Abstract
Rationale
Randomised data in men showed a small but significant reduction in risk of adult-onset asthma among those assigned to aspirin. Results from an observational study in women suggest that frequent use of aspirin decreased the risk of adult-onset asthma. Randomised data in women are lacking.
Objective
To test the effect of 100 mg of aspirin on alternate days or placebo on the risk of adult-onset asthma in the Women's Health Study.
Methods
Post-hoc analyses from a randomised, double-blind, placebo-controlled clinical trial of aspirin and vitamin E in apparently healthy US women with no indication or contraindication to aspirin therapy and free of a history of asthma at study entry.
Measurements
Female health professionals could self-report an asthma diagnosis on yearly questionnaires.
Results
Among 37,270 women without reported history of asthma prior to randomisation and during 10 years of follow-up, there were 872 new reports of asthma diagnosis in the aspirin group and 963 in the placebo group (hazard ratio=0.90; 95% confidence interval=0.82−0.99; P=0.027). This apparent 10% lower relative risk of incident adult-onset asthma among those assigned to aspirin was significantly modified by body mass index, indicating no effect among women with a body mass index of ≥30 kg/m2. There was no significant effect modification by age, smoking status, exercise levels, postmenopausal hormone use, or randomised vitamin E assignment.
Conclusions
In this large, randomised clinical trial of apparently healthy adult women, assignment of 100 mg of aspirin on alternate days reduced the relative risk of newly reported diagnosis of asthma.
Keywords: asthma, aspirin, randomised trial
Over the last decades, asthma has become a growing public health problem and the prevalence and incidence of this respiratory disease continues to increase.1 Explanations for this increase are various including obesity and weight change,2 3 dietary factors 4, changes in early life antigen exposure 5, environmental or geographical factors 6, but may also be due to increased diagnostic testing. Since the increase in asthma incidence was accompanied by a change of utilization pattern of antipyretic and analgesic drugs among children—the use of aspirin decreased due to its linkage with the Reyes Syndrome and as a result the use of other analgesic drugs increased7—it has been suggested that aspirin may be associated with decreased risk of asthma.8
In adult men, results from a recent post-hoc analysis of the Physicians' Health Study, showed that apparently healthy men who were randomised to 325 mg of aspirin every other day had a statistically significant 22% relative risk reduction of newly reported adult-onset asthma diagnosis during a mean of 5 years of follow-up.9 In a large, observational, prospective cohort study, adult women who self-selected for frequent use of aspirin had decreased risk of incident asthma compared with never users after adjusting for a variety of potential confounding factors.10
The Women's Health Study (WHS), a large-scale randomised double blind, placebo-controlled trial of aspirin (100 mg on alternate days) and vitamin E, provided a unique opportunity to test whether low-dose aspirin decreases the risk of newly reported adult-onset asthma diagnosis in apparently healthy women with no indication or contraindication for aspirin therapy.
METHODS
The WHS was a randomised, double-blind, placebo-controlled, two-by-two factorial trial testing the risks and benefits of low-dose aspirin (100 mg every other day supplied by Bayer AG, Leverkusen, Germany), and vitamin E (600 IU every other day supplied by the National Source Vitamin E Association, Washington, DC) in the primary prevention of cardiovascular disease and cancer. All women gave written informed consent and the Institutional Review Board of Brigham and Women's Hospital approved the WHS.
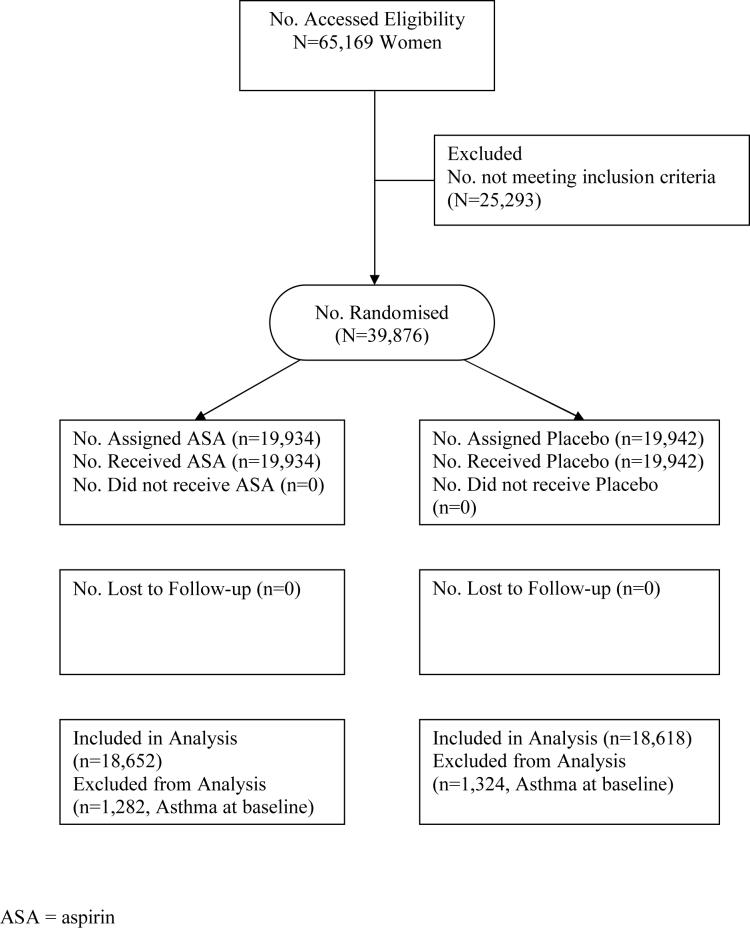
The WHS randomised 39,876 female health professionals between April 1993 and January 1996; of these, 19,934 were assigned to active aspirin and 19,942 to placebo. The results of the trial11-13 and methods used to identify and recruit the study population14 15 have been described in detail previously. In brief, invitational letters were mailed to 1,757,247 female health professionals throughout the United States and Puerto Rico, of whom 453,787 returned the initial study questionnaire. Women were considered eligible if they indicated willingness to participate, were aged 45 years or older without previous history of coronary heart disease, cerebrovascular disease, cancer (except non-melanoma skin cancer), or other major chronic illness. Eligible women also had no history of side effects to any study medications, were not taking aspirin or nonsteroidal anti-inflammatory drug medications more than once a week (or were willing to forego use of these medications during the trial), were not taking anticoagulants or corticosteroids, and were not taking individual supplements of vitamin A, E, or beta-carotene more than once a week. Of women who returned the initial study questionnaire, 65,169 were willing and eligible to participate, and enrolled into a three month run-in period employing all placebo medications, designed to identify a group of participants with a higher likelihood of good long-term compliance. Of these, 39,876 were compliant during the run-in phase and were randomised into the trial (Figure 1).
Figure 1.
Flow diagram of the progress through the phases of the Women's Health Study.
Baseline information was collected by mailed questionnaires that asked about many anthropometric, demographic, clinical, and lifestyle variables. Twice in the first year and yearly thereafter, follow-up questionnaires were sent out asking about compliance with study medication, development of side effects, newly developed diseases that required medical attention including asthma, dates of diagnoses, and personal characteristics and habits.
Ascertainment of asthma
On each of the follow-up questionnaires, the participating female health professionals were specifically asked about newly diagnosed diseases including asthma and the date of diagnosis of these diseases. Asthma was not a pre-specified endpoint of the WHS.
Statistical analysis
We included all reports of asthma from enrolment until the end of the randomised trial on March 31, 2004. We excluded 2,606 participants (1,282 in the aspirin group and 1,324 in the placebo group) who indicated an asthma diagnosis prior to randomization, leaving 37,270 participants without asthma history at baseline. We compared continuous baseline measurements using Student's t test and categorical variables using the chi-square test according to aspirin or aspirin-placebo assignment. Following the intention-to-treat principal, we compared the cumulative incidence of adult-onset asthma over the trial follow-up. We used proportional hazards models to analyze the association between randomised aspirin assignment and time from randomization to date of the reported asthma diagnosis. Furthermore, we assessed whether the effect of aspirin on adult-onset asthma was modified by age (<55, 55−64, ≥65 years), smoking status (never, past, current), body mass index (<25, 25−29.9, ≥30 kg/m2), exercise (rarely, <1/week, 1−3 times/week, ≥4 times/week, postmenopausal hormone use (never, past, current), and randomised vitamin E assignment. We tested statistical significant effect modification by contrasting main effect models to models that also included interaction-term indicators using the likelihood ratio test.
Role of the funding source
The study sponsors had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the paper for publication.
RESULTS
Table 1 summarizes the baseline characteristics of the 37,270 participants without reported history of asthma at study entry, according to randomised aspirin treatment assignment. As expected in this large sample, the baseline characteristics were well balanced between the aspirin and placebo groups.
Table 1.
Baseline characteristics according to randomised aspirin assignment in the Women's Health Study (N=37,270)
| Characteristics | Aspirin (N=18,652) | Placebo (N=18,618) | P Value |
|---|---|---|---|
| Age, years (SD) | 54.7(7.1) | 54.7(7.1) | 0.83 |
| Body mass index, kg/m2(SD) | 26.0(5.0) | 25.9(5.0) | 0.39 |
| BMI >= 30, % | 17.7 | 17.6 | 0.71 |
| History of hypertension, % | 25.7 | 25.4 | 0.51 |
| History of diabetes, % | 2.7 | 2.4 | 0.15 |
| Smoking status, % | |||
| Never | 51.2 | 51.2 | 0.61 |
| Past | 35.7 | 35.5 | |
| Current <15 | 4.8 | 5.0 | |
| Current 15+ | 8.2 | 8.4 | |
| Exercise, % | |||
| Rarely | 38.3 | 38.1 | 0.51 |
| <1/week | 19.5 | 20.3 | |
| 1−3/week | 31.4 | 31.1 | |
| ≥4/week | 10.9 | 10.5 | |
| Alcohol consumption, % | |||
| Rarely | 44.8 | 45.1 | 0.79 |
| 1−3/months | 13.4 | 12.8 | |
| 1−6/week | 31.6 | 31.8 | |
| ≥1/day | 10.2 | 10.3 | |
| Postmenopausal | 55.0 | 54.4 | 0.23 |
| Postmenopausal hormone use | |||
| Never | 50.0 | 50.4 | 0.27 |
| Past | 9.0 | 9.2 | |
| Current | 41.0 | 40.4 |
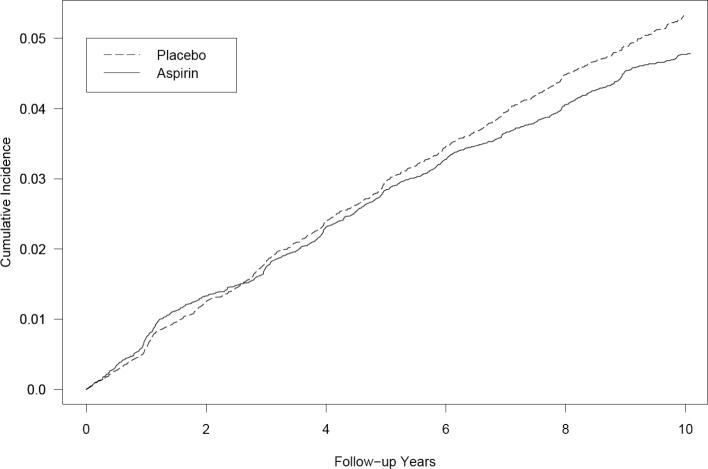
Over a mean follow-up of 9.7 years (362,120 person-years), a total of 1,835 participants reported new diagnoses of asthma, which corresponded to a cumulative incidence of adult-onset asthma of 4.9 percent. There were 872 new diagnoses of asthma reported in the aspirin group and 963 reported in the placebo group (Table 2). This corresponded to a hazard ratio of 0.90 (95% CI, 0.82−0.99; p=0.027) for newly reported adult-onset asthma, representing a 10% relative-risk reduction in favour of aspirin. The cumulative incidence of asthma in the aspirin and placebo groups over follow-up is shown in Figure 2 indicating a constantly decreasing risk of adult-onset asthma in the aspirin group after approximately 6 years of follow-up.
Table 2.
Randomised aspirin assignment and report of newly diagnosed asthma in the Women's Health Study (N=37,270).
| Aspirin Group, n | Placebo Group, n | Hazard Ratio* | 95% CI | P Value | |
|---|---|---|---|---|---|
| N=18,652 (181,467 person years) | N=18,618 (180,652 person years) | ||||
| |
872 |
963 |
0.90 |
0.82−0.99 |
0.027 |
| Stratified Analyses | |||||
| Age, years | 0.37† | ||||
| <55 | 550 | 595 | 0.92 | 0.82−1.03 | 0.16 |
| 55−64.9 | 249 | 268 | 0.92 | 0.78−1.10 | 0.37 |
| 65+ | 73 | 100 | 0.73 | 0.54−0.99 | 0.04 |
| Smoking status | 0.97† | ||||
| Never | 419 | 459 | 0.91 | 0.80−1.04 | 0.16 |
| Past | 322 | 357 | 0.89 | 0.77−1.04 | 0.13 |
| Current | 131 | 146 | 0.91 | 0.72−1.15 | 0.44 |
| Body mass | 0.002† | ||||
| index, kg/m2 | |||||
| <25 | 336 | 406 | 0.83 | 0.71−0.95 | 0.01 |
| 25−29.9 | 258 | 319 | 0.80 | 0.68−0.95 | 0.01 |
| 30+ | 265 | 224 | 1.19 | 0.99−1.42 | 0.06 |
| Exercise | 0.32† | ||||
| Rarely | 347 | 364 | 0.95 | 0.82−1.10 | 0.45 |
| <1/week | 198 | 205 | 1.00 | 0.83−1.22 | 0.97 |
| 1- | 240 | 294 | 0.81 | 0.68−0.95 | 0.01 |
| 3/week | |||||
| ≥4/week | 87 | 100 | 0.84 | 0.63−1.12 | 0.24 |
| Postmenopausal hormone use | 0.89† | ||||
| Never | 387 | 429 | 0.91 | 0.79−1.04 | 0.16 |
| Past | 86 | 92 | 0.96 | 0.71−1.29 | 0.77 |
| Current | 396 | 438 | 0.89 | 0.77−1.01 | 0.08 |
| Vitamin E | 0.57† | ||||
| Active | 428 | 485 | 0.88 | 0.77−1.00 | 0.05 |
| Placebo | 444 | 478 | 0.93 | 0.81−1.05 | 0.25 |
CI, confidence interval
Calculated for Cox proportional hazard models and using the placebo group as reference.
P-value for modification from contrast of a model that included main effects only to a model that additionally included interaction-term indicators using the likelihood ratio test.
Figure 2.
Cumulative incidence of adult-onset asthma during the randomised component of the Women's Health Study
There were no statistically significant differences in the observed effect of aspirin on adult-onset asthma by age, smoking status, levels of exercise, postmenopausal hormone intake, or randomised vitamin E assignment (Table 2). In contrast, body mass index significantly modified the effect of low-dose aspirin on reported asthma diagnosis (p=0.002), indicating no apparent effect of aspirin on asthma incidence for women who had a body mass index of 30 kg/m2 or higher (hazard ratio 1.19, 95% CI, 0.99−1.42). The lack of association was similar when we further categorized high BMI values (data not shown). The effect of aspirin on occurrence of asthma was also significantly modified by linear body mass index (p=0.008).
DISCUSSION
In this large randomised double-blind, placebo-controlled trial among apparently healthy women, assignment of 100 mg of aspirin every other day statistically significantly reduced the relative risk of newly reported adult-onset asthma diagnosis by 10 percent. The magnitude of the reduction was similar across subgroups of age, smoking status, exercise, postmenopausal hormone intake, and randomised vitamin E assignment but was absent among obese.
Our data are consistent with findings in men from a post-hoc analysis of the randomised aspirin component of the Physicians' Health Study,9 which showed a statistically significant 20% relative risk reduction of newly reported adult-onset asthma diagnosis (hazard ratio 0.78, 95% CI, 0.61−1.00, P=0.045) for men who were randomly assigned to 325 mg of aspirin every other day when compared to men assigned to placebo. The smaller effect estimate in the current study may have been due to the lower aspirin dose in the WHS (100 mg every other day) compared to that in the Physicians' Health Study (325 mg every other day). However, gender differences in the association between aspirin and adult-onset asthma may also account for the reduced effect in women.
Our results are also consistent with prior findings from a large prospective observational cohort study of female registered nurses in which women who self-selected for frequent aspirin use (15 days on more per month) had a 40% lower risk of newly reported physician diagnosis of adult-onset asthma compared with women who did not take aspirin.10 The stronger effect size may be due to higher aspirin doses or to residual confounding. However, comparing the attributable incidence rate from the randomised data of the Women's Health Study (5.3/ 10,000 women per year), the randomised data of the Physicians' Health Study (5.9/ 10,000 men per year),9 and the observational data from the Nurses' Health Study (4.4/ 10,000 women per year)10 show very similar results.
Among individuals with existing asthma, aspirin can acutely precipitate bronchospasm in the subset of patients with aspirin intolerant asthma. This intolerance to aspirin affects between 4% to 11% of patients with asthma.16-18 The aetiology of aspirin intolerant asthma is complex and not fully understood, but most evidence points towards an abnormality of arachidonic acid metabolism.19 However, most patients with asthma tolerate aspirin well, and some asthma patients improve when challenged with aspirin or other non-steroidal anti-inflammatory drugs.20-22
The precise biological mechanism by which long-term low-dose aspirin use may reduce the risk of asthma is currently unknown and should be the target of future studies. Several mechanisms that relate to immune-mediating effects of aspirin, which act via cyclooxygenase (COX)-dependent23 and COX-independent pathways, can be envisioned. The effect of inhibiting COX with regard to asthma is likely dependent on the balance between proinflammatory prostaglandin D2, prostaglandin F2α, thromboxane A2 and anti-inflammatory prostaglandin E2 production23 Aspirin irreversibly inhibits the isoenzyme COX-1. COX-1 inhibition can lead to a reduction of prostaglandin E2 and subsequently to a reduction in the synthesis of interferon-γ of Th-1 lymphocytes.24 25 Consistent with this mechanism, aspirin inhibits the activation of NF-kB26 and IL-4 gene expression in T cells. Therapeutic doses of aspirin also inhibit IL-4 and IL-13-induced activation of STAT6 via non-COX dependent pathways.27 Conversely, aspirin promotes STAT1 activation in interferon-γ signalling.28 Aspirin therefore seems to inhibit Th-2 immune responses while promoting Th-1 responses. Since asthma has been associated with cytokine production of the Th-2 phenotype,29 a shift toward a non-asthmatic Th-1 phenotype may lead to reduced asthma susceptibility.
An additional non-COX activity of aspirin, but not of COX-2 inhibitors, is its potent induction of lipoxin A4 and 15-epi-lipoxin A4.30 These novel products of arachadonic acid metabolism have profound anti-inflammatory properties that block airway hyper-responsiveness and pulmonary inflammation in murine models31 and in vivo32 and are currently seen as novel targets for asthma therapies.
We found a statistically significant modification of the effect of aspirin on asthma by body mass index, indicating no effect of low-dose aspirin on adult-onset asthma among obese (body mass index of 30 kg/m2 or above). Although subgroup analyses should be interpreted with caution, it is possible that there was less effect among obese women since respiratory symptoms in obese individuals may represent a different phenotype than asthma in non-obese individuals.33 Furthermore, it can be envisioned that the effects of aspirin among obese are reduced, potentially due to a larger volume distribution. This was also observed for the beneficial effect of aspirin on risk of ischemic stroke in the WHS, which was not apparent for women who were obese.11
The strengths of this trial include its randomised, double-blind, placebo controlled design, large number of participants and outcome events, as well as the high rates of follow-up and compliance. Several limitations should be considered when interpreting our results. Although this large trial had adequate size to detect a small to moderate benefit of low-dose aspirin on incident adult-onset asthma, the trial was not designed to test this specific hypothesis. The occurrence of incident adult-onset asthma was based on self-reports by the participating health care professionals, and thus, random misclassification is possible. We did not collect information on aspirin intolerant asthma in the WHS. However, since the WHS was a trial of aspirin, participants are expected to tolerate aspirin well. Chronic obstructive pulmonary disease (COPD) can be misdiagnosed as adult-onset asthma, even in health professionals.34 Due to the randomised prospective design, however, such misclassification would be expected to result in an underestimation of effect. Supporting this line of reasoning, the effect of aspirin was somewhat stronger among never smokers and weaker among current smokers, who are more likely to develop COPD. In addition, when we censored participants who reported a diagnosis of COPD during follow-up, the results were similar (data not shown). Finally, the WHS was composed of apparently healthy female health professionals aged 45 years and older of whom the majority was white. Thus, generalizability to other populations may be limited.
In summary, 100 mg of aspirin every other day reduced the relative risk of newly reported adult-onset asthma diagnoses in this large randomised trial of apparently healthy women. Although aspirin can worsen symptoms in some patients with asthma, our biologically plausible finding, along with similar results from a large randomised trial in men and observational cohort studies in women, suggests a small benefit of aspirin for the prevention of the development of asthma in adults. However, before public recommendations are provided, results from randomised trials are needed that are specifically designed to test whether low-dose aspirin reduces the risk of asthma.
ACKNOWLEDGEMENTS
We are indebted to the participants in the Women's Health Study for their outstanding commitment and cooperation, to the entire Women's Health Study staff for their expert and unfailing assistance, and to Eunjung Kim for programming assistance.
FUNDING INFORMATION
The Women's Health Study is supported by grants and from the National Heart, Lung, and Blood Institute (HL-43851), and the National Cancer Institute (CA-47988). Dr. Barr was supported by a Robert Wood Johnson Generalist Physician Faculty Scholar Award.
Dr. Kurth had full access to all the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and for the decision to submit for publication.
Footnotes
Clinical Trial Registration Information for the Women's Health Study: url: http://www.clinicaltrials.gov/ct/show/NCT00000479?order=1
Clinicaltrials.gov identifier: NCT00000479
CONFLICT OF INTEREST STATEMENT
Full disclosure statement:
Dr. Kurth has received investigator-initiated research funding from the National Institutes of Health, Bayer AG, McNeil Consumer & Specialty Pharmaceuticals, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety, and received an honorarium from Organon for contributing to an expert panel.
Dr. Barr: has received investigator-initiated research funding from the National Institutes of Health, including as co-investigator on one study for which aspirin was donated by Bayer.
Dr. Gaziano has received investigator-initiated research funding and support as Principal Investigator from National Institutes of Health, BASF, DSM Pharmaceuticals, Wyeth Pharmaceuticals, McNeil Consumer Products and Pliva; received honoraria from Bayer and Pfizer for speaking engagements, and is a consultant for Bayer, McNeil Consumer Products, Wyeth Pharmaceuticals, Merck, Nutraquest and GlaxoSmithKline.
Dr. Buring has received investigator-initiated research funding and support as Principal Investigator from the National Institutes of Health (the National Heart, Lung, and Blood Institute, the National Cancer Institute, and the National Institute of Aging) and Dow Corning Corporation; research support for pills and/or packaging from Bayer Heath Care and the Natural Source Vitamin E Association; honoraria from Bayer for speaking engagements; and serves on an external scientific advisory committee for a study by Procter & Gamble.
REFERENCES
- 1.Maziak W, Behrens T, Brasky TM, Duhme H, Rzehak P, Weiland SK, et al. Are asthma and allergies in children and adolescents increasing? Results from ISAAC phase I and phase III surveys in Munster, Germany. Allergy. 2003;58(7):572–9. doi: 10.1034/j.1398-9995.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 2.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115(5):925–7. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 3.Camargo CA, Jr., Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159(21):2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 4.Shaheen SO, Sterne JA, Thompson RL, Songhurst CE, Margetts BM, Burney PG. Dietary antioxidants and asthma in adults: population-based case-control study. American Journal of Respiratory & Critical Care Medicine. 2001;164(10 Pt 1):1823–8. doi: 10.1164/ajrccm.164.10.2104061. [DOI] [PubMed] [Google Scholar]
- 5.Leynaert B, Neukirch C, Jarvis D, Chinn S, Burney P, Neukirch F. Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood? Am J Respir Crit Care Med. 2001;164(10 Pt 1):1829–34. doi: 10.1164/ajrccm.164.10.2103137. [DOI] [PubMed] [Google Scholar]
- 6.Janson C, Anto J, Burney P, Chinn S, de Marco R, Heinrich J, et al. The European Community Respiratory Health Survey: what are the main results so far? European Community Respiratory Health Survey II. Eur Respir J. 2001;18(3):598–611. doi: 10.1183/09031936.01.00205801. [DOI] [PubMed] [Google Scholar]
- 7.Kogan MD, Pappas G, Yu SM, Kotelchuck M. Over-the-counter medication use among US preschool-age children. JAMA. 1994;272(13):1025–30. [PubMed] [Google Scholar]
- 8.Varner AE, Busse WW, Lemanske RF., Jr. Hypothesis: decreased use of pediatric aspirin has contributed to the increasing prevalence of childhood asthma. Ann Allergy Asthma Immunol. 1998;81(4):347–51. doi: 10.1016/S1081-1206(10)63127-4. [DOI] [PubMed] [Google Scholar]
- 9.Barr RG, Kurth T, Stampfer MJ, Buring JE, Hennekens CH, Gaziano JM. Aspirin and decreased adult-onset asthma: randomized comparisons from the physicians' health study. Am J Respir Crit Care Med. 2007;175(2):120–5. doi: 10.1164/rccm.200603-411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr RG, Wentowski CC, Curhan GC, Somers SC, Stampfer MJ, Schwartz J, et al. Prospective study of acetaminophen use and newly diagnosed asthma among women. American Journal of Respiratory and Critical Care Medicine. 2004;169(7):836–41. doi: 10.1164/rccm.200304-596OC. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 12.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Buring JE, Hennekens CH, Women's Health Study Research Group The Women's Health Study: Summary of the study design. J Myocardial Ischemia. 1992;4(27):27–29. [Google Scholar]
- 15.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 16.Kasper L, Sladek K, Duplaga M, Bochenek G, Liebhart J, Gladysz U, et al. Prevalence of asthma with aspirin hypersensitivity in the adult population of Poland. Allergy. 2003;58(10):1064–6. doi: 10.1034/j.1398-9995.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 17.Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28(4):717–22. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- 18.Vally H, Taylor ML, Thompson PJ. The prevalence of aspirin intolerant asthma (AIA) in Australian asthmatic patients. Thorax. 2002;57(7):569–74. doi: 10.1136/thorax.57.7.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamad AM, Sutcliffe AM, Knox AJ. Aspirin-induced asthma: clinical aspects, pathogenesis and management. Drugs. 2004;64(21):2417–32. doi: 10.2165/00003495-200464210-00004. [DOI] [PubMed] [Google Scholar]
- 20.Szczeklik A, Gryglewski RJ, Nizankowska E. Asthma relieved by aspirin and by other cyclooxygenase inhibitors. Thorax. 1978;33(5):664–5. doi: 10.1136/thx.33.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kordansky D, Adkinson NF, Jr., Norman PS, Rosenthal RR. Asthma improved by nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1978;88(4):508–11. doi: 10.7326/0003-4819-88-4-508. [DOI] [PubMed] [Google Scholar]
- 22.Resta O, Foschino-Barbaro MP, Carnimeo N, Bavoso P, Picca V. Asthma relieved by acetylsalicylic acid and nonsteroid anti-inflammatory drugs. Respiration. 1984;46(1):121–7. doi: 10.1159/000194679. [DOI] [PubMed] [Google Scholar]
- 23.Pang L, Pitt A, Petkova D, Knox AJ. The COX-1/COX-2 balance in asthma. Clin Exp Allergy. 1998;28(9):1050–8. doi: 10.1046/j.1365-2222.1998.00311.x. [DOI] [PubMed] [Google Scholar]
- 24.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146(1):108–13. [PubMed] [Google Scholar]
- 25.Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150(12):5321–9. [PubMed] [Google Scholar]
- 26.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265(5174):956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 27.Perez GM, Melo M, Keegan AD, Zamorano J. Aspirin and salicylates inhibit the IL-4- and IL-13-induced activation of STAT6. J Immunol. 2002;168(3):1428–34. doi: 10.4049/jimmunol.168.3.1428. [DOI] [PubMed] [Google Scholar]
- 28.Chen LC, Kepka-Lenhart D, Wright TM, Morris SM., Jr. Salicylate-enhanced activation of transcription factors induced by interferon-gamma. Biochem J. 1999;342(Pt 3):503–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001;344(5):350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 30.Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del Soldato P, et al. Interaction of a selective cyclooxygenase-2 inhibitor with aspirin and NO-releasing aspirin in the human gastric mucosa. Proc Natl Acad Sci U S A. 2003;100(19):10937–41. doi: 10.1073/pnas.1933204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy S, Volans G. The use of analgesics in patients with asthma. Drug Saf. 2001;24(11):829–41. doi: 10.2165/00002018-200124110-00004. [DOI] [PubMed] [Google Scholar]
- 32.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165(11):1531–5. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 33.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174(2):112–9. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr RG, Herbstman J, Speizer FE, Camargo CA., Jr. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155(10):965–71. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]