Abstract
Animal models are fundamentally important in our quest to understand the genetic, epigenetic, and environmental factors that contribute to human aging. In comparison to humans, relatively short-lived mammals are useful models as they allow for rapid assessment of both genetic manipulation and environmental intervention as related to longevity. These models also allow for the study of clinically relevant pathologies as a function of aging. Data associated with more distant species offers additional insight and critical consideration of the basic physiological processes and molecular mechanisms that influence lifespan. Consistently, two interventions, caloric restriction and repression of the growth hormone (GH)/insulin like growth factor-1/insulin axis, have been shown to increase lifespan in both invertebrates and vertebrate animal model systems. Caloric restriction (CR) is a nutrition intervention that robustly extends lifespan whether it is started early or later in life. Likewise, genes involved in the GH/IGF-1 signaling pathways can lengthen lifespan in vertebrates and invertebrates, implying evolutionary conservation of the molecular mechanisms. Specifically, insulin and insulin-like growth factor 1 (IGF-1)-like signaling and its downstream intracellular signaling molecules have been shown to be associated with lifespan in fruit flies and nematodes. More recently, mammalian models with reduced growth hormone (GH) and/or IGF-1 signaling have also been shown to have extended lifespans as compared to control siblings. Importantly, this research has also shown that these genetic alterations can keep the animals healthy and disease-free for longer periods and can alleviate specific age-related pathologies similar to what is observed for CR individuals. Thus, these mutations may not only extend lifespan but may also improve healthspan, the general health and quality of life of an organism as it ages. In this review, we will provide an overview of how the manipulation of the GH/IGF-axis influences lifespan, highlight the invertebrate and vertebrate animal models with altered lifespan due to modifications to the GH/IGF-1 signaling cascade or homologous pathways, and discuss the basic phenotypic characteristics and healthspan of these models.
GH/IGF-1 axis and aging: the controversy continues
GH synthesis and secretion are well documented to decline with normal aging in all mammalian species studied to date [1–3]. Accordingly, as GH secretion declines there is a concomitant decrease in IGF-1 levels. This well established phenomenon is sometimes referred to as somatopause [4]. In humans as well as other species, a reduction in the GH/IGF-1 axis is correlated with increased percentage of total body and visceral fat, decreased muscle mass, decreased physical fitness, decreased immune function, and physiological declines in estrogen and androgen concentrations [5–7]. Thus, the natural declines in GH and IGF-1 that accompanies age-related degenerative processes implies that the GH/IGF-1 axis may be a causative determinant. To further support this notion, various GH dosing regimens in GH deficient adult populations have been documented to benefit body composition, circulating lipids, fitness and bone density [8–12]. Further, dwarfism that accompanies untreated GH deficiency was reported to significantly shorten median lifespan [13]. These data have led many to embrace the use of GH therapy as an anti-aging regimen.
There is no question that GH treatment is important for the health and well being of GH deficient individuals. However, there is not general agreement (nor sufficient data) regarding the physiological benefits of reestablishing ‘youthful’ GH levels to normal, aging individuals. Thus, a controversy arises over whether this decline could be considered overall beneficial or detrimental to aging and aging-related problems. After all, abnormally high levels of GH, as occurs with acromegaly, increase the incidence of morbidity and mortality in both rodent models and humans [14–17]. However, this level of GH excess does not divulge the impact of restoring more moderate levels of GH on disease progression or aging. While the benefits of GH administration are diverse and well documented, it is also clear that not all clinical outcomes of restoring GH levels are favorable, with even low doses reported to increase diabetes and glucose intolerance in healthy adults [11,18] and to increase mortality in patients that are critically ill [19]. To resolve the debate, one might assume that lifespan data would be the best gauge to appraise the role of this axis on aging. Overwhelmingly, the evidence suggests that a reduction in GH/IGF-1 signaling in vertebrates or its homologous pathways in invertebrates extends lifespan as compared to control or normal siblings. Some data in humans also supports increased longevity with a reduction in this axis [20,21]. These two disparate concepts, namely GH and IGF-I ameliorate functional declines of aging while limiting lifespan, are at the center of the current controversy. Thus, GH, despite having some positive effects, is unlikely to be the answer to our society’s aging woes.
Invertebrate models relevant to GH/IGF-1 axis
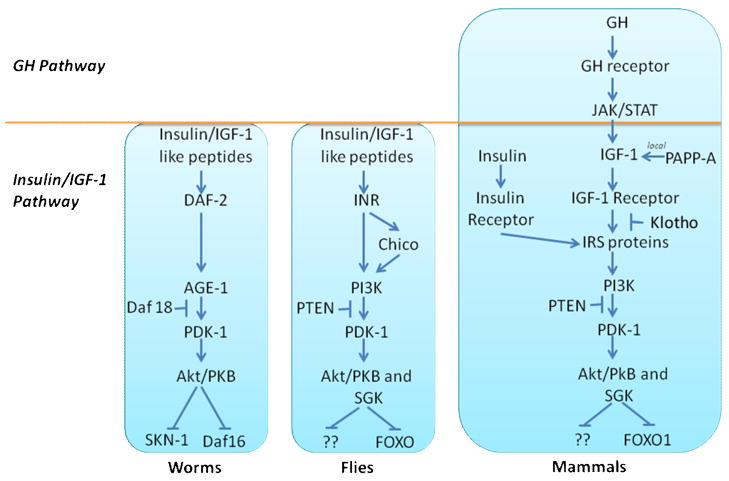
The short lifespans of invertebrate models make them powerful systems to establish molecular pathways that may influence longevity. Mutants in both Caenorhabditis elegans and Drosophila melanogastor have revealed repeatedly that a reduction in intracellular signaling pathways homologous or similar to that induced by insulin or IGF-1 signaling (IIS) increases longevity [22–25]. A comparison of IIS pathways in these species as compared to mammals is offered in Figure 1.
Figure 1. Comparison of GH/IGF-1/insulin signaling pathway in C. elegans, Drosophila and mammals.
A thorough discussion of the IIS genes that are implicated in longevity were comprehensively and recently reviewed [26–28]. Briefly, in C. elegans, genes (and corresponding protein/function in parentheses) involved in IIS include daf-2 (DAF-2 or insulin/IGF-1-like receptor), age-1 (AGE-1 encoding the catalytic subunit of phosphatidylinositol-3 kinase PI3K), pdk-1, akt-1, akt-2, sgk-1 (serine-threonine kinases PDK-1, SGK-1, Akt1 and Akt-2), as well as daf-16 (forkhead transcription factor and the major target of insulin-like signaling in C. elegans) and daf-18 (PTEN, a phosphatase to dephosphorylate phosphatidylinositol (3,4,5)-trisphosphate resulting in a dephosphorylation activity important in inhibition of the AKT signaling pathway). Recently, SKN-1, having distinct but some overlapping functions as the FOXO-like protein DAF-16, was shown to be a second transcription factor inhibited directly by IIS and to influence longevity [29]. Loss of function mutations in daf-2, age-1, pdk-1, akt 1,2 and sgk-1 result in increased lifespan while those in daf-16, daf-18 and skn-1 reduce lifespan. Likewise, in Drosophila, InR (insulin-like receptor), insulin receptor substrate (Chico), PI3K (Dp110 is the catalytic subunit and Dp60 is the regulatory subunit of PI 3-kinase), PTEN, Akt/PKB, and FOXO are components of IIS and particular mutations in many of these genes alter lifespan, albeit sometimes in a tissue specific manner.
Since both invertebrate pathways point to role of IIS in longevity and because of the marked evolutionary conservation of this pathway, it is intriguing to speculate that the pathways have similar influence on longevity in vertebrate species. However, it is important to appreciate that the connection between IIS and longevity in vertebrates is much more complex, having functionally distinct insulin and IGF molecules, multiple receptors and downstream signaling molecules with distinct tissue specific expression profiles, as well as the added dimension of GH expression. Thus, elucidating the similar yet distinct biological activities of GH versus IGF-1 and insulin is of paramount importance. Also, establishing the precise roles of particular intracellular signaling proteins, protein modifications, and tissue and cell specificity of the response to GH, IGF-1, and insulin remains a challenge and a source of intense study.
Mouse models with altered activity in the GH/IGF-1 axis and with increased longevity
Several mouse models with reduced GH and/or IGF-1 signaling have been shown to have extended lifespan as compared to control siblings. Evaluation of these mouse models and new models relevant to this axis, as well as mice with altered GH signaling that do not show increased lifespan, has offered some clues as to factors that contribute to aging. For example, it is clear that the role of the GH/IGF-1 axis overlaps partially, but is distinct from, the effect of CR, a well-recognized means to delay aging [30]. Although the mechanisms linking the GH/IGF-1 axis with delayed aging remain to be determined, there are some commonalities among the long-lived mice, such as reduced IGF-1 signaling, enhanced insulin sensitivity, improved stress resistance, and protection from carcinogenesis, which likely contribute to the improvement in longevity. A comparison of several common traits of long lived mouse models are provided in Table 1. Future studies comparing these mouse models will undoubtedly provide valuable insight into additional factors that contribute to the extension of lifespan.
Table 1.
Phenotypic comparison of mutant mice with reduced activity of GH/IGF-1 axis with calorie restricted micea
| Mouse Model |
Ames dwarfa (Prop1df) |
Snell dwarfa (Pit1dw) |
Ghrhb (lit/lit) |
GHR−/−c | IGF1R+/−d | p66shc −/−e | Klotho transgenic f |
PAPP-A−/−g | IRS-1−/−h | IRS-2+/−i | CRa | GHA transgenic j |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Effect | GH, PRL, TSH deficiency |
GH, PRL TSH deficiency |
GH deficiency |
GHR deficiency/ GH resistance |
Partial IGF-1 resistance |
Stress resistance |
Inhibit IGF-1 and insulin signaling |
Reduction in IGF-1 bio-availability |
Post-IIS receptor signaling |
Post-IIS receptor signaling |
Reduced GH signaling |
|
| % increase in mean lifespan | 49% ♂ | 26% ♂ | 23% ♂ | 26–55% ♂ | 33% ♀ | 30% | 20–31% ♂ | 41% ♂ | 32% ♂ | ↔ or 17% | Varies | Normal |
| 68% ♀ | 42% ♀ | 25% ♀ | 16–38% ♀ | 19% ♀ | 33% ♀ | |||||||
| Plasma levels | ||||||||||||
| GH | ↓ | ↓ | ↓↓ | ↑ | ND | ND | ND | ↔ | ↔ | ↔ | ↓ | ↑ |
| IGF-1 | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↑ | ND | ND | ↔ | ↔ | ↔ | ↓ | ↓ |
| Glucose | ↓ | ↓ | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↓ | ↔ |
| Insulin | ↓ | ↓ | ↓ | ↓↓ | ↔ | ↔ | ↑ | ↔ | ↑ | ↓ | ↓ | ↔ |
| Growth | ||||||||||||
| Body size | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↔ | ↔ | ↔ | ↓↓ | ↓↓ | ↔ to ↑ | ↓ | ↓ |
| % body fat | ↑ | ↑ | ↑↑ | ↑ | ND | ND | ↓↓ | ND | ↓ | ND | ↓ | ↑ |
| Energy Balance | ||||||||||||
| Food Intake | ND | ND | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↑ |
| Metabolic Rate | ↓ | ↓ | ↔ | ↑ | ↔ | ↔ | ↔ | ND | ND | ND | ↓ | ND |
Mice with defects in anterior pituitary function - Snell and Ames Dwarf Mice
Snell mice, as well as dwarf mice from a spontaneous mutation in the same gene generated at Jackson labs, are homozygous for a defect in the Pit-1 (pituitary specific transcription factor 1) gene [31]. The Ames dwarf mouse, so named because it was first reported by researchers at Iowa State University [32], has a recessive point mutation in the PROP-1 (prophet of Pit1 or paired-like homeodomain transcription factor in the Prop-1) gene that encodes a transcription factor that is required for Pit-1 activation [33]. As Pit 1 and Prop-1 proteins are required for differentiation of hormone-specific cell types in the anterior pituitary, both types of homozygous mutant mice lack GH, prolactin, and thyroid stimulating hormone producing cells resulting in severe endocrine deficiency of these three hormones. Likewise, the Ames and Snell mice have similar phenotypes (Table 1). Snell and Ames mice are dwarf and characterized by female sterility and severely reduced circulating levels of insulin, IGF-1, glucose, and thyroid hormones (as reviewed, [34]). Both of these strains of mice are also remarkably long lived and exhibit a greater than 40% increase in longevity. For Ames dwarf, females were reported to have an impressive 68% increase in lifespan (1206 ± 32 days vs. 718 ± 45 days) while males showed a 49% increase in mean lifespan (1076 ± 56 days vs. 723 ± 54 days) [35]. This extension in lifespan for Ames dwarf mice has been consistently reported, varying from 35–69% depending on diet [36–38]. For Snell dwarf mice, the extension in lifespan is less consistent and seems to vary with genetic background, housing and breeding practices of the mice. For Snell dwarfs, one report showed an increased lifespan of 42% with no significant gender difference (1,178 ± 235 days vs. 832 ± 158 days for controls) [39] and another showed a 50% increase in only female mice [40].
Mice with defects in specifically GH function - Lit/Lit and GH Receptor gene disrupted mice (GHR −/−)
Lit/Lit mice have a missense mutation in the extracellular domain of the GH releasing hormone (RH) receptor [41]. As GHRH receptor is specifically targeted, the lit/lit mice are suppressed in GH production but have no apparent defect in prolactin or thyroid stimulating hormone as with the Snell and Ames dwarf mice. Lit/Lit mice share some features of Snell and Ames dwarf mice as they are also dwarf (50–75% smaller than wild-type mice), have reduced serum IGF-1 levels and marked increase in adiposity [42,43]. In a C57BL/6J background, lit/lit mice are longer lived than heterozygous (+/−) controls with a 23% increase in males (1093 ± 186 days versus 886 ± 148 days) and a 25% increase in females (1070 ± 127 days versus 857 ±169 days) [39]. Mice with complete removal of GH action by disrupting or knocking out the GHR/binding protein (BP) gene (termed GHR−/−) were generated in our laboratory nearly a decade ago [44]. These GHR−/− are dwarf, GH resistant or insensitive and are characterized by elevated levels of GH yet markedly reduced levels of IGF-1 [44,45]. Other notable features of these mice include delayed puberty [46,47], decreased fasting insulin and glucose levels [45,48], and diminished pancreatic islet size [48]. The GHR−/− mice also are protected from both diabetes-induced nephropathy [49] and prostate and mammary carcinogenesis [50,51]. While longevity is increased in GHR−/− mice, the increase again varies according to background strain. In a mixed genetic background, GHR−/− mice had an increase in mean lifespan of 55% in males to 38% in females [52]. When GHR−/− mice were backcrossed into the C57BL/6J strain, lifespan was still significantly increased but to a lesser degree with an average increase of 26% and 16% in males and females, respectively [45]. As with the lit/lit mouse, the longevity data in GHR−/− mice implicate the GH/IGF axis and not other pituitary hormones with improving lifespan. Notably, one GHR−/− mouse from a colony managed by Dr. Andrzej Bartke lived 1,819 days and holds the ‘longevity record for laboratory mice’[53].
Mice with defects in IGF-1 or IGF-1 receptor
One common feature of the mouse models with increased longevity mentioned thus far is a very low level of circulating insulin-like growth factor-1 (IGF-1). Likewise, studies conducted on heterogeneous wild-caught mice, wild-caught mice crossed with common inbred strains of mice, and mice selectively bred for small body size all point to a role of reduced IGF-1 levels on extending longevity [54,55]. So what are the aging profiles in genetically homogeneous mice that lack either IGF-1 or its receptor? Regarding targeted genetic modification, mice with a gene disruption in either IGF-1 or the IGF-1 receptor unfortunately die at birth or shortly after, hampering the ability to study their role on longevity [56]. However, mice heterozygous for the IGF-1 receptor gene disruption (IGF1R +/−), which have a 50% reduction in receptor levels, do show a 33% increase in lifespan for females, while male mice show no statistically significant increase in longevity [57]. Interestingly, the female mice have an increased lifespan and are not dwarf, unlike the previous models discussed, suggesting that dwarfism is not a requirement for the extended lifespan. Two other mouse models to be discussed (p66shc −/− mice and KLOTHO transgenic) also shed doubt on the requirement of smaller body size for increased longevity.
Pregnancy-associated plasma protein-A (PAPP-A) is a secreted metalloproteinase that has been reported to degrade inhibitory insulin-like growth factor binding proteins (IGFBPs). Thus, PAPP-A increases IGF-I availability in vascular tissues without altering IGF-1 expression (as reviewed in [58]). PAPP-A KO mice, which presumably have reduced localized IGF-1 bioavailability yet normal circulating levels of IGF-1, were recently shown to have a striking 33% and 41% increase in lifespan for males and females, respectively (Conover, Aging Cell 2007). Interestingly, this extension was not associated with any significant alteration in circulating levels of insulin, glucose, GH, IGF-1 or cholesterol or in level of dietary intake. Thus, these mice are living longer without altered insulin sensitivity and without voluntary calorie reduction. Similar to other mouse models with increased longevity and reduction in GH/IGF axis, these mice displayed a marked reduction in the incidence of spontaneous tumors [59] and vascular disease [60], hinting to the importance of this protein and the GH/IGF-1 system in development of disease pathologies. One potential advantage of PAPP-A KO mice is that they may offer a means to separate the effects of GH directly from those specifically of IGF-I.
Mice with defects in downstream IGF-1 Signaling Molecules
p66shc
Shc proteins mediate growth factor mitogenic actions by activation of the MAPK pathway. Ligand binding and subsequent activation of IGF1R, as well as other growth factor receptors, results in recruitment of p66shc, a specific protein isoform of the proto-oncogene shc locus that functions in the intracellular pathways converting oxidative signals into apoptosis [61], to the receptors where it is phosphorylated and activated. The p66 isoform has recently been suggested to regulate mitochondrial oxidative capacity with its absence increasing the reliance on glycolysis as opposed to oxidative metabolism [62]. Mice lacking this IGF-1 receptor substrate, p66shc −/−, live 30% longer than their normal littermates and have enhanced resistance to oxidative stress [63]. The p66shc −/− mice also are protected from disease progression as they have been shown to have reduced incidences of age-related endothelial dysfunction [64], angiotensin II-induced myocardial damage [65], some behavioral aspects of aging [66], and diabetic glomerulopathy [67], all presumably by reducing oxidative stress. Of note, p66shc −/− mice also confirm that smaller body size is not required for increased longevity..
KLOTHO
The Klotho protein is expressed principally in the distal tubules of the kidney and the choroid plexus in the brain [68,69]. Klotho is a transmembrane protein with homology to B-glycosidases, but evidence also suggests that a fragment of Klotho is found in circulation, implying it may also act as a hormone. In relationship to the GH/IGF-1 axis, the Klotho protein is hypothesized to bind and repress intracellular signals of insulin and IGF-1 [70]. Mice homozygous for a mutation in Klotho exhibited growth retardation, osteoporosis, arteriosclerosis, CNS neuronal alterations and atrophy of the skin, implying accelerated aging [70–72]. Not surprisingly, this gene disruption also results in premature death [69]. In contrast, over expression of Klotho in mice extends lifespan with mice carrying the transgene outliving wild-type controls by 20–31% in males and by 19% in females [70]. In contrast to previously discussed insulin sensitive mouse models with increased lifespan, the Klotho transgenic mice are hyperinsulinemic and insulin resistant. Further, there is evidence that a homologue of Klotho declines in association with human aging [73]. It is important to point out that Klotho has other proposed functions, such as altering Wnt induced intracellular signaling and altering the activity of multiple ion channels. These activities also need to be considered when evaluating the role of Klotho on aging.
IRS-1 and IRS-2
Insulin receptor substrate (IRS) proteins are major substrates of both insulin receptor and IGF receptor kinases and serve as docking proteins between these receptors and a complex network of intracellular signaling pathways. In mammals, there are at least 4 distinct proteins, IRS-1 through IRS-4 with distinct tissue distributions and differences in the signaling capacity [74]. IRS-1 and IRS-2 display differential sensitivities and are more widely expressed than the other IRS proteins. Selman et al. [75] performed longevity studies in homozygous (IRS-1 −/−) and heterozygous (IRS1 +/−) mice as compared to wild-type controls. Female IRS-1 −/− mice displayed a statistically significant increase in longevity (32%) relative to controls. No significant increase in lifespan was noted for males or for heterozygotes of either gender. Features of the longer lived IRS-1 −/− female mice included reduced body mass, normal circulating GH and IGF-1 levels, and some resistance to age-sensitive markers such as motor/neurological function, immune function, bone density and idiopathic dermatitis [75]. Also, these gene disrupted mice had higher circulating insulin and mildly impaired glucose tolerance starting in early adulthood when compared to controls, suggesting that improved insulin sensitivity was not responsible for the enhanced longevity in these mice.
Two separate laboratories have studied the impact of IRS-2 on mouse lifespan, with some inconsistencies in their findings. IRS-2 gene disrupted (IRS-2 −/−) mice have dramatically shortened lifespan, with the reduction more striking in male versus female mice [75]. These mice develop a severe diabetic phenotype due to pancreatic β cell failure [76]. The lifespan of heterozygotes is at the center of the discrepant findings. Selman et al. [75] reported no change in lifespan for heterozygotes (IRS-2+/−) of either gender as compared to control mice. In contrast, Taguchi et al [77] reported that IRS-2+/− mice (presenting data combined for both sexes) live 17% longer than littermate controls. Although IRS 2+/− mice were previously reported to display a relatively normal metabolic phenotype when young [76], Taguchi et al. [77]reported that the mice have slightly increased insulin sensitivity, increased body weight, and no change in food consumption in older IRS-2 +/− mice as compared to wild-type controls. Taguchi et al. [77] also produced mice with conditional disruption of the IRS-2 gene in the brain (bIRS-2 −/−), that resulted in an 18% extension in lifespan, implicating that reduced IRS-2 signaling specifically in the brain was likely to be responsible for the observed extension in lifespan. However, older bIRS-2−/− mice were overweight, hyperinsulinemic, and glucose intolerant when compared with control mice. Also, bIRS-2 −/− mice were more active and displayed greater glucose oxidation during meals, and they displayed stable superoxide dismutase-2 concentrations in the hypothalamus.
Mice with a repression in GH/IGF-1 axis but with no change in longevity
Not all mouse models with reduced GH or IGF-1 levels exhibit improvements in longevity. Mice that express a transgene for a growth hormone antagonist (GHA) represent one such example [78,79]. GHA mice express a molecule that competes with endogenous GH for GHR binding and results in marked reduction of GH-induced intracellular signaling [80] and, as a result, these mice are dwarf and have reduced levels of IGF-1 [78,79]. Unlike the models previously discussed with a reduction in GH/IGF-1 signaling, the GHA transgenic mice are reported to have no extension in lifespan as compared to littermate controls [45]. This model offers an interesting exception to the models previously mentioned. That is, GHA mice are dwarf, have a partial inhibition of GH action and concomitant decrease in IGF-1, but do not exhibit increased longevity. However, there are several key phenotypic differences besides lifespan between GHA and GHR−/− mice. While they have circulating levels of IGF-1 that are significantly lower than control littermates, the reduction is much less pronounced in GHA mice than other models such as the GHR−/− mice (80% decrease in GHR−/− while GHA have only a 20–25% decrease) [45]. Other striking differences between GHR−/− and GHA mice are the weight gain profile and degree of insulin sensitivity. GHA transgenic mice, although dwarf in early months, eventually gain weight that approaches that of control littermates and do not exhibit extreme insulin sensitivity, whereas GHR−/− remain substantially dwarf and notably insulin sensitive even in older mice [45].
Mice with excess in GH/IGF-1 signaling and longevity
Further confirmation that the GH/IGF-1 axis is an essential contributor to lifespan is offered by animal models with an excess in GH and IGF-1 signaling. Mice that express a GH transgene have been generated by multiple independent laboratories. These mice have fairly uniform phenotype regardless of genetic background or species of GH used. That is, GH transgenic mice are larger in size, have reduced adiposity, advanced puberty, elevated IGF-1 and insulin levels and are insulin resistant (as reviewed, [14]). Importantly, lifespan of these GH transgenic mice is drastically reduced (~50%) compared to non-transgenic controls [34,81–83]. In mice, an interesting exception to this phenomenon comes from a recent study that reported overexpression of IGF-I specifically in the heart increased longevity by about 20% and improved several measures of cardiomyocyte health [84]. It is worth noting that cancer tends to be the most common cause of death (70–85% of all deaths) in this and many strains of commonly used laboratory mice, which may have contributed to this discordant finding [85].
Other mammalian models
While the majority of lifespan data in vertebrates has been generated from mice, they are not the only animal models available to assess the impact of the GH/IGF-1 axis on aging. For example, livestock are commonly treated with exogenous growth hormone and other animals, such as rabbits [86] and pigs [87], have been genetically manipulated to express a GH transgene, resulting in acromegalic-like animals. Although these other models share many metabolic features with GH transgenic mice and GH treated or acromegalic humans, information about aging in these animals is not available and more difficult to attain because of their longer lifespans. For this review, we will limit the discussion of lifespan data available for other animal models rodents and dogs.
Other Rodents
Mice have been the model of choice for the study of longevity in part because of their smaller size, shorter lifespans, and ease of genetic manipulation. However, several studies have focused on the use of other rodent models. Data from rats have not been as clear cut or as systemic in the evaluation of the GH/IGF-1 axis on aging although some data does support that repression of this axis may be beneficial for aging. For example, a heterozygous rat model expressing an antisense GH gene shows a modest improvement in lifespan (7–10%). Interestingly, this improvement was not found for rats homozygous for the transgene, as these rats were reported to have lifespans 5 to 10% shorter than normal controls. The homozygous mice were reported to die prematurely from neoplastic diseases and to have immune system alterations as compared to control rats [88]. The dw/dw rats (rats with profound GH deficiency due to an unknown genetic defect that leads to a 95% reduction in pituitary GH [89]) do not have altered lifespan [90]. However, Sonntag et al. [90], using these rats, developed a model with a specific and limited deficiency of GH in adulthood. This was done by treating dw/dw rats with recombinant GH from 4–14 weeks of age and then stopping treatment. These so called adult-onset GH deficient rats did show an increased maximal lifespan by 12%. The adult-onset GH deficient rats also showed improvement in cognitive and cardiac function [91,92]. These results suggest that a specific and limited deficiency of GH and IGF-I may be sufficient to increase lifespan. Rodent models with natural exceptional longevity may also prove useful to provide unique insights not readily available from short-lived models. One such long-living small mammal that has been suggested as useful is the naked mole-rat, which has the longest reported lifespan of any rodent (~28.7 years) [93]. Research tools to study the hormonal milieu of these exceptionally long-lived rodents are not currently available hampering their current use but providing a potential avenue of future exploration.
Dogs
While the domestic dog descended from the gray wolf over 15,000 years ago, it is only within the last 300 or 400 years that the enormous diversification of breeds has occurred. This diversification is largely due to human pressures as opposed to natural selection, leading to unparalleled variations in body mass, metabolism and lifespan within a single species. Mass, for example, can vary from 1.4 kg in the Chihuahua to over 100 kg in the St Bernard. Lifespan also varies tremendously with some studies reporting 8 to >15 years for pet dogs. This genetic diversity poses a unique opportunity to evaluate genes that contribute to lifespan. Repeatedly, lifespan in dogs has been shown to be inversely related to body size [94–96]. Although a cause and effect relationship has not been established, several lines of evidence support a role for the GH/IGF-1 axis. For example, Favier et al. [97] demonstrated that differences in adult body size of medium-sized and giant dog breeds are preceded by differences in GH release although they did not find differences in circulating IGF-I or IGF-II concentrations. Other studies have reported strong positive correlations between body weight of dogs and plasma IGF-1 concentrations [98,99]. More recently, Sutter et al. [100] reported that a single IGF-1 haplotype is a major contributor to size variation in dogs. Although not specifically addressed and more difficult to assess in these longer lived mammals, it is tempting to speculate that this pathway may likewise contribute to aging in dogs.
Healthspan of these animal models
Thus far in this review, lifespan data have been used as the main criterion to evaluate factors that influence the aging process. However, longevity, or the average length of time an organism survives, is not giving adequate attention to the quality of that organism’s existence. The concept of healthspan implies ‘successful aging’ and although no one single definition exists, the term usually refers to life without disability and free of disease. Most long-lived animals would be expected to have some improvement in healthspan, which ultimately enabled them to achieve the longer lifespan. So how do the aforementioned models measure up in terms of healthspan? As the concept of healthspan is quite broad, we will provide a focus on areas that have been most heavily studied. As the mouse models mentioned previously have been subjected to thorough characterization, several common traits emerge as potential mechanisms, including improved insulin sensitivity, improved oxidative stress resistance, reduced incidence of cancer and renal disease, and improved cognitive functioning. On the other hand, musculoskeletal changes, at least in some respects, may be less favorable for healthspan.
Diabetes and insulin sensitivity
Insulin resistance is well documented to be a risk factor for a number of major diseases such as cardiovascular disease and type 2 diabetes mellitus. Likewise, plasma levels of insulin are inversely correlated with survival in humans [101] and insulin sensitivity [20]. Therefore, it is reasonable to hypothesize that a reduction in insulin release and improved insulin sensitivity could be, in part, responsible for increased longevity and improve healthspan by preventing type 2 diabetes and its associated maladies. In 1936, Houssay showed that an “excess of anterior pituitary lobe aggravates the diabetes or produces it in normal animals” and found that the anterior pituitary lobe extract increases the resistance to insulin in normal and hypophysectomized dogs [102]. Thus, the first correlation between a pituitary ‘factor’ inducing insulin resistance was presented. Since then, numerous studies have shown that GH possesses anti-insulin action and is a diabetogenic molecule [103–110]. Therefore, GH would be suspected to generate insulin resistance in tissues, such as adipose, muscle and liver, which respond to both GH and insulin. Indeed, liver [111,112], adipose tissue [113–117] and muscle [115,117–120] have been shown to be impacted by GH to induce insulin resistance. The molecular mechanisms responsible for GH induced insulin resistance is not completely known; however, recent data suggest that GH up regulates the regulatory subunit of PI3 kinase which then down regulates insulin signaling [118,121]. Indeed, several of the long-lived mice, such as GHR−/− mice, have reduced plasma insulin levels and improved insulin sensitivity [48,122]. Since CR GHR−/− mice do not show a further enhancement in longevity, it may suggest that the extreme insulin sensitivity found in these mice may be at an ultimate level for improved lifespan. However other long-lived models do not exhibit improvements in insulin sensitivity. For example, both Klotho transgenic and IRS-1−/− mice exhibit increased insulin resistance despite an improvement in longevity [70,75]. These ‘outliers’ still share a reduction in the signaling downstream of IGF-1 and insulin receptors, albeit by different mechanisms. Given the established cross-talk between the GH/IGF-1 axis and insulin signaling as well as the diabetogenic nature of GH, reduced insulin signaling remains a viable mechanism for the increase in longevity observed with the animals described here.
Tissue-specific gene disruption in mice has also implicated several tissues as key mediators of the extended longevity or altered insulin sensitivity. For example, the fat-specific insulin receptor gene-disrupted (FIRKO) mouse initially displays a normal growth curve, but at 3 months shows a 15–25% decrease in body weight and 50–75% lower fat mass. These lean mice are resistant to the age-related decline in glucose tolerance seen in their non-transgenic littermates and have increased median and maximal lifespan [123]. Finally, the IGF-I gene has been conditionally disrupted in the liver of mice [124]. These mice (termed LID) have ~75% reduction in circulating IGF-I, elevated GH, and a fourfold increase in serum insulin with normal glucose levels. The LID mice do not show increased longevity (Ikeno, personal communication). Interestingly, the insulin insensitivity observed in these mice appears to be mainly at the level of muscle, and treatment with either recombinant human IGF-I or GH-releasing hormone antagonist, which reduces circulating GH levels, increases insulin sensitivity [119]. Also, when LID mice are crossed with GHA mice, insulin resistance disappears [112]. Thus, the insulin resistance seen in the LID mice is due the increased levels of GH.
Resistance to stress
Stress resistance would presumably improve healthspan and contribute to longevity. In invertebrates, reduction in IIS results in resistance to multiple forms of stressors, including oxidants, heat and heavy metals (for recent reviews, see [125–128]. Can stress resistance also be observed in vertebrate models of improved longevity? In addition to other stressors, reactive oxygen species (ROS) and free radicals are involved in a variety of physiological and pathological processes including degenerative diseases. Accumulation of oxidative damage has been implicated in aging and occurs due, in part, to an imbalance between generation rates of ROS and the activity and amount of antioxidant defense systems. There is evidence that several of the mouse models with reduced activity in the GH/IGF-1 axis have improved resistance to oxidative stressors either by improved antioxidant defense or through reduced production of ROS. The p66shc −/− mice display increased resistance to the oxidative stress induced by paraquat, which generates superoxide anions upon cellular intake, and are characterized by a decreased incidence of aging-associated diseases by reportedly reducing oxidative stress [64–67]. Other models also suggest increased tolerance towards oxidative stress in mouse models with reduced activity of the GH/IGF-1 axis. For example, Ames dwarf mice have reduced lipid, protein, and DNA oxidation in brain in comparison to normal siblings [129,130] and increased antioxidant defense in skeletal muscle [131]. Likewise, fibroblasts derived from Snell dwarfs are resistant to a variety of cellular stressors, including UV light, heat, heavy metal exposure and paraquat [132]. Finally, IGF1R+/− mice challenged with paraquat treatment live longer than IGF1R+/+ mice [133]. Data for resistance to oxidative stress is less consistent for GHR−/− mice with these mice showing increased, decreased or no change in oxidative measures [134–136]. Some of the differences observed among the different models not only relate to the tissue/cell type assayed and the type of stressor, but also to the age of the animal. For example, the stress resistance of fibroblasts derived from Snell dwarf mice is dependent on age of the mouse from which the tissue was harvested [137]. Stress resistance has also been observed in long-lived mice with klotho overexpression [138]. However, it should also be noted that the stress response itself has been shown to trigger a suppression of the GH/IGF-1 axis; this suppression has been suggested to be a potential adaptation to shift energy use from growth/proliferation to self- protection from further damage by stressors [139]. Regardless, decreased vulnerability to a variety of stressors is a common theme among tissues and cell types of the long-lived mouse strains and still remains a likely mechanism by which these animals display extended longevity.
Cancer incidence
Available data support that GH/IGF-1 status may influence neoplastic tissue growth; however, this is still a very controversial area. For example, IGF-1 exerts potent effects on key stages of cancer development, having been implicated in many types of cancer (as reviewed, [140–143]); thus, inhibiting IGF-1 signaling has been suggested as an anticancer therapy [144]. On the other hand, the apoptotic influence of IGF-1 on cancer cells has others suggesting IGF-1 a potential target for anticancer therapy [145]. A debate also looms regarding GH therapy and its potential to increase cancer risk as a cancer initiation factor [146]. Animal models with a reduction in the GH/IGF-1 axis and with altered longevity would provide valuable insight into this debate, especially since mice have high mortality rate due to cancers [85]. Indeed, GHR−/− mice have protection from prostate carcinogenesis [51] and estrogen-independent mammary carcinogenesis [50]. Likewise, Ames dwarf mice are reported to have delayed occurrence of total neoplastic lesions and reduced incidence of adenocarcinoma in lung [37]. PAPP-A −/− mice also exhibit a pronounced reduction in cancer incidence. This reduction in cancer incidence is also shared with CR rodents [147] and rat models with reduced GH/IGF signaling[148,149]. Notably, GH treatment in spontaneous dwarf rats, which are protected from mammary carcinogenesis, can be made vulnerable by restoring GH levels, strongly implicating GH and/or IGF-1 in the development of neoplastic tissue and its growth[149]. GHA mice also share some reduction in mammary cancer incidence [150] yet do not have an increase in longevity, indicating that the reduction in cancer incidence may not be the sole factor important in influencing longevity. Recently, humans with GHD or Laron Syndrome have been reported to have lower incidences of cancer [151]. Finally, GH has been reported to be an autocrine cancer causing molecule [152]. Thus, the GH/IGF-1 axis may contribute to cancer propagation or progression which would clearly and negatively impact healthy aging.
Renal Health
Kidney function alters with advancing age due to, or even in the absence of, overt pathology. Many of the mouse models with enhanced longevity also appear to have protection against natural or experimentally-induced forms of kidney damage, which likely improves healthspan. For example, p66shc−/− [67] and GHR−/− [49] mice are protected from diabetes-induced kidney damage. Likewise, mice transgenic for bovine growth hormone develop glomerular lesions characterized by a disproportionate increase in glomerular volume, mesangial sclerosis as well as an increase in glomerular lipid accumulation [153,154]. However, some protection to kidney damage is also offered to GHA mice, which do not reap the benefits in lifespan, suggesting that protection from kidney damage is not sufficient to improve lifespan in this species [155]. Klotho is expressed predominantly in the distal renal tubules of the kidney and, thus, one might expect an important function of this protein in renal health. While little data has been published related to kidney function in the long-lived Klotho transgenic mice, kidney pathologies have been noted for the kidneys of Klotho −/−, which exhibit accelerated aging. These mice have displacement of cotransporters, which function at the proximal renal tubules in reabsorption of phosphate ions, implicating Klotho in calcium homeostasis [138]. Moreover, klotho −/− mice have elevated expression of plasminogen-activator 1 and an increase in renal glomerular fibrin deposition [156]. Accordingly, Klotho expression in humans is greatly suppressed in the kidneys of patients with chronic renal failure [157]. While the molecular mechanisms are not fully resolved, the recent appreciation that Klotho protein binds to fibroblast growth factor receptors and functions as a regulator of fibroblast growth factor 23 (FGF23) signaling will undoubtedly reveal its critical role in the kidney for calcium/phosphate homeostasis (as reviewed, [158]).
Cognitive Function
In humans, aging is associated with a wide spectrum of cognitive ability, ranging from intact cognitive function to clinically manifested dementia that impacts quality of life. Brain aging is typically characterized by changes in cognition function, brain volume, and neuronal function with a loss of synaptic contacts and neuronal apoptosis being common. Neural redundancy and plasticity of brain networks, promoted by mental and physical challenges, promote maintenance of brain activity. GH and IGF-1 are critical players in many aspects of central nervous system development, maintenance and plasticity. How then do the long-lived mouse models compare on basic assessments of cognitive function? Ames dwarf and GHR−/− mice appear to have an apparent delay in mental aging and improvement in some measures of cognitive function [159,160]. This apparent discrepancy may be explained by local production of IGF-1 and subsequent PI3/AKT activation in the hippocampus, reported for Ames dwarf mice [161], that may compensate for low circulating levels of these hormones. Further, hippocampal production of IGF-1 activates an anti-apoptosis signal transduction pathway through PI3/AKT, which contributes to the survival of new neurons [162]. In contrast, excess GH signaling, as with mice expression a GH transgene, exhibit enhanced cognitive function in early life, but earlier-onset of age related declines in cognitive function [163]. The demonstration that brain specific deletion of IRS2−/− influences longevity, also points to the central role of both the brain and insulin-like signaling in lifespan [77]. Much more information will be forthcoming in this area which is a fertile area of research.
Muscloskeletal Health
While there appear to be many benefits to healthspan in decreasing GH/IGF-1 axis, one might expect to find musculoskeletal health to be compromised. For muscle, aging is associated with a decline in skeletal muscle mass, sometimes referred to as “sarcopenia of old age.” There are several underlying mechanisms that have been implicated in contributing to this age-related muscle wasting. In particular, decreased protein synthesis, reduced enzymatic activity (especially in glycolytic and glycogenolytic pathways), a reduction in energy reserves, increased oxidative damage, and changes in ion content likely contribute (reviewed in [164]). GH and IGF-1 have a significant anabolic effect on skeletal muscle and so their decline with aging likely contributes to the decline in muscle mass. For example, GH, in tandem with IGF-I, promotes mitosis, protein synthesis, satellite cell proliferation and nerve sprouting, while preventing apoptosis [165,166]. GHR−/− mice have been reported to have reduced muscle mass [167] and while this decline may influence the lifespan of free-living animals, there is no apparent issue with animals maintained in controlled conditions. There is also evidence that GH influences muscle fiber type [168–170]. Using GHR−/− mice, the Pende laboratory was able to demonstrate that GH action is needed to increase skeletal muscle size by increasing myofiber size but not myofiber number [171]. Further, GH promotes fusion of myoblasts with nascent myotubes leading to increased myonuclear number, and cell fusion independent of IGF-1 [171]. On the other hand, the GH regulates substrate metabolism in muscle by antagonizing insulin-stimulated glucose disposal and by increasing lipolysis. Insulin also has a profound impact on muscle, as skeletal muscle is the principle tissue responsible for insulin-stimulated glucose disposal. Thus, muscle represents a major site responsible for insulin resistance. Recently, GH has been found to affect intracellular insulin signal transduction in muscle [117] and has been reported to induce an increase in the regulatory subunit of PI3 kinase [172]. PI3K has been found to be a potent negative regulator of skeletal muscle insulin signaling that ultimately results in insulin resistance [118,173]. This, coupled with the data of Rabinowitz [115] in which GH induced insulin resistance in the human forearm, reveals the importance of GH action in muscle.
Skeletal health may also be compromised in vertebrates with reduced GH/IGF-1 signaling. Like muscle, normal aging is associated with both quantitative and qualitative changes in bone including alterations in bone architecture, in mineralization, protein content and the accumulations of microfractures. GH and IGF-1 have major effects on growth plate chondrocytes and all bone cells (as reviewed, [174]) The separate effects of GH and IGF-1 on bone growth were established years ago [175,176]. GHR−/− mice and Ames dwarfs were reported to have reduced bone mineral density, bone mineral content, and bone area, but these factors did improve with advancing age, suggesting these dwarf mice have an attenuation of the age-related increase in bone mineral density [177,178]. GHR−/− mice were also shown to have no alteration on trabecular bone modeling [167]. Longer-lived female IRS-1−/− have increased bone volume, trabecular numbers and reduced trabecular segregation despite reports of having osteopenia and low bone turnover [75,179]. PAPP-A−/− mice also exhibit skeletal insufficiency in mass, density, architecture and strength [180]. Klotho, with its role in phosphate calcium homoestasis is clearly important in skeletal health, as its absence results in severe osteoporosis [69], yet it is unclear if the enhancement of lifespan in Klotho transgenic mice comes along with major improvements in bone health. That is, in an inducible Klotho expression system using Klotho −/− mice, researchers were able to alleviate many of the aging-related phenotypes except no improvement in osteopenic changes were observed [181]. Comparison of bone health in these long-lived models, both in early and later life, is warranted to better understand the long-term impact of repressed GH/IGF-1 signaling on bone health.
Reduction in GH/IGF-1 axis is distinct from caloric restriction
CR is recognized as one of the most successful means to delay aging. Interestingly, many phenotypic characteristics of animals subjected to CR are shared by many, but not all, of the animals with repression in the activity of the GH/IGF-1 axis. That is, CR results in reduced body weight, reduced GH and IGF-1 levels, decreased plasma levels of insulin and glucose, reduced fertility, and delayed puberty. Based on these similarities, it is feasible that CR and reductions in the GH/IGF-1 axis may increase lifespan through similar processes. It is important to note that voluntary food restriction in vertebrate models is unlikely to contribute to their increased lifespan. That is, several long-lived mouse models (GHR−/−, Ames dwarf and IRS-1−/− mice) eat more calories than littermate controls when food consumption is normalized to body weight [45,75,182] or eat the same level of calories (PAPP-A−/−, IGF1R+/−) as littermate controls [57,59].
Several studies have attempted to distinguish whether the mechanisms by which CR and GH/IGF-1 lead to increased longevity are distinct or overlapping by using CR on dwarf mouse models. When long lived Ames dwarf mice were subjected to CR, both mean and maximal lifespan were increased as compared to CR in non-dwarf controls or Ames dwarf mice fed ad libidum [36]. Since CR further increased lifespan in this dwarf model, it was suggested that distinct mechanisms were involved. Moreover, specific genes associated with insulin signaling were reported to be altered differently when Ames dwarf mice were subjected to CR [183], further pointing to separate mechanisms. In contrast, similar studies performed using our long lived, dwarf GHR−/− mouse, showed quite different results from those obtained using Ames dwarfs. When GHR−/− mice were subjected to CR, mean lifespan was unchanged in both males and females, and only a slight impact was observed in females when maximal lifespan was reported [30]. Results from this study suggest that more of an overlap exists than previously thought, especially when compared to results for CR in Ames dwarfss. While this may suggest that GH resistance and CR work via similar mechanisms, gene expression profiling still suggests some distinctions. For example, hepatic mRNA levels of IGF-1 are severely reduced while levels of several other genes, including AKT1 and IRS-1, are significantly elevated compared to control mice. CR was not shown to alter expression of these hepatic genes in comparison to mice without CR [184–186]. Recently, a comprehensive study that simultaneously compared hepatic gene expression in Ames, Snell, GHR−/− and lit/lit mice to various CR regimens was reported [187]. Using microarray technology, 43 genes were identified as being related to longevity with 13 genes having similar differential expression patterns in all four dwarf, long-lived strains and another 30 genes that were similarly expressed in both the calorie restricted and the dwarf strains. Numerous other examples of differences in gene expression in other tissues have been reported [184,188–190] suggesting that GHR−/− mice are not mere mimetics of CR animals.
Summary
The similarities in the insulin/IGF-1 and homologous regulatory systems between invertebrates and mammal models with increased longevity suggest that a fundamental mechanism of aging is likely evolutionarily conserved. A common trait shared among the long-lived models described in this review is decreased IGF-I activity. The mechanism for a reduction in IGF-1 differ in these animal models with some resulting from decreased GH action (GHR−/− mice, Lit/Lit mice, rat GH antisense transgenics), in increasing IGF-1 resistance (IGF1R+/− mice, PAPP-A) or from inhibiting IGF-1 signaling (Klotho transgenics, IRS-1 −/− mice). Further, there are no consistent phenotypic outcomes shared by all models with increased lifespan and even disparate results with the same mutant (IRS-2 +/− mice) point to the need for systemic and careful scrutiny of single studies that demonstrate extension of lifespan. While mice are routinely the animal model of choice due to genetic and physiological similiarities to humans as well as the ease of genetic manipulation, the relevance of mouse lifespan and healthspan data to the human condition are still controversial. Regardless, IGF-1 is a potent anabolic hormone that increases cellular metabolism and proliferation, enhances the function of numerous tissues, and participates in glucose homeostasis; therefore, one might expect that various animal models and humans with altered IGF-1 signaling are likely to experience alterations in longevity. A challenge in the future will be to unravel the molecular link or links and the phenotypes that tie these models together and to discern between those pertinent to humans versus those specific to the animal model of choice.
Acknowledgments
DEB is supported by funds from the Diabetes Research Initiative of the Appalachian Rural Health Institute at Ohio University and by funds from the National Institute of Diabetes and Digestive and Kidney Diseases grant K01-DK064905. JJK is supported in part by the State of Ohio’s Eminent Scholars Program, which includes a gift from Milton and Lawrence Goll and from DiAthegen, LLC, by WADA, and by NIH (AG19899, DK075436, CA099904).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sonntag WE, Steger RW, Forman LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980;107:1875–9. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- 2.Crew MD, Spindler SR, Walford RL, Koizumi A. Age-related decrease of growth hormone and prolactin gene expression in the mouse pituitary. Endocrinology. 1987;121:1251–5. doi: 10.1210/endo-121-4-1251. [DOI] [PubMed] [Google Scholar]
- 3.Muller EE, Cella SG, De Gennaro Colonna V, Parenti M, Cocchi D, Locatelli V. Aspects of the neuroendocrine control of growth hormone secretion in ageing mammals. J Reprod Fertil Suppl. 1993;46:99–114. [PubMed] [Google Scholar]
- 4.Toogood AA, O’Neill PA, Shalet SM. Beyond the somatopause: growth hormone deficiency in adults over the age of 60 years. J Clin Endocrinol Metab. 1996;81:460–5. doi: 10.1210/jcem.81.2.8636250. [DOI] [PubMed] [Google Scholar]
- 5.Veldhuis JD, Iranmanesh A, Weltman A. Elements in the pathophysiology of diminished growth hormone (GH) secretion in aging humans. Endocrine. 1997;7:41–8. doi: 10.1007/BF02778061. [DOI] [PubMed] [Google Scholar]
- 6.Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, Pincus S, et al. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1995;80:3209–22. doi: 10.1210/jcem.80.11.7593428. [DOI] [PubMed] [Google Scholar]
- 7.Weltman A, Weltman JY, Hartman ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J Clin Endocrinol Metab. 1994;78:543–8. doi: 10.1210/jcem.78.3.8126124. [DOI] [PubMed] [Google Scholar]
- 8.Amato G, Mazziotti G, Di Somma C, Lalli E, De Felice G, Conte M, Rotondi M, Pietrosante M, Lombardi G, Bellastella A, Carella C, Colao A. Recombinant growth hormone (GH) therapy in GH-deficient adults: a long-term controlled study on daily versus thrice weekly injections. J Clin Endocrinol Metab. 2000;85:3720–5. doi: 10.1210/jcem.85.10.6881. [DOI] [PubMed] [Google Scholar]
- 9.Pincelli AI, Bragato R, Scacchi M, Branzi G, Osculati G, Viarengo R, Leonetti G, Cavagnini F. Three weekly injections (TWI) of low-dose growth hormone (GH) restore low normal circulating IGF-I concentrations and reverse cardiac abnormalities associated with adult onset GH deficiency (GHD) J Endocrinol Invest. 2003;26:420–8. doi: 10.1007/BF03345197. [DOI] [PubMed] [Google Scholar]
- 10.Boguszewski CL, Meister LH, Zaninelli DC, Radominski RB. One year of GH replacement therapy with a fixed low-dose regimen improves body composition, bone mineral density and lipid profile of GH-deficient adults. Eur J Endocrinol. 2005;152:67–75. doi: 10.1530/eje.1.01817. [DOI] [PubMed] [Google Scholar]
- 11.Florakis D, Hung V, Kaltsas G, Coyte D, Jenkins PJ, Chew SL, Grossman AB, Besser GM, Monson JP. Sustained reduction in circulating cholesterol in adult hypopituitary patients given low dose titrated growth hormone replacement therapy: a two year study. Clin Endocrinol (Oxf) 2000;53:453–9. doi: 10.1046/j.1365-2265.2000.01108.x. [DOI] [PubMed] [Google Scholar]
- 12.Beauregard C, Utz A, Schaub AE, Nachtigall L, Biller BM, Miller KK, Klibanski A. Growth Hormone Decreases Visceral Fat and Improves Cardiovascular Risk Markers in Women with Hypopituitarism: a Randomized, Placebo-Controlled Study. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2007-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab. 2003;88:3664–7. doi: 10.1210/jc.2002-021938. [DOI] [PubMed] [Google Scholar]
- 14.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–6. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- 15.Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK. Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 1994;41:95–102. doi: 10.1111/j.1365-2265.1994.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 16.Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–4. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard MC. GH and mortality in acromegaly. J Endocrinol Invest. 2005;28:75–7. [PubMed] [Google Scholar]
- 18.Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. Jama. 2002;288:2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 19.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–92. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 20.Paolisso G, Barbieri M, Bonafe M, Franceschi C. Metabolic age modelling: the lesson from centenarians. Eur J Clin Invest. 2000;30:888–94. doi: 10.1046/j.1365-2362.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- 21.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–42. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–8. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang RAC. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 24.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–6. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 25.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–10. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 26.Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–8. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Gami MS, Wolkow CA. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell. 2006;5:31–7. doi: 10.1111/j.1474-9726.2006.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolkow CA. Identifying factors that promote functional aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:1001–6. doi: 10.1016/j.exger.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–38. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–5. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–33. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 32.Schaible R, Gowen JW. A new dwarf mouse. Genetics. 1961;46:896. [Google Scholar]
- 33.Andersen B, Pearse RV, 2nd, Jenne K, Sornson M, Lin SC, Bartke A, Rosenfeld MG. The Ames dwarf gene is required for Pit-1 gene activation. Dev Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- 34.Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, Bronson RT. Does growth hormone prevent or accelerate aging? Exp Gerontol. 1998;33:675–87. doi: 10.1016/s0531-5565(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 35.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 36.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 37.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–6. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 38.Bartke A, Peluso MR, Moretz N, Wright C, Bonkowski M, Winters TA, Shanahan MF, Kopchick JJ, Banz WJ. Effects of Soy-derived diets on plasma and liver lipids, glucose tolerance, and longevity in normal, long-lived and short-lived mice. Horm Metab Res. 2004;36:550–8. doi: 10.1055/s-2004-825796. [DOI] [PubMed] [Google Scholar]
- 39.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–41. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–30. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 41.Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–32. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- 42.Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 43.Puche RC, Alloatti R, Chapo G. Growth and development of male “little” mice assessed with Parks’ theory of feeding and growth. Growth Dev Aging. 2002;66:71–8. [PubMed] [Google Scholar]
- 44.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–20. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 46.Keene DE, Suescun MO, Bostwick MG, Chandrashekar V, Bartke A, Kopchick JJ. Puberty is delayed in male growth hormone receptor gene-disrupted mice. J Androl. 2002;23:661–8. [PubMed] [Google Scholar]
- 47.Bartke A, Chandrashekar V, Turyn D, Steger RW, Debeljuk L, Winters TA, Mattison JA, Danilovich NA, Croson W, Wernsing DR, Kopchick JJ. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock- out mice. Proc Soc Exp Biol Med. 1999;222:113–23. doi: 10.1046/j.1525-1373.1999.d01-121.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–13. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 49.Bellush LL, Doublier S, Holland AN, Striker LJ, Striker GE, Kopchick JJ. Protection against diabetes-induced nephropathy in growth hormone receptor/binding protein gene-disrupted mice. Endocrinology. 2000;141:163–8. doi: 10.1210/endo.141.1.7284. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Mehta RG, Lantvit DD, Coschigano KT, Kopchick JJ, Green JE, Hedayat S, Christov KT, Ray VH, Unterman TG, Swanson SM. Inhibition of estrogen independent mammary carcinogenesis by disruption of growth hormone signaling. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl138. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Prins GS, Coschigano KT, Kopchick JJ, Green JE, Ray VH, Hedayat S, Christov KT, Unterman TG, Swanson SM. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology. 2005;146:5188–96. doi: 10.1210/en.2005-0607. [DOI] [PubMed] [Google Scholar]
- 52.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–13. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 53.Pilcher HR. Money for old mice. Nature News 2003 [Google Scholar]
- 54.Harper JM, Durkee SJ, Dysko RC, Austad SN, Miller RA. Genetic modulation of hormone levels and life span in hybrids between laboratory and wild-derived mice. J Gerontol A Biol Sci Med Sci. 2006;61:1019–29. doi: 10.1093/gerona/61.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller RA, Chrisp C, Atchley W. Differential longevity in mouse stocks selected for early life growth trajectory. J Gerontol A Biol Sci Med Sci. 2000;55:B455–61. doi: 10.1093/gerona/55.9.b455. [DOI] [PubMed] [Google Scholar]
- 56.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 57.Holzenberger M. The GH/IGF-I axis and longevity. Eur J Endocrinol. 2004;151(Suppl 1):S23–7. doi: 10.1530/eje.0.151s023. [DOI] [PubMed] [Google Scholar]
- 58.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–8. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–9. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 60.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- 61.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, Pawson T, Di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. Embo J. 1997;16:706–16. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, Finkel T. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J Biol Chem. 2006;281:10555–60. doi: 10.1074/jbc.M511626200. [DOI] [PubMed] [Google Scholar]
- 63.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 64.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–95. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 65.Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, Quaini F, Padura IM, Lanfrancone L, Pelicci P, Emanueli C. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005;46:433–40. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- 66.Berry A, Capone F, Giorgio M, Pelicci PG, de Kloet ER, Alleva E, Minghetti L, Cirulli F. Deletion of the life span determinant p66(Shc) prevents age-dependent increases in emotionality and pain sensitivity in mice. Exp Gerontol. 2006 doi: 10.1016/j.exger.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 67.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G. Deletion of p66Shc Longevity Gene Protects Against Experimental Diabetic Glomerulopathy by Preventing Diabetes-Induced Oxidative Stress. Diabetes. 2006;55:1642–50. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 68.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–20. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 69.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 70.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki H, Amizuka N, Oda K, Noda M, Ohshima H, Maeda T. Histological and elemental analyses of impaired bone mineralization in klotho-deficient mice. J Anat. 2008;212:275–85. doi: 10.1111/j.1469-7580.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 73.Arking DE, Krebsova A, Macek M, Sr, Macek M, Jr, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99:856–61. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uchida T, Myers MG, Jr, White MF. IRS-4 mediates protein kinase B signaling during insulin stimulation without promoting antiapoptosis. Mol Cell Biol. 2000;20:126–38. doi: 10.1128/mcb.20.1.126-138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. Faseb J. 2008;22:807–18. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 76.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–4. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 77.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–72. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 78.Chen WY, White ME, Wagner TE, Kopchick JJ. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology. 1991;129:1402–8. doi: 10.1210/endo-129-3-1402. [DOI] [PubMed] [Google Scholar]
- 79.Chen WY, Wight DC, Mehta BV, Wagner TE, Kopchick JJ. Glycine 119 of bovine growth hormone is critical for growth-promoting activity. Mol Endocrinol. 1991;5:1845–52. doi: 10.1210/mend-5-12-1845. [DOI] [PubMed] [Google Scholar]
- 80.Okada S, Chen WY, Wiehl P, Kelder B, Goodman HM, Guller S, Sonenberg M, Kopchick JJ. A growth hormone (GH) analog can antagonize the ability of native GH to promote differentiation of 3T3-F442A preadipocytes and stimulate insulin-like and lipolytic activities in primary rat adipocytes. Endocrinology. 1992;130:2284–90. doi: 10.1210/endo.130.4.1547740. [DOI] [PubMed] [Google Scholar]
- 81.Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–8. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- 82.Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68:71–87. doi: 10.1016/0047-6374(93)90141-d. [DOI] [PubMed] [Google Scholar]
- 83.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–18. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799–806. doi: 10.1111/j.1474-9726.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 85.Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–7. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 86.Costa C, Solanes G, Visa J, Bosch F. Transgenic rabbits overexpressing growth hormone develop acromegaly and diabetes mellitus. Faseb J. 1998;12:1455–60. doi: 10.1096/fasebj.12.14.1455. [DOI] [PubMed] [Google Scholar]
- 87.Wieghart M, Hoover JL, McGrane MM, Hanson RW, Rottman FM, Holtzman SH, Wagner TE, Pinkert CA. Production of transgenic pigs harbouring a rat phosphoenolpyruvate carboxykinase-bovine growth hormone fusion gene. J Reprod Fertil Suppl. 1990;41:89–96. [PubMed] [Google Scholar]
- 88.Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathol. 2002;160:2259–65. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carmignac DF, Wells T, Carlsson LM, Clark RG, Robinson IC. Growth hormone (GH)-binding protein in normal and GH-deficient dwarf rats. J Endocrinol. 1992;135:447–57. doi: 10.1677/joe.0.1350447. [DOI] [PubMed] [Google Scholar]
- 90.Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–32. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 91.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 93.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 94.Galis F, Van der Sluijs I, Van Dooren TJ, Metz JA, Nussbaumer M. Do large dogs die young? J Exp Zoolog B Mol Dev Evol. 2007;308:119–26. doi: 10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Deeb B, Pendergrass W, Wolf N. Cellular proliferative capacity and life span in small and large dogs. J Gerontol A Biol Sci Med Sci. 1996;51:B403–8. doi: 10.1093/gerona/51a.6.b403. [DOI] [PubMed] [Google Scholar]
- 96.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–8. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- 97.Favier RP, Mol JA, Kooistra HS, Rijnberk A. Large body size in the dog is associated with transient GH excess at a young age. J Endocrinol. 2001;170:479–84. doi: 10.1677/joe.0.1700479. [DOI] [PubMed] [Google Scholar]
- 98.Eigenmann JE, Amador A, Patterson DF. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol (Copenh) 1988;118:105–8. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- 99.Tryfonidou MA, Holl MS, Vastenburg M, Oosterlaken-Dijksterhuis MA, Birkenhager-Frenkel DH, van den Brom WE, Hazewinkel HA. Hormonal regulation of calcium homeostasis in two breeds of dogs during growth at different rates. J Anim Sci. 2003;81:1568–80. doi: 10.2527/2003.8161568x. [DOI] [PubMed] [Google Scholar]
- 100.Sutter NB, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–5. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 102.Houssay B. The hypophysis and metabolism. N Engl J Med. 1936;214:961–985. [Google Scholar]
- 103.Bratusch-Marrain PR, Smith D, DeFronzo RA. The effect of growth hormone on glucose metabolism and insulin secretion in man. J Clin Endocrinol Metab. 1982;55:973–82. doi: 10.1210/jcem-55-5-973. [DOI] [PubMed] [Google Scholar]
- 104.Rizza RA, Mandarino LJ, Gerich JE. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes. 1982;31:663–9. doi: 10.2337/diab.31.8.663. [DOI] [PubMed] [Google Scholar]
- 105.Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M, Rizza R. Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. Am J Physiol. 1986;250:E269–73. doi: 10.1152/ajpendo.1986.250.3.E269. [DOI] [PubMed] [Google Scholar]
- 106.Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev. 1987;8:115–31. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- 107.Foss MC, Saad MJ, Paccola GM, Paula FJ, Piccinato CE, Moreira AC. Peripheral glucose metabolism in acromegaly. J Clin Endocrinol Metab. 1991;72:1048–53. doi: 10.1210/jcem-72-5-1048. [DOI] [PubMed] [Google Scholar]
- 108.Clemmons DR. Roles of insulin-like growth factor-I and growth hormone in mediating insulin resistance in acromegaly. Pituitary. 2002;5:181–3. doi: 10.1023/a:1023321421760. [DOI] [PubMed] [Google Scholar]
- 109.Clemmons DR. Role of insulin-like growth factor iin maintaining normal glucose homeostasis. Horm Res 62 Suppl. 2004;1:77–82. doi: 10.1159/000080763. [DOI] [PubMed] [Google Scholar]
- 110.Jorgensen JO, Krag M, Jessen N, Norrelund H, Vestergaard ET, Moller N, Christiansen JS. Growth hormone and glucose homeostasis. Horm Res 62 Suppl. 2004;3:51–5. doi: 10.1159/000080499. [DOI] [PubMed] [Google Scholar]
- 111.Yakar S, Pennisi P, Kim CH, Zhao H, Toyoshima Y, Gavrilova O, LeRoith D. Studies involving the GH-IGF axis: Lessons from IGF-I and IGF-I receptor gene targeting mouse models. J Endocrinol Invest. 2005;28:19–22. [PubMed] [Google Scholar]
- 112.Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J Clin Invest. 2004;113:96–105. doi: 10.1172/JCI200417763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nilsson L, Binart N, Bohlooly YM, Bramnert M, Egecioglu E, Kindblom J, Kelly PA, Kopchick JJ, Ormandy CJ, Ling C, Billig H. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun. 2005;331:1120–6. doi: 10.1016/j.bbrc.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 114.Roupas P, Towns RJ, Kostyo JL. Isolated adipocytes from growth hormone-treated obese (ob/ob) mice exhibit insulin resistance. Biochim Biophys Acta. 1990;1052:341–4. doi: 10.1016/0167-4889(90)90231-2. [DOI] [PubMed] [Google Scholar]
- 115.Rabinowitz D, Klassen GA, Zierler KL. Effect Of Human Growth Hormone On Muscle And Adipose Tissue Metabolism In The Forearm Of Man. J Clin Invest. 1965;44:51–61. doi: 10.1172/JCI105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kloting N, Bluher M. Extended longevity and insulin signaling in adipose tissue. Exp Gerontol. 2005;40:878–83. doi: 10.1016/j.exger.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 117.Jorgensen JO, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund SA, Billestrup N. GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab. 2006;291:E899–905. doi: 10.1152/ajpendo.00024.2006. [DOI] [PubMed] [Google Scholar]
- 118.Barbour LA, Mizanoor Rahman S, Gurevich I, Leitner JW, Fischer SJ, Roper MD, Knotts TA, Vo Y, McCurdy CE, Yakar S, Leroith D, Kahn CR, Cantley LC, Friedman JE, Draznin B. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J Biol Chem. 2005;280:37489–94. doi: 10.1074/jbc.M506967200. [DOI] [PubMed] [Google Scholar]
- 119.Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, Le Roith D. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–8. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- 120.Bramnert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. 2003;88:1455–63. doi: 10.1210/jc.2002-020542. [DOI] [PubMed] [Google Scholar]
- 121.Del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, Kopchick JJ, Friedman JE, Draznin BOM. Growth Hormone Regulation of p85{alpha} Expression and Phosphoinositide 3-Kinase Activity in Adipose Tissue: Mechanism for Growth Hormone-Mediated Insulin Resistance. Diabetes. 2007 doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- 122.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 123.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–4. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 124.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sampayo JN, Gill MS, Lithgow GJ. Oxidative stress and aging--the use of superoxide dismutase/catalase mimetics to extend lifespan. Biochem Soc Trans. 2003;31:1305–7. doi: 10.1042/bst0311305. [DOI] [PubMed] [Google Scholar]
- 126.Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123:765–71. doi: 10.1016/s0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 127.Sorensen JG, Loeschcke V. Studying stress responses in the post-genomic era: its ecological and evolutionary role. J Biosci. 2007;32:447–56. doi: 10.1007/s12038-007-0044-x. [DOI] [PubMed] [Google Scholar]
- 128.Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Exp Gerontol. 2007;42:153–9. doi: 10.1016/j.exger.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 129.Brown-Borg HM, Johnson WT, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Amer Aging Assoc. 2001;24:85–100. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J Amer Aging Assoc. 2002;25:119–122. doi: 10.1007/s11357-002-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev. 2004;125:269–81. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 132.Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. Faseb J. 2003;17:1565–6. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- 133.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 134.Beyea JA, Sawicki G, Olson DM, List E, Kopchick JJ, Harvey S. Growth hormone (GH) receptor knockout mice reveal actions of GH in lung development. Proteomics. 2006;6:341–8. doi: 10.1002/pmic.200500168. [DOI] [PubMed] [Google Scholar]
- 135.Wolf N, Penn P, Pendergrass W, Van Remmen H, Bartke A, Rabinovitch P, Martin GM. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res. 2005;81:276–85. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 136.Hauck SJ, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. 2002;34:481–6. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- 137.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–9. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 138.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–41. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 139.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–43. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 140.Bustin SA, Jenkins PJ. The growth hormone-insulin-like growth factor-I axis and colorectal cancer. Trends Mol Med. 2001;7:447–54. doi: 10.1016/s1471-4914(01)02104-9. [DOI] [PubMed] [Google Scholar]
- 141.Ciampolillo A, De Tullio C, Giorgino F. The IGF-I/IGF-I receptor pathway: Implications in the Pathophysiology of Thyroid Cancer. Curr Med Chem. 2005;12:2881–91. doi: 10.2174/092986705774454715. [DOI] [PubMed] [Google Scholar]
- 142.Larsson O, Girnita A, Girnita L. Role of insulin-like growth factor 1 receptor signalling in cancer. Br J Cancer. 2005;92:2097–101. doi: 10.1038/sj.bjc.6602627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neuhausen SL, Slattery ML, Garner CP, Ding YC, Hoffman M, Brothman AR. Prostate cancer risk and IRS1, IRS2, IGF1, and INS polymorphisms: strong association of IRS1 G972R variant and cancer risk. Prostate. 2005;64:168–74. doi: 10.1002/pros.20216. [DOI] [PubMed] [Google Scholar]
- 144.Clemmons D. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nature Reviews Drug Discovery. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 145.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Nature Reviews Drug Discovery. 2007;6:821–833. [Google Scholar]
- 146.Cohen P, Clemmons DR, Rosenfeld RG. Does the GH-IGF axis play a role in cancer pathogenesis? Growth Horm IGF Res. 2000;10:297–305. doi: 10.1054/ghir.2000.0171. [DOI] [PubMed] [Google Scholar]
- 147.Weindruch R, Naylor PH, Goldstein AL, Walford RL. Influences of aging and dietary restriction on serum thymosin alpha 1 levels in mice. J Gerontol. 1988;43:B40–2. doi: 10.1093/geronj/43.2.b40. [DOI] [PubMed] [Google Scholar]
- 148.Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, Sonntag WE. Growth hormone-deficient dwarf animals are resistant to dimethylbenzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–42. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- 149.Shen Q, Lantvit DD, Lin Q, Li Y, Christov K, Wang Z, Unterman TG, Mehta RG, Swanson SM. Advanced rat mammary cancers are growth hormone dependent. Endocrinology. 2007;148:4536–44. doi: 10.1210/en.2007-0513. [DOI] [PubMed] [Google Scholar]
- 150.Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Br J Cancer. 2001;85:428–30. doi: 10.1054/bjoc.2001.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54–7. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 152.Perry JK, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE. The contribution of growth hormone to mammary neoplasia. J Mammary Gland Biol Neoplasia. 2008;13:131–45. doi: 10.1007/s10911-008-9070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang CW, Striker LJ, Kopchick JJ, Chen WY, Pesce CM, Peten EP, Striker GE. Glomerulosclerosis in mice transgenic for native or mutated bovine growth hormone gene. Kidney Int Suppl. 1993;39:S90–4. [PubMed] [Google Scholar]
- 154.Machado MO, Hirata RD, Sellitti DF, Iotti R, Iotti A, Cusumano AM, Riordan GP, Coschigano KT, Kopchick JJ, Zuhl I, Nguyen N, Hirata MH, Doi SQ. Growth hormone promotes glomerular lipid accumulation in bGH mice. Kidney Int. 2005;68:2019–28. doi: 10.1111/j.1523-1755.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 155.Chen NY, Chen WY, Kopchick JJ. A growth hormone antagonist protects mice against streptozotocin induced glomerulosclerosis even in the presence of elevated levels of glucose and glycated hemoglobin. Endocrinology. 1996;137:5163–5. doi: 10.1210/endo.137.11.8895392. [DOI] [PubMed] [Google Scholar]
- 156.Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost. 2002;28:545–54. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- 157.Negri AL. The klotho gene: a gene predominantly expressed in the kidney is a fundamental regulator of aging and calcium/phosphorus metabolism. J Nephrol. 2005;18:654–8. [PubMed] [Google Scholar]
- 158.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–60. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- 160.Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that middle-aged growth hormone resistant GH-R-KO mice have increased memory retention compared to their normal siblings: Implications for delayed aging (abstract 252). The Endocrine Society’s 82nd Annual Meeting; Toronto, Canada: The Endocrine Society; 2000. p. 70. [Google Scholar]
- 161.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–37. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 162.Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J Gerontol A Biol Sci Med Sci. 2007;62:117–25. doi: 10.1093/gerona/62.2.117. [DOI] [PubMed] [Google Scholar]
- 163.Lemon JA, Boreham DR, Rollo CD. A dietary supplement abolishes age-related cognitive decline in transgenic mice expressing elevated free radical processes. Exp Biol Med (Maywood) 2003;228:800–10. doi: 10.1177/15353702-0322807-05. [DOI] [PubMed] [Google Scholar]
- 164.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–89. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 165.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 166.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 167.Venken K, Moverare-Skrtic S, Kopchick JJ, Coschigano KT, Ohlsson C, Boonen S, Bouillon R, Vanderschueren D. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J Bone Miner Res. 2007;22:72–82. doi: 10.1359/jbmr.060911. [DOI] [PubMed] [Google Scholar]
- 168.Daugaard JR, Laustsen JL, Hansen BS, Richter EA. Growth hormone induces muscle fibre type transformation in growth hormone-deficient rats. Acta Physiol Scand. 1998;164:119–26. doi: 10.1046/j.1365-201X.1998.00409.x. [DOI] [PubMed] [Google Scholar]
- 169.Everitt AV, Terry V, Phillips MJ, Kerry HM, Shorey CD. Morphometric analysis of gastrocnemius muscle fiber size and fiber proportions in the hypophysectomized rat after prolonged administration of growth hormone or thyroxine. Growth Dev Aging. 1996;60:85–93. [PubMed] [Google Scholar]
- 170.Roy RR, Tri C, Grossman EJ, Talmadge RJ, Grindeland RE, Mukku VR, Edgerton VR. IGF-I, growth hormone, and/or exercise effects on non-weight-bearing soleus of hypophysectomized rats. J Appl Physiol. 1996;81:302–11. doi: 10.1152/jappl.1996.81.1.302. [DOI] [PubMed] [Google Scholar]
- 171.Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA, Pende M. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci U S A. 2006;103:7315–20. doi: 10.1073/pnas.0510033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res. 2005;15:324–36. doi: 10.1016/j.ghir.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 173.Barbour LA, Shao J, Qiao L, Leitner W, Anderson M, Friedman JE, Draznin B. Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology. 2004;145:1144–50. doi: 10.1210/en.2003-1297. [DOI] [PubMed] [Google Scholar]
- 174.Bex M, Bouillon R. Growth hormone and bone health. Horm Res 60 Suppl. 2003;3:80–6. doi: 10.1159/000074507. [DOI] [PubMed] [Google Scholar]
- 175.Isaksson OG, Jansson JO, Gause IA. Growth hormone stimulates longitudinal bone growth directly. Science. 1982;216:1237–9. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- 176.Isaksson OG, Lindahl A, Nilsson A, Isgaard J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev. 1987;8:426–38. doi: 10.1210/edrv-8-4-426. [DOI] [PubMed] [Google Scholar]
- 177.Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61:562–7. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- 178.Heiman ML, Tinsley FC, Mattison JA, Hauck S, Bartke A. Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice. Endocrine. 2003;20:149–54. doi: 10.1385/ENDO:20:1-2:149. [DOI] [PubMed] [Google Scholar]
- 179.Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000;105:935–43. doi: 10.1172/JCI9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of Pregnancy-Associated Plasma Protein-A Deletion on the Adult Murine Skeleton. J Bone Miner Res. 2007 doi: 10.1359/JBMR.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–83. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 182.Mattison JA, Wright C, Bronson RT, Roth GS, Ingram DK, Bartke A. Studies of aging in Ames dwarf mice: effects of caloric restriction. J Amer Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J, Przybylski GK, Bartke A. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004;59:784–8. doi: 10.1093/gerona/59.8.b784. [DOI] [PubMed] [Google Scholar]
- 184.Miller RA, Chang Y, Galecki AT, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol Endocrinol. 2002;16:2657–66. doi: 10.1210/me.2002-0142. [DOI] [PubMed] [Google Scholar]
- 185.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–60. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 186.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Bartke A. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40:679–84. doi: 10.1016/j.exger.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 187.Swindell WR. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev. 2008;129:138–53. doi: 10.1016/j.mad.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Argentino DP, Dominici FP, Al-Regaiey K, Bonkowski MS, Bartke A, Turyn D. Effects of long-term caloric restriction on early steps of the insulin-signaling system in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 2005;60:28–34. doi: 10.1093/gerona/60.1.28. [DOI] [PubMed] [Google Scholar]
- 189.Argentino DP, Dominici FP, Munoz MC, Al-Regaiey K, Bartke A, Turyn D. Effects of long-term caloric restriction on glucose homeostasis and on the first steps of the insulin signaling system in skeletal muscle of normal and Ames dwarf (Prop1df/Prop1df) mice. Exp Gerontol. 2005;40:27–35. doi: 10.1016/j.exger.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 190.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Wang Z, Bartke A. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417–29. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–91. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 192.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–6. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 193.Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol. 2003;38:1353–64. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 194.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–51. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 195.Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 196.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–30. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 197.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–41. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin and IGF-1 levels and increased lifespan. Endocrinology. 2003;144:3799–810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 199.Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci U S A. 1990;87:5061–5. doi: 10.1073/pnas.87.13.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]