Abstract
Polybrominated diphenyl ethers (PBDEs) are potentially harmful and persistent environmental pollutants. Despite evidence that soils are a major sink for PBDEs, little is known regarding their behavior in this medium. An environmentally relevant level of a commercial penta-BDE mixture (75 μg kg-1) was added to topsoil and the extractability of three congeners (BDE 47, 99, and 100) was monitored over 10 weeks in planted and unplanted treatments. The extractability of each congener decreased rapidly in the experimental soil due largely to abiotic sorption to soil particles, which was demonstrated by low PBDE recovery from sterilized and dry soils. Monoculture plantings of zucchini and radish did not affect the recovery of PBDEs from soil. However, PBDE recovery from mixed species plantings was nearly 8 times higher than that of unplanted and monoculture treatments, indicating that interspecific plant interactions may enhance PBDE bioavailablity in soil. Evidence for competitive interactions between the two species was revealed by reduced shoot biomass of zucchini plants in mixed treatments relative to pots containing only zucchini. Both plant species accumulated PBDEs in root and shoot tissue (< 5 μg kg-1 plant tissue). PBDE uptake was higher in zucchini and translocation of PBDEs to zucchini shoots was congener-specific. Our results suggest that although abiotic sorption may limit the potential for human exposure to PBDEs in soil, plants may increase the exposure risk by taking up and translocating PBDEs into aboveground tissues and by enhancing bioavailability in soil.
Keywords: brominated flame retardants, polybrominated diphenyl ethers (PBDEs), phytoremediation, bioavailability, zucchini, radish, aging
Introduction
Polybrominated diphenyl ethers (PBDEs) are used around the world as flame retardants. PBDEs have been detected in biological and environmental samples, and burdens have risen rapidly in both areas over the last few decades (1). Human exposure to PBDEs is of concern, as increasing levels of PBDEs have been detected in human blood, breast milk (1), and adipose tissue (2) and a variety of serious health effects from PBDE exposure have been demonstrated in studies of rats and mice (3). The penta-BDE mixture, which consists primarily of tetra- to hexa-BDEs, is of particular interest because of its dominance in many biotic samples (1,4) and to a lesser extent, environmental samples including air (5,6), water (4), soil (7), sediment (1,4) and sewage sludge (8). Although the penta-BDE mixture is banned in Europe and U.S. production has ceased, the environmental release of penta-BDEs is expected to continue from degradation, recycling, and disposal of products made with this and other commercial mixtures (9-11). The lesser-brominated congeners also continue to be produced by biological (12) and photolytic (13,14) breakdown of the deca-BDE mixture, which remains in use by industry.
Airborne congeners of the penta-BDE mixture are likely to accumulate in soil via atmospheric deposition (7). Soil concentrations as high as 76 μg kg-1 have been measured near foam production facilities using the penta-BDE mixture as a flame retardant (9). Although little data is available regarding the concentration and distribution of PBDEs in soil, levels near other production or disposal facilities are likely to approach those found by Hale et al. (9) and urban soils should carry much higher burdens following patterns reported in outdoor air (5-7). In addition, transport models (15,16) and environmental distribution patterns (7) suggest that soils may be the most substantial sink for PBDEs. Little is known, however, about the processes controlling PBDE behaviour in soil. For other halogenated persistent organic pollutants (POPs), sequestration by soil components (17,18), bioavailability (19), and biodegradation (20,21) are important determinants of persistence in soil.
It might be presumed from their structure that PBDE degradation in the environment would be limited (15,16). However, recent studies conducted in solution (not soil), indicate that microbial (22) and photochemical degradation (14) of PBDEs occurs. Relative to soil sorption and availability, it remains unclear how significant chemical or biological degradation is to PBDE persistence in terrestrial environments. Another factor known to impact the availability and degradation of halogenated POPs in soil is planting (21,23-27). Plant effects on PBDEs in soil, including potential PBDE uptake and translocation to aboveground tissues, have not been documented.
The single previously-reported study of penta-BDE behavior in soil did suggest that penta-BDEs may not be as recalcitrant as previously expected (28). Unfortunately, this study was conducted using unrealistically high levels (50,000 μg kg-1) of a single PBDE congener. Thus, information regarding the fate of different PBDE congeners occurring in soil as components of penta-BDE mixtures is another missing piece in our understanding. In order to investigate the fate of penta-BDEs in soil, a commercial penta-BDE mixture was spiked into soil at an environmentally relevant level (75 μg kg-1) and recovery was monitored over time by solvent extraction. The impact of plants on penta-BDE behavior in soil was measured by including planted treatments of radish and zucchini, which have previously demonstrated enhanced degradation (29,30) or uptake of POPs (25,27) respectively. Information regarding the fate of PBDEs in soil and the influence of plants on PBDE persistence will be important in understanding how soil PBDE burdens and associated health risks will change over time.
Experimental Methods
Reagents and standards
All water was deionized (18 MΩ.cm) and prepared by passing through a NanoPure treatment system (Barnstead, Boston, MA). Commercial chemicals were of analytical reagent grade and were used without further purification. Analytical standards were purchased from AccuStandard (New Haven, CT). These included 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99), 2,2′,4,4′,6-pentabromodiphenyl ether (BDE-100). Solvents used for standard preparation and in the soil extractions (hexane, acetone, butanol and propanol) were purchased from Fisher (Fairlawn, NJ). 2-Bromo-m-xylene (Aldrich, St. Louis, MO) was used for a surrogate internal standard and was monitored at the mass of its molecular ion (m/z =185). Internal calibration was utilized and the quantification was based on relative response factors to this internal standard.
Instrumentation
A Hewlett Packard (HP) 5890 Series II Gas Chromatograph (GC) coupled to a Hewlett Packard 5972 Mass Selective Detector (MSD) was utilized in this work. Helium was used as the carrier gas and the column flow rate was 1.5 mL minute-1. An HP-5 (5%phenyl/95%methyl-polysiloxane) capillary column (30m, 0.32 mm ID, 0.25μm film thickness) was used for separation. Chromatographic conditions were taken from those developed by Vonderheide et al. (31) and specific temperatures employed are given in Table 1. Quadrupole mass spectrometry was used to detect and verify analyte identity. The ion source temperature was maintained at 175 °C, and the energy for ionization was 70 eV. The mass spectrometer was equipped with an electron impact ionization source and was operated in the positive ion mode; the mass spectra were collected using selected ion mode. The ions chosen for SIM were the [M]+ and [M+2]+ isotopes of the molecular ions. BDE-47 was monitored at m/z 484 and 486; the pentabrominated congeners were monitored at m/z 406 and 408. Selected ions were those established by Huwe et al. (32). The data obtained was processed using the standard HP ChemStation Software.
Table 1. Instrumental Operating Parameters.
| GC Parameters | |
|---|---|
| Column stationary phase | HP-5 (5% phenyl / 95% methyl-polysiloxane) |
| Carrier gas | Helium (Ultra High Purity) |
| Carrier Gas Flow Rate | 1.5 mL/min |
| Oven temperature program | 100 °C initial ramped at 15 C°/min to 300 °C and held for 2 min |
| Injection volume | 2 μL |
| Injector temperature | 280 °C |
| Injection mode | Splitless |
Characterization of the penta-BDE commercial mixture
The penta-BDE commercial mixture, DE-71, is produced by the Great Lakes Chemical Company. The portion used in this study was donated by Dr. Raoul V. Kuiper, Department of Pathobiology, Faculty of Veterinary Medicine, Utrecht University, Netherlands. Initial characterization was performed through analysis of a highly concentrated solution of the DE-71 under full scan mode. One tetrabrominated (BDE-47), three pentabrominated (BDE-85, 99 and 100) and two hexabrominated (BDE-153 and 154) congeners were found to be present in the commercial mixture. The ratio of the peak areas of the chromatogram were used to approximate the ratio of their concentrations. The percentage of each congener was thereby determined to be as follows: BDE-47: 46.0%; BDE-100: 12.6%; BDE-99: 36.7%; BDE-85: 1.3%; BDE-154: 2.1% and BDE-153: 1.3%.
Soil properties and preparation
A silty clay loam (19% sand, 53% silt, and 27% clay) with a water holding capacity of 50% was used for this study. All soil properties were determined by the Agricultural Analytical Services Laboratory of Pennsylvania State University. The soil contained the following levels of nutrients: nitrate nitrogen (29.8 mg kg-1), ammonium nitrogen (2.5 mg kg-1), phosphate (52.5 mg kg-1), calcium (6720 mg kg-1) and magnesium (417 mg kg-1). Percent organic matter was 1.8 % and pHw was 7.7. Cation exchange capacity (CEC) was 17.3 cmolckg-1. Prior to PBDE-amendment, the pH of the soil was adjusted to 6.8 with sulfuric acid for optimal growth and production of both the radish and zucchini plants.
A small aliquot of soil (1 kg, approximately 10% of final amount) was spiked with the DE-71 penta-brominated commercial mixture (delivered in acetone), mixed thoroughly, and placed under a fume hood for solvent evaporation. Spiked soil was then continuously tumbled with un-spiked soil for two hours at room temperature to insure efficient mixing and bring the soil to a final concentration of 75 μg kg-1 (total penta-BDEs) chosen to best mimic environmental levels determined to date. The initial concentration of PBDEs was verified as part of preliminary extraction comparisons (Table 2). Only three congeners (BDE 47, 99 and 100) were considered throughout the course of this study due to sensitivity constraints at this concentration. Detection limits for our instrumental set-up were established at 5 ppb for each congener; although the hexa-brominated congeners were evident in initial experiments, later results showed that their concentrations were below the detection limits of the analytical scheme. These congeners collectively contributed > 95% of the total penta-BDE concentration in soil (71.5 μg kg-1).
Table 2. Total recovery of BDE-47, 99, and 100 from initial spiked soil using different extraction solvents (n=3, mean ± RSD).
PBDEs were extracted from fresh, moist soil samples and soil samples that were allowed to air dry. Vortexing was employed for sample agitation
| Solvent | Fresh soil (μg kg-1) | Dry soil (μg kg-1) |
|---|---|---|
| Acetone | 72.2 ± 14.5 | 64.4 ± 24.6 |
| Propanol | 10.4 ± 14.6 | 2.28 ± 10.4 |
| Butanol | 6.27 ± 17.2 | 2.74 ± 13.6 |
| 1:1 Hexane:Acetone | 7.11 ± 10.3 | 6.87 ± 16.8 |
Planting
Planting took place within 24 hours of soil amendment. Radish (Hybrid radish, small round red cherriette F1) (Raphanus sativus) and summer squash (Hybrid zucchini squash, yellow gold rush F1) (Cucurbita species) seeds were purchased from Johnny Seeds (Winslow, Maine). Filter paper (#42) was used to line small pots (6 cm × 6 cm × 8 cm) and 180 g (dry wt.) of experimental soil was placed in each. Four different treatments consisting of 10 replicate pots were established. The first 10 replicates were left unplanted; the remaining were planted either with zucchini only, radish only, or a combination of both. Pots with only one species contained either one zucchini plant or five radish plants. Those with a combination of the two contained one zucchini plant and four radish plants. Seeds were surface sterilized and soaked in DI water for two hours prior to placement in the soil pots. Pots were maintained in a controlled growth room (15/20 °C, 8 h dark/16 h light, 520 lx) for 10 weeks and were brought up to 75% of the water holding capacity daily.
Harvesting and PBDE extraction
Plants were harvested after 10 weeks of growth. Plant roots were gently removed from the soil and any remaining plant material was collected. Plants were separated into shoot and root fractions. Each fraction was subsequently washed three times with DI water prior to storage. Plant and soil samples were stored at 4 °C before extraction. Plant root and shoot tissue from each of the 10 planted replicates in each treatment were separately combined and ground with liquid nitrogen in a mortar and pestle. Approximately 0.5 g (dry weight) of shoot tissue was recovered from composite samples and used for extraction of PBDEs. Between 0.l and 0.5 g of tissue was recovered in composite root samples used for plant extraction. Shoot and root samples were placed in 7 ml glass scintillation vials with 3 ml acetone and shaken horizontally for 18 hours at 200 rpm. After shaking, each vial was vortexed for two minutes and after a settling period, the extract was recovered by pipetting and subsequently filtered through glass wool.
Five pots from each treatment were randomly selected for analysis of penta-BDE recovery in soil. Soil from each pot was individually mixed and ground using a mortar and pestle. For each pot, three replicate samples of 5 g each were extracted with 15 mL acetone by vortexing for two minutes in 30 mL glass vials (SMI 2600 multi-tube vortexer). Plant samples from these 5 pots were used to calculate shoot and root biomass. Soil extracts were centrifuged and the supernatant liquid was transferred to a clean vial. All extracts (plant and soil) were taken to dryness by slight warming under nitrogen gas flow and reconstituted with 1 mL of 1:1 hexane:acetone. Before GC-MS analysis, surrogate internal standard was added to each vial.
Aging studies
Parallel aging studies were set up to determine the contribution that sorption to soil constituents would have on PBDE loss over the duration of the experiment. Replicate 5 g (dry weight) samples of PBDE spiked soil were placed in 30 mL glass tubes and then autoclaved 3 separate times for 1 hour at 121 °C. For additional comparison another portion of the PBDE spiked soil was maintained dry (∼ 2% moisture content) to limit microbial activity. Sterile and dry treatments were kept in the dark to account for possible effects of photochemical degradation. At the time of plant and soil harvest, three replicates of both sterile soil and dry soil were placed in a refrigerator (4 °C) and then extracted with acetone in the same manner as was done for pot study soils.
Data analysis
All statistical analyses were performed using SYSTAT© 10.2 software. One-way ANOVA tests were used to determine relationships among planted and unplanted treatments and penta-BDE recovery. Separate ANOVA tests were run for each treatment to determine if percent recovery varied according to individual penta-BDE congeners. Pairwise comparisons were made with post-hoc Tukey tests. Relationships between plant biomass parameters and PBDE levels in soil were analyzed by simple linear regression.
Results and Discussion
The development of analytical methods for the analysis of the PBDEs has progressed rapidly and are reviewed elsewhere (33-35). In this study, extraction parameters for the experimental soil were investigated and optimized. Many authors have used nonpolar solvents (e.g. hexane) because of the extraordinary solubility of the PBDEs (33); others have used a combination of a nonpolar and a polar solvent to allow for maximum matrix infiltration (34,35). Additionally, in this work we explored the use of alkyl alcohols, as these solvents are commonly used as an indicator of bioavailability. Extraction results with different solvents are given in Table 2. Previous studies have noted both losses as well as uptake of compounds from air during the drying step of sample preparation (36). An optimal sequence would therefore not include a soil drying step. Acetone yielded the greatest extraction efficiency of the PBDEs and the yield was negligibly affected by the presence of water (Table 2). It was therefore utilized in the soil extractions performed in this work.
The physical mixing of the extraction solvent with the sample matrix can be accomplished in several ways. Some popular means include mechanical shaking, sonication, and vortexing. Each of these methods was investigated within the criteria of both time and extraction efficiency. Vortexing the sample showed the most reproducible results (74.7 ± 10.9; mean ± RSD). Both mechanical shaking and sonication yielded considerably less precision (125 ± 36.1, and 85.0 ± 34.2, respectively). Vortexing was selected as the physical extraction mechanism due to its accuracy in recovery of initial PBDE burdens (Table 2), better precision, and simplicity.
Penta-BDE recovery in soil
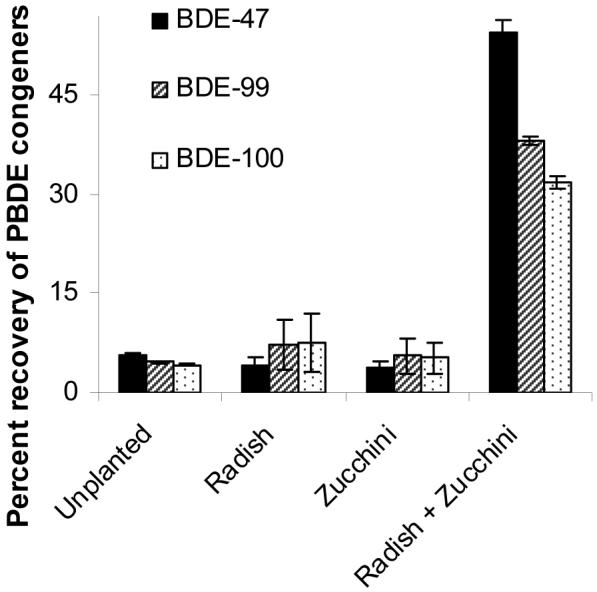
The amount of penta-BDEs recovered in solvent extracts declined rapidly in our experimental system, much like results reported by Litz (28). Total penta-BDEs (including BDE-47, -99, and -100) recovered in unplanted, zucchini, and radish treatments were less than 5 μg kg-1, approximately a 90 % decrease from initial levels. Nearly eight times more total penta-BDEs were recovered in the combined zucchini and radish treatment (Figure 1). Recovery in the mixed species pots was significantly greater than monoculture and unplanted treatments (p<0.0001, pairwise Tukey tests) for all three congeners.
Figure 1.

Total recovery of BDE-47, -99, and -100 in planted and unplanted soils after 10 weeks. Extractability of total penta-BDEs (shown above) and of each individual congeners in mixed species pots (zucchini + radish) was significantly greater than extractability in unplanted and single species pots (p<0.0001, pairwise Tukey tests). Pairwise comparisons between levels of total and individual penta-BDEs recovered in unplanted and single species pots were not statistically significant. Error bars indicate SE (n=5).
While the exact mechanisms of this enhanced recovery are unclear, it may have been the result of interspecific plant interactions in the combined treatments. Competition was evident in mixed zucchini-radish treatments; zucchini shoot biomass collected in mixed treatments was only 60 percent of that recovered in pots with zucchini only (p=0.010, t-test, data not shown). Competition between plants is known to alter root exudate production (37) and any quantitative or qualitative change in exudation could directly impact PBDE recovery by altering sorption to soil particles and/or indirectly by influencing the composition and activity of microbial communities. Exudation also increases during plant stresses such as nutrient limitation, which may arise as a result of competition. Increased exudation may function to enhance nutrient acquisition directly, by chemically enhancing the bioavailability of soil nutrients, or indirectly, by excluding roots of other species from adjacent soil (via allelopathy). Since root biomass in combined zucchini radish treatments was not different from that in radish monoculture (data not shown), it is clear that differences in PBDE concentrations were not driven by root biomass alone. Additionally, PBDE recovery in zucchini and radish monoculture treatments was not correlated with root biomass (data not shown).
Although radish and other plants have been shown in similar studies to enhance degradation of POPs in soil (29), we did not find evidence of any plant enhancement of PBDE dissipation in our experimental soil. This result is not exactly unexpected. The mechanisms controlling the range of possible plant affects on POP dissipation or degradation in soil are still unresolved, and separate studies of halogenated and other POPs yield highly variable results in planted treatments, including enhancement of degradation (26), no effect (38), and inhibition (39). However, because total recovery of PBDEs from soil was not possible, even in the parallel aging studies, we are limited in our ability to detect or compare actual degradation among treatments.
Extractability of individual PBDE congeners, measured as PBDE recovery following solvent extraction at the end of the experiment divided by initial PBDE levels, or % recovery, was equal in radish and zucchini monocultures. However, in unplanted and mixed species planted treatments extractability followed the same trend: BDE-47 > BDE-99 > BDE-100 (Figure 2). These differences may reflect variable degrees of sorption to soil constituents and/or congener specific biotic effects such as microbial degradation or plant uptake.
Figure 2.
Recovery of individual penta-BDE congeners in planted and unplanted soils after 10 weeks. Percent recovery was calculated as the mean recovery of each penta-BDE congener after 10 weeks divided by the initial amount added to the soil at the beginning of the study. In mixed species planted pots, mean % recovery for BDE-47 > BDE-99 > BDE-100 (p<0.02, pairwise Tukey tests). In unplanted pots mean % recovery for BDE-47 > BDE-99 = BDE-100 (p<0.02, pairwise Tukey tests). Mean % recovery of individual congeners for did not differ within single species planted pots. Error bars indicate SE (n=5).
In sterilized soil treatments, the extent of total recovery of BDE-47, -99, and -100 (2.15 ± 0.34 μg kg-1, mean ± SE) was similar to that in non-sterile planted or unplanted soils (Fig. 1), with the exception of the mixed planting treatment. Soil spiked with penta-BDEs but kept dry to minimize biotic effects throughout the experiment also showed a similar magnitude of PBDE recovery at the end of the study (4.15 ± 0.22 μg kg-1). Since biotic and photochemical effects were excluded or severely limited in sterile and dry treatments, the reduced recovery of penta-BDEs in these soils can only be attributed to abiotic sorption processes. Although microbial and photochemical degradation of PBDEs in our non-sterile soils were not directly assessed, comparison of PBDE recovery in sterilized and non-sterile soils suggests that these potential mechanisms for PBDE degradation were likely insignificant relative to abiotic sorption during the ten week experiment. Litz (28) also demonstrated that abiotic sorption to soil components contributes to diminishing PBDE recovery in soil and other POPs are known to be similarly affected by aging processes in soil (40).
Penta-BDE recovery in plant tissues
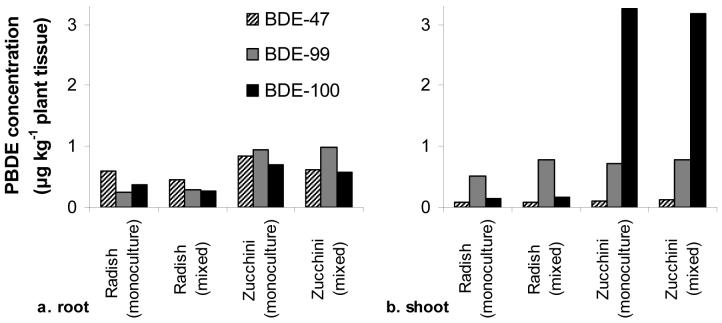
Plant uptake of POPs can also contribute to loss of target compounds from soil (25,27). Our results demonstrate that plants have the ability to take up and accumulate as much as 4 μg total penta-BDEs kg-1 plant tissue. Zucchini roots contained nearly twice as much penta-BDEs as radish roots, and showed a greater ability to translocate and accumulate PBDEs in shoots (Figure 3). Plant uptake and translocation of penta-BDEs was also dependent on the individual congener; translocation to shoot tissue in zucchini increases dramatically with increasing bromine in the penta-BDEs, while the distribution of congeners in root tissue of both species is more comparable (Figure 4 and see supporting information). This preferential translocation may be due to interactions involving distinct stereochemical properties of the congeners and the physiology of uptake and transport within zucchini. If translocation were simply due to passive movement in the transpiration stream of the plant, one might expect that lower brominated congeners with greater water solubility would be more easily transported into above ground tissues, which was not the case. Studies of other persistent organic pollutants also show differences in congener uptake among plant species (27,41). Such differences have been suggested to arise from species specific root morphology, physiology, water and nutrient requirements, and acquisition mechanisms.
Figure 3.
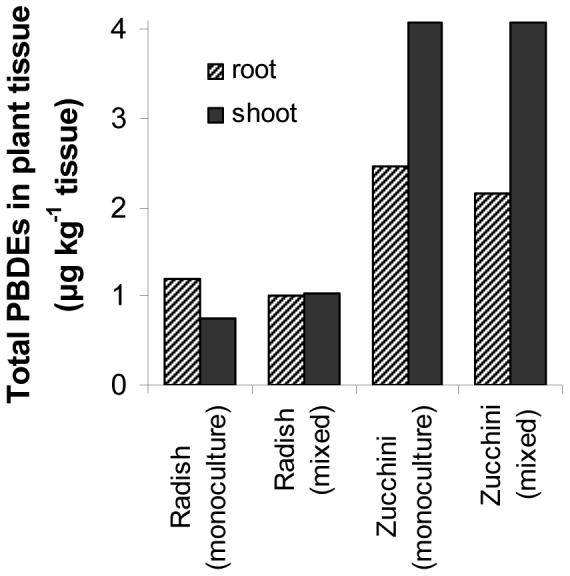
Total penta-BDE (BDE-47, -99, and -100) accumulation in roots and shoots of radish and zucchini from monoculture and mixed species plantings. Due to limited plant biomass per pot (particularly for roots), biomass from each replicate within a treatment was divided into root or shoot biomass and then combined into one composite sample for extraction and analysis (n=1).
Figure 4.
Uptake and translocation of individual penta-BDE congeners in root (a.) and shoot (b.) tissue of radish and zucchini plants. Due to limited plant biomass per pot (particularly for roots), biomass from each replicate within a treatment was divided into root or shoot biomass and then combined into one composite sample for extraction and analysis (n=1).
Although adsorption of PBDEs to root tissue in our study cannot be ruled out, adsorption alone would not be expected to lead to the relative abundance of PBDE congeners observed in root tissues. Specifically, the relative abundance of BDE-100 present in root tissue is much greater than its minor contribution to total PBDE levels in the original DE-71 mixture and spiked soil (Fig. 4 and supporting information).
According to a mass balance analysis, plant uptake of PBDEs by zucchini and radish, though notable, does not represent a sizable portion of the total initial PBDE burden or the quantity of PBDEs that were not recovered is soil. However, because only a fraction of the soil volume is impacted by plant roots and the bioavailable portion within that fraction is also likely to be small, plant uptake of PBDEs may be more efficient or important than a simple mass balance analysis would indicate. The presence of PBDEs in plant tissue also reveals that consumption of plant tissue could be an important route of oral exposure.
Overall fate of penta-BDEs in soil
Abiotic sorption of penta-BDEs to soil constituents appears to be the most important factor in determining the short term fate of PBDEs in soil. However, our results demonstrate that interactions among plant species can have significant effects on the extractability of PBDEs in soil. Increased extractability in mixed zucchini and radish treatments could indicate that a higher fraction of PBDEs in soil is bioavailable. This may have important consequences regarding the long-term fate of PBDEs in soil in natural environments, where plant communities typically consist of multiple species. Enhanced extractability or bioavailability of PBDEs in planted soils also has implications regarding possible microbial degradation of PBDEs, trophic interactions, and human exposure. More information is needed regarding the bioavailability and biodegradability of PBDEs in the environment in order to predict how levels will change over time and to determine the portion of existing PBDE burdens that is ecologically relevant. Environmental concentrations reported for PBDEs may underestimate total or historical burdens if a portion of environmental PBDEs is unavailable to solvent extraction, which appears to be the case in our system. This problem is compounded by the fact that acetone appears to be a much more efficient extraction solvent than other solvents or mixtures previously used in estimation of environmental levels of PBDEs.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Raoul V. Kuiper, Department of Pathobiology, Faculty of Veterinary Medicine, Utrecht University, Netherlands who supplied the DE-71 mixture and Dr. Jason C. White for providing guidance on zucchini planting density. We also acknowledge NIEHS grant #ES04908 for partial funding of this research.
REFERENCES
- (1).Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- (2).Johnson-Restrepo B, Kannan K, Rapaport DP, Rodan BD. Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environ. Sci. Technol. 2005;39:5177–5182. doi: 10.1021/es050399x. [DOI] [PubMed] [Google Scholar]
- (3).Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Oros DR, Hoover D, Rodigari F, Crane D, Sericano J. Levels and distribution of polybrominated diphenyl ethers in water, surface sediments, and bivalves from the San Francisco estuary. Environ. Sci. Technol. 2005;39:33–41. doi: 10.1021/es048905q. [DOI] [PubMed] [Google Scholar]
- (5).Strandberg B, Dodder NG, Basu I, Hites RA. Concentrations and spatial variations of polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air. Environ. Sci. Technol. 2001;35:1078–1083. doi: 10.1021/es001819f. [DOI] [PubMed] [Google Scholar]
- (6).Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC. Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environ. Sci. Technol. 2004;38:34–41. doi: 10.1021/es034705n. [DOI] [PubMed] [Google Scholar]
- (7).Hassanin A, Breivik K, Meijer SN, Steinnes E, Thomas GO, Jones KC. PBDEs in European background soils: levels and factors controlling their distribution. Environ. Sci. Technol. 2004;38:738–745. doi: 10.1021/es035008y. [DOI] [PubMed] [Google Scholar]
- (8).Hale RC, La Guardia MJ, Harvey EP, Gaylor MO, Mainor TM, Duff WH. Persistent pollutants in land-applied sludges. Nature. 2001;412:140–141. doi: 10.1038/35084130. [DOI] [PubMed] [Google Scholar]
- (9).Hale RC, La Guardia MJ, Harvey E, Mainor TM. Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere. 2002;46:729–735. doi: 10.1016/s0045-6535(01)00237-5. [DOI] [PubMed] [Google Scholar]
- (10).Alcock RE, Sweetman AJ, Prevedouros K, Jones KC. Understanding levels and trends of BDE-47 in the UK and North America: an assessment of principal reservoirs and source inputs. Environ. Int. 2003;29:691–698. doi: 10.1016/S0160-4120(03)00120-X. [DOI] [PubMed] [Google Scholar]
- (11).ter Schure AFH, Agrell C, Bokenstrand A, Sveder J, Larsson P, Zegers B. Polybrominated diphenyl ethers at a solid waste incineration plant II: atmospheric deposition. Atmos. Environ. 2004;38:5149–5155. [Google Scholar]
- (12).Kierkegaard A, Balk L, Tjarnlund U, Wit C. A. d., Jansson B. Dietary Uptake and Biological Effects of Decabromodiphenyl Ether in Rainbow Trout (Oncorhynchus mykiss) Environ. Sci. Technol. 1999;33:1612–1617. [Google Scholar]
- (13).Wit C. A. d. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- (14).Eriksson J, Green N, Marsh G, Bergman A. Photochemical decomposition of 15 polybrominated diphenyl ether congeners in methanol/water. Environ. Sci. Technol. 2004;38:3119–3125. doi: 10.1021/es049830t. [DOI] [PubMed] [Google Scholar]
- (15).Palm A, Cousins IT, Mackay D, Tysklind M, Metcalfe C, Alaee M. Assessing the environmental fate of chemicals of emerging concern: a case study of the polybrominated diphenyl ethers. Environ. Pollut. 2002;117:195–213. doi: 10.1016/s0269-7491(01)00276-7. [DOI] [PubMed] [Google Scholar]
- (16).Gouin T, Harner T. Modelling the fate of the polybrominated diphenyl ethers. Environ. Int. 2003;29:717–724. doi: 10.1016/S0160-4120(03)00116-8. [DOI] [PubMed] [Google Scholar]
- (17).Kohl SD, Rice JA. The binding of contaminants to humin: a mass balance. Chemosphere. 1998;36:251–261. [Google Scholar]
- (18).Krauss M, Wilcke W. Sorption strength of persistent organic pollutants in particle-size fractions of urban soils. Soil Sci. Soc. Am. J. 2002;66:430–437. [Google Scholar]
- (19).Morrison DE, Robertson BK, Alexander M. Bioavailability to earthworms of aged DDT, DDE, DDD, and dieldrin in soil. Environ. Sci. Technol. 2000;34 [Google Scholar]
- (20).Davis JW, Gonsior S, Marty G, Ariano J. The transformation of hexabromocyclododecane in aerobic and anaerobic soils and aquatic sediments. Water Res. 2005;39:1075–1084. doi: 10.1016/j.watres.2004.11.024. [DOI] [PubMed] [Google Scholar]
- (21).Donnelly PK, Hedge RS, Fletcher JS. Growth of PCB-degrading bacteria on compounds from photosynthetic plants. Chemosphere. 1994;28:981–988. [Google Scholar]
- (22).Vonderheide AP, Mueller-Spitz SR, Meija J, Welsh GL, Mueller KE, Kinkle BK, Shann JR, Caruso JA. Rapid degradation of brominated flame retardants by soil microorganisms. J. Anal. Atom. Spectrom. 2006 submitted. [Google Scholar]
- (23).Gilbert ES, Crowley DE. Plant compounds that induce polychlorinated biphenyl biodegradation by Arthrobacter sp. strain B1B. Appl. Environ. Microb. 1997;63:1933–1938. doi: 10.1128/aem.63.5.1933-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Koller G, Moder M, Czihal K. Peroxidative degradation of selected PCB: a mechanistic study. Chemosphere. 2000;41:1827–1834. doi: 10.1016/s0045-6535(00)00132-6. [DOI] [PubMed] [Google Scholar]
- (25).Mattina MI, Eitzer BD, Iannucci-Berger W, Lee WY, White JC. Plant uptake and translocation of highly weathered, soil-bound technical chlordane residues: data from field and rhizotron studies. Environ. Toxicol. Chem. 2004;23:2756–2762. doi: 10.1897/03-570. [DOI] [PubMed] [Google Scholar]
- (26).Singer AC, Smith D, Jury WA, Hathuc K, Crowley DE. Impact of the plant rhizosphere and augmentation on remediation of polychlorinated biphenyl contaminated soil. Environ. Toxicol. Chem. 2003;22:1998–2004. doi: 10.1897/02-471. [DOI] [PubMed] [Google Scholar]
- (27).White JC. Differential bioavailability of field-weathered p, p’-DDE to plants of the Cucurbita and Cucumis genera. Chemosphere. 2002;49:143–152. doi: 10.1016/s0045-6535(02)00277-1. [DOI] [PubMed] [Google Scholar]
- (28).Litz N. Some investigations into the behavior of pentabromodiphenyl ether (PeBDE) in soils. J. Plant Nutr. Soil Sc. 2002;165:692–696. [Google Scholar]
- (29).Liste HH, Alexander M. Plant-promoted pyrene degradation in soil. Chemosphere. 2000;40:7–10. doi: 10.1016/s0045-6535(99)00216-7. [DOI] [PubMed] [Google Scholar]
- (30).Dec J, Bollag JM. Use of Plant-Material for the Decontamination of Water Polluted with Phenols. Biotechnol. Bioeng. 1994;44:1132–1139. doi: 10.1002/bit.260440915. [DOI] [PubMed] [Google Scholar]
- (31).Vonderheide AP, Montes-Bayon M, Caruso JA. Development and application of a method for the analysis of brominated flame retardants by fast gas chromatography with inductively coupled plasma mass spectrometric detection. J. Anal. Atom. Spectrom. 2002;17:1480–1485. [Google Scholar]
- (32).Huwe JK, Lorentzsen M, Thuresson K, Bergman A. Analysis of mono- to deca-brominated diphenyl ethers in chickens at the part per billion level. Chemosphere. 2002;46:635–640. doi: 10.1016/s0045-6535(01)00227-2. [DOI] [PubMed] [Google Scholar]
- (33).Eljarrat E, Barcelo D. Sample handling and analysis of brominated flame retardants in soil and sludge samples. Trends Anal. Chem. 2004;23:727–736. [Google Scholar]
- (34).Hyotylainen T, Hartonen K. Determination of brominated flame retardants in environmental samples. Trends Anal. Chem. 2002;21:13–29. [Google Scholar]
- (35).Boer J. d., Allchin C, Law R, Zegers B, Boon JP. Method for the analysis of polybrominated diphenylethers in sediments and biota. Trends Anal. Chem. 2001;20:591–599. [Google Scholar]
- (36).Covaci A, Voorspoels S, Boer J. d. Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples—a review. Environ. Int. 2003;29:735–756. doi: 10.1016/S0160-4120(03)00114-4. [DOI] [PubMed] [Google Scholar]
- (37).Warembourg FR, Roumet C, Lafont F. Interspecific control of non-symbiotic carbon partitioning in the rhizosphere of a grass-clover association: Bromus madritensis-Trifolium angustifolium. J. Exp. Bot. 2004;55:743–750. doi: 10.1093/jxb/erh057. [DOI] [PubMed] [Google Scholar]
- (38).Sung K, Munster CL, Rhykerd R, Drew MC, Corapcioglu MY. The use of vegetation to remediate soil freshly contaminated by recalcitrant contaminants. Water Res. 2003;37:2408–2418. doi: 10.1016/S0043-1354(03)00029-0. [DOI] [PubMed] [Google Scholar]
- (39).Genney DR, Alexander IJ, Killham K, Meharg AA. Degradation of the polycyclic aromatic hydrocarbon (PAH) fluorene is retarded in a Scots pine ectomycorrhizosphere. New Phytologist. 2004;163:641–649. doi: 10.1111/j.1469-8137.2004.01131.x. [DOI] [PubMed] [Google Scholar]
- (40).Alexander M. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ. Sci. Technol. 2000;34:4259–4265. [Google Scholar]
- (41).Lunney AL, Zeeb BA, Reimer KJ. Uptake of weathered DDT in vascular plants: potential for phytoremediation. Environ. Sci. Technol. 2004;38:6147–6154. doi: 10.1021/es030705b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.