Abstract
Notch signaling is critical for the development of the nervous system. Cdk5 is a neuronal kinase involved in neuronal development and phosphorylates a number of neuronal cytoskeletal proteins. To determine the relationship between Notch and cdk5 signaling, we tested the effects of the Notch inhibitor, DAPT (N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester) on cdk5 expression, activity and cytoskeletal protein distribution in the rat cortical neurons in primary cultures. Neurons treated with 10 μM DAPT showed attenuated cdk5 activity in spite of an upregulation of cdk5 protein level, consistent with a phenomenon reported in the cdk5 transgenic mice. Immunoblot and immunofluorescence analyses showed an increased level of cdk5, but not p35. Phospho-tau and phospho-neurofilament showed a shift from axons to cell bodies in DAPT-treated cells. DAPT-induced attenuation of cdk5 activity was restored by overexpression of p35 indicating that it interacted with cdk5 and upregulated nascent cdk5 activity. P35 overexpression also rescued DAPT-induced translocation of phospho-tau and phospho-neurofilament. Immunoprecipitation followed by immunoblotting demonstrated that DAPT does not disrupt cdk5 and p35 interaction. Moreover, DAPT upregulated neurogenin that is negatively regulated by Notch, and downregulated Hes1, a downstream target of Notch, suggesting that Notch signaling in the cortical neurons was disrupted. Semi-quantitative and quantitative RT-PCR (q-PCR) analyses confirmed that DAPT upregulated cdk5 expression at the transcriptional level. These results establish a link between Notch signaling and cdk5 expression regulating neuronal cytoskeletal protein dynamics.
Introduction
Notch signaling plays an important role in the developing vertebrate nervous system. While activation of Notch signaling favors the differentiation toward glial cell types, its inhibition results in neuronal differentiation (Louvi & Artavanis-Tsakonas 2006). Activation of the Notch response is mediated by the Notch intracellular domain, which is cleaved away from the full-length receptor in a two-step proteolytic process, one of which is mediated by a presenilin-secretase complex (Saxena et al. 2001, Selkoe & Kopan 2003). Moreover, γ-secretase inhibitors that have been developed largely as a means to treat Alzheimer’s disease (Annaert & De Strooper 2002, Roberson & Mucke 2006, Tsai et al. 2002) have also been used to inhibit the Notch signaling pathway. One γ-secretase inhibitor, DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester), has been shown to phenocopy various Notch mutations in both zebrafish and Drosophila (Geling et al. 2002, Micchelli et al. 2003). DAPT efficiently blocks the presenilin/γ-secretase complex (Dovey et al. 2001) and, as a consequence, efficiently prevents activation of the Notch response (Geling et al. 2002, Sastre et al. 2001) and enhances neuronal differentiation of embryonic stem cell-derived embryoid bodies (Crawford & Roelink 2007). DAPT has also been shown to inhibit Notch signaling in in vitro studies (Cheng et al. 2003, Li et al. 2006, van den Brandt et al. 2004).
Cyclin-dependent kinase 5 (cdk5) belongs to the family of serine/threonine cyclin-dependent kinase (Meyerson et al. 1992). Cdk5 is found in mitotic cells but its activity is mostly restricted to neuronal cells due to the expression of neuron-specific activators, p35 and p39 (Dhavan & Tsai 2001). Cdk5 knockout mice exhibit defects in organization of the cortex and cerebellum and are embryonically lethal (Ohshima et al. 1996). In addition, regulation and deregulation of cdk5 activity plays an important role in a range of physiological and pathological processes that include involvement in nervous system development and neurodegeneration (Dhavan & Tsai 2001, Shelton & Johnson 2004). Recently, it has been shown that Cdk5 is associated with neuronal differentiation (Cicero & Herrup 2005). Cdk5 phosphorylates a large number of proteins, including the neurofilaments and tau (Ackerley et al. 2003, Bu et al. 2002, Pant et al. 1997, Shea et al. 2004b).
Since Notch signaling and regulated cdk5 activity play important roles in the development of the nervous system, the question arises if these two processes are linked at some point. In this study, we took advantage of DAPT to inactivate Notch signaling in the rat cortical neurons. We show that DAPT causes upregulation of cdk5 expression, however, leading to attenuated cdk5 activity in the cortical neurons. A consequent change in localization of phospho-tau and phospho-neurofilament-H is observed in the neurons as opposed to their normal distribution in the untreated cells. DAPT-induced suppression of cdk5 activity can be rescued by ectopic expression of p35 that is accompanied by a reversal of the cell body localization of phospho-tau and phospho-neurofilament. In addition, we demonstrate that cdk5 upregulation by DAPT occurs at the transcriptional level, a finding that establishes a potential link between Notch signaling and cdk5 gene expression.
Materials and methods
Materials
Antibodies to Cdk5 (J-3, C-8) and p35 (C-19), used at a dilution of 1:500, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-tau-S199/202 and Tau-5 monoclonal antibodies were from BioSource International (Camarillo, CA) and used at 1:1000 and 1:500 dilutions, respectively. AT8 antibody was purchased from Innunogenetics (Ghent, Belgium) and used at 1:500. Alpha-tubulin antibody from Sigma-Aldrich (St. Louis, MO) was used at 1:2000. Secondary horseradish peroxidase-conjugated antibodies were obtained from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom) and used at 1:2000. Secondary fluorescence-conjugated Oregon Green and Texas Red antibodies (Invitrogen, Carlsbad, CA) were used at 1:400. Anti-NF200 antibody and NGF were obtained from Sigma-Aldrich. RT97, a phospho-NF-H antibody was a gift from Drs. R. A. Nixon and Veeranna (Nathan Kline Institute for Psychiatric Research, Orangeburg, NY).
Cell cultures and treatment
Primary cultures of rat cortical neurons were prepared from E-18 rat fetuses as described previously (Zheng et al. 2003). After 7 days in culture, neurons were treated with 10 μM DAPT or only DMSO for 24 h. Rat hippocampal neuronal cultures were prepared from embryonic E-18 rat embryos at a density of 100,000 cells/ml on polyornithine- and fibronectin-coated coverslips as described previously (Zheng et al. 2007).
Immunoblotting
Western blot analyses of cell lysates prepared from the cortical neuron lysates were performed as described previously (Zheng et al. 2002). In brief, cortical neurons were harvested by scraping from dishes and lysed in ice-cold lysis buffer and incubated for 30 min on ice. After centrifugation for 20 min at 13,000 × g at 4°C, the protein concentrations of the supernatants were determined using bicinchoninic acid protein reagent. An equal amount of total protein (25 μg of protein/lane) was resolved on a 4-20% SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. This membrane was incubated in blocking buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.1% (vol/vol) Tween 20 (TTBS) plus 5% dry milk (wt/vol) for 1 h at room temperature. This was followed by incubation overnight at 4°C in primary antibodies: anti-Cdk5 (1:500), anti-p35 (1:500), anti-tubulin (1:2000), phospho-tau (AT8; 1:500) and total tau (1:1000), phospho-NF-H (RT97; 1:5000) and anti-NF-H (1:2000), phospho- or phospho-independent Erk1/2 antibodies (1:2000 and 1:1000), anti-cleaved caspase-3 (1:1000). The membranes were then washed four times in TTBS (5 min/each). This was followed by incubation in secondary antibody (goat anti-mouse or goat anti-rabbit IgG [H+L]-horseradish peroxidase conjugate at a dilution of 1:3000) for 2 h at room temperature. Western blots were analyzed using the GE Healthcare enhanced chemiluminescence kit following the manufacturer’s instructions. Quantitative assay of antigen expression was based on density measurements of protein bands using ImageJ software (http://rsb.info.nih.gov/ij/).
Transient transfection of cortical neurons
Cortical neuronal cultures were prepared and plated as described earlier. Neurons were transfected with pCDNA3-p35 using Lipofectamine 2000 (Invitrogen, USA) following the manufacturer’s instructions.
Immunocytochemical analyses
Immunofluorescence was performed as described previously (Zheng et al. 2003). In brief, cortical neurons were grown on glass coverslips coated with poly-L-lysine. Cells were washed twice in phosphate-buffered saline (PBS) and fixed for 30 min at room temperature in 4% (wt/vol) paraformaldehyde in PBS, permeabilized in 0.1% (vol/vol) Triton X-100 in PBS for 20 min, blocked with 5% (vol/vol) fetal bovine serum-PBS for 30 min, and then probed with primary antibodies: phospho-Erk (1:100), AT8 (1:500), anti-Cdk5 (1:50), RT97 (1:500), and anti-NF-H (1:50). Antibody was diluted in blocking solution at room temperature for 1 h. After washing in PBS (three times for 15 min each), the cells or coverslips were incubated with Oregon Green- and Texas Red-conjugated secondary antibodies at 1:400 for 1 h at room temperature, followed by three PBS washes, and mounted in aqueous medium. Fluorescent images were observed using 63 X oil immersion objective on a Zeiss LSM510 laser-scanning confocal microscope. Images were combined using Zeiss LSM510 image software and managed in Adobe Photoshop (Adobe Systems, Mountain View, CA).
Immunoprecipitation and cdk5 kinase assay
Immunoprecipitations and kinase assays were performed as described previously (Veeranna et al. 1998, Zheng et al. 2002).
Semi-quantitative RT-PCR
Total RNA was extracted using phenol-chloroform (TRIzol reagent, Invitrogen, Carlsbad, CA). cDNA was prepared using the First Strand Synthesis kit (Roche, USA). Semi-quantitative amplification was performed using the following primers: 5′-GGCACCTACGGAACTGTGTT-3′ (forward), and 5′-CACAATCTCAGGGTCCAGGT-3′ (reverse) for rat cdk5, 5′-TGACCTGTCTGTACCTCTCC-3′ (forward) and 5′-AGTCGCTTCTTGTCCTCCTG-3′ (reverse) for rat p35, 5′-GCACAGAAAGTCATCAAAGCC-3′ (forward) and 5′-GTTCATGCACTCGCTGAAG-3′ (reverse) for Hes1, and forward 5′-GCCAGCGATACAGAGTCCTG-3′ and reverse-5′-CCCTAGTGGTACGGGATGAA-3′ for neurogenin (Ngn). Rat GAPDH primers used as control are 5′-GACATGCCGCCTGGAGAAAC-3′ (forward) and 5′-AGCCCAGGATGCCCTTTAGT-3′ (reverse).
Quantitative RT-PCR (qPCR)
Total RNA was extracted using phenol-chloroform (TRIzol reagent, Invitrogen, Carlsbad, CA). cDNA was prepared using the First Strand Synthesis kit (Roche, USA). For the qPCR, the iQ SYBR Green kit was used (Bio-Rad, Hercules, CA). The 2-CT method was used to determine the relative gene expression (Livak & Schmittgen 2001). The GAPDH gene was the internal control for all qPCR experiments. The experiments were repeated in triplicates, and the mean values with SD are presented. For cdk5 qPCR, the primers used are as follows: forward 5′- AGCCTTTGGTATCCCAGTCC - 3′, and reverse 5′- TCCTCTTCAGCTGGTCATCC - 3′.
Results
Effect of DAPT on cdk5 protein expression
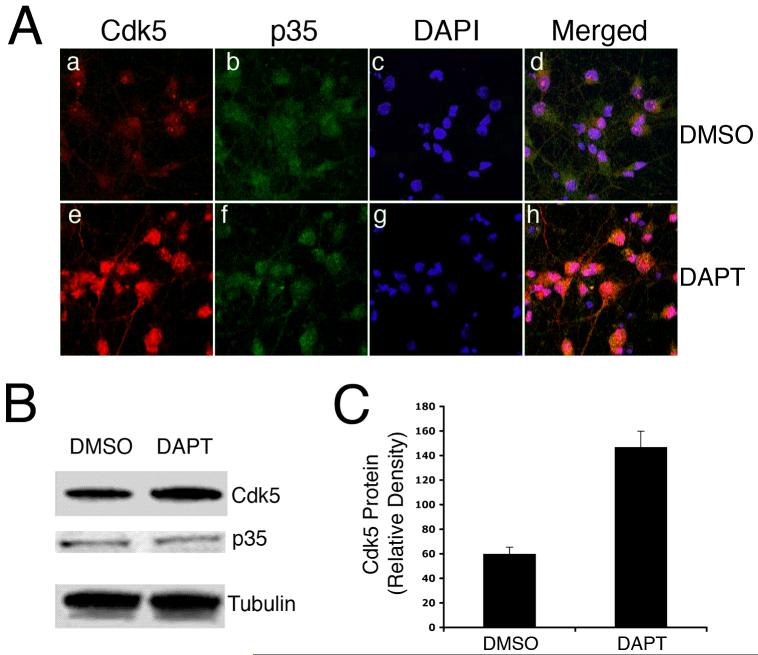
Several studies have used DAPT, a γ-secretase inhibitor, to mimic Notch signaling impairment. In this study, we examined the effect of DAPT on cdk5 expression and activity in order to determine if cdk5 and Notch, both being critical signaling components in neuronal development and survival, are linked in anyway. In the present study, rat cortical neurons were treated for 24 hours with 10 μM DAPT. Immunocytochemical studies demonstrated that compared to the control DMSO-treated neurons (Fig. 1A a), cdk5 was upregulated in the neurons treated with DAPT (Fig. 1 A e). However, there was no significant change in the p35 level between the control DMSO-treated neurons (Fig. 1A b) and DAPT-treated neurons (Fig. 1A f). The nuclear staining with DAPI for these groups of neurons is shown in Fig. 1A c and g, while overlap of cdk5 and p35 expressions is shown in Fig. 1A (d and h). Consistent with these observations, immunoblot analyses showed a significant increase in the cdk5 protein level while p35 and α-tubulin levels remained unaltered (Fig. 1B, C).
Figure 1.
DAPT induces upreguation of cdk5. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with either DMSO or 10 μM DAPT for 24h. Cells were lysed for immunoblotting or fixed for immunocytochemistry (ICC). (A) Fixed cells were immunostained for cdk5 (a, e) and p35 (b, f). Nuclei are stained with DAPI (c, g). Immunostaining for cdk5, p35 and DAPI are merged (d, h). (B) A representative immunoblot shows cdk5, p35 levels in the DMSO- and DAPT-treated neurons. Alpha-tubulin levels are shown as loading controls. (C) Quantitation of cdk5 expression in the control, DMSO- treated and DAPT-treated cells. Data are derived from three separate experiments.
DAPT downregulates cdk5 activity and activates Erk1/2
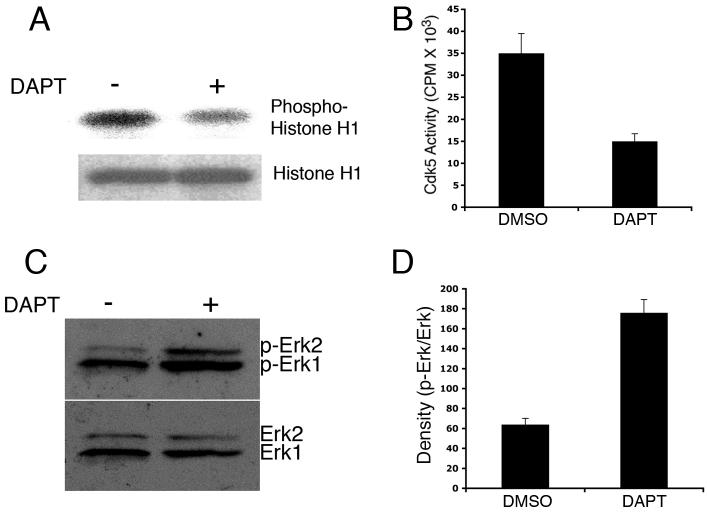
Cdk5 overexpression does not directly correlate with its catalytic activity since the activator p35 appears to be the limiting factor. To examine whether DAPT-induced cdk5 overexpression alters cdk5 activity in the primary neurons, we assayed for cdk5 catalytic activity. Kinase activity assays revealed that although DAPT induced cdk5 expression, cdk5 activity was downregulated (∼ 3-fold) in the neurons compared to that in the control, DMSO-treated neurons (Fig. 2 A, B). This is consistent with a previous report showing cdk5 transgenic mice with 40% reduction in cdk5 catalytic activity in the brain (Tanaka et al. 2001).
Figure 2.
DAPT downregulates cdk5 activity and activates MAPK (Erk1/2). (A) Cdk5 kinase activity is shown for DMSO- and DAPT-treated neuronal extracts in a representative autoradiogram. Upper panel shows the autoradiogram of phosphorylated Histone H1 by the immunoprecipitated cdk5 from the lysates. Lower panel shows Coomassie blue staining of the Histone H1 substrate used for the kinase assay. (B) Densitometric analyses of phosphorylated Histone H1 from three different autoradiograms show the difference in cdk5 activity between DMSO- and DAPT-treated neurons. (C) A representative immunoblot shows p-Erk (upper panel) and total Erk levels in cortical neurons treated with DMSO- and DAPT.
In a previous study, we demonstrated that cdk5 inhibits the MAPK pathway in NGF-stimulated PC12 cells by phosphorylating MEK1 (Sharma et al. 2002). It has been shown that Erk p42/44 MAPK regulates NF anterograde transport by NF C-terminal phosphorylation (Chan et al. 2004), and cdk5 induced inhibtion of MAPK activity inhibits anterograde axonal transport of neurofilaments (Moran et al. 2005). Here, we explored whether downregulation of cdk5 activity by DAPT resulted in a change in MAPK activity. To this end, immunoblot analyses of DMSO- and DAPT-treated cortical neuron lysates revealed an upregulation of p-Erk1/2 in DAPT-treated cells (Fig. 2 C, upper panel and Fig. 2 D). Equal loading was confirmed as shown by the presence of equivalent levels of total Erk1/2 (Fig. 2 C, lower panel).
Tau accumulates in cell bodies of DAPT-treated neurons
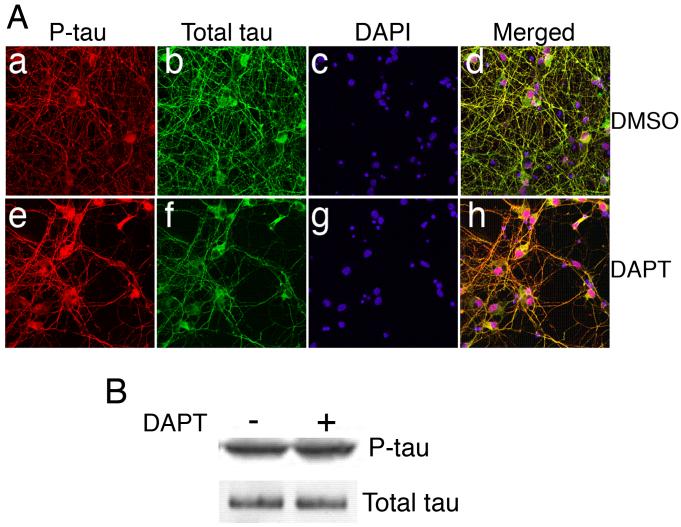
Since DAPT induced a suppression of cdk5 activity albeit through a mechanism that upregulates cdk5 protein level, we further tested whether the downstream effects of reduced cdk5 activity did occur. Based on prior studies that cdk5 phosphorylates a large number of proteins, including the neurofilaments and tau (Ackerley et al. 2003, Bu et al. 2002, Pant et al. 1997, Shea et al. 2004a), we hypothesized that DAPT by attenuating cdk5 activity could therefore affect the cytoskeletal proteins in terms of their phosphorylation state and subsequent distribution due to elevated Erk1/2 activity (Sharma et al. 2002). Immunocytochemical studies demonstrated that the distribution of phospho-tau (p-tau) was significantly altered (Fig. 3A). Significant accumulation of p-tau level in the soma occurred in DAPT-treated neurons (3A e) as compared to the control, DMSO-treated neurons (Fig. 3A a). Total tau expression is shown in Fig. 3A b and f, respectively. DAPI staining of the nuclei are shown in Fig. 3A c and g. Merged images are presented in Fig. 3A d and h. Immunoblot analyses demonstrated that in DAPT-treated primary neurons there was a slight increase of the p-tau level (Fig. 3B). We have shown in our earlier report that inhibition of cdk5 activity by treating cortical neurons with roscovitine results in the accumulation of p-tau in the soma (Zheng et al. 2007). In this case, however, roscovitine does not induce a change in cdk5 protein level but elevates Erk1/2 activity. Therefore, it is intriguing that attenuation of cdk5 activity by upregulating its expression level has a similar effect on p-tau distribution as observed by inhibiting cdk5 activity without any change in cdk5 protein level. How different the two ways of suppressing cdk5 activity can be, is well documented by loss of cdk5 activity in mice (Tanaka et al. 2001). For instance, p35-null mice have reduced cdk5 activity without any change in the cdk5 protein level and these mice have defects in cortical lamination, and show seizures and adult lethality (Chae et al. 1997). On the other hand, cdk5 transgenic mice show decreased cdk5 activity with an increase in cdk5 protein level and these mice are normal (Tanaka et al. 2001). The present study further supports previous studies that cdk5 crosstalk is one of the major factors regulating neuronal behavior (Rudrabhatla et al. 2008). It is important to note that not only the reduction in cdk5 activity, but also how that reduction comes about, is relevant for a specific biological outcome. This in itself is a critical parameter when it comes to choosing agents for therapeutic use.
Figure 3.
DAPT affects distribution of tau protein. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with either DMSO (a-d) or 10 μM DAPT (e-h) for 24 h. Cells were lysed for immunoblotting or fixed for immunocytochemistry (ICC). (A) Fixed cells were immunostained for phospho-tau (p-tau) (a, e) and total tau (b, f). Nuclei are stained with DAPI (c-g). Merged image of p-tau, total tau and DAPI staining of DMSO-treated cells is shown (d) and that DAPT-treated cells (h) are shown. (B) Immunoblot shows p-tau and total tau levels in the DMSO- and DAPT-treated neurons.
Neurofilament-H shifts to soma from axons in neurons treated with DAPT
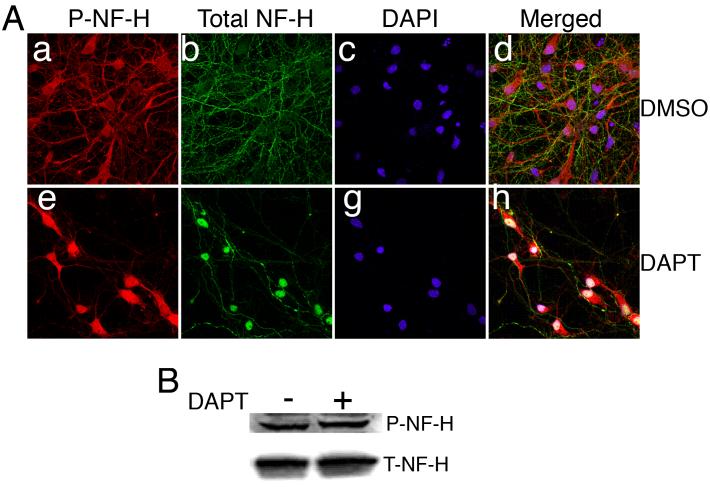
Similar studies also showed that total neurofilament (NF-H) expression in the control DMSO-treated and DAPT-treated cells did not change (Fig. 4A a, e), but phospho-N-FH (p-NF-H) accumulated in the soma accompanied by a decrease in axon localization in the neurons treated with DAPT (Fig. 4A e) in contrast to the DMSO-treated cells (Fig. 4A b). DAPI staining for the nuclei (Fig. 4A c, g) and the overlap of total-NF-H, P- NF-H is shown in Fig. 4A d, h. Immunoblot analyses demonstrated that DAPT-treated neurons showed a slight increase in P-NF-H level (Fig. 4B). These results reflect a scenario seen in the neurons treated with the cdk5 inhibitor, roscovitine, described earlier in our report, where inhibition of cdk5 activity resulted in the accumulation of p-tau and p-NF-H in the cell bodies (Zheng et al. 2007).
Figure 4.
DAPT affects distribution of high molecular weight neurofilament (NF-H) proteins. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with either DMSO (a-d) or 10 μM DAPT (e-h) for 24 h. Cells were lysed for immunoblotting or fixed for immunocytochemistry (ICC). (A) Fixed cells were immunostained for phospho-NF-H using R97 antibody (a, e) and total NF-H using anti-NF-H antibody (b, f). Nuclei are stained with DAPI (c, g). Merged image of p-NF-H, total NF-H and DAPI staining of DMSO-treated cells is shown (d) and that DAPT-treated cells (h) are shown. (B) Immunoblot shows p-NF-H and total NF-H levels in the DMSO- and DAPT-treated neurons.
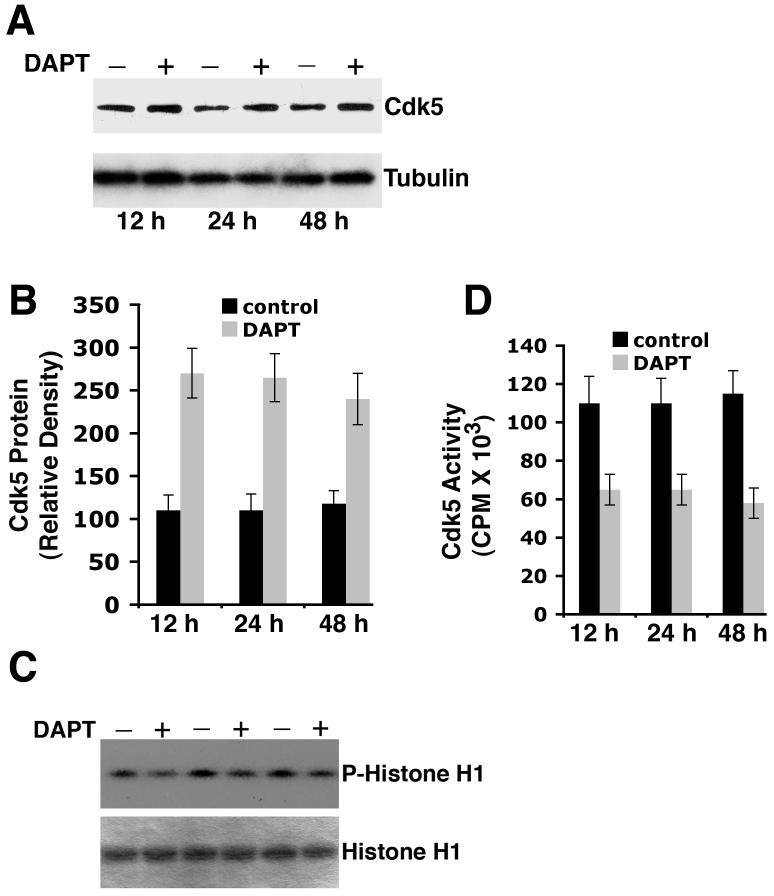
Effect of long-term treatment of neurons with DAPT
Although a 24 h time point was chosen to see if DAPT had any effect on the cortical neuron survival, it was imperative to elucidate its effect over a defined period of time. Neurons were treated with DMSO or DAPT from 12 - 48 h. This time-course experiment revealed that a significant upregulation in the cdk5 protein level occurred as early as 12 h after DAPT treatment (Fig. 5A, B). Immunoblotting of the protein extracts with anti-tubulin antibody was performed to indicate total protein loads in each lane (Fig. 5A, bottom panel). Densitometric analyses of the immunoblot for cdk5 demonstrated that DAPT-induced cdk5 overexpression remains unaltered from 12-48 h of treatment (Fig. 5 B). Cdk5 activity remained at a lower level during this period of time (Fig. 5C, D). Significant suppression of cdk5 activity occurred as early as 12 h after DAPT treatment and the level of attenuation remained unaltered until 48 h (Fig. 5D).
Figure 5.
DAPT induces cdk5 expression and causes reduction in cdk5 activity. (A) A time course experiment using immunoblotting shows DAPT induction of cdk5 expression in the cortical neurons as early as in 12 h of treatment. Equal total protein loading is shown by α-tubulin expression. (B) Densitometric analyses of three separate experiments show significant changes in cdk5 expression between the control, DMSO-treated neurons and DAPT-treated neurons (12-48 h). (C) A time course experiment shows cdk5 activity in the control, DMSO-treated cells and DAPT-treated cells at 12, 24 and 48 h. Upper panel shows the autoradiogram of the gels with phosphorylated Histone H1 that was used as a substrate in the kinase reaction, bottom panel shows the Coomassie blue-stained Histone H1, the source of the autoradiogram, in the SDS-PAGE gel. (D) Scintillation counting of the phospho-Histone H1 protein cut out from the SDS-PAGE gels after autoradiography shows the relative extent of Histone H1 phosphorylation representing cdk5 activity in the control, DMSO- treated and DAPT-treated cells. Data are derived from three separate experiments.
Effect of p35 overexpression on DAPT-induced Tau and NF-H translocation
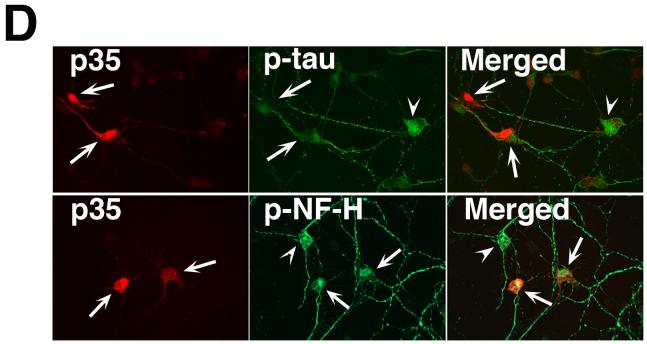
Since DAPT induced an increase in cdk5 protein expression accompanied by the downregulation of cdk5 catalytic activity that is reminiscent of what happens in cdk5 transgenic mice (Tanaka et al., 2001), we attempted to overexpress p35 in the neurons in order to activate the nascent cdk5, produced by DAPT treatment. Cortical neurons were transfected with pcDNA3-p35 plasmid and 24 h post-transfection, DAPT was added. After 24 h of DAPT addition, neurons were processed for immunolocalization of p-tau and p-NF-H. First, lysates prepared from these cells showed an increased expression of p35 (Fig. 6A). It is important to note that this particular exposure time of the western blot does not show the endogenous p35 level in the vector-transfected neurons (Fig. 6A, upper panel), although overexposed films show the endogenous p35 levels (data not shown). As expected, cdk5 level increased in the DAPT-treated vector-transfected neurons and also in the p35-transfected neurons compared to their control, DMSO-treated counterparts (Fig. 6A, bottom panel). DAPT caused attenuation of cdk5 activity while p35 overexpression increased cdk5 activity (Fig. 6B, upper panel). Interestingly, in p35-overexpressing neurons cdk5 activity further increased significantly in presence of DAPT (Fig. 6B). Quantitative differences of the cdk5 activities in these experimental groups obtained by scintillation counting of the phospho-Histone H1 cut from the stained SDS-PAGE gels following autoradiography are shown (Fig. 6C). These results suggested that cdk5/p35 association is not disrupted by DAPT treatment and more importantly the nascent cdk5 induced by DAPT can be activated by the overexpressed p35. Whether the rescue of cdk5 activity in DAPT-treated neurons by p35 overexpression did have an impact on p-tau and p-NF-H localization was examined by immunocytochemistry. P35 overexpression did reverse DAPT-induced localization of p-tau to the soma (Fig. 6D, upper panel, arrows), thus relocalizing p-tau to the neurites (Fig. 6D, upper panel, arrowhead). A partial rescue of DAPT-induced p-NF-H localization to the cell body was evident in p35-overexpressing neurons (Fig. 6D, lower panel, arrow) as compared to the neurons not overexpressing p35 (Fig. 6D, lower panel, arrowhead). A partial rescue of DAPT-induced cell body accumulation of p-NF-H is considered significant in the context that p-NF-H translocation to the cell body upon DAPT treatment is much more extensive in comparison to that seen for p-tau (Figs. 3A and 4A, bottom panels). These results indicate that DAPT-induced attenuation of cdk5 activity is, in fact, responsible for the cellular distribution of p-tau and p-NF-H.
Fig. 6.
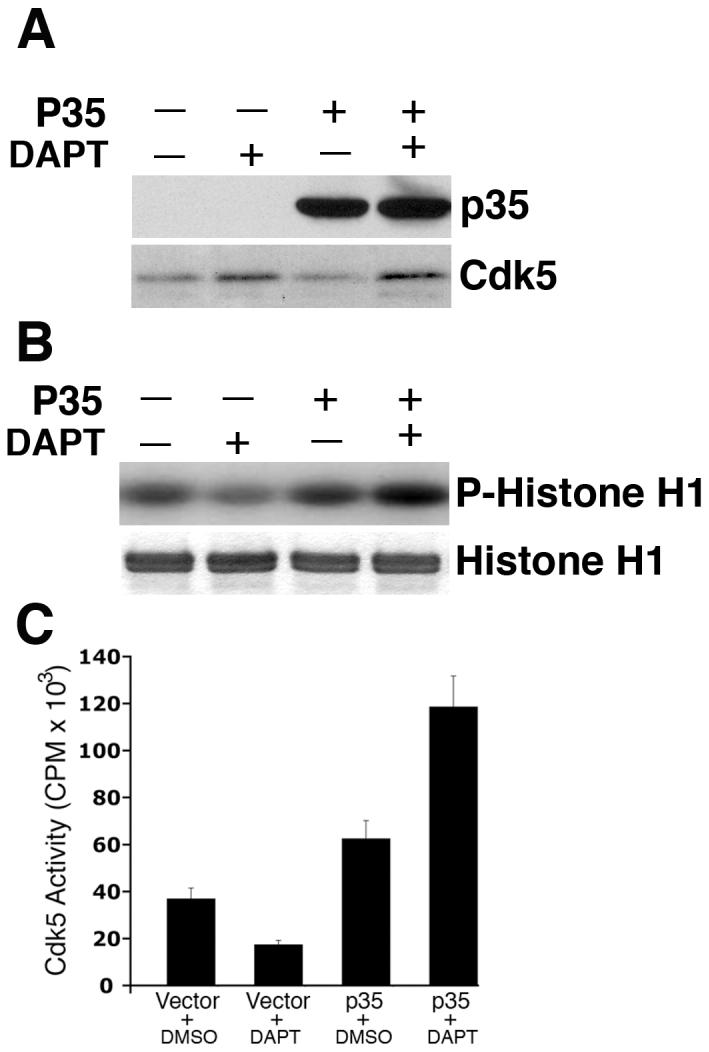
DAPT-induced attenuation of cdk5 activity and relocalization of p-tau and p-NF-H is reversed upon p35 overexpression. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium. Neurons were transfected with either pCDNA3.1 vector or pCDNA3.1-p35 plasmid. Twenty four hours post-transfection, cells were treated with DAPT. Cells were processed 24 h after DAPT treatment for immunoblot analyses using anti-p35 antibody to detect p35 expression. (A) Immunoblot shows significant increase in p35 levels in the cells transfected with pCDNA3-p35 compared to that in the empty vector-transfected cells. Note that although in this exposure time of the film, endogenous p35 in the vector-transfected neurons is not detectable, a longer exposure shows the presence of endogenous p35 in the first two lanes, where signals of the overexpressed p35 in the last two lanes distort the blot to have a clean figure for presentation. The bottom panel shows the cdk5 expression level in the vector- transfected and p35-transfected cells treated with either DMSO or DAPT. (B) Overexpression of p35 by transfection rescues DAPT-induced downregulation of cdk5 activity. Upper panel shows the autoradiogram of phosphorylated Histone H1 substrate used in the kinase assays; bottom panel shows the source of the autoradiogram, the Coomassie blue-stained Histone H1 in the SDS-PAGE gel. (C) Scintillation counting of the phospho-Histone H1 protein cut out from the SDS-PAGE gels after autoradiography shows the relative extent of Histone H1 phosphorylation representing cdk5 activity in the empty vector-transfected and p35-transfected neurons treated with either DMSO or DAPT. Data are derived from three separate experiments. (D) Immunocytochemical analyses were also performed to detect p-tau and p-NF-H in DAPT-treated and p35-transfected E-18 rat cortical neurons. Immunostaining for p35 (red) and p-tau (green, upper panel) or p-NF-H (green, lower panel) demonstrates that p35 overexpression can reverse the DAPT-induced translocation of p-tau and p-NF-H to the soma (arrows). Neurons overexpressing p35 (arrows) show relatively less p-tau (arrows) staining in the soma compared to the untransfected neuron (arrowhead). Partial rescue (from the soma) occurred for p-NF-H upon p35 transfection (bottom panel). Neurons overexpressing p35 (arrows) show relatively less p-NF-H staining in the soma (arrows) compared to the untransfected neuron (arrowhead).
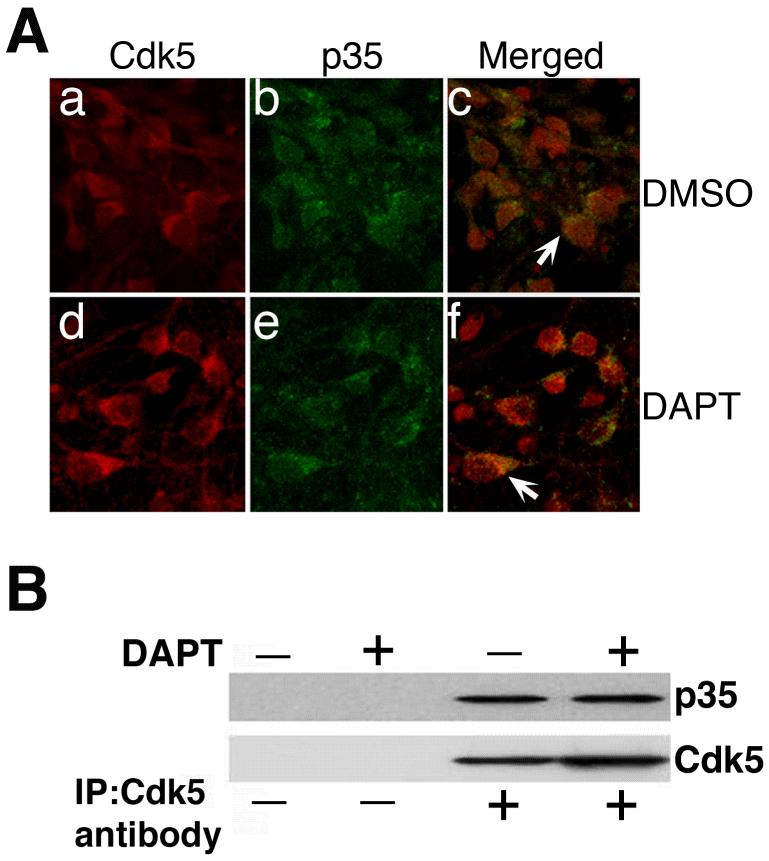
Effect of DAPT on endogenous cdk5/p35 interaction
Since DAPT suppressed cdk5 activity in the neurons, in which, cdk5 expression was upregulated and p35 expression remained unchanged, we suspected that DAPT could disrupt cdk5/p35 interaction contributing to the observed attenuation of cdk5 activity. In order to test this hypothesis, we analyzed the immunocytochemistry data that reveals the expression of cdk5 and p35 upon DAPT treatment. The results demonstrated that in both the control DMSO- and DAPT-treated cells, cdk5 colocalized with p35 (Fig. 7A, arrows). Whether cdk5 and p35 interaction remained unperturbed in these cells in presence of DAPT was further analyzed by co-immunoprecipitation assays followed by immunoblotting. The immunoprecipitates obtained from the lysates of neurons treated with DMSO or DAPT for 24 h, using the cdk5 antibody, were immunoblotted and probed with either anti-p35 antibody or anti-cdk5 antibody. The results demonstrated that p35 remained bound to cdk5 in the DAPT-treated neurons as in the control, DMSO-treated neurons (Fig. 7B). These results indicate that DAPT-induced cdk5 retains the ability to bind to p35 in the neurons and are consistent with what is observed in the cdk5 transgenic mice where the overexpressed cdk5 retains its binding ability to p35 (Tanaka et al. 2001). Despite cdk5’s binding to p35 remaining unperturbed in the cdk5 transgenic mice as well as in DAPT-treated neurons, why in both, a reduction in cdk5 activity occurs remains an enigma. It is possible that overexpressing cdk5 singularly without its activator may induce some conformational changes in the existing cdk5/p35 complex in the neurons, thus masking the active catalytic site. This assumption is further supported by the results where p35 overexpression overrides DAPT-induced suppression of cdk5 activity. In this case, the nascent excess cdk5 binds to the exogenous p35, potentially relieving the inhibitory effect of the unbound cdk5 on the endogenous cdk5/p35 complex.
Figure 7.
DAPT does not disrupt cdk5/p35 interaction. (A) In neurons after 24 h of DAPT treatment, cdk5 colocalizes with p35 comparably (indicated by arrows) in the control, DMSO-treated (a-c) and DAPT-treated cells (d-f). (B) Neurons were treated with DMSO or DAPT for 24 h. Cell lysates were prepared and one milligram of total protein from each sample was immunoprecipitated using the anti-cdk5 antibody. The control groups included lysates with no antibody for immunoprecipitation. The immunoprecipitates were resolved in an SDS-PAGE gel, immunoblotted for detection of p35 using an anti-p35 antibody or anti-cdk5 antibody.
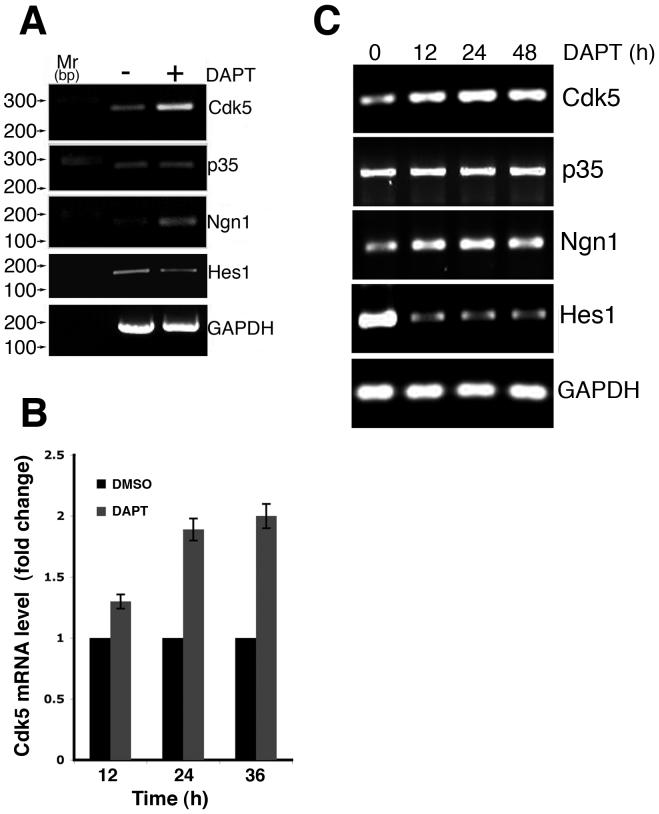
Regulation of cdk5 and Notch-response genes by DAPT
Based on the above results, we proposed that Notch might regulate cdk5 expression. Whether the observed increase in cdk5 protein level was due to an increase in cdk5 at the transcriptional level was verified by semi-quantitative RT-PCR analyses. In DAPT-treated primary neurons, cdk5 transcripts were upregulated ∼ 2-fold over that of the DMSO-treated control neurons (Fig. 8A). It has been shown that Notch signaling maintains its expressing cells in an undifferentiated state, while neighboring Delta-positive cells express the neuronal specification factor neurogenin (Ngn1) (Blader et al. 1997) and generate neuroblasts. DAPT treatment results in an increase in the number of Ngn1-positive cells in zebrafish (Geling et al. 2002). In this study, we monitored neurogenin (Ngn) expression in the cortical neurons. Ngn is a transcription factor that is upregulated when Notch signaling is inhibited. Our results demonstrated an increase in Ngn expression in the DAPT-treated neurons suggesting that Notch signaling was disrupted, while control GAPDH transcripts remained unchanged (Fig. 8A). Additionally, DAPT-induced downregulation of Hes1 (Fig. 8A) supports that Notch signaling was disrupted. There was no change in p35 transcript level upon DAPT treatment (Fig. 8A). In addition, quantitative PCR (qPCR) was performed to quantitate the cdk5 mRNA level in DAPT-treated neurons compared to the DMSO-treated control neurons. The results showed a significant increase in the cdk5 mRNA level in DAPT-treated cells occurring as early as 12 h of DAPT treatment (1.3 ± 0.059 fold) (Fig. 8B). The increase of cdk5 level at 24 h through 48 h of DAPT treatment further augmented the expression level of cdk5 mRNA (∼ 2 fold). Using semi-quantitative RT-PCR analyses in a time course experiment demonstrated the regulation of cdk5, Hes1 and Ngn1 by DAPT as early as 12 h after treatment (Fig. 8C). However, p35 transcript levels remained unchanged as did the control GAPDH transcripts (Fig. 8C). These results demonstrated that inhibition of Notch signaling by DAPT specifically results in enhanced transcription of cdk5. Cdk5 gene regulation has not been extensively studied although cdk5 at the protein level has been a theme of numerous studies, especially in terms of its kinase activity. Therefore, regulation of cdk5 expression as a Notch response would be a critical factor in explaining a number of neuronal functions that cdk5 plays in the nervous system ranging from neuron development, apoptosis to nervous system disorders.
Figure 8.
DAPT upregulates cdk5 expression at the transcriptional level. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with either DMSO or 10 μM DAPT. Total RNA was isolated from the neurons and RT-PCR was performed using the First Strand cDNA synthesis Kit (Roche, USA). Transcript levels of cdk5, p35, and Notch-responsive genes, neurogenin (Ngn), and Hes1 are shown. GAPDH transcript levels are shown as controls. The data represent one of three separate experiments. (A) Semi-quantitative RT-PCR analyses show changes in transcript levels of the various genes as indicated in neurons at 24 h of DAPT treatment (+) as compared to the DMSO-tretaed control (-). (B) Quantitative PCR analyses show the relative levels of cdk5 mRNA expression in DMSO- and DAPT-treated neurons from 12 h to 48 h of treatment. (C) Semi-quantitative RT-PCR analyses show the expression of indicated genes in the neurons treated with DAPT from 0 - 48 h of treatment.
Discussion
Notch-Delta signaling is thought to mediate most lateral inhibitory interactions necessary for patterning of neural cells (Lewis 1996, Lowell et al. 2000). Canonical Notch signaling is active in lateral inhibition and depends upon DSL (Delta/Serrate (Jagged)/Lag) ligand-regulated binding of the extracellular domain of Notch (Chitnis & Kintner 1996). Binding of DSL ligands to Notch allows access of a presenilin/γ-secretase complex to cleave and release the Notch internal cytoplasmic domain (NICD). Then NICD translocates to the nucleus and forms a transcriptional activation complex with CSL/RBP-jK and Mastermind and positively regulates transcription of Notch target genes, such as the Hes genes, and negatively regulates the Ngn1 gene (Mumm et al. 2000, Selkoe & Kopan 2003).
On the other hand, cdk5, a predominantly neuronal kinase has been shown to play a critical role in a variety of neuronal processes like migration, survival, and neurotransmission. Deregulated cdk5 has been implicated in neurodegenerative diseases while therapies based on γ-secreatse inhibitors like DAPT are being assessed to treat these diseases (Annaert & De Strooper 2002, Roberson & Mucke 2006, Tsai et al. 2002). In this report, our goal was to study the effect of Notch inhibition on cdk5-regulated processes. These studies were designed, first to see if a γ-secretase inhibitor affects cdk5 kinase activity, and second, to examine if Notch inhibition does have any effect on cdk5. DAPT is a γ-secretase inhibitor and therefore a Notch signaling inhibitor.
Interestingly, DAPT treatment upregulated cdk5 protein level in the rat cortical neurons indicating that Notch inhibition may regulate cdk5 expression. The increased cdk5 level resulted in reduced kinase activity, not surprisingly, since cdk5 transgenic mice brain shows a reduction in cdk5 activity (Tanaka et al. 2001). These results also led to the assumption that the neuronal cytoskeletal proteins would be modified as cdk5 activity is attenuated by DAPT.
In DAPT-treated neurons, a profound change in the localization of phosphorylated cytoskeletal proteins p-tau and p-NF-H, a shift from neurites to cell bodies, was observed. These observations are similar to the results obtained by treating the cells with cdk5 inhibitor, roscovitine (Zheng et al. 2007). Moreover, our results are consistent with studies showing accumulation of phosphorylated NF proteins in the soma associated with reduced cdk5 activity and Erk1/2 hyperactivation in cdk5 knockout brain stem neurons (Sharma et al. 2002) and a redistribution of phosphorylated cytoskeletal proteins in p35 null mouse brain as well (Hallows et al. 2003). In stress-induced neurons undergoing apoptosis (Zhang & Johnson 2000) and in neurodegenerative disorders (Buee et al. 2000), abnormal accumulation of hyperphosphorylated tau and NF proteins occurs in cell bodies. The use of DAPT to reduce β-amyloid accumulation has led to the assumption that this compound has a potential for therapies of the Alzheimer’s disease (Dovey et al. 2001, El Mouedden et al. 2006). In this context, our findings are critically important since p-tau and p-NF-H shift from the axons to the soma that can serve as a primer to induce apoptosis. Our results show that DAPT modulates cytoskeletal protein redistribution similar to that in cortical neurons treated with roscovitine. It is noteworthy that although the biological consequences are similar, inhibition of cdk5 activity by DAPT occurs in a very different way than that by roscovitine. What causes a 40% reduction in cdk5 activity in the cdk5 transgenic mice (Tanaka et al. 2001) seems more likely the pathway DAPT exercises as well to attenuate cdk5 activity. This notion is based on the fact that DAPT induces upregulation of cdk5 transcript and protein levels. As in the transgenic mice, we show that DAPT-induced cdk5 is capable of binding to p35. There is no clear explanation to justify yet why cdk5 transgenic mice show decreased cdk5 activity. Similarly, our current results are equally inadequate to provide an explanation as to how DAPT attenuates cdk5 activity. We speculate that overexpression of unpartnered cdk5 in the cells mask the catalytic site of the existing cdk5/p35 complex. Considering that a molar excess of cdk5 alone could hinder the active site of the existing cdk5/p35 complex, a rescue of the endogenous cdk5 activity was achieved by ectopic expression of p35. These results along with coimmunoprecipitation assays confirmed that DAPT does not disrupt cdk5/p35 interaction. P35 overexpression also rescued DAPT-induced p-tau and p-NF-H translocation suggesting that the exogenous p35 partnered with the DAPT-induced cdk5, activated it, and consequently reversed the abnormal localization of these two neuronal cytoskeletal proteins.
An important observation in this report, however, is the transcriptional upregulation of cdk5 by DAPT. DAPT-treated neurons that showed disruption of Notch signaling evidenced by the downregulation of Hes1 and upregulation of Ngn, not only showed an increase in the cdk5 protein level, but also showed an increase in the level of cdk5 transcripts. Whether Notch directly regulates cdk5 promoter or its effect is indirect via other signaling pathways needs further analyses of the cdk5 gene and the regulatory elements present in its promoter. Previous reports have shown upregulation of cdk5 and p35 at the transcriptional level by retinoic acid during neuronal differentiation (Lee & Kim 2004) and upregulation of p35 during 1,25-Dihydroxyvitamin D3-induced myeloid cell differentiation (Chen et al. 2000, Chen et al. 2004). In both of these cases, cdk5 activity was upregulated. Furthermore, Fas (CD95), a lymphocyte receptor has been shown to upregulate p35 at the transcriptional level by activating Erk (Desbarats et al. 2003), although the report doesn’t show its effect on cdk5 catalytic activity. Our studies reveal a unique occurrence where the catalytic activity of cdk5 is attenuated by its overexpression. This is the first report that establishes a link between Notch signaling and cdk5 expression, which we believe will be fundamental to our understanding and future studies of cdk5 gene regulation.
Acknowledgements
This work was supported by the National Institutes of Health (NIH)-National Institute of Neurological Disorders and Stroke (NINDS) intramural funds.
References
- Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, Shaw CE, Miller CC. Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol. 2003;161:489–495. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaert W, De Strooper B. A cell biological perspective on Alzheimer’s disease. Annu Rev Cell Dev Biol. 2002;18:25–51. doi: 10.1146/annurev.cellbio.18.020402.142302. [DOI] [PubMed] [Google Scholar]
- Blader P, Fischer N, Gradwohl G, Guillemot F, Strahle U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development. 1997;124:4557–4569. doi: 10.1242/dev.124.22.4557. [DOI] [PubMed] [Google Scholar]
- Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci. 2002;22:6515–6525. doi: 10.1523/JNEUROSCI.22-15-06515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chan WK, Dickerson A, Ortiz D, et al. Mitogen-activated protein kinase regulates neurofilament axonal transport. J Cell Sci. 2004;117:4629–4642. doi: 10.1242/jcs.01135. [DOI] [PubMed] [Google Scholar]
- Chen F, Rao J, Studzinski GP. Specific association of increased cyclin-dependent kinase 5 expression with monocytic lineage of differentiation of human leukemia HL60 cells. J Leukoc Biol. 2000;67:559–566. doi: 10.1002/jlb.67.4.559. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang Q, Wang X, Studzinski GP. Up-regulation of Egr1 by 1,25-dihydroxyvitamin D3 contributes to increased expression of p35 activator of cyclin-dependent kinase 5 and consequent onset of the terminal phase of HL60 cell differentiation. Cancer Res. 2004;64:5425–5433. doi: 10.1158/0008-5472.CAN-04-0806. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development. 2003;130:5031–5042. doi: 10.1242/dev.00697. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Kintner C. Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development. 1996;122:2295–2301. doi: 10.1242/dev.122.7.2295. [DOI] [PubMed] [Google Scholar]
- Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TQ, Roelink H. The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling. Dev Dyn. 2007;236:886–892. doi: 10.1002/dvdy.21083. [DOI] [PubMed] [Google Scholar]
- Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- El Mouedden M, Vandermeeren M, Meert T, Mercken M. Reduction of Abeta levels in the Sprague Dawley rat after oral administration of the functional gamma-secretase inhibitor, DAPT: a novel non-transgenic model for Abeta production inhibitors. Curr Pharm Des. 2006;12:671–676. doi: 10.2174/138161206775474233. [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows JL, Chen K, DePinho RA, Vincent I. Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J Neurosci. 2003;23:10633–10644. doi: 10.1523/JNEUROSCI.23-33-10633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim KT. Induction of cyclin-dependent kinase 5 and its activator p35 through the extracellular-signal-regulated kinase and protein kinase A pathways during retinoic-acid mediated neuronal differentiation in human neuroblastoma SK-N-BE(2)C cells. J Neurochem. 2004;91:634–647. doi: 10.1111/j.1471-4159.2004.02770.x. [DOI] [PubMed] [Google Scholar]
- Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- Li H, Yu B, Zhang Y, Pan Z, Xu W, Li H. Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;341:320–325. doi: 10.1016/j.bbrc.2005.12.182. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. Embo J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Esler WP, Kimberly WT, Jack C, Berezovska O, Kornilova A, Hyman BT, Perrimon N, Wolfe MS. Gamma-secretase/presenilin inhibitors for Alzheimer’s disease phenocopy Notch mutations in Drosophila. Faseb J. 2003;17:79–81. doi: 10.1096/fj.02-0394fje. [DOI] [PubMed] [Google Scholar]
- Moran CM, Donnelly M, Ortiz D, Pant HC, Mandelkow EM, Shea TB. Cdk5 inhibits anterograde axonal transport of neurofilaments but not that of tau by inhibition of mitogen-activated protein kinase activity. Brain Res Mol Brain Res. 2005;134:338–344. doi: 10.1016/j.molbrainres.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant AC, Veeranna, Pant HC, Amin N. Phosphorylation of human high molecular weight neurofilament protein (hNF-H) by neuronal cyclin-dependent kinase 5 (cdk5) Brain Res. 1997;765:259–266. doi: 10.1016/s0006-8993(97)00561-1. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer’s disease. Science. 2006;314:781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrabhatla P, Kanungo J, Zheng Ya-Li, Amin ND, Kesavapany S, Pant HC. Cyclin-dependent protein kinase 5 (Cdk5) modulates signal transduction pathways regulating neuronal survival. In: Ip NY, Tsai L-H, editors. Cyclin Dependent Kinase 5 (Cdk5) Springer Sci. Buis Media; 2008. [Google Scholar]
- Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena MT, Schroeter EH, Mumm JS, Kopan R. Murine notch homologs (N1-4) undergo presenilin-dependent proteolysis. J Biol Chem. 2001;276:40268–40273. doi: 10.1074/jbc.M107234200. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Sharma P, Veeranna, Sharma M, Amin ND, Sihag RK, Grant P, Ahn N, Kulkarni AB, Pant HC. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:528–534. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]
- Shea TB, Yabe JT, Ortiz D, Pimenta A, Loomis P, Goldman RD, Amin N, Pant HC. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J Cell Sci. 2004a;117:933–941. doi: 10.1242/jcs.00785. [DOI] [PubMed] [Google Scholar]
- Shea TB, Zheng YL, Ortiz D, Pant HC. Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J Neurosci Res. 2004b;76:795–800. doi: 10.1002/jnr.20099. [DOI] [PubMed] [Google Scholar]
- Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem. 2004;88:1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Veeranna, Ohshima T, et al. Neuronal cyclin-dependent kinase 5 activity is critical for survival. J Neurosci. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JY, Wolfe MS, Xia W. The search for gamma-secretase and development of inhibitors. Curr Med Chem. 2002;9:1087–1106. doi: 10.2174/0929867023370185. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Voss K, Schott M, Hunig T, Wolfe MS, Reichardt HM. Inhibition of Notch signaling biases rat thymocyte development towards the NK cell lineage. Eur J Immunol. 2004;34:1405–1413. doi: 10.1002/eji.200324735. [DOI] [PubMed] [Google Scholar]
- Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Johnson GV. Tau protein is hyperphosphorylated in a site-specific manner in apoptotic neuronal PC12 cells. J Neurochem. 2000;75:2346–2357. doi: 10.1046/j.1471-4159.2000.0752346.x. [DOI] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Amin ND, Albers W, Pant HC. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Kanungo J, Kesavapany S, Amin N, Grant P, Pant HC. Cdk5 Modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell. 2007;18:404–413. doi: 10.1091/mbc.E06-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Veeranna, Pant HC. Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J Biol Chem. 2003;278:24026–24032. doi: 10.1074/jbc.M303079200. [DOI] [PubMed] [Google Scholar]