, Chen W, Mercer SL, Coop A, France CP. Discriminative stimulus effects of gamma-hydroxybutyrate: role of training dose. J Pharmacol Exp Ther. 2006b;317:409–417. doi: 10.1124/jpet.105.096909. [DOI] [PubMed] [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, et al. Discriminative stimulus effects of gamma-hydroxybutyrate (GHB) in pigeons: involvement of diazepam-sensitive and -insensitive GABAA receptors and of GABAB receptors. J Pharmacol Exp Ther. 2004;308:904–911. doi: 10.1124/jpet.103.056093. [DOI] [PubMed] [Google Scholar]
- Koek W, France CP. Cataleptic effects of gamma-hydroxybutyrate (GHB) and baclofen in mice: mediation by GABA(B) receptors, but differential enhancement by N-methyl-d-aspartate (NMDA) receptor antagonists. Psychopharmacology. 2008;199:191–198. doi: 10.1007/s00213-008-1160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Khanal M, France CP. Synergistic interactions between ‘club drugs’: gamma-hydroxybutyrate and phencyclidine enhance each other’s discriminative stimulus effects. Behav Pharmacol. 2007a;18:807–810. doi: 10.1097/FBP.0b013e3282f18d45. [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology. 2007b;192:407–414. doi: 10.1007/s00213-007-0718-y. [DOI] [PubMed] [Google Scholar]
- Laborit H. Sodium 4-hydroxybutyrate. Int J Neuropharmacol. 1964;32:433–451. doi: 10.1016/0028-3908(64)90074-7. [DOI] [PubMed] [Google Scholar]
- Laborit H, Jouany JM, Gerard J, Fabiani F. Summary of an experimental and clinical study on a metabolic substrate with inhibitory central action: sodium 4-hydroxybutyrate. Presse Med. 1960;68:1867–1869. [PubMed] [Google Scholar]
- Lanza M, Fassio A, Gemignani A, Bonanno G, Raiteri M. CGP 52432: a novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol. 1993;237:191–195. doi: 10.1016/0014-2999(93)90268-m. [DOI] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Baker LE, Poling A. Gamma-hydroxybutyrate (GHB) reduces operant behavior without impairing working memory in rats responding under fixed-consecutive-number schedules. Pharmacol Biochem Behav. 2008;88:205–212. doi: 10.1016/j.pbb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, Morris-Kukoski C, Schaff JE, Deakin A. Further evidence of in vitro production of gamma-hydroxybutyrate (GHB) in urine samples. Forensic Sci Int. 2007;169:152–156. doi: 10.1016/j.forsciint.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Lee T, Chen S, Su S, Yang S. Baclofen intoxication: report of four cases and review of the literature. Clin Neuropharmacol. 1992;15:56–62. [PubMed] [Google Scholar]
- LeTourneau JL, Hagg DS, Smith SM. Baclofen and gamma-hydroxybutyrate withdrawal. Neurocrit Care. 2008;8:430–433. doi: 10.1007/s12028-008-9062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Stokes SA, Woeckener A. A tale of novel intoxication: seven cases of gamma-hydroxybutyric acid overdose. Ann Emerg Med. 1998;31:723–728. doi: 10.1016/s0196-0644(98)70231-8. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Kunz I, Greminger P, Speich R, Kupferschmidt H. Clinical features of gamma-hydroxybutyrate and gamma-butyrolactone toxicity and concomitant drug and alcohol use. Drug Alcohol Depend. 2006;81:323–326. doi: 10.1016/j.drugalcdep.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lingenhoehl K, Brom R, Heid J, Beck P, Froestl W, Kaupmann K, et al. γ-hydroxybutyrate is a weak agonist at recombinant GABAB receptors. Neuropharmacology. 1999;38:1667–1673. doi: 10.1016/s0028-3908(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Liu B, Hattori N, Jiang B, Nakayama Y, Zhang NY, Wu B, et al. Single cell RT-PCR demonstrates differential expression of GABAC receptor rho subunits in rat hippocampal pyramidal and granule cells. Brain Res Mol Brain Res. 2004;123:1–6. doi: 10.1016/j.molbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Lobina C, Agabio R, Reali R, Gessa GL, Colombo G. Contribution of GABAA and GABAB receptors to the discriminative stimulus produced by gamma-hydroxybutyric acid. Pharmacol Biochem Behav. 1999;64:363–365. doi: 10.1016/s0091-3057(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Macias AT, Hernandez RJ, Mehta AK, MacKerell AD, Jr, Ticku MK, Coop A. 3-Chloropropanoic acid (UMB66): a ligand for the gamma-hydroxybutyric acid receptor lacking a 4-hydroxyl group. Bioorg Med Chem. 2004;12:1643–1647. doi: 10.1016/j.bmc.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Maitre M, Hechler V, Vayer P, Gobaille S, Cash CD, Schmitt M, et al. A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties. J Pharmacol Exp Ther. 1990;255:657–663. [PubMed] [Google Scholar]
- Mamelak M. Alzheimer’s disease, oxidative stress and gammahydroxybutyrate. Neurobiol Aging. 2007;28:1340–1360. doi: 10.1016/j.neurobiolaging.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Scharf MB, Woods M. Treatment of narcolepsy with gamma-hydroxybutyrate. A review of clinical and sleep laboratory findings. Sleep. 1986;9:285–289. doi: 10.1093/sleep/9.1.285. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Intravenous self-administration of gamma-hydroxybutyric acid in drug-naïve mice. Eur Neuropsychopharmacology. 1998;8:293–296. doi: 10.1016/s0924-977x(97)00087-4. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Fattore L, Cossu G, Fratta W. Rewarding properties of gamma-hydroxybutyric acid: an evaluation through place preference paradigm. Psychopharmacology. 1997;132:1–5. doi: 10.1007/s002130050312. [DOI] [PubMed] [Google Scholar]
- Maxwell R, Roth RH. Conversion of 1,4-butanediol to -hydroxybutyric acid in rat brain and in peripheral tissue. Biochem Pharmacol. 1972;21:1521–1533. doi: 10.1016/0006-2952(72)90301-2. [DOI] [PubMed] [Google Scholar]
- McDaniel CH, Miotto KA. Gamma hydroxybutyrate (GHB) and gamma butyrolactone (GBL) withdrawal: five case studies. J Psychoactive Drugs. 2001;33:143–149. doi: 10.1080/02791072.2001.10400479. [DOI] [PubMed] [Google Scholar]
- McDonough M, Kennedy N, Glasper A, Bearn J. Clinical features and management of gamma-hydroxybutyrate (GHB) withdrawal: a review. Drug Alcohol Depend. 2004;75:3–9. doi: 10.1016/j.drugalcdep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Coop A, France CP, Winger G, Woolverton WL. Evaluation of the reinforcing and discriminative stimulus effects of 1,4-butanediol and gamma-butyrolactone in rhesus monkeys. Eur J Pharmacol. 2003;466:113–120. doi: 10.1016/s0014-2999(03)01486-9. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Muschaweck NM, Maeda DY, Coop A, Ticku MK. Binding characteristics of the γ-hydroxybutyric acid receptor antagonist [3H](2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acid in the rat brain. J Pharmacol Exp Ther. 2001;299:1148–1153. [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Baclofen induces catatonia in rats. Neuropharmacology. 1987;26:1419–1423. doi: 10.1016/0028-3908(87)90108-0. [DOI] [PubMed] [Google Scholar]
- Metcalf BR, Stahl JM, Allen JD, Woolfolk DR, Soto PL. Discrimination of gamma-hydroxybutyrate an ethanol administered separately and as a mixture in rats. Pharmacol Biochem Behav. 2001;70:31–41. doi: 10.1016/s0091-3057(01)00579-2. [DOI] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5:1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Miotto KA, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: patterns of use, effects and withdrawal. Am J Addict. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- Nakamura RK, Myslobodsky MS, Coppola R, Johannesen-Conway J, Mirsky AF. Effects of gamma-hydroxybutyrate on the performance of monkeys in a Go/No-go visual discrimination task. Behav Brain Res. 1987;26:19–27. doi: 10.1016/0166-4328(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Pedraza C, Martin M, Manzaneque JM, Davila G, Maldonado E. Tiapride-induced catalepsy is potentiated by gamma-hydroxybutyric acid administration. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:835–844. doi: 10.1016/s0278-5846(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Balster RL. GHB: a new and novel drug of abuse. Drug Alcohol Depend. 2001;63:1–22. doi: 10.1016/s0376-8716(00)00191-5. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Koella WP. Inhibition of nigral and neocortical cells by gamma-hydroxybutyrate: a microiontophoretic investigation. Eur J Pharmacol. 1979;53:359–364. doi: 10.1016/0014-2999(79)90460-6. [DOI] [PubMed] [Google Scholar]
- Osorio I, Davidoff RA. γ-hydroxybutyric acid is not a GABA-mimetic agent in the spinal cord. Ann Neurol. 1979;6:111–116. doi: 10.1002/ana.410060206. [DOI] [PubMed] [Google Scholar]
- Pardi D, Black J. Gamma-hydroxybutyrate/sodium oxybate Neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20:993–1018. doi: 10.2165/00023210-200620120-00004. [DOI] [PubMed] [Google Scholar]
- Poggioli R, Vitale G, Colombo G, Ottani A, Bertolini A. Gamma-hydroxybutyrate increases gastric emptying in rats. Life Sci. 1999;64:2149–2154. doi: 10.1016/s0024-3205(99)00163-0. [DOI] [PubMed] [Google Scholar]
- Poldrugo F, Addolorato G. The role of γ-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies. Alcohol Alcohol. 1999;34:15–24. doi: 10.1093/alcalc/34.1.15. [DOI] [PubMed] [Google Scholar]
- Poldrugo F, Snead OC., 3rd 1,4 Butanediol, γ-hydroxybutyric acid and ethanol: relationships and interactions. Neuropharmacology. 1984;23:109–113. doi: 10.1016/0028-3908(84)90226-0. [DOI] [PubMed] [Google Scholar]
- Quang LS, Colombo G, Lobina C, Maccioni P, Orru A, Gessa GL, et al. Evaluation from the withdrawal syndrome from gamma-hydroxybutyric acid (GHB), gamma-butyrolactone (GBL), and 1,4-butanediol (1,4-BD) in different rat lines. Ann NY Acad Sci. 2006;1074:545–558. doi: 10.1196/annals.1369.055. [DOI] [PubMed] [Google Scholar]
- Queva C, Bremner-Danielsen M, Edlund A, Ekstrand AJ, Elg S, Erickson S, et al. Effects of GABA agonists on body temperature regulation in GABA(B(1))−/− mice. Br J Pharmacol. 2003;140:315–322. doi: 10.1038/sj.bjp.0705447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybon JJ, Boje KMK. Pharmacokinetics and pharmacodynamics of gamma-hydroxybutyric acid during tolerance in rats: effects of extracellular dopamine. J Pharmacol Exp Ther. 2007;320:1252–1260. doi: 10.1124/jpet.106.113886. [DOI] [PubMed] [Google Scholar]
- Robinson DM, Keating GM. Sodium oxybate: a review of its use in the management of narcolepsy. CNS Drugs. 2007;21:337–354. doi: 10.2165/00023210-200721040-00007. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Ashton CH, Gilvarry E, Young AH. Liquid ecstasy: a new kid on the dance floor. Br J Psychiatry. 2004;184:104–106. doi: 10.1192/bjp.184.2.104. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Preliminary report on the metabolism of γ-butyrolactone and γ-hydroxybutyric acid. Biochem Pharmacol. 1965;14:177–178. doi: 10.1016/0006-2952(65)90073-0. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Evidence that central nervous system depression by 1,4-butanediol in mediated through a metabolite, gamma-hydroxybutyrate. Biochem Pharmacol. 1968;17:735–739. doi: 10.1016/0006-2952(68)90010-5. [DOI] [PubMed] [Google Scholar]
- Roth RH, Levy R, Giarman NJ. Dependence of rat serum lactonase upon calcium. Biochem Pharmacol. 1967;16:596–598. doi: 10.1016/0006-2952(67)90110-4. [DOI] [PubMed] [Google Scholar]
- Sanguineti VR, Angelo A, Frank MR. GHB: a home brew. Am J Drug Alcohol Abuse. 1997;23:637–642. doi: 10.3109/00952999709016901. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Di Filippo M, Nardi K, Calabresi P. Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr Pain Headache Rep. 2007;11:343–351. doi: 10.1007/s11916-007-0216-2. [DOI] [PubMed] [Google Scholar]
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–166. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Brown D, Woods M, Brown L, Hirschowitz J. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J Clin Psychiatry. 1985;46:222–225. [PubMed] [Google Scholar]
- Schlicker K, Boller M, Schmidt M. GABAC receptor mediated inhibition in acutely isolated neurons of the rat dorsal lateral geniculate nucleus. Brain Res Bull. 2004;63:91–97. doi: 10.1016/j.brainresbull.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser. 1988;4:161–175. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Milteer R, Lebeau MA. Drug-facilitated sexual assault (‘date rape’) South Med J. 2000;93:558–561. [PubMed] [Google Scholar]
- Scrima L, Hartman PG, Johnson FH, Jr, Thomas EE, Hiller FC. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13:479–490. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- Serra S, Colombo G, Melis S, Vacca G, Carai MAM, Gessa GL. Gamma-hydroxybutyric acid versus alcohol preference in Sardinian alcohol-preferring rats. Alcohol Alcohol. 2002;37:128–131. doi: 10.1093/alcalc/37.2.128. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Foddi C, Concas A, Biggio G. Failure of gamma-hydroxybutyrate to alter the function of the GABAA receptor complex in the rat cerebral cortex. Psychopharmacology. 1991;104:351–355. doi: 10.1007/BF02246035. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, France CP, Koek W. Neuroleptic-like effects of gamma-hydroxybutyrate: interactions with haloperidol and dizocilpine. Eur J Pharmacol. 2004;483:289–293. doi: 10.1016/j.ejphar.2003.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, France CP. Streptozotocin-induced diabetes differentially modulates haloperidol- and gamma-hydroxybutyric acid (GHB)-induced catalepsy. Eur J Pharmacol. 2005;517:64–67. doi: 10.1016/j.ejphar.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Shearman GT, Lal H. Generalization and antagonism studies with convulsant, GABAergic and anticonvulsant drugs in rats trained to discriminate pentylenetetrazol from saline. Neuropharmacology. 1980;19:473–479. doi: 10.1016/0028-3908(80)90055-6. [DOI] [PubMed] [Google Scholar]
- Shelton KL. Substitution profiles of N-methyl-D-aspartate antagonists in ethanol-discriminating inbred mice. Alcohol. 2004;34:165–175. doi: 10.1016/j.alcohol.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Sircar R, Basak A. Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning. Pharmacol Biochem Behav. 2004;79:701–708. doi: 10.1016/j.pbb.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Smith KM. Drugs used in acquaintance rape. J Am Pharm Assoc. 1999;39:519–525. doi: 10.1016/s1086-5802(16)30472-7. [DOI] [PubMed] [Google Scholar]
- Smith MA, Gergans SR, Lyle MA. The motor-impairing effects of GABAA and GABAB agonists in gamma-hydroxybutyrate (GHB)-treated rats: Cross-tolerance to baclofen but not flunitrazepam. Eur J Pharmacol. 2006;552:83–89. doi: 10.1016/j.ejphar.2006.08.080. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd Gamma hydroxybutyrate in the monkey IV Dopaminergic mechanisms. Neurology. 1978;28:1179–1182. doi: 10.1212/wnl.28.11.1179. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd The ontogeny of [3H]γ-hydroxybutyrate and [3H]GABAB binding sites: relation to the development of experimental absence seizures. Brain Res. 1994;659:147–156. doi: 10.1016/0006-8993(94)90874-5. [DOI] [PubMed] [Google Scholar]
- Snead OC., 3rd Relation of the [3H]γ-hydroxybutyric acid (GHB) binding site to the γ-aminobutyric acidB (GABAB) receptor in rat brain. Biochem Pharmacol. 1996;52:1235–1243. doi: 10.1016/0006-2952(96)00477-7. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Bearden LJ. Naloxone overcomes the dopaminergic, EEG, and behavioral effects of gamma-hydroxybutyrate. Neurology. 1980;30:832–838. doi: 10.1212/wnl.30.8.832. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Liu CC. Gamma-hydroxybutyric acid binding sites in rat and human brain synaptosomal membranes. Biochem Pharmacol. 1984;33:2587–2590. doi: 10.1016/0006-2952(84)90629-4. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Liu CC. GABAA receptor function in the γ-hydroxybutyrate model of generalized absence seizures. Neuropharmacology. 1993;32:401–409. doi: 10.1016/0028-3908(93)90163-w. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Nichols AC. γ-Hydroxybutyric acid binding sites: evidence for coupling to a chloride anion channel. Neuropharmacology. 1987;26:1519–1523. doi: 10.1016/0028-3908(87)90173-0. [DOI] [PubMed] [Google Scholar]
- Sprince H, Joesephs JA, Jr, Wilpizeski CR. Neuropharmacological effects of 1,4-butanediol and related congeners compared with those of gamma-hydroxybutyrate and gamma-butyrolactone. Life Sci. 1966;5:2041–2052. [Google Scholar]
- Stillwell ME. Drug-facilitated sexual assault involving gamma-hydroxybutyric acid. J Forensic Sci. 2002;47:1133–1134. [PubMed] [Google Scholar]
- Tremblay H, Godbout R, Girodias V, Schmitt M, Bourguignon JJ. Effects of gamma-hydroxybutyrate on ventral tegmental unit activity in the rat: considerations on rem sleep control. Sleep Res Online. 1998;1:152–158. [PubMed] [Google Scholar]
- United States Federal Register. Placement of gamma-butyrolactone in List I of the Controlled Substances Act (21 U.S.C. 802(34)). Drug Enforcement Administration, Department of Justice. Final rule. Fed Regist. 2000a;65:21645–21647. [PubMed] [Google Scholar]
- United States Federal Register. Schedules of controlled substances: addition of gamma-hydroxybutyric acid to Schedule I. Drug Enforcement Administration, Department of Justice. Final rule. Fed Regist. 2000b;65:13235–13238. [Google Scholar]
- Van Sassenbroeck DK, De Paepe P, Belpaire FM, Boon PA, Buylaert WA. Tolerance to the hypnotic and electroencephalographic effect of gamma-hydroxybutyrate in the rat: pharmacokinetic and pharmacodynamic aspects. J Pharm Pharmacol. 2003;55:609–615. doi: 10.1211/002235703765344513. [DOI] [PubMed] [Google Scholar]
- Varela M, Nogué S, Orós M, Miró Ò. Gamma hydroxybutyrate use for sexual assault. Emerg Med J. 2004;21:255–256. doi: 10.1136/emj.2002.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayer P, Maitre M. Gamma-hydroxybutyrate stimulation of the formation of cyclic GMP and inositol phosphates in rat hippocampal slices. J Neurochem. 1989;52:1382–1387. doi: 10.1111/j.1471-4159.1989.tb09183.x. [DOI] [PubMed] [Google Scholar]
- Vickers MD. Gammahydroxybutyric acid. Int Anesthesiol Clin. 1969;7:75–89. doi: 10.1097/00004311-196900710-00007. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, et al. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology. 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Griffiths RR, Ator NA. Self-administration of the gamma-hydroxybutyrate (GHB) prodrug gamma-butyrolactone (GBL) in baboons. Abstract for the CPDD 70th Annual Scientific Meeting; 2008. [accessed Aug 28, 2008]. http://www.cpdd.vcu.edu/ [Google Scholar]
- Wellendorph P, Hog S, Greenwood JR, de Lichtenberg A, Nielsen B, Frolund B, et al. Novel cyclic gamma-hydroxybutyrate (GHB) analogs with high affinity and stereoselectivity of binding to GHB sites in rat brain. J Pharmacol Exp Ther. 2005;315:346–351. doi: 10.1124/jpet.105.090472. [DOI] [PubMed] [Google Scholar]
- Werewattanachai N, Towiwat P, Unchern S, Maher TJ. Neuropharmacological profile of tetrahydrofuran in mice. Life Sci. 2007;80:1656–1663. doi: 10.1016/j.lfs.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Winter JC. The stimulus properties of γ-Hydroxybutyrate. Psychopharmacology. 1981;73:372–375. doi: 10.1007/BF00426468. [DOI] [PubMed] [Google Scholar]
- Winters WD, Spooner CE. Various seizure activities following gamma-hydroxybutyrate. Int J Neuropharmacol. 1965;4:197–200. doi: 10.1016/0028-3908(65)90034-1. [DOI] [PubMed] [Google Scholar]
- Wong G, Skolnick P, Katz JL, Witkin JM. Transduction of a discriminative stimulus through a diazepam-insensitive gamma-aminobutyric acid A receptor. Eur J Pharmacol. 1993;178:29–36. [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Winger G, Woods JH, Gerak LR, France CP. Evaluation of the reinforcing and discriminative stimulus effects of γ-hydroxybutyrate in rhesus monkeys. Drug Alcohol Depend. 1999;54:137–143. doi: 10.1016/s0376-8716(98)00153-7. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ali S, Ahmadian G, Liu CC, Wang YT, Gibson KM, et al. γ-Hydorxybutyric acid (GHB) and γ-aminobutyric acidB receptor (GABABR) binding sites are distinctive from one another: molecular evidence. Neuropharmacology. 2004;47:1146–1156. doi: 10.1016/j.neuropharm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Wu H, Zink N, Carter LP, Mehta AK, Hernandez RJ, Ticku MK, et al. A tertiary alcohol analog of gamma-hydroxybutyric acid as a specific gamma-hydroxybutyric acid receptor ligand. J Pharmacol Exp Ther. 2003;305:675–679. doi: 10.1124/jpet.102.046797. [DOI] [PubMed] [Google Scholar]
- Wüllner U, Klockgether T, Schwarz M, Sontag KH. Behavioral actions of baclofen in the rat ventromedial thalamic nucleus: antagonism by delta-aminovalerate. Brain Res. 1987;422:129–136. doi: 10.1016/0006-8993(87)90547-6. [DOI] [PubMed] [Google Scholar]
- Xie X, Smart TG. γ-hydroxybutyrate hyperpolarizes hippocampal neurons by activating GABAB receptors. Eur J Pharmacol. 1992;212:291–294. doi: 10.1016/0014-2999(92)90347-7. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yu B, Gallagher JP. Different subtypes of GABAB receptors are present at pre- and postsynaptic sites within the rat dorsolateral septal nucleus. J Neurophysiol. 1999;81:2875–2883. doi: 10.1152/jn.1999.81.6.2875. [DOI] [PubMed] [Google Scholar]