Abstract
Objective
To compare daily cranberry juice cocktail to placebo during pregnancy on asymptomatic bacteriuria (ASB) and symptomatic urinary tract infections (UTIs).
Study Design
188 women were randomized to cranberry or placebo in three treatment arms: A: Cranberry three times daily (n=58), B: Cranberry at breakfast, then placebo at lunch and dinner (n=67), C. Placebo three times daily (n=63). After 27.7% (52/188) of the subjects were enrolled, the dosing regimens were changed to twice daily dosing to improve compliance.
Results
There were 27 UTIs in 18 subjects in this cohort: 6 in 4 subjects in Group A, 10 in 7 subjects in Group B, and 11 in 7 subjects in Group C, p=0.71. There were 57% and 41% reductions in the frequency of ASB and all UTIs in the multiple daily dosing group, however, this study was not sufficiently powered at the alpha 0.05 level (CI 0.14–1.39 and 0.22–1.60, respectively, incidence rate ratios). 73/188 (38.8%) subjects withdrew, most for gastrointestinal upset.
Conclusion
These data suggest there may be a protective effect of cranberry ingestion against ASB and symptomatic UTIs in pregnancy. Further studies are planned to evaluate this effect.
Keywords: Urinary tract infection, asymptomatic bacteriuria, cranberry juice, pregnancy
Introduction
Asymptomatic bacteriuria (ASB) in pregnancy has an estimated prevalence of 5–12%,1–4 and is associated with a variety of adverse perinatal outcomes, including preterm delivery and low birth weight.5–8 A primary goal in the detection and treatment of ASB during pregnancy is the reduction of risk of acute pyelonephritis. Preterm births may be associated with acute pyelonephritis.1,4
There has been little progress in this area over the past several decades. The development of alternative methods for preventing asymptomatic bacteriuria and subsequent pyelonephritis would represent a major advancement in prenatal care.
Cranberry juice and encapsulated powders are commonly used to prevent or treat urinary tract infections (UTIs). Scientific evidence to support the use of cranberry for the prevention/treatment of UTIs is limited by lack of focus on reproductive age women, identification of symptomatic UTIs following daily cranberry juice ingestion, and inadequate assessment of dosing regimens and duration of therapy. Most importantly, there are no data regarding the efficacy of daily cranberry ingestion in pregnancy for the prevention of ASB.9–10
Using a hypothesis that a strategy to prevent the development of ASB in pregnancy could lead to improved pregnancy outcomes by reducing preterm births, we undertook this investigation to provide preliminary data on the effect of daily cranberry juice cocktail ingestion on the frequency of ASB and other UTIs in pregnancy. Outcomes studied included the incidence of ASB, tolerability and side effect profile of daily cranberry juice ingestion, and patient adherence to dosing regimens.
Materials and Methods
Study Population
Eligible pregnant subjects at less than 16 weeks’ gestation presented initially for prenatal care at University of California, Irvine Medical Center or Long Beach Memorial Medical Center. Institutional review board approval was obtained at both institutions. Subjects were excluded for the following: previous underlying medical condition including diabetes mellitus, renal failure, sickle cell disease, chronic hypertension, chronic renal disease; previous or current antimicrobial therapy at the time of screening or within two weeks of screening; and known urologic abnormalities. Each subject also had a pre-treatment urine culture performed to ensure absence of ASB. Written informed consent was obtained.
Study Methods
Women were followed through delivery and the immediate puerperium. Each subject was contacted weekly for six weeks via telephone by a research coordinator, inquiring about compliance, tolerance, and side effects to the daily juice regimen. For those subjects who reported poor compliance or tolerance, we offered more frequent research coordinator contact or clinic follow-up. Follow-up clinic visits occurred concurrently with their monthly prenatal care visits. In addition, subjects maintained dietary diaries, in which they placed labels from the juice bottles they consumed and recorded side effects.
At the follow-up visits, a clean-catch urine specimen was collected for dipstick urinalysis. If positive for leukocyte esterase and nitrites, reflex microscopy and culture and susceptibility were performed. Subjects were queried for symptoms of urinary tract infection or preterm labor.
All subjects were instructed to ingest one 240 mL bottle, containing either cranberry juice or placebo, at each meal (three-times daily) until delivery. We instructed subjects not to consume cranberry products other than those for study. We also educated them about UTIs and hygiene practices to aid in the prevention of UTIs, including adequate fluid intake, frequent voids, and voiding after coitus. They were also educated about the importance of compliance with recommended therapies.
Randomization
Using a computer-generated randomization table, women were randomized to receive active CJC with each meal, CJC at breakfast followed by placebo at lunch and dinner, or placebo at each meal. Randomization was stratified by site. After 52 subjects were enrolled, the dosing frequency was reduced to twice daily because of a high withdrawal rate and poor tolerability of the thrice daily dosing regimen. The main side effects were gastrointestinal. Thus, the protocol was modified to CJC twice daily (breakfast and dinner), CJC at breakfast and placebo at dinner, or placebo twice daily. In addition, we permitted a modification of the cranberry dosing schedules to allow for step-down dosing to once daily for those with moderate to severe gastrointestinal disturbance.
The identities of the treatment assignments were not known to the subjects, research coordinators or investigators, and unblinding did not occur until termination of the investigation.
Study Product
Low calorie CJC beverage containing 27% cranberry juice was supplied by Fisher BioServices Corporation in collaboration with Ocean Spray Cranberries, Inc. (OS). It was formulated to meet research needs under contract with the National Center for Complimentary and Alternative Medicine (NCCAM, NOT-CA-02-014) following competitive award, and although not specifically commercially available, is similar in composition to Ocean Spray low calorie CJC found in retail stores. The CJC was sweetened with sucralose (Splenda™). A Drug Master File for this research grade low calorie CJC is on file with the United States Food and Drug Administration. Berries from Vaccinium macrocarpon Aiton were used. Each dosage consisted of 240 ml CJC with a mean proanthocyanidin concentration of 106 mg per bottle. The CJC was stored under refrigerated conditions.
The placebo beverage was formulated by Ocean Spray Cranberries, Inc. to mimic the flavor (including sugar and acid profile) and color of the cranberry beverage. There were no cranberry ingredients in the placebo beverage. It was bottled in the identical polypropylene bottles used for the active beverage and was also stored under refrigerated conditions.
Outcome Measures
The primary outcome measure was the number of cases of bacteriuria, which was defined as having a urine culture with 100,000 or more of a single uropathogen (measured as colony forming unit [CFU] per mL). ASB was defined as urine cultures consistent with bacteriuria without symptoms. Acute cystitis was diagnosed in subjects with symptoms of dysuria, urinary frequency, and/or urinary urgency and found to have urine cultures consistent with bacteriuria. Acute pyelonephritis was diagnosed in subjects with flank pain, fevers (temperature greater than 100.4°F), chills, nausea and/or vomiting, with urinalyses and/or urine cultures indicative of bacteriuria. We defined a treatment failure as any case of bacteriuria, acute cystitis or acute pyelonephritis. Those women with treatment failures continued drinking the investigational juice through delivery.
We used standardized treatments for ASB, acute cystitis and acute pyelonephritis. Generally, for ASB and acute cystitis, cephalexin 500 mg four times per day for 7 days was prescribed, and for acute pyelonephritis, intravenous cefazolin 1 or 2 g four times per day was given until the subject was two days without fever. Parenteral gentamicin could be added based on clinical response. Subjects with acute pyelonephritis were subsequently treated with oral cephalexin 500 mg four times per day to complete a minimum of 10-days of antibiotic therapy. Any subject with ASB or a symptomatic urinary tract infection continued with juice therapy during treatment. Cultures were repeated within two weeks of treatment completion to assess eradication of bacteria. We anticipated 20 to 30% of women would require a second course of a different antibiotic based on susceptibility testing.
At each monthly visit compliance was assessed using two measures, including the dietary diaries (described above), and a self-reported assessment of percentage compliance with the dosing schedule.
Sample size and data analysis
Because an efficacy trial was not feasible with the available resources and because the data were lacking by which to support a larger trial, we aimed to perform a pilot trial to generate preliminary data for the design of a large-scale clinical trial. Additional outcome measures included effective resolution of ASB with antibiotic treatment, side effects, recurrence rates of ASB, and preterm delivery with its associated neonatal morbidities. Toxicities, side effects, tolerability, and compliance were reviewed by a pre-appointed, data safety monitoring committee at 4 times during the study period: 6,12,18 months, and at study termination.
We used SAS STATA SE, version 10.0 (StataCorp, College Station, TX) for data management and analysis. ANOVA was used for continuous variables. Chi Square or Fisher’s Exact tests were performed for categorical variables. Poisson regression was performed to obtain incidence rate ratios for the number of urinary tract infections. We also compared the time to first diagnosis of ASB using Kaplan-Meier plots and log-rank tests. The data analyses were performed based on intent-to-treat.
Results
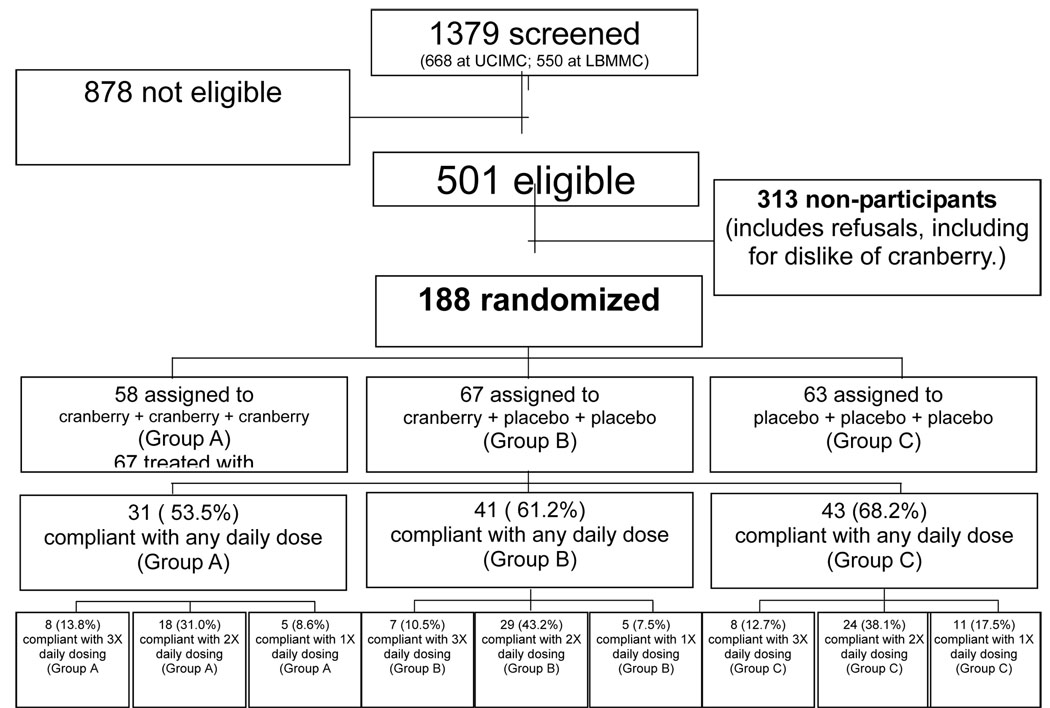
From July 2005 through July 2007, a total of 188 women were enrolled in this pilot investigation (Figure 1). There were no differences in demographic characteristics (Table 1).
Figure 1.
Participant flow chart for cranberry for the prevention of asymptomatic bacteriuria in pregnancy
Table 1.
Demographics by Group
| Group A (C,C,C) (n=58) |
Group B (C,P,P) (n=67) |
Group C (P,P,P) (n=63) |
P* | |
|---|---|---|---|---|
| Maternal Age (yr) | 25.8 ± 5.6 | 27.7 ± 5.4 | 25.6 ± 5.0 | 0.058 |
| Gravida | ||||
| 1 | 16 (27.6%) | 12 (17.9%) | 27 (42.9%) | |
| 2 | 14 (24.1%) | 21 (31.3%) | 15 (23.8%) | |
| ≥ 3 | 28 (48.3%) | 34 (50.8%) | 21 (33.3%) | 0.034 |
| Para | ||||
| 0 | 22 (37.9%) | 18 (26.9%) | 30 (47.6%) | |
| 1 | 17 (29.3%) | 23 (34.3%) | 22 (34.9%) | |
| ≥ 2 | 19 (32.8%) | 26 (38.8%) | 11 (17.5%) | 0.053 |
| Ethnicity† | ||||
| Non-Hispanic White | 11 (18.9%) | 11 (16.4%) | 10 (15.9%) | |
| Hispanic White | 40 (68.9%) | 45 (67.2%) | 47 (74.6%) | |
| Non-Hispanic Black | 3 (5.2%) | 6 (8.9%) | 4 (6.4%) | |
| Asian/Other | 4 (6.9%) | 5 (7.5%) | 2 (3.2%) | 0.89 |
| Site of Enrollment | ||||
| LBMMC | 23 (39.7%) | 28 (41.8%) | 26 (41.3%) | |
| Santa Ana (UCI) | 25 (43.1%) | 24 (35.8%) | 27 (42.9%) | |
| Manchester (UCI) | 10 (17.2%) | 15 (22.4%) | 10 (15.9%) | 0.84 |
| Insurance Status | ||||
| Government | 44 (75.9%) | 53 (79.1%) | 50 (79.4%) | |
| Private/Other | 14 (24.1%) | 14 (20.9%) | 13 (20.6%) | 0.88 |
| Yrs School | ||||
| 0–8 yrs | 8 (13.8%) | 8 (11.9%) | 4 (6.3%) | |
| 9–12 yrs | 33 (56.9%) | 41 (61.2%) | 40 (63.5%) | |
| ≥ 13 yrs | 17 (23.3) | 18 (26.9%) | 19 (13.2%) | 0.72 |
| Employment Status | ||||
| Employed | 25 (41.4%) | 27 (40.3%) | 26 (41.3%) | |
| Not Employed | 34 (58.6%) | 40 (59.7%) | 37 (58.7%) | 0.99 |
| History of Prior UTI | ||||
| Yes | 17 (29.3%) | 18 (26.9%) | 20 (31.8%) | |
| No | 41 (70.7%) | 49 (73.1%) | 43 (68.2%) | 0.83 |
ANOVA for continuous variables, Fisher’s Exact or X2 for categorical variables.
There were no Hispanic Black subjects in the study population.
LBMMC: Long Beach Memorial Medical Center; UTI: Urinary tract infection
There were a total of 27 UTIs in this cohort. There was one case of Enterobacter faecalis cystitis in a woman in Group B, three cases of pyelonephritis due to Escherichia coli (2 in Group A, 1 in Group B), with the remainder of cases attributed to ASB (Table 2). Five women had more than one UTI. There was a trend toward fewer UTIs, both asymptomatic and symptomatic, in those women who received multiple daily doses of CJC compared to those women who received placebo. This trend persisted with single daily dosing of CJC, although the magnitude of the difference was less (Table 3).
Table 2.
Types of urinary tract infections with uropathogens
| Treatment Group | ||||
|---|---|---|---|---|
| Asymptomatic bacteriuria | Total No. of UTIs | Group A (C,C,C) | Group B (C,P,P) | Group C (P,P,P) |
| Escherichia coli | 9 | 1* | 3+,++ | 3 |
| Citrobacter freundii | 5 | 1^ | ||
| Group B Streptococcus | 3 | 1 | 2 | |
| Proteus mirabilis | 3 | 1** | 1 | |
| Other | 3 | 1 | 2 | |
| Total | 23 | |||
| Symptomatic bacteriuria (Cystitis) | ||||
| Enterobacter cloacae | 1 | 1 | ||
| Total | 1 | |||
| Pyelonephritis | ||||
| Escherichia coli | 3 | 2* | 1++ | |
| Total | 3 | |||
| Total subjects with UTIs | 4 (6.9%) |
7 (10.4%) |
7 (11.1%) |
|
Notes: Group A (C,C,C):
One subject with E. coli ASB also developed E. coli pyelonephritis;
One subject with two bouts of Proteus mirabilis ASB. Group B (C,P,P):
One subject with two bouts of E. coli ASB;
One subject with two bouts of E. coli ASB also developed E. coli pyelonephritis. Group C (P,P,P):
One subject with five bouts of Citrobacter freundii ASB.
Table 3.
Incidence rate ratios for number of cases of asymptomatic bacteriuria and urinary tract infections (Intent-to-treat)
| Asymptomatic bacteriuria | All urinary tract infections | |
|---|---|---|
| IRR [95% CI]* | IRR [95% CI]* | |
| Group A (C,C,C) | 0.43 [0.14–1.39] | 0.59 [0.22–1.60] |
| Group B (C,P,P) | 0.85 [0.34–2.08] | 0.85 [0.36–2.01] |
| Group C (P,P,P) | 1.0 | 1.0 |
Poisson.
More women in the once daily dosing group and the placebo group were likely to have at least one UTI during the study compared to the multiple daily dosing group (7/67 [10.4%], 7/63 [11.1%] versus 4/58 [6.9%], respectively, p=0.71, Fisher’s exact test). Similar results were seen in evaluating only those UTIs due to enteric bacteria (5/67 [7.5%], 5/63 [7.9%], and 3/58 [5.2%], respectively, p=0.83), and the trend toward reduction in ASB alone for multiple daily cranberry juice cocktail dosing (incidence rate ratio 0.43 [95% CI 0.14–1.39], as well as for single daily cranberry juice cocktail dosing (incidence rate ratio 0.85 [95% CI 0.34–2.08] persisted.
Compliance and tolerability were considerable obstacles during this investigation. Actual dosing regimens, despite the change from thrice to twice daily dosing did not differ between groups with 50.7% (34/67) of Group A,39.7% (23/58) of Group B, and 55.5% (35/63) of Group C consuming placebo juice once or twice daily, p=0.45. Compliance rates differed between groups: 65.7±30.9% in Group A, 78.7±29.2% in Group B, and 76.9±24.9% in Group C of total doses prescribed for duration of participation were consumed, p=0.03. 73/188 (38.8%) subjects could not complete the study and withdrew, most for gastrointestinal upset including nausea, vomiting, diarrhea and dislike of taste (44 of 73). There were fewer withdrawals after the dose change was made (50/136, 36.8%) versus before (23/52, 44.2%, p=0.35).
Evaluating the cohort on an intent-to-treat basis, the median number of days in study was 152.5 [IQR 56–183] for Group A, 158 [IQR 61–181] for Group B, and 171 [IQR 76–185] for Group C, p=0.26. For those who completed the study protocol, the median number of days in study was 183 [IQR 161–195] for Group A (n=41), 177 [IQR 165–185] for Group B (n=31), and 182 [IQR 169–192] for Group C (n=43), p=0.29. For those who withdrew, 56 [IQR 21–77] for Group A (n=27), 56 [IQR 30–90] for Group B (n=26), and 55.5 [IQR 28.5–80] for Group C (n=20), p=0.85.
There were no differences between the groups with regards to obstetrical or neonatal outcomes (Table 4). No preterm deliveries less than 34 weeks’ occurred in women with UTIs during this investigation.
Table 4.
Obstetrical and Neonatal Outcomes
| Group A (C,C,C) (N=58) |
Group B (C,P,P) (N=67) |
Group C (P,P,P) (N=63) |
P* | |
|---|---|---|---|---|
| Gestational age at delivery | 38.7 ± 3.0 | 38.2 ± 3.6 | 38.8 ± 2.5 | |
| (wk) | (n=55) | (n=58) | (n=57) | 0.027 |
| Preterm delivery < 37 weeks | ||||
| Yes | 6 (10.9%) | 11 (19.0%) | 4 (7.0%) | |
| No | 49 (89.1%) | 47 (81.0%) | 53 (93.0%) | |
| (n=55) | (n=58) | (n=57) | 0.15 | |
| Preterm delivery < 34 weeks | ||||
| Yes | 2 (3.6%) | 4 (6.9%) | 2 (3.5%) | |
| No | 53 (96.4%) | 54 (93.1%) | 55 (96.5%) | |
| (n=55) | (n=58) | (n=57) | 0.73 | |
| Route of Delivery | ||||
| Spontaneous vaginal delivery | 36 (66.7%) | 41 (70.7%) | 40 (70.2%) | |
| Instrumented vaginal delivery | 5 (9.3%) | 4 (6.9%) | 2 (3.5%) | |
| Cesarean/Cesarean hysterectomy | 13 (24.0%) | 13 (22.4%) | 15 (26.3%) | |
| (n=54) | (n=58) | (n=57) | 0.80 | |
| Birth weight (gm) | 3270 ± 522 | 3296 ± 591 | 3423 ± 644 | 0.31 |
| Low birth weight | ||||
| Yes | 4 (6.9%) | 4 (6.0%) | 2 (3.2%) | |
| No | 54 (93.1%) | 63 (94.0%) | 61 (96.3%) | 0.72 |
| 1 min Apgar <7 | ||||
| Yes | 3 (5.5%) | 5 (8.8%) | 2 (3.5%) | |
| No | 50 (94.5%) | 52 (91.2%) | 55 (96.5%) | |
| (n=53) | (n=57) | (n=57) | 0.52 | |
| 5 min Apgar <9 | ||||
| Yes | 4 (7.5%) | 5 (8.8%) | 5 (8.8%) | |
| No | 49 (92.5%) | 52 (91.2%) | 52 (91.2%) | |
| (n=53) | (n=57) | (n=57) | 1.0 | |
| Admission to NICU | ||||
| Yes | 3 (5.7%) | 7 (11.9%) | 6 (10.5%) | |
| No | 50 (94.3%) | 52 (88.1%) | 48 (89.5%) | |
| (n=53) | (n=59) | (n=57) | 0.51 | |
ANOVA for continuous variables, Fisher’s Exact or X2 for categorical variables.
Discussion
Our investigation is provides support for a unique approach toward the reduction of asymptomatic bacteriuria in pregnancy and its associated adverse perinatal outcomes. The standard of obstetrical care remains screening for asymptomatic bacteriuria and treatment if the diagnosis is made.12 However, the current recommendations for screening may not apply to those women with poor compliance or late entry to prenatal care, and do not address issues related to provider error or optimal post-treatment surveillance. The option to combine routine urinary screening with a non-harmful foodstuff, cranberry, to reduce the risk of gestational bacteriuria and its attendant potential complications is attractive for both public health and cost considerations, especially when factoring in the expense of caring for a premature newborn and subsequent costs related to lifelong disability that often affects survivors of premature birth.
The mechanism by which cranberry may prevent urinary tract infection is unknown, although there is a growing body of evidence that proanthocyanidins, or condensed tannins, components in many berry products, inhibit the adhesion of piliated enteric bacteria such as E.coli, to the uroepithelium.13,14
In the 2001 Cochrane Library review of cranberry for the prevention and treatment of UTI, the authors felt that there was preliminary evidence supporting its efficacy but that the published trials suffered from major limitations including lack of control groups, small sample sizes, lack of controlled diets or dietary assessment, inappropriate analysis of data for drop-outs or withdrawals, and lack of blinding.9 Importantly, this Cochrane review noted that there were no investigations of the role of cranberry in preventing UTI in young patients or in pregnant women. This review included only five trials in which description of the product was lacking in all. Outcome measures were also varied, with some researchers focusing on bacteriuria and pyuria, and others on symptomatic UTIs. Largely, the appropriate product, dose, duration of intervention, and mechanism(s) of action were unknown or not clearly elucidated. The same authors of the Cochrane review published updates in 200710,11 and concluded that upon meta-analysis of four high-quality randomized controlled trials,15–18 cranberry products significantly reduced the incidence of symptomatic UTIs in 12 months (RR 0.66, 95% CI 0.47–0.92) compared with placebo or control, particularly in women with recurrent UTIs. These studies involve primarily women15,16, subjects with spinal cord injury,17 or the elderly.18 To date, no investigations into the effect of cranberry on urinary tract infection in pregnant women have been published.
Similar to our own clinical trial, withdrawals or losses to follow-up are significant in other published studies, as high as 47% or more.10,18,19 This has led some to suggest that drinking considerable amounts of cranberry juice over a long period such as the duration of pregnancy, may not be acceptable.10 The possibility exists, however, that different cranberry formulations such as capsules or tablets will have better efficacy and improved compliance. Stothers reported year-long compliance rates of 70 to 100% with cranberry capsules and lesser rates with cranberry juice in a trial evaluating the clinical and cost effectiveness of cranberry for uroprotection.15 The nausea and vomiting of pregnancy certainly was a poor prognosticator for compliance during this investigation, and any gastrointestinal symptoms related to intolerability to the juice or placebo may have exacerbated or been exacerbated by physiologic changes in pregnancy. We altered the treatment regimens after approximately one-third of the subjects were randomized, due to concerns about compliance. There was a mild improvement in retention of compliant subjects in the investigation after this alteration was made. As there are in vitro data to suggest that the antiadhesion activity of cranberry juice on fimbriated E. coli persists for ten hours after ingestion, twice daily dosing of cranberry juice may be adequate for prevention of UTIs in pregnancy.20
We acknowledge the limitations of this investigation including its small sample size and the lack of bioassay for compliance. As expected, compliance and tolerability to the cranberry juice product were limitations, and resulted in over 30% drop-out rate. As a result of these difficulties, a change in the dosing regimens was required in the midst of the study. A minor weakness is that the provision of additional follow-up for those subjects with poor tolerance or compliance to the juice could have introduced bias in their ultimate clinical outcomes. Conclusive information about the benefits of daily cranberry juice ingestion can only be gleaned from larger clinical trials in which consistent treatments are applied. Additional evidence will soon be available from other NCCAM-supported clinical trials in which the same cranberry juice and placebo products were used.
Acknowledgments
Financial disclosure: This investigation was supported by the National Institute of Diabetes, and Digestive and Kidney Diseases (NIDDK) R21DK65827-01, and the National Center for Complementary and Alternative Medicine (NCCAM) NOT-CA-02-014. Clinical Trials Registration NCT00093938.
Abbreviation Key
- ANOVA
Analysis of variance
- ASB
Asymptomatic bacteriuria
- CFU
Colony forming unit
- CJC
Cranberry juice cocktail
- E.coli
Escherichia coli
- IQR
Interquartile range
- NCCAM
National Center for Complementary and Alternative Medicine
- NIDDK
National Institute of Diabetes, Digestive and Kidney Diseases
- UTI
Urinary Tract Infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation: Multiple, eight-ounce doses of cranberry juice cocktail, administered daily, appear to reduce the frequency of asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy.
References
- 1.Duff P. Pyelonephritis in pregnancy. Clin Obstet Gynecol. 1984;27:17. doi: 10.1097/00003081-198403000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Stenqvist K, Dahlen-Nilsson I, Lidin-Janson G, Lincoln K, Oden A, Rignell S, et al. Bacteriuria in pregnancy: Frequency and risk of acquisition. Am J Epidemiol. 1989;129:372. doi: 10.1093/oxfordjournals.aje.a115140. [DOI] [PubMed] [Google Scholar]
- 3.Patterson TF, Andriole VT. Detection, significance, and therapy of bacteriuria in pregnancy. Update in the managed health care era. Infect Dis Clin North Am. 1997;11:593. doi: 10.1016/s0891-5520(05)70375-5. [DOI] [PubMed] [Google Scholar]
- 4.Gilstrap LC, 3rd, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581. doi: 10.1016/s0889-8545(05)70219-9. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Oyarsun E, Mazor M, Sitori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol. 1989;73:576. [PubMed] [Google Scholar]
- 6.Kincaid-Smith P, Buller M. Bacteriuria in pregnancy. Lancet. 1965;1:395. doi: 10.1016/s0140-6736(65)90001-2. [DOI] [PubMed] [Google Scholar]
- 7.McGrady GA, Daling JR, Peterson DR. Maternal urinary tract infection and adverse fetal outcomes. Am J Epidemiol. 1985;121:377. doi: 10.1093/oxfordjournals.aje.a114009. [DOI] [PubMed] [Google Scholar]
- 8.Kass EH. Bacteriuria and pyelonephritis of pregnancy. Arch Intern Med. 1960;105:194. doi: 10.1001/archinte.1960.00270140016003. [DOI] [PubMed] [Google Scholar]
- 9.Jepson RG, Mihaljevic L, Craig J. Cranberries for preventing urinary tract infections. The Cochrane Database of Syst Rev. Cochrane. 2001;(3) doi: 10.1002/14651858.CD001321. CD001321. [DOI] [PubMed] [Google Scholar]
- 10.Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. 2007;51:738. doi: 10.1002/mnfr.200600275. [DOI] [PubMed] [Google Scholar]
- 11.Jepson RG, Craig J. Cranberries for the preventing urinary tract infection. The Cochrane Database of Syst Rev. Cochrane. 2008 Jan 23;(1) doi: 10.1002/14651858.CD001321.pub4. CD001321. [DOI] [PubMed] [Google Scholar]
- 12.US Preventive Services Task Force. Screening for asymptomatic bacteriuria. [Accessed March 2008];Guide to clinical preventive servies. (2nd edition). 1996 http://www.ahcpr.gov/clinic/uspstfix.htm.
- 13.Foo L, Lu Y, Howell AB, Vorsa N. A-type proanthrocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prods. 2000;63:1225. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 14.Gupta K, Chou MY, Howell A, Wobbe C, Grady R, Stapleton AE. Cranberry products inhibit adherence of P-fimbriated Escherichia Coli to primary cultured bladder and vaginal epithelial cells. J Urol. 2007;177:2357. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558. [PubMed] [Google Scholar]
- 16.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Kaskela M, Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spin Cord Med. 2004;27:35. doi: 10.1080/10790268.2004.11753728. [DOI] [PubMed] [Google Scholar]
- 18.McMurdo ME, Bissett LY, Price RJ, Phillips G, Crombie IK. Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing. 2005;34:256. doi: 10.1093/ageing/afi101. [DOI] [PubMed] [Google Scholar]
- 19.Foda MM, Middlebrook PF, Garfield CT, Potvin G, Wells G, Schilling JF. Efficacy of cranberry in prevention of urinary tract infections in a susceptible pediatric population. Can J Urol. 1995;2:98. [PubMed] [Google Scholar]
- 20.Howell AB, Foxman B. Cranberry juice ingestion and adhesion of antibiotic-resistant uropathogens. JAMA. 2002;23:187. doi: 10.1001/jama.287.23.3082. [DOI] [PubMed] [Google Scholar]