SUMMARY
Interleukin-8 (IL-8) promotes neutrophil-mediated host defense through its chemoattractant and immunostimulatory activities. The Group A Streptococcus (GAS) protease SpyCEP (also called ScpC) cleaves IL-8, and SpyCEP expression is strongly upregulated in vivo in the M1T1 GAS strains associated with life-threatening systemic disease including necrotizing fasciitis. Coupling allelic replacement with heterologous gene expression, we show that SpyCEP is necessary and sufficient for IL-8 degradation. SpyCEP decreased IL-8-dependent neutrophil endothelial transmigration and bacterial killing, the latter by reducing neutrophil extracellular trap formation. The knockout mutant lacking SpyCEP was attenuated for virulence in murine infection models, and SpyCEP expression conferred protection to coinfecting bacteria. We also show that the zoonotic pathogen Streptococcus iniae possesses a functional homolog of SpyCEP (Cepl) that cleaves IL-8, promotes neutrophil resistance, and contributes to virulence. By inactivating the multifunctional host defense peptide IL-8, the SpyCEP protease impairs neutrophil clearance mechanisms, contributing to the pathogenesis of invasive streptococcal infection.
INTRODUCTION
Group A Streptococcus (GAS) is a leading human pathogen responsible for a wide range of diseases ranging from simple pharyngitis and impetigo to life-threatening, invasive conditions such as necrotizing fasciitis (NF) and toxic shock syndrome (TSS). The worldwide burden of invasive GAS infection is estimated at over 650,000 cases and 150,000 deaths annually (Carapetis et al., 2005). While GAS strains of many genotypes are capable of producing invasive infections, strains representing one globally disseminated M1T1 clone have persisted for over 20 years as the most prevalent sterile site isolates (Chatellier et al., 2000; Cleary et al., 1998; Cockerill et al., 1997; Murono et al., 1999), as corroborated by the nine surveillance centers of the United States Centers for Disease Control Emerging Infections Program Network each year from 1997 though 2005 (http://www.cdc.gov/ncidod/dbmd/abcs).
Sumby et al. recently provided a genome-wide analysis using expression microarrays to characterize fundamental differences in the transcriptome of M1T1 GAS isolates isolated from invasive disease cases versus those associated with mucosal site infection (Sumby et al., 2006). This comprehensive analysis showed that a frameshift mutation in the gene covS, encoding the sensor kinase component of an important GAS global transcriptional regulator (Dalton et al., 2006; Mitrophanov et al., 2006), resulted in striking effects on GAS gene expression including the upregulation of several proven or candidate virulence factors. A second most highly upregulated gene (25-fold) in the invasive GAS transcriptional profile was that encoding SpyCEP (S. pyogenes cell envelope protease). SpyCEP (also known as ScpC and PrtS) is a predicted 1645 amino acid protein that contains a C-terminal LP(X)TG cell wall anchor, and it was recently identified as a protease capable of degrading and functionally inactivating the major human CXC chemokine interleukin-8 (IL-8) (Edwards et al., 2005; Hidalgo-Grass et al., 2006).
IL-8 is a multifunctional host defense protein with a prominent role in recruitment and activation of neutrophils. Not only does IL-8 act as potent chemoattractant (Kunkel et al., 1991), it also tethers itself to the luminal surface of the microvasculature providing a stop signal to rolling neutrophils (DiVietro et al., 2001; Middleton et al., 1997). SpyCEP purified from an M81 GAS NF isolate was found to specifically cleave the IL-8 C terminus between a glutamine residue at position 59 and an arginine residue at position 60, eliminating its chemotactic properties (Edwards et al., 2005); the murine chemokine MIP-2 was cleaved at the same site. Independent studies in a GAS M14 NF isolate (Hidalgo-Grass et al., 2006) revealed that the same protease also efficiently degraded the murine CXC chemokines KC and MIP-2 both in vitro and in vivo, impairing mouse neutrophil recruitment to a greater extent than the related GAS protease ScpA, which specifically targets the human complement-derived chemoattractant C5a (Cleary et al., 1992; Ji et al., 1996).
Due to its marked upregulation in the invasive transcriptional profile, we hypothesized that SpyCEP played an important role in M1T1 GAS resistance to phagocytic clearance and animal virulence. To test this hypothesis, we deleted the gene encoding SpyCEP, cepA, in an invasive M1T1 NF isolate through precise, in-frame allelic replacement. This loss of function reagent is coupled with heterologous expression of SpyCEP in the non-pathogenic Gram-positive Lactococcus lactis to probe potential pathogenic roles of the chemokine protease in promoting GAS resistance to human neutrophil killing, adherence and invasion of epithelial cells, and virulence in murine models of NF and upper respiratory tract infection.
RESULTS
Mutagenesis and Heterologous Expression of SpyCEP
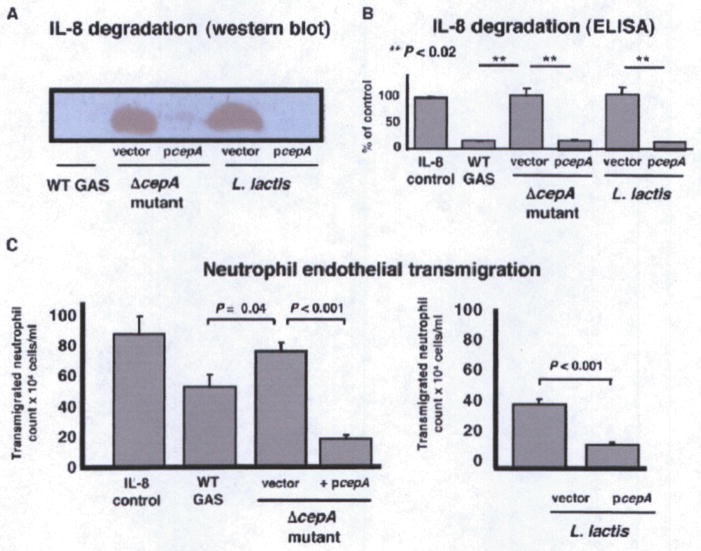
A strategy for precise, in-frame allelic replacement of the cepA gene with a chloramphenicol acetyltransferase (cat) antibiotic resistance gene cassette was executed successfully in M1T1 GAS strain 5448, an isolate from a patient with NF and TSS, yielding mutant 5448ΔcepA (Figure S1A). The intact M1T1 cepA gene was also cloned in the Escherichia coli-streptococcal shuttle expression vector pDCerm (Jeng et al., 2003), generating pcepA, for use in complementation analysis and heterologous expression of the protease in Lactococcus lactis. Western blot analysis detected the presence of SpyCEP in culture supernatants of the WT M1T1 GAS parent strain, loss of expression in the isogenic ΔcepA mutant, and overexpression of SpyCEP upon complementation of the mutant or expression in L. lactis using the high-copy number pcepA plasmid (Figure S1B). SDS-PAGE showed generation of the 6 kD SpyCEP IL-8 cleavage product after coincubation of the supernatants from the WT GAS parent strain, complemented ΔcepA mutant, and L. lactis expressing pcepA with IL-8, but the cleavage product was absent in the ΔcepA mutant or L. lactis containing the empty vector control (Figure S1C). Western blot analysis (Figure 1A) and IL-8 ELISA (Figure 1B) confirmed IL-8 protease activity of the corresponding strains expressing SpyCEP and its absence in strains lacking the protease. These studies confirm generation of loss-of-function and gain-of-function bacterial reagents with alterations in IL-8 protease activity for analysis of functional interactions with human neutrophils. The ΔcepA mutant did not differ from the parent strain in expression of M1 protein (Figure S2A), zone of β-hemolysis surrounding colonies on blood agar plates (not shown), DNase activity (Figure S2B), nor susceptibility to the cationic antimicrobial peptide polymyxin B (MIC = 28 μg/ml for both).
Figure 1. SpyCEP Is Necessary and Sufficient for IL-8 Degradation and Impairment of Neutrophil Recruitment.
(A and B) SpyCEP expression was manipulated by targeted mutagenesis of GAS (loss of function), complementation, and heterologous expression in L. lactis (gain of function). Presence of SpyCEP correlates with IL-8 protease activity by anti-IL-8 western blot (A) and ELISA (B). Error bars represent mean ± standard deviation.
(C) SpyCEP degradation of IL-8 impairs human neutrophil transmigration across an endothelial monolayer. Recombinant IL-8 was used as a positive control. Transmigration indicates migration of neutrophils across the endothelium. Transwell experiments were performed in triplicate and repeated three times; pooled data are shown ± SEM. “Vector” refers to empty plasmid pDCerm to serve as control for complementation analysis.
SpyCEP Impairs Neutrophil Endothelial Transmigration
In response to the chemotactic signal of IL-8, neutrophils will migrate directionally across a human microvascular endothelial cell monolayer, a process we and others have modeled in vitro using a transwell filter insert system (Doran et al., 2003; Edwards et al., 2005; Yao et al., 1996). Human microvascular endothelial cells (hBMEC) were propagated on transwells until tight junctions were established, living bacteria placed in the bottom well along with a fixed concentration of recombinant IL-8, and freshly isolated human neutrophils added to the upper well. Neutrophil transmigration of the endothelial monolayer toward the GAS ΔcepA mutant and untransformed L. lactis was significantly greater than observed with the WT GAS parent strain, complemented ΔcepA mutant or L. lactis expressing pcepA (Figure 1C). These studies confirm that GAS expression of the IL-8 protease can retard directional migration of human neutrophils, and consequently serve to delay the kinetics of the innate immune response.
SpyCEP Promotes Resistance to Neutrophil Killing
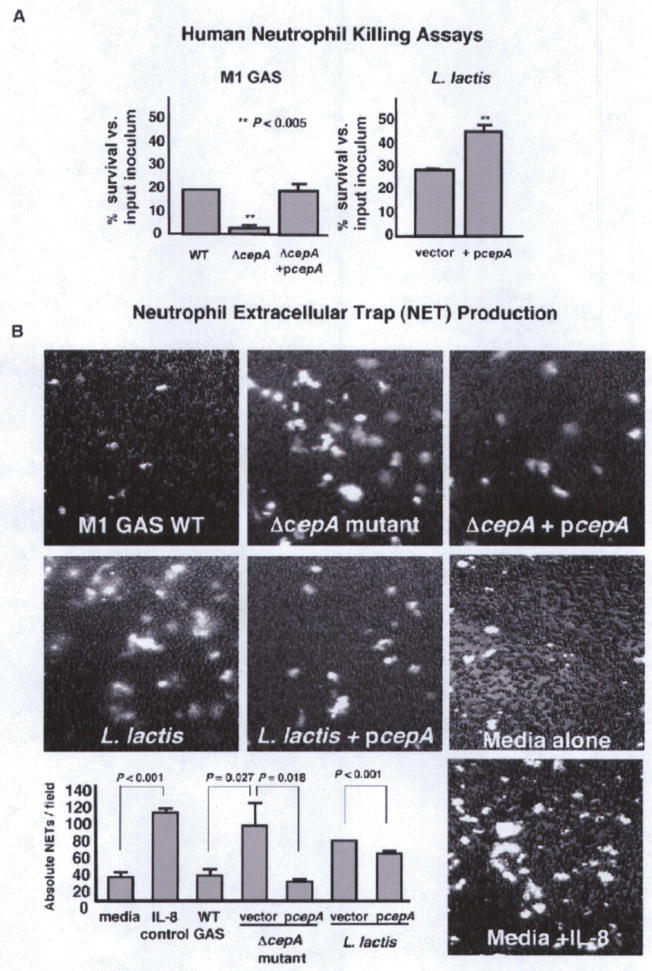
The very strong upregulation of cepA expression in the invasive M1T1 GAS transcriptome suggested potential benefits to bacterial survival beyond interference with neutrophil recruitment. IL-8, formerly known as neutrophil activating peptide-1, is produced in large amounts by neutrophils themselves upon encountering bacteria (Bazzoni et al., 1991; Strieter et al., 1992), and in an autocrine fashion can exert stimulatory effects on oxidative burst function and release of granule enzymes amplifying bactericidal activity (Simms and D’Amico, 1997; Thelen et al., 1988). We assessed the contribution of SpyCEP to bacterial survival in a human neutrophil opsonophagocytic killing assay. WT parent M1T1 GAS survived neutrophil killing 6-fold more than ΔcepA mutant bacteria (p < 0.005); WT levels of neutrophil resistance were restored by complementation of the mutant with the pcepA expression vector (Figure 2A, left). Heterologous expression of SpyCEP in L lactis increased survival of the transformed bacterium in the neutrophil killing assay by ~50% (p < 0.005) (Figure 2A, right). Consistent with prior results (Hidalgo-Grass et al., 2006). SpyCEP did not promote survival in human whole blood (Figure S3A), suggesting its action to be predominantly exerted at localized tissue sites of infection.
Figure 2. SpyCEP Promotes GAS Resistance to Neutrophil Killing.
(A) SpyCEP contributes to GAS survival and in creases Lactococcus lactis survival upon coincubation with human neutrophils, experiments performed in triplicate and repeated three times with similar results; representative experiment ± SEM.
(B) The presence of SpyCEP reduces IL-8-mediated induction of neutrophil extracellular traps (NETs) in response to bacterial exposure. Shown are fluorescent images of NETs stained with Sytox Orange and overlaid upon brightfield image of neutrophils. Also shown are quantitative enumeration of NETs; experiments performed in triplicate and repeated three times with similar results; representative experiment ± SEM, two-sided t test.
SpyCEP Reduces Neutrophil Production of Extracellular Traps
Apart from their phagocytic function, neutrophils can efficiently capture and kill microbes in the extracellular space by extrusion of a matrix of DNA and histones known as neutrophil extracellular traps, or NETs, which ensnare bacteria and subject them to microbicidal effectors including elastase and myeloperoxidase (Brinkmann et al., 2004). M1T1 GAS express DNases including the phage-encoded Sda1 that contribute to NET degradation and immune evasion (Buchanan et al., 2006; Sumby et al., 2005; Walker et al., 2007). NETs are induced by exposing human neutrophils to IL-8 (Gupta et al., 2005). We thus hypothesized that the IL-8 degradation by SpyCEP could reduce NET formation and thus bacterial killing, suggesting another mechanism by which the IL-8 protease could impair host defense function. Using a fluorescent assay for real-time visualization of NETs (Buchanan et al., 2006), we confirmed the capacity of the chemokine to stimulate NET formation by human neutrophils (Figure 2B). We then visualized NET formation elicited by our isogenic bacterial strains in the presence of the inhibitor G-actin to block DNase-mediated NET degradation. As shown in Figure 2B, NETs were significantly more abundant in human neutrophils incubated with the ΔcepA mutant than with the isogenic WT parent strain or the complemented mutant; conversely, NET production was diminished in neutrophils exposed to L. lactis expressing SpyCEP compared to L. lactis transformed with empty vector control. A significant contribution of SpyCEP to M1 GAS survival was maintained when phagocytosis was inhibited with cytochalasin D, confirming a role in the pathogen’s resistance to extracellular neutrophil killing mechanisms (Figure S3B). Furthermore, a significant contribution of SpyCEP to GAS neutrophil survival was also maintained when NET degradation was inhibited with G-actin (Figure S3B). This indicates SpyCEP action affects NET production rather than NET degradation, consistent with the observation that WT and ΔcepA mutant GAS exhibit similar DNase activity (Figure S2B). Also consistent with the hypothesis that SpyCEP blocks NET production by ablating IL-8-induced NET formation, addition of excess exogenous IL-8 to the assay eliminated the differences in NET induction between WT and ΔcepA mutant strains (Figure S3C).
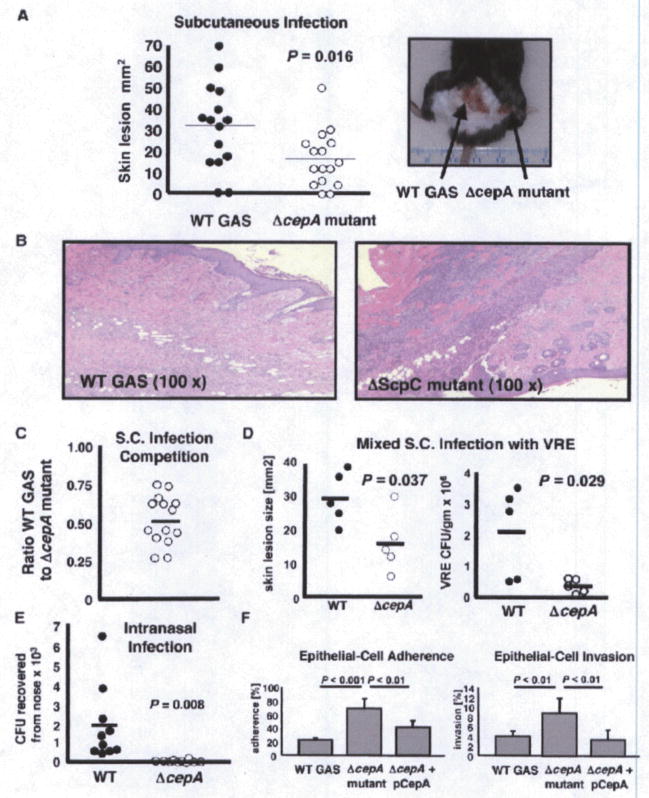
SpyCEP Contributes to GAS Virulence in a Murine Necrotic Skin Infection Models
A murine subcutaneous challenge model can be used to examine the contribution of specific bacterial phentoypes to the virulence of M1T1 GAS in vivo (Buchanan et al., 2006; Datta et al., 2005; Sumby et al., 2005). Using inbred C57BI6 mice, we injected one shaved flank with 5 × 107 cfu of the WT M1T1 GAS parent strain and the contralateral flank with an equivalent inoculum of the isogenic ΔcepA mutant. As shown in the graph and representative photo of Figure 3A, the mean lesion size generated by the ΔcepA mutant was ~50% that of the WT lesions (p < 0.02). Histopathologic analysis showed increased neutrophil recruitment in lesions generated by the ΔcepA mutant compared to the WT parent strain (Figure 3B), confirming earlier indications that SpyCEP can impair murine neutrophil recruitment in vivo due to its shared activity against murine C-X-C chemokines KC and MIP-2 (Hidalgo-Grass et al., 2006).
Figure 3. SpyCEP Contributes to GAS Virulence in a Murine Infection Models.
(A) C57B16 mice were injected subcutaneously with equivalent inocula of the WT M1 GAS strain (left flank) and the isogenic ΔcepA mutant (right flank) and skin lesion progression measured for 4 days; a representative photograph of skin lesions observed is shown.
(B) Representative histology (H&E stain) biopsies from skin lesions showing increased neutrophil recruitment at the ΔcepA mutant infection site (100× magnification).
(C) In a competition experiment, WT M1 GAS and isogenic ΔcepA mutant were injected simultaneously s.c. injection and bacteria enumerated after 4 days; ratio WT M1 GAS to isogenic ΔcepA mutant is shown.
(D) GAS SypCEP expression supports survival in vivo of vancomycin resistance Enterococcus faecalis (VRE) in a mixed infection model.
(E) SpyCEP contributes to GAS mucosal infection in an intranasal inoculation model.
(F) SpyCEP reduces GAS epithelial cell adherence and invasion. Elimination of SpyCEP increases the adherence and invasion of HEp-2 human pharyngeal epithelial cells by M1 GAS. Experiments were performed in triplicate and repeated three times with similar results; representative experiment is shown ± standard deviation.
We next asked whether SpyCEP protection against neutrophil killing was only afforded to individual bacterial cells themselves expressing the surface-anchored IL-8 protease (action in cis), or whether SpyCEP-mediated chemokine degradation could protect other vulnerable bacteria in the local environment (action in trans). To address this question, we performed a coinfection experiments in which an equal concentration of WT M1 GAS and isogenic ΔcepA mutant were inoculated in the same lesion site. When lesions were biopsied and harvested 4 days later, no survival advantage of the WT strain versus the mutant was detected (Figure 3C), indicating the WT strain could rescue the virulence defect of the mutant bacteria, presumably by decreasing neutrophil recruitment, phagocytic ability, and NET formation at the local site of infection. To extend this line of investigation, we adopted a model of polymicrobial necrotizing infection, using a vancomycin-resistant isolate of the nosocomial pathogen Enterococcus faecalis (VRE), a frequent isolate from mixed etiology wound and necrotizing soft tissue infections including Fournier’s gangrene (Kuo et al., 2007; Ou et al., 1993). Necrotic skin lesions produced by the WT M1 GAS + VRE were significantly larger than those produced by the GAS ΔcepA mutant + VRE, and it was shown that the presence of the WT strain (with SpyCEP expression) promoted survival of VRE (in vivo and in trans) in the mixed infection model (Figure 3D).
SpyCEP Contributes to GAS Virulence following Intranasal Infection
As GAS is also an important cause of upper and lower respiratory tract infections, we next assessed the contribution of SpyCEP to virulence following intranasal inoculation. We observed the WT M1 strain to have a marked advantage in establishing upper respiratory tract infection compared to the ΔcepA mutant (Figure 3E). In contrast to subcutaneous infection, this challenge model involves interaction of GAS with epithelial cells. This raises the possibility that in addition to immune resistance, changes in epithelial cell binding of the ΔcepA mutant compared to WT GAS could contribute to the observed in vivo attenuation, especially since cepA shares ~30% sequence identity and ~50% sequence similarity with the GAS C5a peptidase, ScpA, which may function as a GAS epithelial cell invasin (Purushothaman et al., 2004). We therefore examined the contribution of SpyCEP to GAS adherence to and invasion of cultured HEp-2 human pharyngeal epithelial cells. The isogenic ΔcepA mutant was found to be ~3-fold more adherent (p < 0.001) and ~2-fold more invasive (p < 0.01) than the GAS WT parent strain; complementation of the mutant with the pcepA expression plasmid reversed the adherence and invasion phenotype to nearly WT levels (Figure 3F). These results show that in contrast to ScpA. SpyCEP does not act as a GAS epithelial cell adhesin or invasin. An underlying mechanism may be found in the recent observation that GAS is capable of triggering IL-8 release from Hep-2 cells, and that the cytokine can promote GAS entry into cells (Nobbs et al., 2007); SpyCEP degradation of IL-8 would interfere with this pathway. In sum, we conclude that chemokine and neutrophil mediated host defense are operating in the nasal infection model, and whatever contribution SpyCEP contributes to reduce GAS epithelial adherence is outweighed by its ability to blunt immune clearance in vivo.
The Zoonotic Pathogen a aa Possesses a Functional SpyCEP Homolog
The (β-hemolytic species Streptococcus iniae is an important pathogen in wild and aquacultured fish, where it can produce a fatal meningoencephalitis (Agnew and Barnes, 2007). S. iniae is also recognized as an emerging zoonotic pathogen of humans, producing invasive cellulitis in fish handlers (Weinstein et al., 1997). By whole genome pyrosequencing and bioinformatic analysis, we identified an S. iniae gene encoding a 1631 amino acid (aa) candidate protein sharing 54.4% aa identity and 66.2% aa similarity to SpyCEP of GAS, which we named Cepl (cell envelope proteinase of S. iniae) (Figure S4). The genomic position of cepl is consistent with cepA in GAS–downstream from a lactate oxidase gene, IctO (previously characterized in S. iniae) (Gibello et al., 1999). and upstream of genes with high similarity to spy_0421 and metG of M1 GAS (Ferretti et al., 2001). Like GAS, S. iniae is virulent in the murine subcutaneous infection model (Fuller et al., 2001). Targeted plasmid integrational mutagenesis of the cepl gene in virulent S. iniae strain K288 was performed (Figure S5A). Compared to the WT parent strain, the S. iniae Δcepl mutant lost the ability to degrade IL-8 (Figure S5B), showed increased sensitivity to human neutrophil killing (Figure S5C). and generated smaller lesions in the murine subcutaneous infection model (Figure S5D). Thus, a functional homolog of SpyCEP is present in at least one additional bacterial species, where it contributes as it does for GAS to chemokine degradation, neutrophil resistance and disease pathogenesis.
DISCUSSION
The prominence of GAS among bacteria able to cause serious systemic infections even in previously healthy humans reflects the diverse capacities of this pathogen to avoid eradication by phagocyte defenses of the innate immune system (Kwinn and Nizet, 2007; Voyich et al., 2004). Resistance to neutrophil killing involves the antiphagocytic properties of classical GAS virulence factors such as the surface M protein and hyaluronic acid capsule and cytolytic action of the pore-forming toxins streptolysin S and streptolysin O. In the globally disseminated GAS M1T1 clone, accumulation of a larger repertoire of virulence factors such as the streptococcal inhibitor of complement (SIC) protein and the phage-encoded DNase Sda1 provide added capabilities to resist effective opsonization and escape extracellular killing in NETs, finding corroboration in epidemiologic associations to severe disease including NF.
In the present study, we establish molecular Koch’s postulates for the contribution of IL-8 protease SpyCEP to impaired neutrophil recruitment and resistance to neutrophil killing, including single gene complementation analysis and heterotogous expression studies. M1T1 expression of SpyCEP had the anticipated effect of delaying neutrophil endothelial transmigration and recruitment to the tissues and promoting virulence (Edwards et al., 2005; Hidalgo-Grass et al., 2006; Sumby et al., 2008). These results find clinical correlation in the striking paucity of neutrophils at the site of infection that was observed in a subset of human cases of GAS NF consistent with a primary deficit in chemoattractant function (Cockerill et al., 1998; Hidalgo-Grass et al., 2004). Likewise, in a baboon model of GAS soft-tissue infection, the risk of septicemia and mortality was inversely correlated to the magnitude of neutrophil influx at the site of infection (Taylor et al., 1999). However, in advanced human necrotic disease, prominent neutrophil infiltrates ultimately accumulate in association with proliferating bacteria (Dahl et al., 2002), as could be expected since additional microbial components (e.g., fMLP) and host inflammatory response proteins (e.g.. C5a, platelet activating factor) possess strong neutrophil chemoattractant properties (Wagner and Roth, 2000).
Our data suggest the proteolytic inactivation of IL-8 by SpyCEP can exert additional effects to impair neutrophil killing of M1T1 GAS even in late stage necrotic lesions. By eliminating IL-8-induced NET production, SpyCEP can synergize with the M1T1 DNase Sda1 (Buchanan et al., 2006; Sumby et al., 2005) to impair NET-mediated extracellular bacterial killing. The cumulative effect of these activities is consistent with our observation that GAS expression of SpyCEP was necessary and sufficient to promote bacterial survival in killing assays with isolated neutrophils, and for GAS to protect non-SpyCEP producing bacteria from neutrophil killing in vivo (Figure 2). The specificity of our assays was aided by the achievement of single gene replacement of cepA (independent of scpA) and single gene complementation that were technically unsuccessful, respectively, in other recent informative studies of the GAS IL-8 protease activity (Hidalgo-Grass et al., 2006; Sumby et al., 2008). Nevertheless, it is likely that bacterial and mouse strain specificities influence the observed patterns of disease progression, since inflammatory lesion size may also reflect a contribution from host neutrophils that could be augmented in the absence of SpyCEP chemokine degradation (Sumby et al., 2008).
The gene encoding SpyCEP was one of the most highly differentially expressed upon comparison of the transcriptional profile of invasive M1T1 GAS isolates versus similar clonal strains isolated from the pharyngeal mucosa (Sumby et al., 2006). In simple colonization, our results indicate SpyCEP may decrease epithelial cell adherence and, therefore, its expression would not be favored. The observed 25-fold upregulation of cepA expression in the invasive transcriptional profile of M1T1 GAS strains is consistent with our findings linking SpyCEP expression to resistance to neutrophil killing, a selective pressure induced uniquely in vivo. Our discovery and analysis of a functional SpyCEP homolog in S. iniae may likewise explain the human disease potential of this zoonotic pathogen. Strategies directed at neutralizing SpyCEP activity could prove useful as an adjunctive therapy to invasive GAS infection, acting not to kill the bacterium directly, but rather to render it susceptible to our natural innate defenses.
EXPERIMENTAL PROCEDURES
Bacterial Strains
WT GAS M1T1 strain 5448 was originally isolated from a patient with NF and TSS (Chatellier et al., 2000), S. iniae strain K288 was isolated from the brain of a moribund hybrid striped bass at the Kent SeaTech aquaculture facility (Buchanan at al., 2005). Also used were vancomycin-resistant Enterococcus fecalis (VRE) (ATCC 51299) and nonpathogenic L. lactis strain NZ9000. GAS, S. iniae., L. lactis, and VRE were grown in Todd-Hewitt broth (THB) (Difco, BD Diagnostics) or TH agar plates, using 1 μg/ml chloramphenicol (Cm) or 2 μg/ml erythromycin (Em) selection where appropriate. E. coli TOP 10 (Invrtrogen) were grown in Luria Bertani (LB) broth or LB agar plates using 100 μg/ml ampicillin, 500 μg/ml Em, or 5 μ/ml Cm selection. GAS and L. lactis were made competent for transformation by electroporation by growth in THB + 0.6% glycine. For use in neutrophils, whole blood, epithelial cell, and mouse challenge studies, bacteria were grown to logarithmic phase in THB (OD600 = 0.4 = ~2 × 108 cfu/ml), pelleted, washed, and resuspended in PBS or tissue culture media at the desired concentration.
Allelic Exchange Mutagenesis of GAS
PCR was used to amplify upstream and downstream DNA fragments from the M1 GAS 5448 chromosome immediately flanking the cepA gene. Primers pairs used were(1)cepA-upF (5′ ggtataagacggggtcaaca-3′, 526 bp upstream of the cepA start codon) with cepA-upR (5′-ggtggtatatccagtgatttttttctccatctga taccctcctaaatgtt-3′; immediately upstream of the cepA start site + 30 bp extension corresponding to 5′ end of the cat gene) and (2) cepA-downF (5′-tactgcgatgagtggcagggcggggcgtaataacaaagcgcaaagagaca-3′; immediately downstream of the cepA stop codon + 30 bp extension corresponding to the 3′ end of the cat gene) with cepA-downR (5′-gtcacatcagcagagctgtt-3′; 526 bp downstream of the cepA stop codon). PCR was performed using the two amplicons and an amplicon of the cat gene to yield a fusion product in which cat replaced cepA precisely in GAS chromosomal context. This PCR product was TA cloned into the Gateway entry vector, pCR8/GW/TOPO (Invitrogen), then subcloned into pKODestErm, a derivative of pHY304 constructed to allow L–R combination of gene cassettes, thus generating knockout vector pKO-cepA. This plasmid was transformed into GAS M1 and single recombination events identified at 37°C under Em + Cm selection. Selection was relaxed by serial passage at 30°C without antibiotics and double-crossover events identified by screening for colonies with CmR but EmS phenotype (Figure S1 A). Precise, inframe allelic exchange of cepA with cat in the GAS 5448ΔcepA mutant was confirmed by PCR analysis. M protein dot blot analysis was performed on serial 10-fold dilutions of GAS cells using antibiodies directed at the N-terminal domain of the M1 protein (Chatellier et al., 2000). Polymyxin B MIC was determined on logarthmic phase bacteria after 24 hr incubation with the peptide at 37°C. DNase activity was tested by coincubating the supernatants of logarithmic phase bacteria (OD 0.4) with calf thymus DNA (1 μg/μl) and buffer (300 mM Tris, 3 mM CaCl2. 3mM MgCl2) for 15 min. The reaction was stopped by adding 0.33 M EDTA and the extent of DNA degradation visualized by electrophoresis on a 1 % agarose gel.
Complementation Studies and Heterologous Expression of a A
The M1 GAS cepA gene and flanking was amplified by PCR from the GAS chromosome using primers cepA-upF and cepA-downR, TA cloned into the Gateway-TOPO vector, then subcloned using L–R recombinase into pDestErm, a version of pDCerm (Jeng et al., 2003) engineered to contain L–R recombination sequences in its multiple cloning site, generating expression vector pcepA. This plasmid was electroporated into the GAS 54484ΔcepA mutant and L. lactis strain NZ900 and transformants identified by EmR phenotype, and confirmed by PCR and restriction analysis. Plasmid pDestErm was introduced into the corresponding strains as an empty vector control.
Mutagenesis of Cepl
Whole genome pyrosequencing (454 Life Sciences) of the virulent S. iniae isolate K288 generated short sequence contigs with high BLAST similarity to the GAS cepA gene (Hidalgo-Grass et al., 2006), Using preliminary public data from the Baylor College of Medicine Human Genome Sequencing Center S. iniae genome sequencing project (http://www.hgsc.bcm.tmc.edu), we identified the full putative S. iniae cepl gene and designed primers for mutagenesis. The midde third of S. iniae cepl was amplified by PCR using primers cepl-1632F (5′-aggtcgtttacgatttgaaag-3′) + cepl-3284R (5′-tcttcaaccatataatagaag-3′). The 1653 bp amplicon was cloned into pCR8GW/TOPO and then subcloned via LR recombination into pKODestErm, as described for generation of the GAS pKO-cepA knockout plasmid. Electrocompetent WT S. iniae K288 were transformed with the pKO-cepl plasmid. Positive EmR transformant colonies growing at 30°C were temperature shifted to 37°Cto select for bacteria that underwent a single-crossover event in which the pKO-cepl was linearized into the chromosome, disrupting the native cepl gene (Figure S5A). Insertion mutants were confirmed through PCR analysis. For comparative analyses with the EmR cepl mutant, EmR was introduced into WT K288 through transformation with the empty pDestErm complementation vector (Locke et al., 2007). For functional assays, S. iniae strains were grown at 30° Con THA agar or in THB liquid media containing 5μg/ml Em.
Western Blot and ELISA Analysis
For IL 8 detection, bacteria were grown to midlogarithmic phase, washed with PBS, and resuspended in RPMI + 0.1 % fetal cal serum (FCS). Human IL-8 (R&D Systems) was added to the bacterial suspensions at final concentration of 1 mg/ml. After 4 hr (GAS) and 24 hr (S. iniae) incubation at 37°C, the samples were loaded and separated on a 4%–12% Tris-tricine in MES buffer Invitrogen) and transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk + 0.2% Tween-20. Detection used primary antibody mouse anti-human-monoclonal IL-8 (R&D Systems), secondary antibody peroxidase-conjugated sheep anti-mouse (GE Healthcare UK Limited), and detected using the Supersignal West Pico Chemiluminiscent System (Pierce). For detection of SpyCEP protein by GAS and L. lactis strains, rabbit polyclonal antibody raised against a recombinant protein representing residues 35–587 of the pre-pro SpyCEP enzyme sequence at 1:1000 dilution was employed and developed using Protein G-peroxidase (Sigma) and the ECL system. To monitor IL-8 cleavage. 10 μl of cell-free supernatant from an overnight culture of each bacterial strain was coincubated with an equal volume of human IL-8 (final concentration 5 μg/mL) at 37° C. Samples were then electrophoresed on a 12% Bis-Tris SDS PAGE gel (Invitrogen) in MES buffer and stained using the colloidal blue staining kit (Invitrogen). Quantitative ELISA for IL-8 was performed using the Quantikine kit (R&D Systems) as described previously (Hidalgo-Grass et al., 2004).
Endothelial Transcytosis Assay
Transmigration of human neutrophils across polar human brain microvascular endothelial cell (hBMEC) monolayers was assessed using an adaptation of a published assay (Chen et al., 2006) Briefly, polar hBMEC monolayers were established on collagen-coated TranswellTM plates (6.5 mm diameter, 3 mm pore size. Biocoat, BD Biosciences). Human neutrophils were purified from healthy donors using the PolymorphPrepTM system (Axis-Shield, Fresnius) and 1×106 cells added to the upper well of the chamber. Midlog phase GAS or L. lactis, (1× 107 cfu/ml) were pre-incubated with 70 ng/ml of recombinant human IL-8 for 2 hr at 37°C, then added to the lower well in 600 μl volume. Recombinant human IL-8 (40 ng) was added to the lower well as a positive control. Plates were incubated for 150 min at 37°C in 5% CO2 before direct enumeration of neutrophils transcytosing to the lower well in a counter chamber (average of 4 high-power fields).
Neutrophil and Whole-Blood Killing Assays
Neutrophils were isolated from the blood of healthy volunteers as above. Logarithmic phase bacteria were preopsonized in 80% autologous human plasma for 30 min at 37° C, added to neutrophils at final multiplicity of infection (MOI) = 2.5 bacteria/neutrophil, then incubated for 30 min at 37 °C with orbital rotation. In stated assays, G-actin 100 μg/ml, to inhibit DNase activity, or cytochalasin D 10 μg/ml, to inhibit phagocytosis, were added to the neutrophils during 10 min preincubation before addition of bacteria. Neutrophils were then lysed with sterile H2O and serial dilutions plated on THA for enumeration of surviving bacterial cfu. For visualization of NETs, bacteria were mixed with neutrophils at MOI = 0.1 in the presence of G-actin 100 μg/ml for 5 min to inhibit GAS DNase activity (Buchanan et al., 2006). 0.1 mM Sytox orange stain (Molecular Probes. Invitrogen) for extracellular DNA added, and the mixture immediately visualized without fixation or washes using a Nikon TE200 inverted fluorescent microscope with image capture by CCD camera. In stated assays exogenous IL-8 was added at a final concentration of 2.5 μg/μL. For quantification NETs were enumerated by counting three transects of three independent wells after staining with Sytox orange. For whole-Wood killing, phlebotomy was performed on healthy volunteers, and 104 cfu of midlog phase GAS in 100 μl were added to 300 μl whole blood. Aliquots were plated on THB agar after 15 and 30 min for enumeration of surviving GAS cfu.
Epithelial Cell Adherence and Invasion Assays
HEp-2 human pharyngeal epithelial cells were cultured in RPMI media + 10% FCS, split into 24- well plates, and allowed to grow to confluence for 48 hr prior to assays. Using MOI = 5 bacteria/cell, cellular adherence and antibiotic protection (invasion) assays were performed as described (Timmer et al., 2006). All assays were performed in quintuplicate and repeated three times with similar results. For live cell imaging, HEp-2 cells were incubated with bacteria as described for the adherence assay; however, the bacteria were prestained for 30 min using Fluorescein isothiocyanate Isomer I (Fluka, Sigma-Alderich). At the end of the assay, wells were treated for 10 min with the fish antimicrobial peptide moronecidin (Lauth et al., 2002) at final concentration 4 μM to kill all extracellular bacteria and Sytox Orange added to final concentration 0.1 μM for staining of dead cells. Cells were visualized using a Nikon TE200 inverted microscope with appropriate fluorescent filters and images captured with a CCD camera.
Mouse Infection Models
GAS and S.iniae virulence was tested using an established murine model of necrotizing skin infection (Buchanan et al., 2006; Nizet et al., 2001). Briefly, logarithmic phase GAS or S. iniae were resuspended in PBS, mixed 1:1 with sterile Cytodex beads (Sigma, St. Louis, MO). An inoculum of 5 × 107 cfu of WT GAS or S. iniae was injected subcutaneously into one flank of 10- to 12-week-old C57BI6 mice (Charles River Laboratories, CA). and simultaneously an equivalent inoculum of the isogenic ΔcepA (Δcepl) mutant was injected into the contralateral flank, allowing each mouse (N = 16, GAS; N = 6, S. iniae) to serve as its own control. The size of developing necrotic lesions was monitored daily for 4 days, at which time mice were euthanized and histopathology performed on formalin-fixed biopsy samples. Differences in lesion size were analyzed using the paired Student’s t test. For a co-infection competition model, 5 × 107 cfu of a 50:50 mix of WT GAS and isogenic ΔcepA GAS mutant was injected subcutaneously into the shaved flank of C57BI6N5 mice (N = 14); on day 4, the skin was collected in order to enumerate the surviving bacteria with strain determination by antibiotic selection and PCR confirmation. For the polymicrobial infection model, 2.5 × 107 cfu of WT GAS or the isogenic ΔcepA GAS mutant were mixed with an equivalent number of VRE and injected subcutaneously into the shaved flank of C57BI6 mice (N = 5); on day 4 the skin was collected in order to enumerate the surviving bacteria. For upper respiratory tract mucosal infection, 10- to 12-week-old C57BI6 mice (N = 10 per group) were infected intranasally with 1 × 108 cfu of WT GAS or isogenic ΔcepA GAS mutant, respectively. After 4 days, the mice were euthanized, nose excised and homogenized, and the surviving intranasal bacteria enumerated. Differences were analyzed using an unpaired Student’s t test.
Acknowledgments
This research was supported by an U.S.-Israel Binational Science Foundation Grant (E.H. and V.N.). NIH grant AI077780 (V.N.), Swiss National Foundation Grant PBZHB-108365 (A.S.Z.) and UK Medical Research Council (C.E.T.). We thank the UCSD Histopathology Core facility for assistance with histology studies and Reto Schuepbach for useful suggestions on experimental design.
Footnotes
Supplemental Data include five figures and Supplemental References and can be found online at http://www.cellhostandmicrobe.com/cgi/content/full/4/2/170/DC1/.
References
- Agnew W, Barnes AC. Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet Microbiol. 2007;122:1–15. doi: 10.1016/j.vetmic.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interteukin 8. J Exp Med. 1991;173:771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reicnard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1536. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Stannard JA, Lauth X, Ostland VE, Powell HC, Westerman ME, Nizet V. Streptococcus iniae phosphoglucomutase is a virulence factor and a target for vaccine development. Infect Immun. 2005;73:6935–6944. doi: 10.1128/IAI.73.10.6935-6944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Carapetis JR, Steer AC, Mulhoiland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- Chatellier S, Ihendyane N, Kansal RG, Khambaty F, Basma H, Norrby-Teglund A, Low DE, McGeer A, Kotb M. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect Immun. 2000;68:3523–3534. doi: 10.1128/iai.68.6.3523-3534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkemagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Cleary PP, Prahbu U, Dale JB, Wexter DE, Handley J. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect Immun. 1992;60:5219–5223. doi: 10.1128/iai.60.12.5219-5223.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary PP, LaPenta D, Vessela R, Lam H, Cue D. A globally disseminated M1 subcione of group A streptococci differs from other sub-clones by 70 kilobases of prophage DNA and capacity for high-frequency intracellular invasion. Infect Immun. 1998;66:5592–5597. doi: 10.1128/iai.66.11.5592-5597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill FR, 3rd, MacDonald KL, Thompson RL, Roberson F, Kohner PC, Besser-Wiek J, Manahan JM, Musser JM, Schlievert PM, Talbot J, et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA. 1997;277:38–43. [PubMed] [Google Scholar]
- Cockerill FR, 3rd, Thompson RL, Musser JM, Schlievert PM, Talbot J, Holley KE, Harmsen WS, Iistrup DM, Kohner PC, Kim MH, et al. Molecular, serological, and clinical features of 16 consecutive cases of invasive streptococcal disease. Southeastern Minnesota Streptococcal Working Group. Clin Infect Dis. 1998;26:1448–1458. doi: 10.1086/516376. [DOI] [PubMed] [Google Scholar]
- Dahl PR, Pernciaro C, Holmkvist KA, O’Connor MI, Gibson LE. Fulminant group A streptococcal necrotizing fasciitis: clinical and pathologic findings in 7 patients. J Am Acad Dermatol. 2002;47:489–492. doi: 10.1067/mjd.2002.120536. [DOI] [PubMed] [Google Scholar]
- Dalton TL, Hobb RI, Scott JR. Analysis of the role of CovR and CovS in the dissemination of Streptococcus pyogenes in invasive skin disease. Microb Pathog. 2006;40:221–227. doi: 10.1016/j.micpath.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Datta V, Myskowski SM, Kwinn LA, Chiem DN, Varki N, Kansal RG, Kotb M, Nizet V. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol Microbiol. 2005;56:681–695. doi: 10.1111/j.1365-2958.2005.04583.x. [DOI] [PubMed] [Google Scholar]
- DiVietro JA, Smith MJ, Smith BR, Petruzzelli L, Larson RS, Lawrence MB. Immobilized IL-8 triggers progressive activation of neutrophils rolling in vitro on P-selectin and intercellular adhesion molecule-1. J Immunol. 2001;167:4017–4025. doi: 10.4049/jimmunol.167.7.4017. [DOI] [PubMed] [Google Scholar]
- Doran KS, Liu GY, Nizet V. Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RJ, Taylor GW, Ferguson M, Murray S, Rendell N, Wrigley A, Bai Z, Boyle J, Finney SJ, Jones A, et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J Infect Dis. 2005;192:783–790. doi: 10.1086/432485. [DOI] [PubMed] [Google Scholar]
- Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, et al. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci USA. 2001;98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JD, Bast DJ, Nizet V, Low DE, de Azavedo JC. Streptococcus iniae virulence is associated with a distinct genetic profile. Infect Immun. 2001;69:1994–2000. doi: 10.1128/IAI.69.4.1994-2000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibello A, Collins MD, Dominguez L, Fernandez-Garayzabal JF, Richardson PT. Cloning and analysis of the L-lactate utilization genes from Streptococcus iniae. Appl Environ Microbiol. 1999;65:4346–4350. doi: 10.1128/aem.65.10.4346-4350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Dan-Goor M, Maly A, Eran Y, Kwinn LA, Nizet V, Ravins M, Jaffe J, Peyser A, Moses AE, et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet. 2004;363:696–703. doi: 10.1016/S0140-6736(04)15643-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Mishalian I, Dan-Goor M, Belotserkovsky I, Eran Y, Nizet V, Peled A, Hanski E. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. EMBO J. 2006;25:4628–4637. doi: 10.1038/sj.emboj.7601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J Bacteriol. 2003;185:1208–1217. doi: 10.1128/JB.185.4.1208-1217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, McLandsborough L, Kondagunta A, Cleary PP. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel SL, Standiford T, Kasahara K, Strieter RM. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991;17:17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- Kuo CF, Wang WS, Lee CM, Liu CP, Tseng HK. Foumier’s gangrene: ten-year experience in a medical center in northern Taiwan. J Microbiol Immunol Infect. 2007;40:500–506. [PubMed] [Google Scholar]
- Kwinn LA, Nizet V. How group A Streptococcus circumvents host phagocyte defenses. Future Microbiol. 2007;2:75–84. doi: 10.2217/17460913.2.1.75. [DOI] [PubMed] [Google Scholar]
- Lauth X, Shike H, Burns JC, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, Nizet V, Taylor SW, Shimizu C, et al. Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. J Biol Chem. 2002;277:5030–5039. doi: 10.1074/jbc.M109173200. [DOI] [PubMed] [Google Scholar]
- Locke JB, Colvin KM, Varki N, Vicknair MR, Nizet V, Buchanan JT. Streptococcus iniae β-hemolysin streptolysin S is a virulence factor in fish infection. Dis Aquat Organ. 2007;76:17–26. doi: 10.3354/dao076017. [DOI] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Mitrophanov AY, Churchward G, Borodovsky M. Control of Streptococcus pyogenes virulence: Modeling of the CovR/S signal transduction system. J Theor Biol. 2006;246:113–128. doi: 10.1016/j.jtbi.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murono K, Fujita K, Saijo M, Hirano Y, Zhang J, Murai T. Emergence and spread of a new clone of M type 1 group A Streptococcus co-incident with the increase in invasive diseases in Japan. Pediatr Infect Dis J. 1999;18:254–257. doi: 10.1097/00006454-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisil J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Nobbs AH, Shearer BH, Drobni M, Jepson MA, Jenkinson HF. Adherence and internalization of Streptococcus gordonii by epithelial cells involves beta1 integrin recognition by SspA and SspB (antigen I/II family) polypeptides Cell. Microbiol. 2007;9:65–83. doi: 10.1111/j.1462-5822.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- Ou LF, Yeh FL, Fang RH, Yu KW. Bacteriology of necrotizing fasciitis: a review of 58 cases. Zhonghua Yi Xue Za Zhi (Taipei) 1993;51:271–275. [PubMed] [Google Scholar]
- Purushothaman SS, Park HS, Cleary PP. Promotion of fibronectin independent invasion by C5a peptidase into epithelial cells in group A Streptococcus. Indian J Med Res. 2004;119(Suppl):44–47. [PubMed] [Google Scholar]
- Simms HH, D’Amico R. Studies on polymorphonuclear leukocyte bactericidal function: the role of exogenous cytokines. Shock. 1997;7:84–89. doi: 10.1097/00024382-199702000-00002. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS, Chensue SW, Kunkel SL. Cytokine-induced neutrophil-derived interleukin-8. Am J Patrol. 1992;141:397–407. [PMC free article] [PubMed] [Google Scholar]
- Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA. 2005;102:1679–1684. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby P, Zhang S, Whitney AR, Falugi F, Grandi G, Graviss EA, Deleo FR, Musser JM. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect Immun. 2008;76:978–985. doi: 10.1128/IAI.01354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Jr, Bryant AE, Blick KE, Hack E, Jansen PM, Kosanke SD, Stevens DL. Staging of the baboon response to group A streptococci administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response patterns. Clin Infect Dis. 1999;29:167–177. doi: 10.1086/520147. [DOI] [PubMed] [Google Scholar]
- Thelen M, Peveri P, Kernen P, von Tscharner V, Walz A, Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988;2:2702–2706. [PubMed] [Google Scholar]
- Timmer AM, Kristian SA, Datta V, Jeng A, Gillen CM, Walker MJ, Beall B, Nizet V. Serum opacity factor promotes group A streptococcal epithelial cell invasion and virulence. Mol Microbiol. 2006;62:15–25. doi: 10.1111/j.1365-2958.2006.05337.x. [DOI] [PubMed] [Google Scholar]
- Voyich JM, Braughton KR, Sturdevant DE, Vuong C, Kobayashi SD, Porcella SF, Otto M, Musser JM, DeLeo FR. Engagement of the pathogen survival response used by group A Streptococcus to avert destruction by innate host defense. J Immunol. 2004;173:1194–1201. doi: 10.4049/jimmunol.173.2.1194. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13:981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- Weinstein MR, Litt M, Kertesz DA, Wyper P, Rose D, Coulter M, McGeer A, Facklam R, Ostach C, Willey BM, et al. Invasive infections due to a fish pathogen, Streptococcus iniae. S iniae. Study Group. N Engl J Med. 1997;337:589–594. doi: 10.1056/NEJM199708283370902. [DOI] [PubMed] [Google Scholar]
- Yao L, Lowy FD, Berman JW. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect Immun. 1996;64:3407–3409. doi: 10.1128/iai.64.8.3407-3409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]