Abstract
Background
Controversy persists over the benefits of pneumococcal polysaccharide vaccine (PPV) in at-risk adults. We studied PPV, protein-conjugate pneumococcal vaccine (PCV), or immunologic ‘priming’ with PCV followed by ‘boosting’ with PPV in adults who recovered from pneumococcal pneumonia.
Methods
Subjects received PPV followed in 6 months by PCV, or vice-versa. IgG to capsular polysaccharide and opsonophagocytic killing activity (OPK) were studied at baseline, 4–8 weeks and 6 months after each vaccination.
Results
PPV and PCV stimulated similar IgG levels and OPK at 4–8 weeks. Six months post-PPV, antibody declined to baseline but remained modestly elevated post-PCV. PCV given 6 months post-PPV stimulated modest IgG increases that failed to reach post-PPV peaks. In contrast, PPV 6 months after PCV caused dramatic increases in IgG and OPK to all polysaccharides, consistent with a booster effect. Six months after the second vaccination, however, IgG and OPK in all patients fell precipitously, returning toward original baseline levels.
Conclusions
In high-risk subjects, the effect of PPV is short-lived; PCV stimulates a more prolonged response. PPV as a booster following PCV causes early antibody rises, but IgG declines rapidly thereafter, consistent with induction of suppressor cells or tolerance. Protein vaccines may be needed for high-risk adults.
Keywords: Pneumococcus, Polysaccharide, Vaccine, Protein-conjugate, Pneumonia
Introduction
Several lines of evidence show that pneumococcal polysaccharide vaccine (PPV) protects recipients against pneumococcal infection [1–5]. Some studies, however, have not confirmed these findings [6, 7] and, while most authorities agree that PPV is protective at some level [5, 7, 8], controversy continues to surround the issue [9, 10]. Even staunch advocates of PPV [5] agree that a better vaccine and/or a better vaccination strategy is needed to protect persons at highest risk of pneumococcal disease.
Protein-conjugate pneumococcal vaccine (PCV) has been brilliantly effective in preventing invasive pneumococcal disease in children [11, 12] and, via the so-called ‘herd effect’ [13], in adults [12]. Attempts to show that PCV might elicit better antibody responses than PPV have found modest differences in healthy adults and few or no differences in elderly subjects or those with HIV infection and low CD4 counts [14–16]. Jackson et al [17] recently showed that, one year after PCV, antibody levels in healthy older adults were proportional to the dose and were greater than those observed after PPV. The attractive suggestion [5, 15, 16, 18] that conjugate, followed by non-conjugate, vaccine would lead to a priming/boosting effect, culminating in substantially higher antibody levels than either vaccine alone was supported in studies of patients with Hodgkin disease [18] or HIV-infection [19], both of which reported antibody levels only 3–6 weeks after the second vaccination. Other investigations have failed to support this hypothesis [15–17, 20], perhaps due, in part, to the doses used and/or the dosing schedule.
In this study, we tested the hypothesis that PCV followed by PPV might provide a useful strategy for vaccinating a high-risk population, namely, adults who had survived pneumococcal pneumonia.
Methods
Patients
Between October, 2002 and August, 2006, patients hospitalized at the Michael E. DeBakey VA Medical Center, Houston for pneumococcal pneumonia were asked, after their medical condition stabilized, to participate in this study. The diagnosis of pneumococcal pneumonia was based on clinical and radiologic evidence for pneumonia together with either of the following: (a) ≥1 blood culture yielding S. pneumoniae; or (b) a sputum sample showing >10 inflammatory cells per epithelial cell on microscopic examination with clear predominance of Gram positive cocci in pairs and chains by Gram stain, and a culture yielding many pneumococci with no other likely pathogenic bacterium being identified [21, 22].
By the time we began this study, a great proportion of all veterans at MEDVAMC had received PPV. Accordingly, prior pneumococcal vaccination was not a criterion for exclusion. Patients who remained medically unstable or were thought to be unlikely to survive for one year were not asked to participate. Those who gave written consent (approved by the Institutional Review Board, Baylor College of Medicine) generally received their first dose of vaccine while they were still in the hospital.
Study design
Baseline sera were obtained, and patients were randomized to receive either: (a) 0.5 ml PPV (Pneumovax®) which contains CPS from 23 pneumococcal serotypes; or (b) 0.5 ml PCV (Prevnar®) given at each of two sites. Both vaccines were given intramuscularly. Prevnar contains protein conjugated CPS from S. pneumoniae types 4, 6B, 9V, 14, 18C, 19F, and 23F which are all among the 23 CPS included in Pneumovax. Twice the pediatric dosage was chosen as a dose greater than that used for children but still practicable; in fact, this dose was subsequently shown to be optimal for vaccinating healthy adults [17]. Serum was obtained 4–8 weeks and 6 months later. At 6 months, patients were given the vaccine they had not received the first time, and serum was again obtained 4–8 weeks and 6 months thereafter. For the purposes of this manuscript, we call patients who received PPV followed by PCV Group 1, and those who received PCV followed by PPV, Group 2.
ELISA
IgG to 7 capsular polysaccharides (CPS) common to PPV and PCV was assayed by a "sandwich" type ELISA as we have described previously [23]. Sera were pre-absorbed with 5 µg/ml pneumococcal cell-wall polysaccharide (Statens Seruminstitut, Copenhagen) and 5 µg/ml type 22F CPS (ATCC, Rockville MD) for 30 min at room temperature. Dilutions of a laboratory reference serum (Pool 89-SF, FDA, Bethesda) were included on every ELISA plate. All sera from each individual subject were studied concurrently.
OPK
The capacity of each serum to opsonize S. pneumoniae types 6B, 14, 19F and 23F, for ingestion and killing by phagocytes was determined by incubating bacteria in serum and then exposing them in vitro to HL-60 cells [24]; results are reported as an opsonophagocytic killing [OPK] index, the reciprocal of the serum dilution that led to 50% uptake and killing of pneumococci during 1 hr of incubation.
Statistics
IgG levels (µg/ml) and OPK index levels were converted to log10, and geometric means were calculated. Because data were not normally distributed, differences were compared using Wilcoxon rank analysis with a two-tailed test. Significance is reported at P ≤0.05 level and power at 80%. P values presented for multiple comparisons pertain to every individual comparison in the data set. Categorical variables were compared using two-tailed Chi square or Fisher’s exact test.
RESULTS
Subjects
Eighty-one subjects consented to participate, were randomized to Group 1 (PPV followed by PCV, 44 patients) or Group 2 (PCV followed by PPV, 37 patients), and provided a baseline serum. As is expected in a study of middle-aged persons who have survived pneumococcal pneumonia, the attrition rate was substantial. Of the 81 subjects, 7 died and 8 were transferred to remote nursing facilities without a follow-up serum being obtained. Only those 66 who returned at 4–8 weeks to provide a post-vaccine serum sample were included in the final data analysis. All were male. The average ages for Groups 1 and 2 were 64.3 and 62.7, respectively. Underlying conditions including alcohol consumption, cigarette use, chronic pulmonary disease, cancer, diabetes, congestive heart failure, liver disease, and HIV infection were similar in the two groups (Table 1). Seventy-six percent of all patients had received pneumococcal vaccine prior to enrollment in this study, with no difference between Group 1 and Group 2 (Table 1). Forty-seven of the 66 subjects returned at 6 months to provide blood and receive the second vaccine; of the 19 who did not return, 8 died and 11 were lost to follow-up. Forty-one provided blood 4–8 weeks later and 26 completed the study with a final blood sample 6 months after the second vaccination. In order to exclude the possibility that a bias resulted from the attrition of patients, we compared demographics and comorbidities as specified in Table 1 for those who completed the study and those who died or were lost to study; there were no differences (P>.02 for all comparisons).
Table 1.
Comparison of epidemiologic characteristics of the groups
| GROUP 1 | GROUP 2 | ||
|---|---|---|---|
| (PPV/PCV) | (PCV/PPV) | P value | |
| n=34 | n=32 | (<0.05) | |
| Age | 64.3 | 62.7 | 0.7 |
| Gender (Male) | 34 (100%) | 32 (100%) | 1.0 |
| Race | |||
| W | 21 (61.7%) | 21 (65.6%) | 0.8 |
| AA | 12 (35.3%) | 10 (31.3%) | 0.8 |
| H | 1 (2.9%) | 1 (3.1%) | 1.0 |
| COPD | 13 (38.2%) | 16 (50.0%) | 0.5 |
| Cancer | 7 (20.5%) | 7 (21.9%) | 1.0 |
| Diabetes | 11 (32.4%) | 6 (18.8%) | 0.3 |
| CHF | 3 (8.8%) | 6 (18.8%) | 0.3 |
| Liver disease | 8 (23.5%) | 8 (25.0%) | 1.0 |
| HIV | 1 (2.9%) | 4 (12.5%) | 0.2 |
| Splenectomy | 0 (0%) | 1 (3.1%) | 0.5 |
| Alcohol | 14 (41.2%) | 9 (28.1%) | 0.3 |
| Smoking | 26 (76.5%) | 27 (84.4%) | 1.0 |
| Previously Vaccinated | 24 (70.6%) | 26 (76.5%) | 0.4 |
Antibody levels at enrollment
Geometric mean IgG to CPS before the initial dose of vaccine (called ‘baseline’ in this report) was <1 µg/ml for 5 of the 7 CPS tested and was essentially identical in patients randomized to the 2 vaccination groups (Figure 2 and Figure 3; P>0.2 for all comparisons). OPK indices for the four serotypes studied were also similar at baseline in both groups (Figure 4; P>0.1 for all comparisons). Interestingly, these baseline antibody levels were similar in subjects who had received and those who had not received PPV in the preceding 5 years (data not shown; P>0.2 for all CPS).
Figure 2.
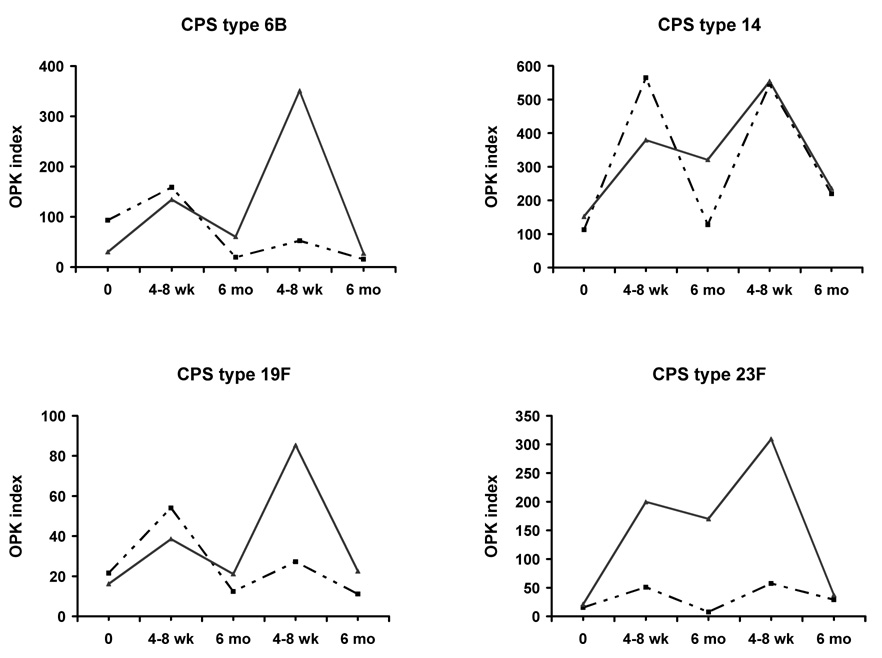
Opsonic activity reported as OPK index for four CPS at each point of the study. Group 1 (PPV followed by PCV) is shown by dashed lines and Group 2 (PCV followed by PPV) by solid lines.
Figure 3.
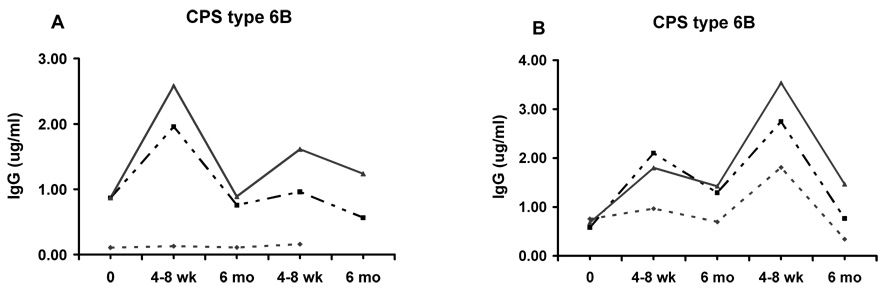
IgG after patients received PPV followed by PCV (Panel A) or vice-versa (Panel B), with patients stratified according to prior vaccination status. Dotted line: those who received pneumococcal polysaccharide vaccine within the previous year; dashed line: those vaccinated 1 – 5 years previously; and solid line: those vaccinated > 5 years previously or never vaccinated. Representative data are shown for CPS 6B; similar responses were observed with the other 3 CPS studied. None of the patients in Group 1, who received PPV in the previous year (Panel A) had a 6 month follow-up serum. Small numbers of subjects in some groups precluded statistical analysis.
Antibody 4–8 weeks post-vaccination
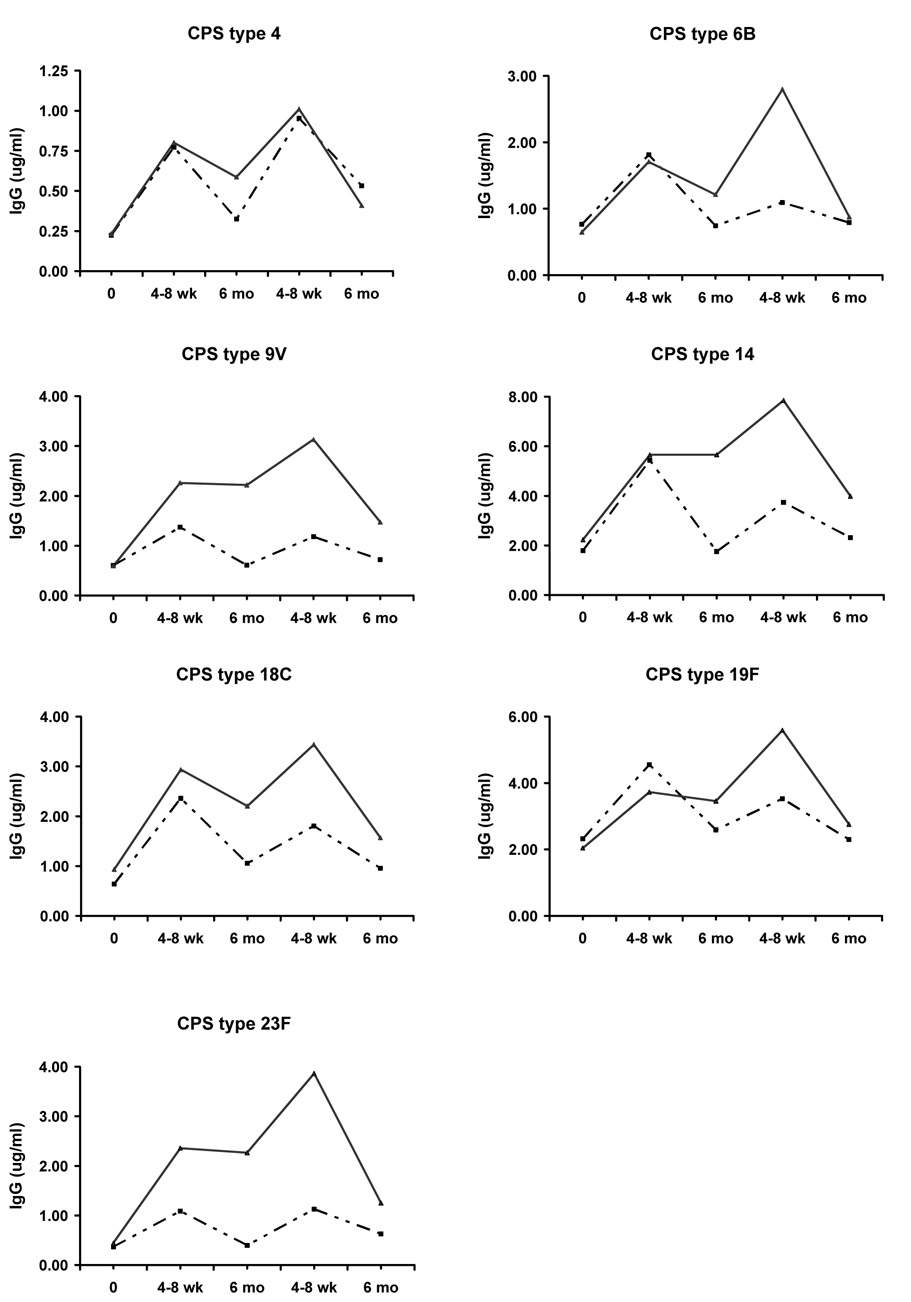
Four to 8 weeks post-vaccination (Table 2 and Figure 1), mean IgG levels exceeded baseline for all 7 CPS in both groups of subjects (P<0.03 for all comparisons). IgG levels were similar in Group 1 (recipients of PPV) and Group 2 (recipients of PCV), with P>0.2 for CPS 4, 6B, 14, 18C and 19F; P=0.1 for CPS 9V and P=0.06 for CPS 23F).
Table 2.
Serum IgG to capsular polysaccharides of S. pneumoniae*
| Serotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 18C | 19F | 23F | ||
| Group 1 (PPV followed by PCV) | ||||||||
| Baseline | n†=33 | 0.22 | 0.76 | 0.61 | 1.79 | 0.64 | 2.32 | 0.37 |
| 4–8 wk post-PPV | n=34 | 0.77 | 1.81 | 1.37 | 5.43 | 2.36 | 4.55 | 1.09 |
| 6 mo post-PPV | n=21 | 0.32 | 0.74 | 0.61 | 1.75 | 1.05 | 2.59 | 0.40 |
| 4–8 wk post-PCV | n=22 | 0.95 | 1.09 | 1.18 | 3.73 | 1.80 | 3.53 | 1.13 |
| 6 mo post-PCV | n=14 | 0.53 | 0.79 | 0.72 | 2.31 | 0.96 | 2.30 | 0.63 |
| Group 2 (PCV followed by PPV) | ||||||||
| Baseline | n=29 | 0.23 | 0.65 | 0.59 | 2.23 | 0.93 | 2.04 | 0.44 |
| 4–8 wk post-PCV | n=32 | 0.80 | 1.71 | 2.26 | 5.66 | 2.93 | 3.72 | 2.36 |
| 6 mo post-PCV | n=19 | 0.59 | 1.21 | 2.22 | 5.66 | 2.20 | 3.46 | 2.27 |
| 4–8 wk post-PPV | n=19 | 1.01 | 2.80 | 3.13 | 7.85 | 3.44 | 5.59 | 3.86 |
| 6 mo post-PPV | n=12 | 0.41 | 0.88 | 1.47 | 3.99 | 1.57 | 2.76 | 1.25 |
Data are presented as geometrical mean IgG (µg/ml) at each stage of study for patients who received pneumococcal polysaccharide vaccine (PPV) followed by protein-conjugate pneumococcal vaccine (PCV) (Group 1) or those who received PCV followed by PPV (Group 2).
n= numbers of subjects at each time point. Apparent discrepancies in the numbers of subjects at different time points reflect either (a) the loss or contamination of some sera; or (b) the fact that some patients miss one follow-up appointment but return for another, for example, failing to return for a 4–8 wk follow up serum but returning at 6 months to provide serum and receive a second vaccination.
Figure 1.
IgG to capsular polysaccharides (in µg/ml) is shown for Group 1 (patients who received PPV followed, 6 months later, by PCV, dashed lines) and Group 2 (patients who received PCV followed, 6 months later, by PPV, solid lines). Each panel shows results for a different CPS.
Except for type 6B in Group 1, mean OPK index in both groups of subjects and for all CPS rose ≥2-fold 4–8 weeks after the initial vaccination (Table 3 and Figure 2). The increases were significant for CPS 14, 19F and 23F in Group 1, and for all serotypes in Group 2 (P<0.02). Comparing Groups 1 and 2, there were no significant differences except for a higher OPK titer to CPS 23F in Group 2 (P=0.04). Taken together, these data show that patients who recently recovered from pneumococcal pneumonia had similar quantitative and qualitative antibody responses to PPV or PCV 4–8 weeks post-vaccination.
Table 3.
Serum opsonophagocytic killing of S. pneumoniae*
| Serotype | |||||
|---|---|---|---|---|---|
| 6B | 14 | 19F | 23F | ||
| Group 1 (PPV followed by PCV) | |||||
| Baseline | n†=28 | 93 | 113 | 22 | 15 |
| 4–8 wk post-PPV | n=31 | 159 | 565 | 54 | 51 |
| 6 mo post-PPV | n=20 | 20 | 128 | 12 | 8 |
| 4–8 wk post-PCV | n=20 | 53 | 545 | 27 | 57 |
| 6 mo post-PCV | n=14 | 16 | 220 | 11 | 29 |
| Group 2 (PCV followed by PPV) | |||||
| Baseline | n=22 | 30 | 152 | 16 | 21 |
| 4–8 wk post-PCV | n=30 | 135 | 379 | 39 | 200 |
| 6 mo post-PCV | n=17 | 60 | 321 | 21 | 170 |
| 4–8 wk post-PPV | n=17 | 351 | 554 | 85 | 309 |
| 6 mo post-PPV | n=11 | 27 | 236 | 23 | 37 |
Geometrical mean for opsonophagocytic killing (OPK) index at each stage of study for patients who received PPV followed by PCV (Group 1) or PCV followed by PPV (Group 2).
n= numbers of subjects at each time point. Fewer sera were included at each time point in this assay than in the ELISA, because they could not be studied due to presence of antibiotics.
Antibody 6 months post-vaccination
Six months after vaccination, however, in Group 1 patients, IgG levels to all 7 CPS returned essentially to baseline levels (Table 2 and Figure 1; P≥0.11 for all comparisons to baseline). In Group 2, IgG levels remained significantly higher than baseline for all CPS (P≤0.04), and significantly exceeded levels in Group 1 subjects for CPS 9V, 14 and 23F (P≤0.02).
OPK activity followed a similar pattern; levels returned to baseline for all serotypes in Group 1 but remained ≥2-fold greater than baseline for 3 of 4 serotypes in Group 2 (Table 3 and Figure 2), with differences vs. baseline significant for serotypes 6B, 19F and 23F (P≤0.01). These results suggest that, in these high-risk patients, very little antibody to CPS persists for 6 months after PPV, whereas there is a greater degree of persistence following PCV.
Antibody 4–8 weeks post second vaccination
At the 6-month visit, patients were given whichever vaccine they had not received initially. In Group 1, PCV caused modest increases in IgG that did not reach levels observed 4–8 wk post-PPV for 5 CPS and that equaled or slightly exceeded the earlier peak for 2 CPS (Table 2 and Figure 1). In contrast, in Group 2, PPV stimulated substantial further increases in IgG, although the increases were not significant, reflecting the persistence of higher IgG 6 months after initial PCV. Importantly, at this 4–8 week time point, mean IgG levels were greater in Group 2 than in Group 1 (P=0.02 for CPS 6B, 9V and 23F).
In Group 1, OPK indices doubled 4–8 weeks after the second vaccination (Table 3 and Figure 2) with significant increases for all serotypes (P≤0.02), but generally did not reach the initial 4–8 week post-PPV peak. In Group 2 patients, the OPK index increased substantially after the second vaccine (P≤0.04 for CPS 6B, 14 and 19F). This peak after vaccination with PPV exceeded the initial 4–8 week post-PCV peak in every instance, although these differences were not significant (P≥0.10). Comparing Groups 1 and 2, OPK index after the second vaccination was >3-fold higher in Group 2 for CPS 6B, 19F and 23F (differences not significant, P≥0.08). Taken together, these IgG and OPK results 4–8 weeks after the second vaccine appeared to support the hypothesis that patients who had been ‘primed’ with PCV exhibited a ‘booster’ response to PPV.
Antibody 6 months post second vaccination
Six months after revaccination, IgG levels in both groups of subjects strikingly declined, approaching original baseline levels (Table 2 and Figure 1), although, in Group 1, IgG to serotype 4, and in Group 2, IgG to serotypes 4, 9V and 23F remained significantly higher than baseline (P≤0.04). At this time 6 months after the second vaccination, there were no significant differences between Groups 1 and 2. Final OPK index also returned nearly to baseline and were essentially identical in Groups 1 and Group 2 (Table 3 and Figure 2). These data, showing precipitous declines in IgG and OPK following a second dose of pneumococcal vaccine in all subjects, suggest the induction of a suppressor effect (see Discussion). In order to exclude the possibility of bias resulting from the loss of subjects during the course of the study, we analyzed results at each time point using data only from those 26 patients who completed the study; results were similar (data not shown).
Stratification of antibody responses based on prior vaccine status
Vaccine-related information at our medical center is highly reliable; a yearly review is mandated at clinic visits, and all vaccine-related data are documented in a specially designated field in the computerized medical record. In order to determine whether vaccine responses were affected by prior vaccine status, patients were stratified into three groups, based on having previously received PPV as follows: never or >5 years; <5 years but >1 year; or <1 year. In this group of at-risk patients, baseline levels of IgG were similar in the three groups. Patients who had received PPV within the preceding year had distinctly lower IgG responses to revaccination with PPV and somewhat lower responses to PCV than those who had never been given PPV or had received it >5 years previously. IgG levels for those who had been vaccinated 1–5 years previously were intermediate; results for a representative CPS (6B) are shown in Figure 3. Similar responses were seen in OPK index (data not shown).
Discussion
This study examined possible benefits of PCV, PPV, or sequential administration of these two vaccines in adults whose high risk for pneumococcal pneumonia was documented by their having just recovered from that disease. PCV has been remarkably successful in infants and young children whose immature immune systems do not respond to PPV, eliciting antibody and protecting against invasive pneumococcal disease [11, 25]. In contrast, PCV in healthy or immune-compromised adults stimulates IgG levels that, 4–8 weeks post-vaccine, equal or only modestly exceed those following PPV [14–18]. We hypothesized that PCV might lead to better antibody responses than PPV, albeit for a limited number of vaccine serotypes, in our high-risk patients. We found that IgG levels and opsonic activity in serum increased after vaccination and, at 4–8 weeks, were similar in recipients of PPV or PCV. Six months after vaccination, however, IgG and OPK levels had returned to baseline in recipients of PPV; in contrast, although levels fell in those vaccinated with PCV, they remained measurably greater than baseline,, as recently reported in healthy elderly adults [17].
A further hypothesis of our work was that sequential vaccination with PCV followed by PPV might elicit a priming/boosting response, as had been described in patients with Hodgkin disease [18] or HIV-infection [19]. Other studies have failed to demonstrate this effect [15, 16, 20], perhaps due to vaccine dose, preparation or schedule. Our PCV dose was subsequently shown to be optimal in older adults [17], and our boosting dose was higher than in some earlier studies. In Group 1 patients, administration of PCV 6 months after PPV caused antibody at 4–8 weeks to increase but not to exceed the original, post-PPV peak. This finding is consistent with Heidelberger’s [26, 27] meticulous observations on responses to polysaccharide antigens and indicates the lack of participation by long-lived T-helper cells in response to polysaccharide antigens. In contrast, in Group 2 patients who received PCV followed by PPV, IgG and OPK levels rose to greater levels 4–8 weeks after PPV, consistent with a priming/boosting effect suggestive of participation by amplifying B or T cells [28].
The major contribution of our study, however, is in the long-term follow-up of our subjects. Six months after the second vaccination, antibody in both groups fell precipitously, returning nearly to original, baseline levels. Previous investigations of a priming/boosting effect [17–19] only studied subjects 3–8 weeks after the boost. Our findings suggest the induction of a long-lasting suppressor effect [28–30] rather than a simple waning of the antibody response since, in Group 2 subjects, IgG levels originally rose and then declined rapidly after the second dose of vaccine. We hypothesize that, in elderly subjects, induction of a population of relatively long-lived memory suppressor T regulatory cells is responsible for the observed suppression and may be intrinsic to the immune response to polysaccharide antigens [31]. This interpretation is supported by our important observation that subjects who had received PPV within a year of enrolling in the study had almost no response to PPV or PCV, whereas IgG levels increased in proportion to time elapsed since, or in the absence of, prior vaccination. Alternatively, repeated administration of antigen may deplete the existing pool of sensitized lymphocytes in a classically described immunologic tolerance [28], but If this mechanism were responsible, we do not think there should be such a nice initial response 4–8 weeks after the boosting dose.
The results of this study have both clinical and basic implications. First, they raise serious question about the value of administering pneumococcal vaccine routinely to patients who have recovered from pneumococcal pneumonia, a policy that has been elevated to the status of a quality indicator at many medical centers. They also suggest that, in these patients, a priming/boosting strategy with PCV followed by PPV is unlikely to provide protective benefit, at least with the presently available conjugate vaccine. At a more basic level, they support the hypothesis that the magnitude of the antibody response to polysaccharide antigens is determined by the interplay of amplifying and suppressive B or T cells or by a balance between generation and depletion of sensitized B cells [26–28]. Except in infants, multiple doses of polysaccharide, whether pure or protein-conjugated, have long been known to exert a suppressive effect [26, 32]. Infants may respond well to several doses of PCV because of the delayed ontogeny of the regulatory suppressor T cells. Although other mechanisms, such as immunological paralysis or tolerance [33, 34], or genetically mediated [35] or acquired [36] failure [36] to respond to polysaccharide antigens might be responsible for our observed results, adequate responses at 4–8 weeks oppose these possibilities and favor induction of a suppressor effect. This suppressive effect appears to wane after 5 years; interestingly, in a recent study [37] showing that previously vaccinated older adults respond well to a repeat dose of PPV, the mean time between the first and second dose was 5.4 years.
There are several limitations of this study. (1) We only studied a single population of adults who are susceptible to pneumococcal infection, namely those who had pneumococcal pneumonia and survived, and only a small proportion of these patients survived to the final analysis. Furthermore, the majority of these patients had previously received pneumococcal vaccine, which could possibly indicate that they constitute a poorly-responding subgroup. A recent study of conjugate followed by polysaccharide pneumococcal vaccine in HIV-infected children 1–2 year follow-up [38] did not find evidence for suppression; as noted above, this difference could reflect the immaturity of the regulatory T cell population in children. Otherwise healthy adults might respond differently to vaccination. (2) Since no subjects received only PCV, we can not exclude the possibility that we simply detected a waning antibody response when we gave PPV after PCV; this explanation seems unlikely considering how well antibody had persisted for 6 months after PCV alone and how striking the decline was 6 months after the putative boosting dose. (3) We did not include a group that received only two doses of PCV, for example, at 6-month intervals [28]; such a study is now in progress in our laboratory. Notable strengths of the present study include the availability of an exceptionally accurate vaccine history thanks to reliability of MEDVAMC automated records, measurement both of IgG levels and OPK activity, and the 6-month follow-up after vaccination.
Based solely on our findings, one might still propose a vaccination strategy that relies solely on conjugate vaccine for at-risk adults. There are at least two problems with such an approach: (1) presently known techniques of protein-conjugation limit the number of CPS that can be included in a vaccine; in accord with current vaccine recommendations, PPV might still need to be given to cover a greater range of serotypes [39, 40]; and (2) the emergence of ‘replacement types’ of S. pneumoniae [41, 42] may severely limit the long-term efficacy of conjugate vaccines.
In conclusion, our findings suggest that a vaccination strategy based on repeated doses of pneumococcal polysaccharide, including ‘priming’ with PCV and ‘boosting’ with PPV is not likely to be beneficial in adults who are most at risk, such as those who have recently recovered from pneumococcal pneumonia. Depletion of sensitized B-cells or induction of long-lived T regulator suppressor cells may be responsible, and this phenomenon may be an intrinsic property of the response to polysaccharides in adults These results question the long-term value of administering PPV to persons who have recovered from pneumonia. They further reinforce the need to develop protein vaccines for high-risk adults, for example, those derived from pneumolysin, from surface-exposed pneumococcal surface proteins A or C [43], or from other proteins newly recognized by microarray analysis [44, 45] in order to protect our highest risk populations from pneumococcal disease.
ACKNOWLEDGEMENTS
The authors are indebted to Dawn Reimer and Robert Burton for their excellent technical assistance. Conjugate vaccine was provided for this study by Wyeth Pharmaceuticals.
SUPPORT: This work was funded by the Department of Veterans Affairs through the Merit Review Program (Dr. Musher) and the NIH (AI-69695) (Dr. Nahm). Wyeth Pharmaceuticals provided conjugate vaccine.
Footnotes
POTENTIAL CONFLICT OF INTEREST
- Daniel M. Musher: From 1997 through 2002 Dr. Musher’s laboratory received funding from Merck for participating in an investigator-designed, multicenter study of pneumococcal polysaccharide vaccine.
- Adriana M. Rueda: No conflict
- Moon H. Nahm: No conflict
- Edward A. Graviss: No conflict
- Maria C. Rodriguez-Barradas: No conflict
This work was presented, in part, at the 44th Annual Meeting of the Infectious Diseases Society of America, October, 2006 Toronto, Ontario, Canada. Abstract No. 483
References
- 1.Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med. 1980;303:549–552. doi: 10.1056/NEJM198009043031003. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 3.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 4.Musher DM, Rueda-Jaimes AM, Graviss EA, Rodriguez-Barradas MC. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004–1008. doi: 10.1086/507699. [DOI] [PubMed] [Google Scholar]
- 5.Fedson DS, Musher DM. Pneumococcal vaccine. In: Plotkin SA, Orenstein WB, editors. Vaccines. Fourth ed. Philadelphia: W. B. Saunders; 2003. pp. 529–588. [Google Scholar]
- 6.Simberkoff MS, Cross AP, Al-Ibrahim M, et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;315:1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 7.Dear K, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2003;(Issue 4) doi: 10.1002/14651858.CD000422. article # CD000422. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: Recommendations of the Advisory Committee on Immunization Proctices (ACIP) MMWR Morb Mortal Wkly Rep. 1997;46:1–18. [Google Scholar]
- 9.Conaty S, Watson L, Dinnes J, Waugh N. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine. 2004;22:3214–3224. doi: 10.1016/j.vaccine.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Lipsky BA, Hirschmann JV. Pneumococcal polysaccharide vaccines do not protect the elderly from pneumococcal infections. Neth J Med. 2004;62:33–35. [PubMed] [Google Scholar]
- 11.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of pneumococcal conjugate vaccine introduction on drug-resistant Streptococcus pneumoniae. New England J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 13.Musher DM. Pneumococcal vaccine--direct and indirect ("herd") effects. N Engl J Med. 2006;354:1522–1524. doi: 10.1056/NEJMe068038. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed F, Steinhoff MC, Rodriguez-Barradas MC, Hamilton RG, Musher DM, Nelson KE. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis. 1996;173:83–90. doi: 10.1093/infdis/173.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Shelly MA, Jacoby H, Riley GJ, Graves BT, Pichichero M, Treanor JJ. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feikin DR, Elie CM, Goetz MB, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine. 2001;20:545–553. doi: 10.1016/s0264-410x(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LA, Neuzil KM, Nahm MH, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2007;25:4029–4037. doi: 10.1016/j.vaccine.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Chan CY, Molrine DC, George S, et al. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin's disease. J Infect Dis. 1996;173:256–258. doi: 10.1093/infdis/173.1.256. [DOI] [PubMed] [Google Scholar]
- 19.Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine. 2000;19:886–894. doi: 10.1016/s0264-410x(00)00232-2. [DOI] [PubMed] [Google Scholar]
- 20.Powers DC, Anderson EL, Lottenbach K, Mink CM. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J Infect Dis. 1996;173:1014–1018. doi: 10.1093/infdis/173.4.1014. [DOI] [PubMed] [Google Scholar]
- 21.Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore) 2000;79:210–221. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39:165–169. doi: 10.1086/421497. [DOI] [PubMed] [Google Scholar]
- 23.Wernette CM, Frasch CE, Madore D, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10:514–519. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KH, Yu J, Nahm MH. Efficiency of a pneumococcal opsonophagocytic killing assay improved by multiplexing and by coloring colonies. Clin Diagn Lab Immunol. 2003;10:616–621. doi: 10.1128/CDLI.10.4.616-621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eskola J, Black S, Shinefield H. Pneumococcal conjugate vaccines. In: Plotkin SA, Orenstein WA, editors. Vaccines. Fourth Edition ed. Philadelphia: Saunders; 2003. pp. 589–624. [Google Scholar]
- 26.Heidelberger M, MacLeod CM, diLapi MM. The human antibody response to simultaneous injection of six specific polysaccharides of pneumococcus. J Exp Med. 1948;88:359–372. doi: 10.1084/jem.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidelberger M, DiLapi MM, Siegel M, Walter AW. Persistence of antibodies in human subjects injected with pneumococcal polysaccharides. J. Immunol. 1950;65:535–541. [PubMed] [Google Scholar]
- 28.O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 29.Taylor CE, Stashak PW, Caldes G, et al. Activation of antigen-specific suppressor T cells by B cells from mice immunized with type III pneumococcal polysaccharide. J Exp Med. 1983;158:703–717. doi: 10.1084/jem.158.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor CE, Baker PJ. Production of soluble suppressor factor by spleen cells from mice immunized with type III pneumococcal polysaccharide. J Immunol. 1985;135:2551–2556. [PubMed] [Google Scholar]
- 31.Heilmann C. Vaccination-induced activation of human blood T cells suppressing pneumococcal polysaccharide-specific B cells. Acta Pathol Microbiol Immunol Scand [C] 1987;95:65–69. doi: 10.1111/j.1699-0463.1987.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 32.Felton LD, Ottinger B. Pneumococcus polysaccharide as a paralyzing agent on the mechanism of immunity in white mice. J. Bacteriol. 1942;43:94–105. [Google Scholar]
- 33.Pichichero ME. Immunological paralysis to pneumococcal polysaccharide in man. Lancet. 1985;2:468–471. doi: 10.1016/s0140-6736(85)90401-5. [DOI] [PubMed] [Google Scholar]
- 34.Weigle WO. Different types of immunological unresponsiveness. Adv Exp Med Biol. 1973;29:357–362. doi: 10.1007/978-1-4615-9017-0_52. [DOI] [PubMed] [Google Scholar]
- 35.Musher DM, Groover JE, Watson DA, et al. Genetic regulation of the capacity to make immunoglobulin G to pneumococcal capsular polysaccharides. J Investig Med. 1997;45:57–68. [PubMed] [Google Scholar]
- 36.Romero-Steiner S, Musher DM, Cetron MS, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 37.Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine. 2003;22:96–103. doi: 10.1016/s0264-410x(03)00521-8. [DOI] [PubMed] [Google Scholar]
- 38.Abzug MJ, Pelton SI, Song LY, et al. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:920–929. doi: 10.1097/01.inf.0000237830.33228.c3. [DOI] [PubMed] [Google Scholar]
- 39.Austrian R. Pneumococcus: The first one hundred years. Rev Infect Dis. 1981;3:183–189. doi: 10.1093/clinids/3.2.183. [DOI] [PubMed] [Google Scholar]
- 40.Alanee SR, McGee L, Jackson D, et al. Association of serotypes of Streptococcus pneumoniae with disease severity and outcome in adults: an international study. Clin Infect Dis. 2007;45:46–51. doi: 10.1086/518538. [DOI] [PubMed] [Google Scholar]
- 41.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–472. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez BE, Hulten KG, Lamberth L, Kaplan SL, Mason EO., Jr Streptococcus pneumoniae serogroups 15 and 33: an increasing cause of pneumococcal infections in children in the United States after the introduction of the pneumococcal 7-valent conjugate vaccine. Pediatr Infect Dis J. 2006;25:301–305. doi: 10.1097/01.inf.0000207484.52850.38. [DOI] [PubMed] [Google Scholar]
- 43.Moore QC, 3rd, Johnson L, Repka M, McDaniel LS. Immunization with PspA incorporated into a poly(ethylene oxide) matrix elicits protective immunity against Streptococcus pneumoniae. Clin Vaccine Immunol. 2007;14:789–791. doi: 10.1128/CVI.00082-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basset A, Thompson CM, Hollingshead SK, et al. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect Immun. 2007;75:5460–5464. doi: 10.1128/IAI.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giefing C, Meinke AL, Hanner M, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2007 doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]