Abstract
Recent studies have indicated several therapeutic applications for δ opioid agonists and antagonists. To exploit the therapeutic potential of δ opioids developing a structural basis for the activity of ligands at the δ opioid receptor is essential. The conformationally sampled pharmacophore (CSP) method (Bernard et al., JACS, 125: 3103–3107, 2003) is extended here to obtain quantitative models of δ opioid ligand efficacy and affinity. Quantification is performed via overlap integrals of the conformational space sampled by ligands with respect to a reference compound. Iterative refinement of the CSP model identified hydrophobic groups other than the traditional phenylalanine residues as important for efficacy and affinity in DSLET and ICI 174,864. The obtained models for a structurally diverse set of peptidic and non-peptidic δ opioid ligands offer good predictions with R2 values > 0.9 and the predicted efficacy for a set of test compounds was consistent with the experimental value.
Introduction
Treatments of severe and chronic pain often depend on the use of opioid analgesics, which are generally effective by their action at the μ opioid receptor.1, 2 However, the use of μ opioids are accompanied by adverse effects including life threatening incidents such as respiratory depression3 and constipation.4 In addition, the development of tolerance and dependence5 complicate the therapeutic use of these drugs. Thus, there is a need for the development of effective medications, which lack serious side effects. Since the identification of the enkephalins 6, 7 and the δ opioid receptors, δ opioid ligands have been pursued as analgesic agents and numerous studies have investigated the biological processes involving the δ opioid receptor system. This has led to the discovery of several potential therapeutic applications for δ opioid ligands such as the treatment of substance abuse and immunosuppression, among others.8–10 It has also been found that co-administration of a δ-antagonist with a μ-agonist reduces the development of tolerance and dependence to the μ agonist11, 12 and a slower development of tolerance has been observed with the administration of a peptide with the dual profile of μ-agonism and δ-antagonism.13 Thus, efforts are also being made to develop novel analgesics with this dual profile of μ agonism and δ antagonism.14
The rational design of drugs for a specific target is greatly aided by structural information of the receptor. However, in the absence of an experimentally obtained 3D structure of the receptor, drug development methods must rely on information obtainable from known ligands of the receptor. This is the case with the G-protein coupled δ opioid receptor15, 16 for which no experimental structure is available. While computational 3D models of the δ opioid receptor have been developed17–23, a large number of studies have focused on developing structure-activity relationships (SAR) based on the study of known δ opioid ligands24 via use of both pharmacophore25–35 and QSAR36–41 models. The former method typically involves identification of low energy conformations of the ligands of interest followed by structural alignment and identification of common features that are predictive of biological activity. While these studies have advanced our understanding of δ opioid SAR the information is typically restricted to congeneric series of compounds and omit consideration of conformational changes that may occur upon binding of ligands to their target receptor 36–41.
To overcome the limitations of traditional methods of pharmacophore development an approach involving extensive conformational sampling of ligands followed by the use of all sampled conformers in the pharmacophore model was developed27, 28. The method, which is referred to as a conformationally sampled pharmacophore (CSP), was applied to the study of non-peptidic δ opioid ligands27 and peptidic δ opioid ligands28 resulting in models distinguishing δ opioid agonists from antagonists. The inclusion of all sampled conformers of the ligands in the model accounts for the inherent dynamic nature of molecules and the nature of their interaction with bio-molecules, as molecules at room temperature possess kinetic energy sampling a variety of conformations other than just the lowest energy conformation(s)42. More importantly, the favorable interaction with the receptor may enable a molecule to overcome the conformational strain associated with assuming a higher energy conformation and hence the bound conformation of a molecule need not be among the low energy conformers of the unbound molecule43. Accordingly the inclusion of all conformers increases the probability of including the bioactive conformer in the model. The importance of conformational sampling and the use of higher energy conformers in addition to the low energy ones has gained increasing attention,44, 45 and the utility of the CSP method is emphasized by its consideration in studies by workers in the field46–52 including its application for pharmacophore development of compstatin analogues53.
Here we describe an extension of the CSP method into quantitative models predicting the efficacy and the affinity of δ opioid ligands. Subtypes of the δ opioid receptor (i.e. δ1 and δ2) have been proposed, but are not invoked in the current model as their existence remains a matter of debate10. The overlap in conformational space, as defined by various combinations of geometric parameters for different ligands with respect to a reference ligand, are quantified and then used as parameters for prediction of ligand efficacies and affinities using multiple regression models. The utility of the approach is shown in the identification of novel functional groups on selected peptides that are essential for biological activity and ligand affinity.
Computational Methods
Conformational sampling of peptidic and non-peptidic δ opioid ligands was achieved by utilizing molecular dynamics (MD) simulations54 using the program CHARMM55, 56. Initially, the δ opioid ligands (Figure 1 & Table 1) were modeled using Sybyl 6.257 and energy minimized to a gradient of 0.05 kcal/molÅ using the Tripos force field. Each molecule was then subjected to 200 steps of Adopted Basis Newton Raphson minimization in CHARMM using the Merck-Molecular Force Field (MMFF)58, 59 prior to MD simulations. Conformational sampling for the non-peptidic ligands (Figure 1) was carried out with 10 ns MD simulations at 300 K with snapshots saved every 100 integration time steps for analysis. Sampling for the peptidic ligands (Table 1) was carried out using replica exchange MD simulations60 which involved 10 ns simulations with four replicas between 300 and 400 K using an exponential scale; 300, 330, 363 and 400 K. For each replica, simulations were carried out for 100 integration time steps following which the coordinates were saved for analysis and an exchange of replicas was attempted. Conformations from all 4 replicas were used in the analysis. For all MD simulations, Langevin dynamics61 were performed with an integration time step of 0.002 ps including SHAKE of all covalent bonds involving hydrogens62 and aqueous solvation was treated via the Generalized Born Continuum Solvent Model (GBSW)63, 64. The physiologically relevant protonation states of the ligands were used in the study, which in the case of some of the non-peptidic ligands involved multiple configurations of the proton on the basic nitrogen, as previously performed27.
Figure 1.
Non-peptidic δ opioid ligands used in the development of the quantitative efficacy and affinity models. The pharmacophore groups A in green, B in red and N in blue. Compounds 8 and 9 were included only for affinity modeling and compounds 10 - 13 were used as external tests for efficacy prediction.
Table 1.
Peptidic δ opioid ligands used in the development of CSP.
|
The pharmacophore points are the protonated nitrogen (N) on Tyr1, the centroid of the phenolic group (A) on Tyr1 (green) and the centroid of the hydrophobic group (B) (red). For 17 and 18 the various groups considered as the hydrophobic moiety are shown in red.
Angles and distances between the three pharmacophoric points (Figure 1 & Table 1) were measured for all conformations of the δ opioid ligands obtained from the MD simulations. Geometric data from all configurations for the non-peptidic ligands with multiple protonation states were combined for analysis. All possible combinations of distances with angles were then utilized to obtain 2D probability distributions of the pharmacophoric parameters for each ligand. These probability distributions were obtained with a bin size of 0.1 Å and 1° for the distances and angles, respectively. Overlap coefficients, OC, for the 2D pharmacophoric parameters were calculated using equation 1:
| (1) |
where P represents the normalized probability at pixel ij from the 2D distributions for compounds k (i.e. the reference compound) and l.
Overlap coefficients were then utilized as independent variables in regression analysis with respect to the reported biological activity and affinity (i.e. the dependent variables, Table S.1, supporting information) using the program Excel. For model development compounds 1–9 in Figure 1, and 14–18 in Table 1, were used as the training set; compounds 10–13 were used as test molecules. 1 and 7 are structurally almost identical with the only difference being the methoxy substituent in 7 as opposed to the hydroxyl substituent in 1 on the aromatic ring (Figure 1). This similarity in structure results in very high overlaps in the pharmacophoric parameters and preliminary regression analysis indicated a bias in the models due to 7 dominating the regression fits. This compound was therefore excluded during the initial phase of development of the quantitative model; however, once preliminary models were developed 7 was reintroduced into the analysis and is included in the final models. The OC were calculated for all 9 distance-angle pairs for each compound with respect to the reference compound. Combinations of the different distance-angle OC for all compounds were then used in multiple regression analyses to fit the experimental efficacies for the ligands. From these regression analysis sets of OC values that yielded the highest correlation coefficients, (R2 > 0.9), with suitable P-values (<0.05) for the coefficients were determined and subjected to further analysis.
Results and Discussions
To generate a quantitative CSP model involving the 2D pharmacophoric parameters, a set of δ opioid ligands with efficacies and affinities determined under identical experimental conditions were selected for the training set65. The selected data set involved the binding and G-protein activation by a set of peptidic and non-peptidic δ opioid ligands in C6 glioma cell lines stably transfected with the δ opioid receptor from rat for efficacy measurements, and the displacement of radiolabeled 4 for determination of ligand affinities. Table S.1 of the supplemental information presents the list of ligands and the experimental data extracted from Table 1 of Clarke et al65. Since the reported ligand efficacies were obtained based on the maximum stimulation of [35S]GTP-γS binding with respect to that by the δ opioid ligand 1, evaluation of the OC pharmacophoric parameters were carried out using 1 as the reference compound. In addition, the influence of the identity of the reference compound was studied by individually using 7 and 6 as the reference compound.
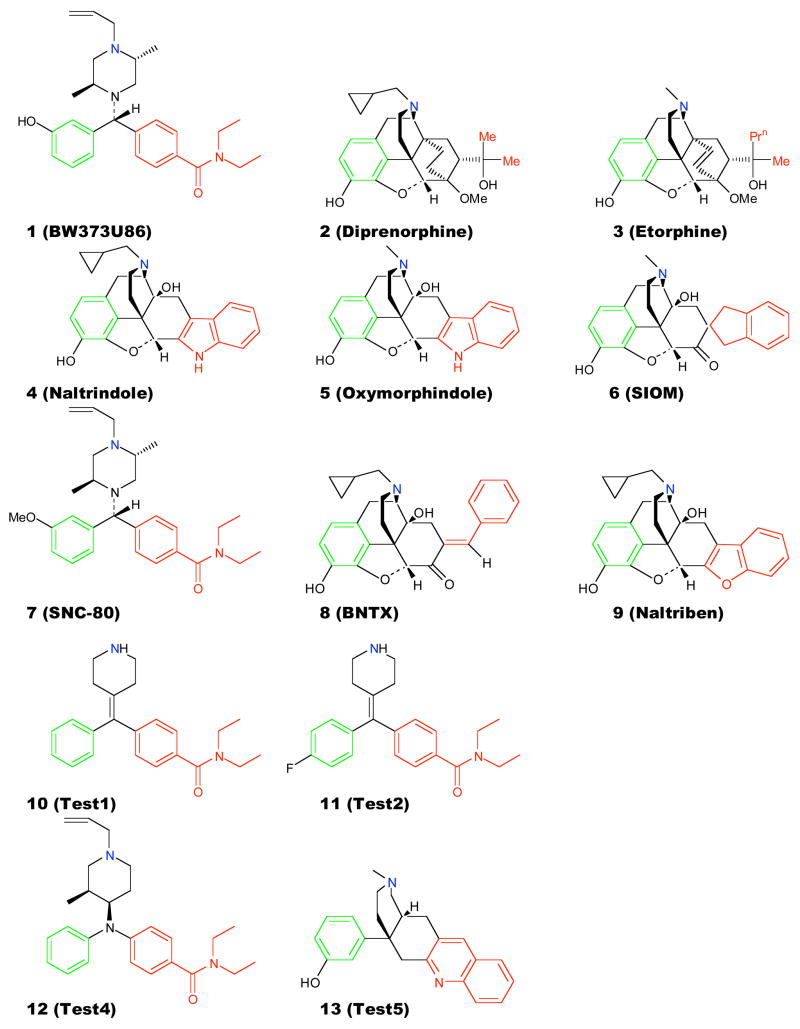
The CSP method is based on relating the regions of conformational space sampled by the ligands to their biological activity, with the conformational space defined as the distributions of the distances and angles between the pharmacophoric points (Figure 1 & Table 1) obtained from all accessible conformations of the respective ligands. Shown in Figure 2 are selected 2D conformational distributions for the non-peptidic ligands 1, 4 and 7. As may be seen, the overlap of the distributions of 1 and 7 (Figure 2A), both agonists, is high, while that of 1 and 4 (Figure 2B), an antagonist, is low. This is the basis for the qualitative 2D CSP that discriminates δ-opioid agonists from antagonists27, 28. To extend the approach to a quantitative method, the extent of overlap of the distributions between compounds may be obtained using equation 1. In the case of the distributions shown in Figures 2A and 2B, the computed OC values are 0.77 and 0.00, respectively. By obtaining the OC values for all compounds in a training set with respect to a reference compound, those values may be regressed against the biological data, yielding a quantitative model.
Figure 2.
2D probability distributions and calculated OC for, A) 1 (red) and 7 (green), and B) 1(red) and 4 (blue).
Development of δ-opioid ligand efficacy model
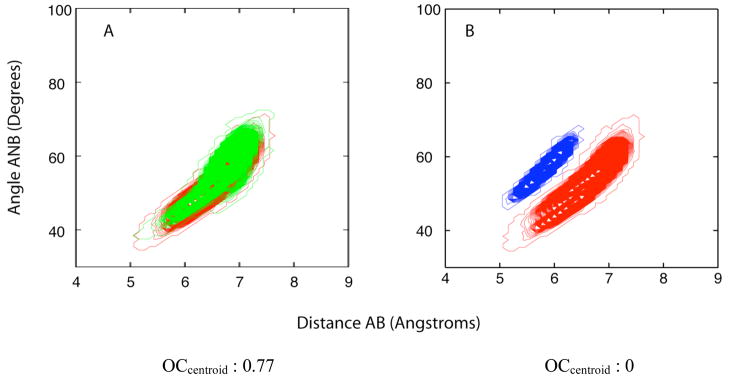
Development of the quantitative CSP used the following pharmacophore points (Figure 1 & Table 1); the basic nitrogen (N), and centroids of the aromatic ring (A) and hydrophobic (B) moieties in the δ opioid ligands (Centroid) (Figure 3A), as previously performed27, 28. This yields three distances and three angles, from which nine possible 2D pharmacophore parameters are obtained. In lieu of the centroids for pharmacophore points A and B, the atoms that were the maximum distance apart between the A and B groups were also used to define the respective pharmacophore points (MaxD) (Figure 3B). This was achieved by computing the maximum (AB) distance between all non-hydrogen atoms of the aromatic group, A, and of the hydrophobic group, B, for all saved conformers of a compound and then selecting the two relevant atoms for the computation of the remaining distances, AN and BN, and angles ANB, NAB and NBA, (Figure 3B). Additionally, in the calculation of the OC values, overlaps were also measured by equal weighting (Centroid= or MaxD=) of all points in conformational space. This was performed by using a binary measure of occupancy for the summation of points occupied by a compound and the reference compound (i.e. 1 if sampled, 0 if never sampled), normalized by the total number of points occupied. Development of the quantitative efficacy model involved compounds 1–7 and 14–18. The appropriate definition of the B group in d opioid ligands with multiple hydrophobic residues or groups, such as 17 and 18, were obtained as a result of refinement of the predictive models as discussed below with the successive inclusion of these ligands in the quantitative model. Initially, 17 was included in model development with Phe as the hydrophobic B group. Refinement of the model involved consideration of the Leu residue in 17 as the B group followed by inclusion of 18 in the training set, where the N-terminal allyl substitutents were individually considered as the B group. The OC values for the 9 2D pharmacophoric parameters used in the initial model for the various ligands are presented in Tables S.2 and S.3 of the supporting information. In the majority of cases finite OC values are obtained; however, with some agonists, such as 2, 3, 5 and 17, and the antagonist 4 zero OC values were obtained.
Figure 3.
Examples of centroid and maximum distance based calculations of pharmacophoric parameters shown for 4. The pharmacophore groups A in green, B in red and N in blue. The lines in magenta indicate the type of measurement A) Centroid B) Maximum A-B distance (MaxD).
The 9 2D OC parameters based on the different definitions of the pharmacophore points, Centroid and MaxD, with and without equal weighting of conformers, were then used in multiple regression analysis with respect to the efficacy data. Initial analysis selected those models with high R2 values (Table S.4 supporting information), showing the best models to include pharmacophoric parameters based on equal weighting of all conformers for both the centroid (Centroid=) and maximum A to B distance (MaxD=) measurements. Further evaluation of these models was performed using P-values to estimate the significance of the individual OC parameters in these models (Table S.5, supporting information). It was seen that the Centroid= combination of the NA-NBA and BN-NAB 2D pharmacophoric parameters resulted in a R2 value of 0.898 with P-values < 0.05 for both OC parameters. However, 2 and 5, which have low, but significant experimental activity (Table S.1), show no overlap in either of these parameters with respect to 1 (Table S.2ii), suggesting that these parameters may not be indicative of the requirements for activity at the δ opioid receptor. Alternatively, the AB-NBA + NA-NBA MaxD= combination gives an R2 value of 0.936 with P-values <0.001 for both parameters (Table S.5ii). However, detailed analysis of the selected OC parameters revealed that for the highly active δ opioid ligand 17, both of the selected OC parameters were zero with respect to 1, (Table S.3ii). While this may indicate limitations in the model, with 17 there is a second functional group on the peptide that may act as the hydrophobic B group, leucine, rather than the phenylalanine side chain that is traditionally accepted as the hydrophobic group. Thus this group was tested as the hydrophobic B group, with the resultant OC values giving reasonable overlaps for all nine parameters (Table S.6, supporting information).
The recalculated OC values for 17 were utilized in additional regression analysis (Table S.7, supporting information). The best correlations were seen with the parameters obtained using the maximum A to B distance (MaxD) with probability based OC values giving R2 values > 0.92, with the AB-NBA and NA-NBA MaxD distance angle combination yielding the best R2. Importantly, AB-NBA and NA-NBA MaxD combination had significant coefficients (p<0.05) supporting the validity of the model (Table 2). Thus, the application of the quantitative 2D CSP method predicts that the hydrophobic moiety responsible for δ activity in 17 is the side-chain of the Leu moiety rather than the traditional Phe side-chain.
Table 2.
Model properties, selected pharmacophore points and comparison of observed and predicted relative efficac ies of δ opioid ligands with 1 as the reference molecule.
| Model, R2 = 0.978 | Y-intercept | AB-NBA | NA-NBA | |
|---|---|---|---|---|
| Coefficients | 0.072 | 3.375 | 11.026 | |
| P-values | 0.0431 | 0.0004 | 0.0007 | |
| Biological Activity | OC Values | |||
| Compound | Experimental | Predicted | AB-NBA | NA-NBA |
| 2 | 0.08 | 0.1 | 0.007492 | 0 |
| 3 | 0.36 | 0.27 | 0.057046 | 0.000529 |
| 4 | 0 | 0.08 | 0.000996 | 0 |
| 5 | 0.12 | 0.08 | 0.001019 | 0 |
| 6 | 0.18 | 0.22 | 0.044116 | 0.000063 |
| 14 | 0.59 | 0.56 | 0.074764 | 0.021763 |
| 15 | 0.59 | 0.59 | 0.070327 | 0.025072 |
| 16 | 0.8 | 0.8 | 0.084515 | 0.040306 |
| 17 | 0.9 | 0.93 | 0.180618 | 0.022434 |
Model obtained using multiple regression with the maximum A to B distance based calculation of OC.
Detailed analysis of the AB-NBA and NA-NBA MaxD based model is presented in Table 2. In accord with the high R2, 0.978, and significant P values, the model nicely predicts the activity of all the compounds in the training set. Examination of the 2D parameters AB-NBA and NA-NBA shows that all compounds have overlap with the reference 1, for the AB-NBA distance angle parameter. On the other hand the low efficacy compounds, 2 and 5, and the antagonist 4 have no overlap for the NA-NBA parameter. It is also seen that the AB-NBA parameter is most directly correlated with the efficacies with compounds having higher overlaps being more active, indicating that the structural features associated with this parameter are probably the most significant determinants of δ opioid activity. Notable is the ability of the model to predict the difference between the peptidic ligands 15 and 16. These ligands only differ by the presence of the chlorine atom on Phe3, suggesting that the present approach is capable of predicting changes in activity associated with subtle structural changes in a ligand. The ligands for which the model makes the poorest prediction is 4, which is a full antagonist (i.e. no discernable biological activity in the applied experimental assay), although the model predicts it to have a low, but nonzero efficacy. The structural similarity between 4 and 5 (Figure 1), results in almost identical OC values for the two compounds and seems to be the cause for the predicted activity for 4. However, N-cyclopropylmethyl substituents as in 4 tend to give lower opioid efficacy than N-methyl substituents,8 an effect not accounted for in the present model; future models will incorporate this substituent to further fine-tune the model.
Structurally both distance AB and angle NBA involve the hydrophobic pharmacophoric moiety, B, indicating the relevance of this group for δ-opioid activity. The fact that the maximum distance based criterion is the best predictor of the ligand efficacies also indicates that the spatial extent of the B group influences the activity, as does its orientation with respect to the other pharmacophoric groups. This is consistent with previous observations by us27, 28 and others35, 66, 67. In addition it was seen that for the 2 parameter combinations of AB-NBA with the remaining 2D parameters involving either the AB or BN distance with any angle gave R2 values > 0.9, further indicating the significance of the hydrophobic group, B (Table S.7, supporting information).
Impact of reference compound
1 is a full δ agonist, and since experimental efficacy data were available with reference to this compound, the initial development of a quantitative model used it as the reference compound. To test if the selection of the reference compound would impact the resulting model, the quantitative 2D CSP was performed using two alternate compounds as the reference. 7 was selected as it is structurally similar to 1. The second 6, was selected as it is a weak agonist with low efficacy and is also structurally different from 1.
OC values based on 7 as the reference differ significantly from those with respect to 1 (data not shown). This difference is an outcome of using all conformations of the ligands with the CSP method, which accounts for the inherent dynamics of molecules, whereby though 7 and 1 are almost structurally identical, the difference in conformations sampled becomes evident. While this may complicate the refinement of the pharmacophore, it indicates that the CSP method goes beyond the biases associated with structural alignment techniques used typically in pharmacophore development by not limiting the conformations considered to only those that satisfy predetermined scaffolds.
With 7 as the reference, regression analyses were performed for various combinations of 2D pharmacophoric parameter OC, as above, yielding a number of combinations with good correlation. The best predictions of efficacy R2 > 0.9 were seen with the MaxD parameters (Table S.8, supporting information) similar to that seen with 1, and the use of the probability based weighting again provided better predictions. Several of these models had R2 values > 0.9, although the best model, (AB-NBA + NA-NBA, R2=0.946) had a large P value for the AB-NBA term. Of the remaining AB-NBA models with R2 > 0.9 (Table S.8) the P values were all < 0.05 indicating good reliability in the model. Thus, with 7 as the reference compound predictive models are obtained, with the AB-NBA parameter being the most relevant for biological activity. However, the second parameter differed from that of the model with 1 as the reference indicating that subtle differences in the models are obtained with different reference compounds.
With 6 as the reference compound the OC values were very different from those using 1 (data not shown), as expected due to the structural differences in the two compounds. On performing multiple regression analyses, reasonable R2 values (i.e. > 0.9) were obtained only with combinations involving three or more 2D pharmacophoric parameters (Table S.9, supporting information). Interestingly, R2 values > 0.9 were obtained only for the MaxD= based parameter where, unlike for 1 or 7 as reference compounds, equal weighting of the conformers gave better predictions. Of the top models the two best with R2 > 0.96, NA-NBA, BN-NAB, NA-ANB and NA-NBA, NA-NAB, BN-NBA, were also the only two with P values < 0.05 for all three parameters..
For the 6 based model the two significant differences with respect to the models based on 1 and 7 was the lack of the term AB-NBA and the use of equal weighting of the MaxD term in the best models. In the use of all sampled conformations of a molecule in pharmacophore development, one may expect a highly active compound to populate regions of conformational space that are relevant to receptor activation to a greater extent than a compound with low activity. As a result, for a low-efficacy reference compound both the regions of overlap relevant to activity and the population of those regions may be much smaller such that the ability of the compound to sample that region of conformational space is more relevant rather than details of the extent and probability of sampling active conformations. This difference may lead to equal weighting leading to more predictive models when using a low-efficacy compound as the reference. Concerning the lack of inclusion of the AB-NBA term in the best models, this term was included in one model with R2 > 0.9 (Table S.9) and since the other best models contain both the NA, BN and various angle terms the AB-NBA related information is implicitly in the model. Thus, the use of a low-efficacy compound as reference does allow for the development of a predictive model. However, because of the probable limitations in sampling conformational regions related to receptor activation in low efficacy compounds it is suggested that higher efficacy compounds serve as better reference compounds.
Inclusion of 18 in the training set
As mentioned above the peptide antagonist 18 possesses multiple hydrophobic groups including, the Phe and Leu residues, and the allyl substituents on the amino group that may influence the nature of the interaction of this peptide with the δ opioid receptor. In addition this peptide is different from the other peptidic ligands in that it has a tertiary amino group that forms one of the pharmacophoric points, which could also be a cause for the antagonistic nature of this peptide. Due to the structural differences in this ligand and the different hydrophobic groups that could serve as the pharmacophoric B group it was not included in the initial training set. As mentioned above the assumption of the Phe residue as the hydrophobic group in 17 was found to be inconsistent and therefore, model development was extended to include 18 with Leu5 as well as the allyl substituents on the N considered, in addition to Phe4, being considered as the required hydrophobic B group.
Overlap calculations for each of the possible groups in 18 was undertaken with respect to 1 with the resulting OC values reported in Table S.10 of the supporting information. The different B group definitions offered variable degrees of overlap for the pharmacophoric parameters with the B1 (Phe4) and B4 (Leu5) definitions providing overlaps for all parameters. The B2 and B3 (allyl substituents) definitions gave finite overlaps in only a few parameters including the AB-NBA and NA-NBA parameters and, in some instances the NA-ANB parameter. In addition, due to the symmetry of the allyl groups, they were considered as one group and the OC values calculated. Regression analyses were then performed with 18 in the training set using each set of pharmacophoric parameters for the different B group definitions individually. Only in case of the MaxD based parameters without equal weighting were regression models with R2 values > 0.9 obtained, (Table S.11, supporting information). This observation is consistent with the results without 18 (see above). Interestingly it was observed that reasonable fits (R2 > 0.85) could be obtained with each of the B group definitions for 18, although the best models were obtained with the allylic substituents as the B group. Once again all models with R2 value > 0.9 involved AB-NBA and the combination with BN-NBA providing the best R2 value of 0.95 with acceptable P-values for the regression parameters. Thus, the quantitative CSP model can be extended to include 18 with the model suggesting that the hydrophobic B moieties may be the allyl substituents on the tertiary amino group, which also acts as the essential basic N on the compound.
Inclusion of 7 and 18 in the final model
7 is structurally very similar to the reference compound, 1, which resulted in very high OC values such that it dominated the regression fits during model development, leading to its exclusion from the initial fitting step. With the change in definitions of the hydrophobic B group for 17 and the multiple hydrophobic groups in 18 and the identification of the MaxD OC parameters as the best predictor of activity, an attempt was made to extend the model to include both 7 and 18 using 1 as the reference compound. For these analyses the hydrophobic groups identified for 18, viz. Phe4 (B1), the two allylic amino substituents (B2-3) and Leu5 (B4) were evaluated separately with the use of the Leu5 residue as the hydrophobic group for 17. Regression analyses were performed iteratively as described above using combinations of the OC values obtained from the MaxD parameters yielding multiple combinations with R2 values > 0.9 and P-values < 0.05 for the regression coefficients. In all cases the regression constant was found to be insignificant and hence to obtain the final model regression analyses were performed once again restricting the intercept to zero. The resulting combinations, with higher R2 values, obtained using different B group definitions for 18 are shown in Table 3. As seen in the table, irrespective of the B group used most of the combinations giving good correlations are the same, in contrast to the result seen above in the model that omitted 7. While more combinations yielding high R2 values are seen with the Phe4 group (data not shown) as the hydrophobic moiety, the best R2 value 0.973 is obtained with the use of either the Leu5 residue or the allylic substituents as the B group. The two best R2 models were compared with the experimentally obtained efficacy values as shown in Tables 4 and 5 and Figures 4A and 4B. The best R2 values in both cases (B2-3 or B4) are with the AB-ANB+AB-NBA+BN-NAB combination and the coefficients for this model are also very similar, suggesting that either of these hydrophobic groups could provide the required hydrophobic interactions with the receptor for 18. In the final model all combinations involve the AB-NBA parameter, and the best combination in all three cases include the BN-NAB parameter as well, suggesting that the structural features associated with these two parameters are the most important descriptors for predicting δ opioid efficacies.
Table 3.
Statistical analysis of the three best regression models for different definitions of the hydrophobic B group in 18 obtained with MaxD based OC and the inclusion of 7 in the training set.
| 2D Pharmacophoric Parameter | R2 | Coefficient 1 | Coefficient 2 | Coefficient 3 |
|---|---|---|---|---|
| ICI-B1 | ||||
| AB-NAB + AB-NBA + BN-NAB | 0.945 | 6.8053 | 5.4185 | −11.6207 |
| 2.36E-05 | 2.95E-06 | 2.08E-06 | ||
| AB-NAB + AB-NBA + BN-ANB | 0.944 | 9.1739 | 5.2474 | −13.4950 |
| 1.26E-05 | 3.74E-06 | 2.34E-06 | ||
| AB-ANB + AB-NAB + AB-NBA | 0.939 | −8.1779 | 3.9389 | 5.2830 |
| 3.18E-06 | 0.0002 | 4.86E-06 | ||
| ICI-B2-3 | ||||
| AB-ANB + AB-NBA + BN-NAB | 0.973 | −20.4463 | 5.0678 | 17.0941 |
| 1.35E-06 | 3.32E-07 | 9.52E-06 | ||
| AB-ANB + AB-NAB + AB-NBA | 0.963 | −8.2981 | 4.0780 | 5.2779 |
| 4.43E-07 | 3.51E-05 | 7.46E-07 | ||
| AB-ANB + AB-NBA + BN-ANB | 0.957 | −14.4864 | 5.2995 | 10.3428 |
| 4.53E-06 | 1.34E-06 | 6.78E-05 | ||
| ICI-B4 | ||||
| AB-ANB + AB-NBA + BN-NAB | 0.973 | −20.4520 | 5.0703 | 17.0959 |
| 1.43E-06 | 3.49E-07 | 1E-05 | ||
| AB-ANB + AB-NAB + AB-NBA | 0.961 | −8.2618 | 4.0408 | 5.2812 |
| 5.25E-07 | 4.2E-05 | 8.86E-07 | ||
| AB-NBA + BN-NBA + NA-NBA | 0.960 | 3.3760 | −21.5598 | 17.4879 |
| 0.0007 | 8.57E-05 | 0.0006 |
Multiple regression was performed without a constant. The values for the regression coefficients and the corresponding P-values are listed for the different 2D pharmacophoric parameter OC combinations.
Table 4.
Model properties, selected pharmacophore points and comparison of observed and predicted relative affinities of δ opioid ligands with 1 as the reference molecule.
| Model, R2 = 0.973 | AB-ANB | AB-NBA | BN-NAB | ||
|---|---|---|---|---|---|
| Coefficients | −20.4463 | 5.0678 | 17.0941 | ||
| P-values | 1.35E-06 | 3.32E-07 | 9.52E-06 | ||
| Biological Activity | OC Values | ||||
| Compound | Experimental | Predicted | AB-ANB | AB-NBA | BN-NAB |
| 2 | 0.08 | 0.04 | 0 | 0.007492 | 0 |
| 3 | 0.36 | 0.29 | 0 | 0.057046 | 0.000019 |
| 4 | 0 | 0.01 | 0 | 0.000996 | 0.000008 |
| 5 | 0.12 | 0.01 | 0 | 0.001019 | 0.000008 |
| 6 | 0.18 | 0.22 | 0.001138 | 0.044116 | 0.001433 |
| 7 | 1.02 | 1.02 | 0.877801 | 0.906859 | 0.840738 |
| 14 | 0.59 | 0.63 | 0.008131 | 0.074764 | 0.024471 |
| 15 | 0.59 | 0.48 | 0.001921 | 0.070327 | 0.009454 |
| 16 | 0.8 | 0.80 | 0.064178 | 0.084515 | 0.098641 |
| 17 | 0.9 | 0.94 | 0.000554 | 0.180618 | 0.002153 |
| 18 | 0 | 0.01 | 0 | 0.00226 | 0 |
Model obtained using multiple regression without a constant. 7 and 18 were included in the training set with both the allylic amino substituents (B2/B3) in 18 as the pharmacophoric B group.
Table 5.
Model properties, selected pharmacophore points and comparison of observed and predicted relative efficacies of δ opioid ligands with 1 as the reference molecule.
| Model, R2 = 0.973 | AB-ANB | AB-NBA | BN-NAB | ||
|---|---|---|---|---|---|
| Coefficients | −20.4520 | 5.0703 | 17.0959 | ||
| P-values | 1.43E-06 | 3.49E-07 | 1E-05 | ||
| Biological Activity | OC Values | ||||
| Compound | Experimental | Predicted | AB-ANB | AB-NBA | BN-NAB |
| 2 | 0.08 | 0.04 | 0 | 0.007492 | 0 |
| 3 | 0.36 | 0.29 | 0 | 0.057046 | 0.000019 |
| 4 | 0 | 0.01 | 0 | 0.000996 | 0.000008 |
| 5 | 0.12 | 0.01 | 0 | 0.001019 | 0.000008 |
| 6 | 0.18 | 0.22 | 0.001138 | 0.044116 | 0.001433 |
| 7 | 1.02 | 1.02 | 0.877801 | 0.906859 | 0.840738 |
| 14 | 0.59 | 0.63 | 0.008131 | 0.074764 | 0.024471 |
| 15 | 0.59 | 0.48 | 0.001921 | 0.070327 | 0.009454 |
| 16 | 0.8 | 0.80 | 0.064178 | 0.084515 | 0.098641 |
| 17 | 0.9 | 0.94 | 0.000554 | 0.180618 | 0.002153 |
| 18 | 0 | 0.03 | 0.041485 | 0.039248 | 0.039496 |
Model obtained using multiple regression without a constant. 7 and 18 were included in the training set with the Leu5 (B4) residue in 18 as the pharmacophoric B group.
Figure 4.
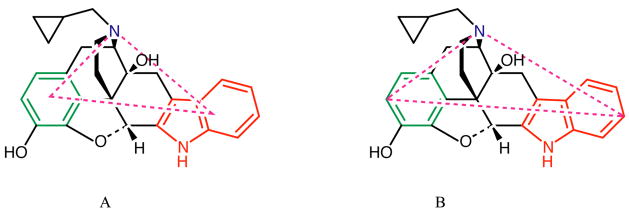
Quantitative conformationally sampled δ opioid pharmacophore (CSP) models. A) Efficacy model based on the MaxD parameter with the Leu5 residue in 17 and the allylic amino substituent in 18 as the pharmacophoric B group (see Table 4 for original data). B) Efficacy model based on the MaxD parameter with the Leu5 residue in 17 and the Leu5 residue in 18 as the pharmacophoric B group (see Table 5 for original data). C) Affinity model for high efficacy δ opioid ligands with the Leu5 residue in 17 as the pharmacophoric B group (see Table 6 for original data). D) Affinity model for low efficacy δ opioid ligands with the allylic amino substituent in 18 as the pharmacophoric B group (see Table 7 for original data). Affinity models developed using the natural logarithms of experimental values.
Determination of a common bioactive conformation of the δ opioid ligands
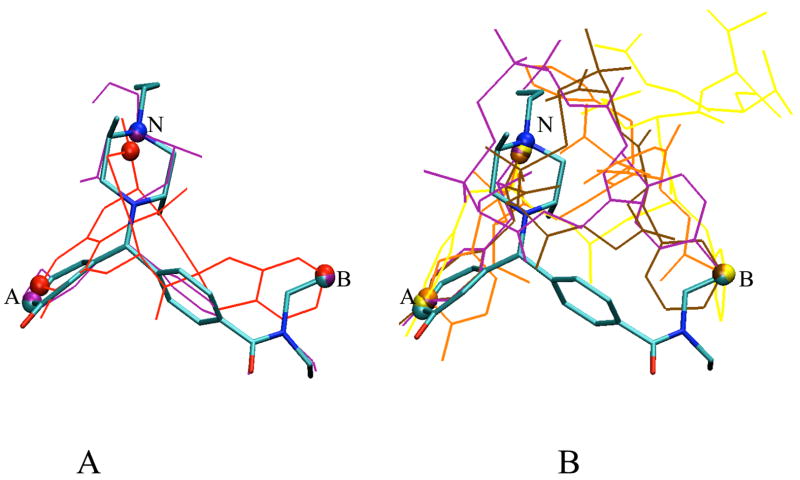
The δ opioid efficacy model includes the OC of the pharmacophoric parameters AB-ANB, AB-NBA and BN-NAB with respect to the reference compound 1. Therefore, utilizing these parameters the regions common to all δ opioid ligands (i.e. regions of common intersection or overlap) in these parameters were identified. As can be seen from the δ opioid efficacy model, Table 5, the non-peptides 6 and 7, and the peptides 14, 15, 16 and 17, have finite OC values for all three parameters and in the determination of a common bioactive conformation, regions common to these ligands as well as 1 were identified. While multiple conformations were obtained for each compound, the superimposition of one selected conformation for each ligand is shown in Figure 5 with 1 as the reference. As can be seen from the superimposition of the conformers for 6 and 7, Figure 5a, the pharmacophore points match very well for 7, as expected, with slight difference in the orientation of the 6 conformer. With regard to the peptidic ligands, Figure 5b, the pharmacophore points superimpose quite well, with the same conformer of 1. Importantly, in the common conformations these pharmacophore points are all exposed to the environment consistent with an essential role in interacting with the receptor. However, there appears to be no significant trend in the orientation of the other residues in these peptides. Thus, the application of the CSP method permits the identification of conformations that satisfy pharmacophoric criteria suggesting probable bioactive forms of the δ opioid ligands. In addition the presence of both peptide and non-peptide conformers that satisfy the same pharmacophoric criterion may indicate similar modes of receptor activation for peptides and non-peptides.
Figure 5.
Superimposition of conformations for A) non-peptidic ligands and B) peptidic ligands identified based on the δ opioid efficacy model. The reference compound 1 is colored based on atom type in bond format. The three atoms defining the pharmacophore points (A, B & N using MaxD criterion) are shown as spheres. Remaining structures as wireframe in the following colors: A) 6 in red and 7 in purple. B) 14 in yellow, 15 in brown, 16 in purple and 17 in orange.
Development of δ opioid ligand affinity model
The utility of the quantitative CSP method in the development of a model describing δ opioid ligand affinities was evaluated by using the experimentally obtained Ki values based on the displacement of radiolabelled 465, which was therefore also used as the reference for this analysis. This evaluation involved the study of the high efficacy δ agonists (1, 3, 7 & 14–18) as one class and the partial agonists (2, 5 & 6) and antagonists (8, 9 & 18) as a second class (Table S1 of supporting information). Efforts to develop a unified affinity model for both high and low efficacy ligands were unsuccessful (data not shown). OC pharmacophoric parameters were calculated with respect to 4 as it has the highest affinity for the δ receptor in the dataset as well as being the ligand used in the displacement assay. All possible definitions of the pharmacophoric B group for 17 and 18 were utilized for the modeling of ligand affinities. Regression analyses were performed using the OC values as the independent variables (Table S.12 and S.13, supporting information) and the log of the Ki values as the dependent variable. Obtained regression models with R2 > 0.9 were then examined for the significance of the determined regression parameters P-value < 0.05.
For the high efficacy δ agonists it was observed that the MaxD parameters based on the pharmacophore groups A and B provide models with good predictability of the ligand affinities. As mentioned above, for 17 the two definitions of the pharmacophoric B group, Phe4 and Leu5, were explicitly evaluated; with Phe4, while satisfactory R2 values were obtained, the binding affinity for 17 was always overestimated and P-values < 0.05 were not obtained, indicating this group to be inappropriate for the prediction of affinity, consistent with the efficacy models presented above. Table 6 and Figure 4C present the optimal model for the prediction of affinities of the high efficacy ligands. The high R2 of 0.987 and low P-values support the validity of the model and all predicted activities are within the reported 95% confidence limits, except for 3, for which the binding affinity is underestimated, and 14, for which it is overestimated. The model based on the MaxD definition includes the OC parameters from the AB-ANB, AB-NBA and NA-NAB terms. Notably, the AB-NBA parameter, for which all ligands have finite OC (Table S.13), and the AB-ANB parameter, which are important for δ opioid ligand efficacy model both contribute to the best affinity model. Clearly, the structural features associated with these terms play essential roles in the interaction of the compounds with the δ opioid receptor.
Table 6.
Model properties, selected pharmacophore points and comparison of observed and predicted affinities of high-efficacy δ opioid agonists with 4 as the reference molecule.
| Model, R2 = 0.987 | Y-intercept | AB-ANB | AB-NBA | NA-NAB | |
|---|---|---|---|---|---|
| Coefficients | 4.0162 | 23.8041 | 18.9059 | −51.6319 | |
| P-values | 0.0005 | 0.0065 | 0.0313 | 0.0016 | |
| Binding Affinity | OC values | ||||
| Compound | Experimental | Predicted | AB-ANB | AB-NBA | NA-NAB |
| 1 | 0.45 (0.38–0.54) | 0.48 | 0 | 0.000996 | 0.092267 |
| 3 | 40(36–44) | 56 | 0 | 0.000001 | 0 |
| 7 | 60(45–80) | 54 | 0 | 0.000165 | 0.000632 |
| 14 | 81(65–99) | 52 | 0.093061 | 0.032653 | 0.056057 |
| 15 | 487(447–531) | 510 | 0.080481 | 0.092059 | 0.02786 |
| 16 | 135(105–174) | 168 | 0.170757 | 0.081941 | 0.087233 |
| 17 | 93(80–109) | 83 | 0.003827 | 0.068911 | 0.019242 |
The Leu5 residue (B2) in 17 was used as the pharmacophoric B group. Regression analysis was performed using the natural log of reported experimental values.
Regression analysis was next performed for the low efficacy δ opioid ligands, with all possible definitions for the hydrophobic moiety 18 used for model development. Using the Phe4 residue as the hydrophobic B group in 18, models with R2 value > 0.9 and P values < 0.05 for the coefficients were obtained with the centroid based parameters and equal weighting of the conformers (Centroid=). However, these models did not give accurate affinity predictions with the predicted values being beyond the 95% reported experimental confidence intervals. With the use of the Leu5 group as the pharmacophoric group, no models with significant correlation were identified. On the other hand with the use of the allylic substituents, B2 or B3 as the B group, models with R2 value > 0.9 were obtained with the MaxD based parameters. All models included the AB-NBA parameter; however on examining the predicted affinities with the experimental values for the models with B2 as the B group, affinities for most of the compounds were outside the 95% confidence intervals for the experimental value. With the B3 group as hydrophobic moiety and equal weighting of conformations (MaxD=), the AB-NAB+AB-NBA+BN-NAB combination yielded a model with R2 value 0.97 with P values < 0.05 for all regression parameters, but in this case affinities for 2 and 18 were outside the experimental range. As with the efficacy prediction, the combined use of both allylic groups in determining the hydrophobic pharmacophore point was evaluated, and only one model was obtained with R2 value > 0.9 and P-values < 0.05 for the regression parameters. This model involved a combination of the AB-NAB, AB-NBA and BN-NAB MaxD= OC values, and gave an R2 value of 1 with P-values well below 0.05 (Table 8). A comparison of the predicted and experimental values (Figure 4D) shows that this model gives very accurate predictions for all the low efficacy δ opioid ligand affinities.
Table 8.
Prediction of efficacy of test compounds using the CSP based δ opioid efficacy model. The reported experimental efficacy values were normalized with respect to the common reference compound 7.
| Biological Activity | ||
|---|---|---|
| Compound | Experimental | Predicted |
| 10a | 1.1 | 2.07 |
| 11a | 0.96 | 1.68 |
| 12b | 0.89 | 0.49 |
| 13c | 0.15 | 0.03 |
: from Wei et al [ 6a and 6b ]
: from Thomas et al [ (−)-23a ]
: from Burkey et al [ TAN-67 ]
The AB-NBA parameter is also seen to be important for the affinity of the low efficacy δ opioid ligands. In addition, the BN-NAB parameter that was important in efficacy prediction is important for the affinity of the low efficacy ligands. From the previously published CSP models used for qualitative analysis of δ opioid agonists and antagonists27, 28 it was observed that 2D combinations of the BN distance with the angle parameters showed the best discrimination, and the inclusion of the BN-NAB parameter in both the efficacy model and the model for the affinities of low efficacy ligand suggest that this parameter may differentiate between the high and low efficacy δ ligands. The importance of the hydrophobic B group in δ opioid ligands is thus observed for both efficacy and affinity models, including its contribution to the AB and BN distances, with the latter consistent with previous conclusions based on the qualitative δ opioid CSP models27, 28.
Prediction of efficacy on test compounds
To more rigorously test the final efficacy model it was used to predict the activity of four test compounds (Figure 1) obtained from literature with reported experimental results68–70. Compounds 10, 11 and 12 were selected as they effective agonists that are structurally similar to the reference compound, 1, while 13 was selected as it has a structure that differs from those included in the training set as well a being a low efficacy ligand. Applying the same simulation protocol as used for the other compounds and defining the pharmacophore groups as shown in Figure 1, the efficacy for each ligand was obtained using the developed δ opioid efficacy model (Table 4), with the resulting predictions along with the experimental data shown in Table 8. As 7 was common to the three studies from which the test compounds were selected as well as part of the present study, experimental values were normalized with respect to 7. Analysis of Table 8 shows the predictions to be consistent with the experimental values. The overall ordering of the activities is in agreement with experiment, though differences in the absolute values exist. The predicted efficacies for compounds 10 and 11 are much higher than the experimental values reported while those for 12 and 13 are underestimated. Compounds 10 and 11, as mentioned have similar structural features as compound 7. However, an important difference is the presence of an alkene bond in these compounds that restricts the conformational flexibility of these molecules particularly with respect to the relative orientation of the pharmacophoric point N. This conformational restraint gives higher overlap values in the BN-NAB parameter resulting in an over estimation of the efficacy. While these results indicate that additional refinement of the model may be achieved via the inclusion of conformationally restricted agonists in the training set, differences in experimental methods may have an impact. For example, the use of membranes in some studies68, 69, as opposed to the transfected C6 glioma cell data used in the present work could contribute to some of the discrepancy in predicted versus experimental value. Compounds 12 and 13 are predicted to be less efficacious in comparison to 7, which is consistent with the experimental data. The predicted efficacy of 0.03 for 13 agrees well with the reported efficacy of 0.1570 with respect to 7. The extent of agreement is similar to 5, which had a predicted efficacy of 0.01 versus an experimental value of 0.12 (Table 4). Thus, the developed CSP efficacy model effectively predicts 13 to be a relatively poor agonist, although it appears that the model is limited in accurately separating low efficacy agonists from full antagonists, as discussed above. Overall, the quality of the predictions, while not ideal, indicates the utility of the developed model as well as pointing towards the path toward improvements in the model.
Conclusions
The CSP approach includes all sampled conformers of a ligand in the development of a pharmacophore thus maximizing the probability of including the receptor bound conformations in the model. The application of this method to the study of δ opioid ligands including both non-peptides and peptides resulted in qualitative pharmacophore models capable of distinguishing δ opioid agonists from antagonists27, 28. The present study extends the application of the CSP method to allow quantitative predictions of ligand efficacies and affinities using regression analyses of overlap coefficients of the 2D pharmacophoric parameters. An important aspect of the CSP method is that it does not require conformational alignment of the molecules, but rather includes all conformers in the analysis and the essential pharmacophoric parameters are verified during refinement. This allows for the development of models that span a diverse range of structures, including both peptidic and non-peptidic ligands. In addition, the approach appears to be able to distinguish substituent effects as both the efficacy and affinity models are able to distinguish 15 from 16, which differ only by the single chlorine atom. Similarly with the test compounds 10 and 11, which differ in just a fluorine substituent, the relative efficacies68 of these ligands are predicted correctly. The application of the model for the prediction of the efficacy of other external compounds 12 and 13, indicated them to be weaker agonist compared to 7, consistent with experimental data69, 70.
Application of the CSP approach to 17 and 18, led to the reevaluation of the identity of the hydrophobic B group essential for δ opioid activity. Traditionally, the Phe4 side chain is considered to be the hydrophobic moiety24. However, with 17 the side chain of the Leu5 residue yielded better models of both efficacy and affinity. Similarly, with 18 it was seen that groups other than the Phe4 side chain could serve as the hydrophobic B group, with the present results indicating that the allylic substituents on the basic nitrogen in this linear peptide to fulfill that role. It is hoped that the present observation will motivate the design of novel ligands to test the hypothesis that alternate hydrophobic groups are substituting for Phe4.
Use of either 1 or 7, both high efficacy ligands, as the reference for calculation of the overlap coefficients indicated the AB-NBA parameter as the primary predictor of δ opioid activity. However, the lower efficacy 6 as the reference compound yielded a model where the NA-NBA parameter had better correlation with δ opioid ligand efficacies. Use of a lower activity compound as the reference required a third parameter to yield a predictive model apparently due to the lower population of active conformations sampled by the reference compound. This is also the probable cause that the OC values based on equal weighting to all conformational points is required for efficacy prediction. These observations show that the success of the quantitative CSP approach is not dependent on the selected reference compound, but the use of high efficacy or high affinity compounds is preferable.
The obtained quantitative models (Figure 4), indicating the importance of the hydrophobic B group are consistent with the qualitative observations from previous studies27, 28, 35, 66, 67. This is based on the contribution of terms including the AB distance in the best efficacy and affinity models and the importance of the BN distance in the affinity model of the low efficacy ligands, suggesting a role of this pharmacophoric parameter in discriminating low and high efficacy ligands. In addition, it is seen that the overall dimensions of the molecules are important since the best predictions of activity and affinity are obtained using the MaxD based calculation of OC. The development of the quantitative efficacy model further enabled the determination of the probable bioactive conformations of the d opioid ligands, where both peptide and non-peptide agonists were found to satisfy identical pharmacophoric requirements suggesting similar binding modes for the two classes of compounds.
While the limited availability of suitable experimental data does not permit further refinement of the models at present, this study indicates the applicability of the CSP approach for pharmacophore development. The present study involves a set of structurally diverse molecules, including both peptides and non-peptides, and does not rely on molecular alignment techniques for determination of pharmacophoric groups. Thus the method does not require the use of a rigid reference compound and even the use of a structurally flexible ligand as the reference (i.e. 1) yields models with high predictability of δ opioid ligand efficacies and affinities. The quantitative CSP approach is therefore suggested to be appropriate for general application in ligand-based drug development methods for structurally diverse ligands.
Supplementary Material
Biological data used for efficacy and affinity modeling. Overlap coefficients for all pharmacophoric parameters for each compound for efficacy and affinity models. Statistical analysis of initial efficacy models. Efficacy models with 7 or 6 as reference compounds, and efficacy models for various B group definitions for 18. This material is available free of charge via the Internet at http://pubs.acs.org.
Table 7.
Model properties, selected pharmacophore points and comparison of observed and predicted affinities of low efficacy δ opioid ligands with 4 as the reference molecule.
| Model, R2 = 1.000 | Y-intercept | AB-NAB | AB-NBA | BN-NAB | |
|---|---|---|---|---|---|
| Coefficients | −33.8161 | −807.593 | 99.81508 | 737.9552 | |
| P-values | 8.08E-05 | 8.58E-05 | 7.37E-05 | 8.78E-05 | |
| Binding Affinity | OC values | ||||
| Compound | Experimental | Predicted | AB-NAB | AB-NBA | BN-NAB |
| 2 | 1.6(1.5–1.8) | 1.5 | 0 | 0.343025 | 0 |
| 5 | 6.5(6.0–7.1) | 6.3 | 0.901773 | 0.910539 | 0.912037 |
| 6 | 8.4(7.3–9.6) | 8.4 | 0.46239 | 0.500997 | 0.486971 |
| 8 | 0.079(0.069–0.086) | 0.079 | 0.091803 | 0.188357 | 0.11738 |
| 9 | 0.037(0.034–0.042) | 0.038 | 0.894354 | 0.908654 | 0.897235 |
| 18 | 37(29–47) | 39 | 0 | 0.375375 | 0 |
Both allylic amino substituents (B2/B3) in 18 were used for the pharmacophoric B group. Regression analysis was performed using the natural log of reported experimental values.
Acknowledgments
The authors wish to acknowledge Dr. Michael Lee and Dr. James Polli for useful discussions. Appreciation to NIDA (DA13583 and DA19634), NIH (DK67530) and the Computer Aided Drug Design Center, School of Pharmacy, University of Maryland, Baltimore is also expressed for financial and computational support.
Footnotes
Abbreviations: OC, overlap coefficient; Centroid, pharmacophore parameters calculated using centroids of groups of atoms; MaxD, pharmacophore parameters calculated using maximum distance between groups of atoms; Centroid=, pharmacophore parameters calculated using centroids of groups of atoms with equal weighting of all confomers; MaxD=, pharmacophore parameters calculated using maximum distance between groups of atoms with equal weighting of all confomers
References
- 1.Martin TJ, Eisenach JC. Pharmacology of Opioid and Nonopioid Analgesics in Chronic Pain States. J Pharmacol Exp Ther. 2001;299:811–817. [PubMed] [Google Scholar]
- 2.Coop A, MacKerell AD., Jr The future of opioid analgesics. Am J Pharm Educ. 2003;66:153–156. [Google Scholar]
- 3.White JM, Irvine RJ. Mechanisms of Fatal Opioid Overdose. Addiction. 1999;94:961–972. [PubMed] [Google Scholar]
- 4.Pappagallo M. Incidence, prevalence and management of opioid bowel dysfunction. Am J Surg. 2001;182:S11–S18. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti S, Wang L, Tang WJ, Gintzler AR. Chronic Morphine Augments Adenylyl Cyclase Phosphorylation: Relevance to Altered Signaling during Tolerance/Dependence. Mol Pharmacol. 1998;54:949–953. doi: 10.1124/mol.54.6.949. [DOI] [PubMed] [Google Scholar]
- 6.Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258(5536):577–80. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 7.Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous Opioid Peptides: Multiple Agonists and Receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 8.Dondio G, Ronzoni S, Petrillo P. Non-peptide δ opioid agonists and antagonists. Exp Opin Ther Patents. 1997;7(10):1075–1098. [Google Scholar]
- 9.Dondio G, Ronzoni S, Petrillo P. Non-peptide δ opioid agonists and antagonists (Part II) Exp Opin Ther Patents. 1999;9(4):353–374. [Google Scholar]
- 10.Coop A, Rice KC. Role of δ-Opioid Receptors in Biological Processes. Drug News Perspect. 2000;13:481–487. [PubMed] [Google Scholar]
- 11.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective Blockage of Delta Opioid Receptors Prevents Development of Tolerance and Dependence in MIce. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 12.Hepburn MJ, Little PJ, Gingras j, Kuhn CM. Differential Effects of Naltrindole on Morphine-Induced Tolerance and Physical Dependence on Rats. J Pharmacol Exp Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- 13.Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TMD, Lemieux C, Chung NN, Coderre TJ. The Opioid μAgonist/δAntagonist DIPP-NH2[ψ] Produces a Potent Analgesic Effect, No Physical Dependence, and Less Tolerance than Morphine in Rats. J Med Chem. 1999;42:3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- 14.Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In Vivo Pharmacological Characterization of SoRI 9409, a Nonpeptidic opioid μ-Agonist/δ-Antagonist That Produces Limited Antinociceptive Tolerance and Attenuates Morphine Physical Dependence. J Pharmacol Exp Ther. 2001;297:597–605. [PubMed] [Google Scholar]
- 15.Satoh M, Minami M. Molecular Pharmacology of the Opioid Receptors. Pharmacol Ther. 1995;68(3):343–364. doi: 10.1016/0163-7258(95)02011-x. [DOI] [PubMed] [Google Scholar]
- 16.Knapp RJ, Malatynska E, Collins N, Fang L, Wang JY, Hruby VJ, Roeske WR, Yamamura HI. Molecular Biology and Pharmacology of Cloned Opioid Receptors. FASEB J. 1995;9:516–525. doi: 10.1096/fasebj.9.7.7737460. [DOI] [PubMed] [Google Scholar]
- 17.Metzger TG, Ferguson DM. On the role of extracellular loops of opioid receptors in conferring ligand selectivity. FEBS Lett. 1995;375:1–4. doi: 10.1016/0014-5793(95)01185-h. [DOI] [PubMed] [Google Scholar]
- 18.Metzger TG, Paterlini MG, Portoghese PS, Ferguson DM. Application of the Message-Address Concept to the Docking of Naltrexone and Selective Naltrexone-Derived Opioid Antagonists into Opioid Receptor Models. Neurochem Res. 1996;21:1287–1294. doi: 10.1007/BF02532369. [DOI] [PubMed] [Google Scholar]
- 19.Alkorta I, Loew GH. A 3D model of the delta opioid receptor and ligand-receptor complexes. Protein Eng. 1996;9(7):573–83. doi: 10.1093/protein/9.7.573. [DOI] [PubMed] [Google Scholar]
- 20.Pogozheva ID, Lomize AL, Mosberg HI. Opioid Receptor Three-Dimensional Structures from Distance Geometry Calculations with Hydrogen Bonding Constraints. Biophys J. 1998;75:612–634. doi: 10.1016/S0006-3495(98)77552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filizola M, Carteni-Farina M, Perez JJ. Molecular modeling study of the differential ligand-receptor interaction at the mu, delta and kappa opioid receptors. J Comput Aided Mol Des. 1999;13(4):397–407. doi: 10.1023/a:1008079823736. [DOI] [PubMed] [Google Scholar]
- 22.Filizola M, Laakkonen L, Loew GH. 3D modeling, ligand binding and activation studies of the cloned mouse delta, mu, and kappa opioid receptors. Protein Eng. 1999;12(11):927–42. doi: 10.1093/protein/12.11.927. [DOI] [PubMed] [Google Scholar]
- 23.Mosberg HI. Complementarity of delta opioid ligand pharmacophore and receptor models. Biopolymers. 1999;51(6):426–439. doi: 10.1002/(SICI)1097-0282(1999)51:6<426::AID-BIP5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Bernard D, Coop A, MacKerell AD., Jr Computer-Aided Drug Design: Structure-Activity Relationships of Delta Opioid Ligands. Drug Design Reviews - Online. 2005;2(4):277–291. [Google Scholar]
- 25.Huang P, Kim S, Loew G. Development of a common 3D pharmacophore for delta-opioid recognition from peptides and non-peptides using a novel computer program. Journal of Computer-Aided Molecular Design. 1997;11(1):21–28. doi: 10.1023/a:1008067209563. [DOI] [PubMed] [Google Scholar]
- 26.Coop A, Jacobson AE. The LMC Opioid Reconition Pharmacophore: Comparison of SNC80 and Oxymorphindole. Bioorganic & Medicinal Chemistry Letters. 1999;9:357–362. doi: 10.1016/s0960-894x(98)00745-8. [DOI] [PubMed] [Google Scholar]
- 27.Bernard D, Coop A, MacKerell AD., Jr 2D conformationally sampled pharmacophore: A ligand-based pharmacophore to differentiate delta opioid agonists from antagonists. J Am Chem Soc. 2003;125(10):3101–3107. doi: 10.1021/ja027644m. [DOI] [PubMed] [Google Scholar]
- 28.Bernard D, Coop A, MacKerell AD., Jr Conformationally Sampled Pharmacophore for Peptidic δ Opioid Ligands. J Med Chem. 2005;48:7773–7780. doi: 10.1021/jm050785p. [DOI] [PubMed] [Google Scholar]
- 29.Wilkes BC, Schiller PW. Theoretical Conformational-Analysis of a Mu-Selective Cyclic Opioid Peptide Analog. Biopolymers. 1987;26(8):1431–1444. doi: 10.1002/bip.360260817. [DOI] [PubMed] [Google Scholar]
- 30.Wilkes BC, Schiller PW. Theoretical Conformational-Analysis of the Opioid Delta-Antagonist H-Tyr-Tic-Phe-Oh and the Mu-Agonist H-Tyr-D-Tic-Phe-Nh2. Biopolymers. 1994;34(9):1213–1219. doi: 10.1002/bip.360340909. [DOI] [PubMed] [Google Scholar]
- 31.Temussi PA, Salvadori S, Amodeo P, Bianchi C, Guerrini R, Tomatis R, Lazarus LH, Picone D, Tancredi T. Selective Opioid Dipeptides. Biochem Biophys Res Commun. 1994;198(3):933–939. doi: 10.1006/bbrc.1994.1133. [DOI] [PubMed] [Google Scholar]
- 32.Lomize AL, Pogozheva ID, Mosberg HI. Development of a model for the delta-opioid receptor pharmacophore.3. Comparison of the cyclic tetrapeptide, Tyr-c[D-Cys-Phe-D-Pen]OH with other conformationally constrained delta-receptor selective ligands. Biopolymers. 1996;38(2):221–234. doi: 10.1002/(SICI)1097-0282(199602)38:2%3C221::AID-BIP8%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Mosberg HI, Lomize AL, Wang CG, Kroona H, Heyl DL, Sobczykkojiro K, Ma WL, Mousigian C, Porreca F. Development of a Model for the Delta-Opioid Receptor Pharmacophore.1. Conformationally Restricted Tyr(1) Replacements in the Cyclic Delta-Receptor Selective Tetrapeptide Tyr-C[D-Cys-Phe-D-Pen]Oh (Jom-13) J Med Chem. 1994;37(25):4371–4383. doi: 10.1021/jm00051a015. [DOI] [PubMed] [Google Scholar]
- 34.Mosberg HI, Omnaas JR, Lomize A, Heyl DL, Nordan I, Mousigian C, Davis P, Porreca F. Development of a Model for the Delta-Opioid Receptor Pharmacophore.2. Conformationally Restricted Phe(3) Replacements in the Cyclic Delta-Receptor Selective Tetrapeptide Tyr-C[D-Cys-Phe-D-Pen]Oh (Jom-13) J Med Chem. 1994;37(25):4384–4391. doi: 10.1021/jm00051a016. [DOI] [PubMed] [Google Scholar]
- 35.Brandt W. A Uniform Molecular Model of Delta Opioid Agonist and Antagonist Pharmacophore Conformations. J Comput Aided Mol Des. 1998;12:615–621. doi: 10.1023/a:1008003421291. [DOI] [PubMed] [Google Scholar]
- 36.Schullery SE, Mohammedshah T, Makhlouf H, Marks EL, Wilenkin BS, Escobar S, Mousigian C, Heyl DL. Binding to delta and mu opioid receptors by deltorphin I/II analogues modified at the Phe(3) and Asp(4)/Glu(4) side chains: a report of 32 new analogues and a QSAR study. Bioorg Med Chem. 1997;5(12):2221–2234. doi: 10.1016/s0968-0896(97)00163-6. [DOI] [PubMed] [Google Scholar]
- 37.Schullery SE, Rodgers DW, Tripathy S, Jayamaha DE, Sanvordekar MD, Renganathan K, Mousigian C, Heyl DL. The role of backbone conformation in deltorphin II binding: A QSAR study of new analogues modified in the 5-, 6-positions of the address domain. Bioorg Med Chem. 2001;9(10):2633–2642. doi: 10.1016/s0968-0896(01)00183-3. [DOI] [PubMed] [Google Scholar]
- 38.Heyl DL, Schullery SE, Renganathan K, Jayamaha MN, Rodgers DW, Traynor JR. pK(a) and volume of residue one influence delta/mu opioid binding: QSAR analysis of tyrosine replacement in a nonselective deltorphin analogue. Bioorganic & Medicinal Chemistry. 2003;11(17):3761–3768. doi: 10.1016/s0968-0896(03)00329-8. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers DW, Renganathan K, Jayamaha MN, Schullery SE, Heyl DL. QSAR analysis of ring-substituted tyrosine replacements at the N-terminus of a nonselective deltorphin analog. Biopolymers. 2003;71(3):372–372. [Google Scholar]
- 40.Peng Y, Keenan SM, Zhang Q, Kholodovych V, Welsh WJ. 3D-QSAR Comparative Molecular Field Analysis on Opioid Receptor Antagonists: Pooling Data from Different Studies. Journal of Medicinal Chemistry. 2005;48:1620–1629. doi: 10.1021/jm049117e. [DOI] [PubMed] [Google Scholar]
- 41.Peng Y, Keenan SM, Zhang Q, Welsh WJ. 3D-QSAR comparative molecular field analysis on delta opioid receptor agonist SNC80 and its analog. J Mol Graph Model. 2005;24:25–33. doi: 10.1016/j.jmgm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 42.McQuarrie DA. Statistical Mechanics. Harper Collinns Publishers; New York: 1976. pp. 452–466. [Google Scholar]
- 43.Nicklaus MC, Wang S, Driscoll JS, Milne GWA. Conformational changes of small molecules binding to proteins. Bioorganic & Medicinal Chemistry. 1995;3(4):411–428. doi: 10.1016/0968-0896(95)00031-b. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert KM, Skawinski WJ, Misra M, Paris KA, Naik NH, Buono RA, Deutsch HM, Venanzi CA. Conformational analysis of methylphenidate: comparison of molecular orbital and molecular mechanics methods. J Comput Aided Mol Des. 2004;18:719–738. doi: 10.1007/s10822-004-7610-1. [DOI] [PubMed] [Google Scholar]
- 45.Misra M, Banerjee A, Dave RN, Venanzi CA. Novel Feature Extraction technique for Fuzzy Relational Clustering of a Flexible Dopamine Reuptake Inhibitor. J Chem Inf Model. 2005;45:610–623. doi: 10.1021/ci049708d. [DOI] [PubMed] [Google Scholar]
- 46.Kane BE, Svensson B, Ferguson DM. Molecular recognition of opioid receptor ligands. Aaps Journal. 2006;8(1):E126–E137. doi: 10.1208/aapsj080115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert KM, Venanzi CA. Hierarchical clustering analysis of flexible GBR 12909 dialkyl piperazine and piperidine analogs. Journal Of Computer-Aided Molecular Design. 2006;20(4):209–225. doi: 10.1007/s10822-006-9046-2. [DOI] [PubMed] [Google Scholar]
- 48.Fiorentino A, Pandit D, Gilbert KM, Misra M, Dios R, Venanzi CA. Singular value decomposition of torsional angles of analogs of the dopamine reuptake inhibitor GBR 12909. Journal Of Computational Chemistry. 2006;27(5):609–620. doi: 10.1002/jcc.20371. [DOI] [PubMed] [Google Scholar]
- 49.Codd EE, Carson JR, Colburn RW, Dax SL, Desai-Krieger D, Martinez RP, McKown LA, Neilson LA, Pitis PM, Stahle PL, Stone DJ, Streeter AJ, Wu WN, Zhang SP. The novel orally active delta opioid RWJ-394674 is biotransformed to the potent Mu opioid RWJ-413216. Journal Of Pharmacology And Experimental Therapeutics. 2006;318(3):1273–1279. doi: 10.1124/jpet.106.104208. [DOI] [PubMed] [Google Scholar]
- 50.Chang C, Swaan PW. Computational approaches to modeling drug transporters. European Journal Of Pharmaceutical Sciences. 2006;27(5):411–424. doi: 10.1016/j.ejps.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 51.von Korff M, Steger M. GPCR-tailored pharmacophore pattern recognition of small molecular ligands. Journal Of Chemical Information And Computer Sciences. 2004;44(3):1137–1147. doi: 10.1021/ci0303013. [DOI] [PubMed] [Google Scholar]
- 52.Dean PM, Lloyd DG, Todorov NP. De novo drug design: Integration of structure-based and ligand-based methods. Current Opinion In Drug Discovery & Development. 2004;7(3):347–353. [PubMed] [Google Scholar]
- 53.Mallik B, Morikis D. Development of a Quasi-Dynamic Pharmacophore Model for Anti-Complement Peptide Analogues. J Am Chem Soc. 2005;127:10967–10976. doi: 10.1021/ja051004c. [DOI] [PubMed] [Google Scholar]
- 54.van Gunsteren WF, Berendsen HJC. Computer Simulation of Molecular Dynamics: Methodology, Applications, and Perspectives in Chemistry. Angew Chem Int Ed Engl. 1990;29:992–1023. [Google Scholar]
- 55.Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 56.MacKerell ADJ, Brooks B, Brooks CL, Nilsson L, Roux B, Won Y, Karplus M. CHARMM: The Energy Function and Its Parameterization with an Overview of the Program. Vol. 1. John Wiley & Sons; Chichester: 1998. pp. 271–277. [Google Scholar]
- 57.SYBYL. Tripos Associates: St. Louis, MO 63144; Tripos Associates: St. Louis, MO 63144. [Google Scholar]
- 58.Halgren TA. The Merck Molecular Force Field: I. Basis, Form, Scope, Parameterization and Performance of MMFF94. J Comput Chem. 1996;17:490–519. [Google Scholar]
- 59.Halgren TA. Merck Molecular Force Field: II. MMFF94 van der Waals and Electrostatic Parmeters for Intermolecuolar Interactions. J Comput Chem. 1996;17:520–552. [Google Scholar]
- 60.Feig M, Karanicolas J, Brooks CL., III MMTSB Tool Set: enhanced sampling and multiscale modeling methods for applications in structural biology. Journal of Molecular Graphics and Modelling. 2004;22(5):377–395. doi: 10.1016/j.jmgm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Allen MP, Tildesley DJ. Computer Simulation of Liquids. Oxford University Press; New York: 1989. [Google Scholar]
- 62.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- 63.Im W, Lee MS, Brooks CL., III Generalized Born Model with a Simple Smoothing Function. J Comp Chem. 2003;24(14):1691–1702. doi: 10.1002/jcc.10321. [DOI] [PubMed] [Google Scholar]
- 64.Im W, Feig M, Brooks CL., III An Implicit Membrane Generalized Born Theory for the Study of Structure, Stability, and Interactions of Membrane Proteins. Biophys J. 2003;85:2900–2918. doi: 10.1016/S0006-3495(03)74712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark MJ, Emmerson PJAM, Akil H, Woods JH, Portoghese PS, Remmers AE, Medzihradsky F. Opioid Efficay in a C6 Glioma Cell Line Stably Expressing the Delta Opioid Receptor. J Pharmacol Exp Ther. 1997;283:501–510. [PubMed] [Google Scholar]
- 66.Portoghese PS, Sultana M, Moe ST, Takemori AE. Synthesis of Naltrexone-Derived δ-Opiod Antagonists. Role of the δ Address Moiety. J Med Chem. 1994;37:579–585. doi: 10.1021/jm00031a006. [DOI] [PubMed] [Google Scholar]
- 67.Schiller PW, Weltrowska G, Berezowska I, Nguyen TMD, Wilkes BC, Lemieux C, Chung NN. The TIPP opioid peptide family: Development of delta antagonists, delta agonists, and mixed mu agonist/delta antagonists. Biopolymers. 1999;51(6):411–425. doi: 10.1002/(SICI)1097-0282(1999)51:6<411::AID-BIP4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 68.Wei ZY, Brown W, Takasaki B, Plobeck N, Delorme D, Zhou F, Yang H, Jones P, Gawell L, Gagnon H, Schmidt R, Yue SY, Walpole C, Payza K, St-Onge S, Labarre M, Godbout C, Jakob A, Butterworth J, Kamassah A, Morin PE, Projean D, Ducharme J, Roberts E. N, N-Diethyl-4-(phenylpiperidin-4-ylidenemethyl)benzamide: A Novel, Exceptionally Selective, Potent Opioid Receptor Agonist with Oral Bioavailability and Its Analogues. J Med Chem. 2000;43(21):3895–3905. doi: 10.1021/jm000229p. [DOI] [PubMed] [Google Scholar]
- 69.Thomas JB, Herault XM, Rothman RB, Atkinson RN, Burgess JP, Mascarella SW, Dersch CM, Xu H, Flippen-Anderson JL, George CF, Carroll FI. Factors Influencing Agonist Potency and Selectivity for the Opioid Receptor Are Revealed in Structure-Activity Relationship Studies of the 4-[(N-Substituted-4-piperidinyl)arylamino]-N, N-diethylbenzamides. J Med Chem. 2001;44(6):972–987. doi: 10.1021/jm000427g. [DOI] [PubMed] [Google Scholar]
- 70.Burkey TH, Ehlert FJ, Hosohata Y, Quock RM, Cowell SM, Hosohata K, Varga E, Stropova D, Li X, Slate C, Nagase H, Porreca F, Hruby VJ, Roeske WR, Yamamura HI. The Efficacy of δ-Opioid Receptor-Selective Drugs. Life Sciences. 1998;62:1531–1536. doi: 10.1016/s0024-3205(98)00102-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Biological data used for efficacy and affinity modeling. Overlap coefficients for all pharmacophoric parameters for each compound for efficacy and affinity models. Statistical analysis of initial efficacy models. Efficacy models with 7 or 6 as reference compounds, and efficacy models for various B group definitions for 18. This material is available free of charge via the Internet at http://pubs.acs.org.