SUMMARY
The protozoan parasite Giardia lamblia utilizes arginine deiminase (gADI) to produce energy from free L-arginine under anaerobic conditions. In this work, we demonstrate that in addition to its known role as a metabolic enzyme, it also functions as a pepidtyl-arginine deiminase converting protein-bound arginine into citrulline. gADI specifically binds to and citrullinates the arginine in the conserved CRGKA tail of variant-specific surface proteins (VSPs) affecting both antigenic switching and antibody mediated cell death. During encystation gADI translocates from the cytoplasm to the nucleus and appear to play a regulatory role in the expression of encystation specific genes. gADI is also sumoylated, which may modulate its activity. Our findings reveal a dual role played by gADI and define novel regulatory pathways used by Giardia for survival.
Keywords: arginine deiminase, citrullination, sumoylation, antigenic variation, encystation, gene regulation
INTRODUCTION
Giardia lamblia is an ubiquitous unicellular parasite of humans and other vertebrates that commonly causes diarrhea and gastrointestinal upset (Adam, 2001). Giardia trophozoites undergo fundamental biological changes in response to adverse environmental conditions. To survive outside the host's intestine, Giardia trophozoites differentiate into infective cysts, which are then released with the feces and are responsible for the transmission of the disease among susceptible hosts. Another mechanism of adaptation is antigenic variation (a process by which the parasite continuously switches its major surface molecules), allowing the parasite to evade the host's immune response during trophozoite colonization of the gut (Nash, 2002). Only one variant-specific surface protein (VSP), from a pool of approximately 250 genes present in the parasite's genome, is expressed on the surface of Giardia trophozoites at any point in time (Morrison et al., 2007). These antigens are membrane proteins with common characteristics, such as their variable N-terminal extracellular region, a well-conserved carboxyl terminus that consists of a hydrophobic intramembranous domain that anchors the VSPs to the parasite surface, as well as a perfectly conserved 5 amino acid (CRGKA) C-terminal tail located in the cytoplasm (Mowatt et al., 1991; Mowatt et al., 1994). However, the biological purpose of the conserved elements in VSPs and their role in antigenic variation are still unknown.
The function of the CRGKA tail of VSPs is unclear but recent evidence indicates that it is important in VSP biology. It has been previously shown that many VSPs are palmitoylated (Hiltpold et al., 2000; Papanastasiou et al., 1997b), and we recently established that palmitic acid binds specifically to the Cys of the CRGKA tail of the VSPs, thus controlling the segregation of these proteins to detergent-resistant domains at the plasma membrane (Touz et al., 2005). Consequently, VSP mutants unable to bind palmitic acid showed abnormal membrane segregation and avoided VSP-specific antibody cytotoxicity, performing an essential function in the control of VSP-mediated signaling and processing (Touz et al., 2005). These results, taken together, prompted us to investigate whether the CRGKA cytoplasmic tail contains other post-translational modifications or interacting proteins involved in the control of signaling.
In this work, we found that Giardia arginine deiminase (gADI) bound specifically to the CRGKA sequence. Prior studies in Giardia lamblia determined that gADI catalyzes the irreversible catabolism of arginine to citrulline in the arginine dehydrolase pathway (ADH) (Schofield et al., 1990) and serves as an important source of energy (Schofield et al., 1990). This pathway has been regarded as being restricted to prokaryotic organisms. On the other hand, higher eukaryotes utilize nitric oxide synthase (NOS) to convert free L-arginine into citrulline and nitric oxide (NO), and use peptidyl-arginine deiminases (PADs) to deiminate protein-bound arginine and convert it to citrulline (citrullination) by a Ca2+ dependent way. This post-translational modification has a major impact on the structure and function of the target protein (Vossenaar et al., 2003). We discovered that the function of arginine deiminase in Giardia goes beyond energy production since it also functions as a peptidyl-arginine deiminase. We present evidence indicating that gADI plays an essential role in the control of antigenic variation via VSPs citrullination and influences the process of encystation. Our findings on the participation of gADI in unusual post-translational modification mechanisms during Giardia survival are discussed.
RESULTS
Giardia arginine deiminase interacts with VSPs
Almost all VSP genes encode the type I integral membrane proteins that have a five-amino acid cytoplasmic tail, CRGKA. Because this cytoplasmic sequence is highly conserved, we examined the possibility of CRGKA interaction with regulatory proteins. Pull-down assays using the synthesized peptide NH2-HHHHHHCRGKA-COO− (His6-CRGKA) and further LC-MS/MS analysis showed an associated protein corresponding to the enzyme arginine deiminase (GGD 112103) in both GS/H7 and WB/1267 clones (Fig. 1A). The 85 kDa band corresponds to the modified whole protein (see below), and the ∼13 kDa band to the protein C-terminus, respectively (Fig. 1A). In addition, using the yeast two-hybrid system we determined that the entire VSPH7 (H7-BD) and VSP1267 (1267-BD), but not VSP1267 lacking the cytoplasmic tail CRGKA (1267– tail-BD), interacted with gADI (gADI-AD) thus validating the VSP/gADI interaction found by the peptide pull-down assays (Fig. 1B).
Figure 1. Arginine deiminase (gADI) is associated with variant-specific surface proteins (VSPs) thorough their cytoplasmic tail.
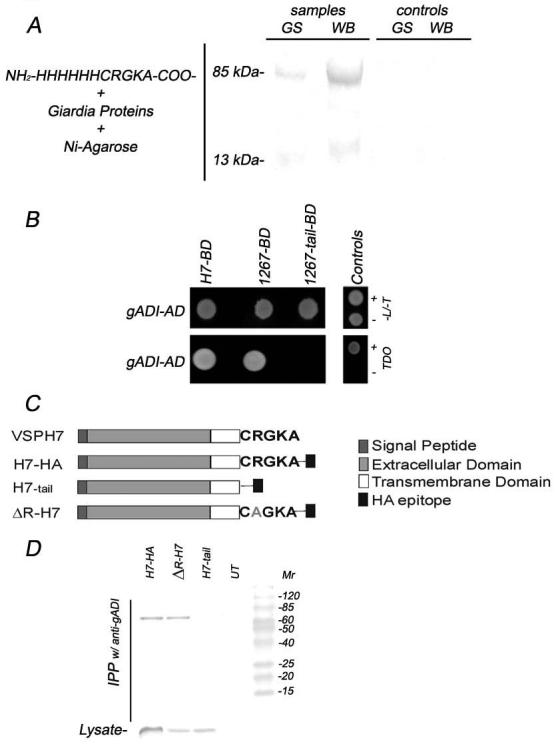
(A) Schematic representation of the pull-down assays (left). SDS-PAGE and Coomassie staining show an ∼85 kDa and an ∼13 kDa protein from both GS/H7 (GS) and WB/1267 (WB) isolates. Identification of these bands as gADI was performed by LC/MS-MS. Controls without peptide fail to pull down any protein (right).
(B) Two-hybrid interactions were detected by the ability of yeast cells (AH109) to grow on selective plates (TDO). In the upper left panel, the expression of VSPH7-BD, VSP1267-BD, 1267-tail-BD (VSP1267 lacking its cytoplasmic tail), and gADI-AD is revealed by the presence of white colonies in the –L/-T medium. Interaction of gADI-AD with both VSPH7-BD and VSP1267-BD is shown in the bottom left panel by the growth of yeast colonies in TDO plates. No interaction between gADI and 1267-tail-BD is observed. Controls of the methodology include ESCP-BD/gMuA-AD interaction (+) and emptyBD/gADI-AD vector (−). –L/-T: SD yeast medium lacking Leu and Trp. TDO stands for Triple Dropout Medium: SD/–His/–Leu/–Trp.
(C) Schematic representation of VSPH7 and transgenic VSPH7 proteins. VSPH7 ORF contains a signal peptide, an extracellular domain, a transmembrane domain (TM), and a cytoplasmic tail. H7-HA has a HA epitope sequence at the C-terminus, and the H7-tail possesses the HA epitope right after H7's TM. ΔR-H7 is VSPH7 containing a point mutation of the R residue of its cytoplasmic tail.
(D) H7-HA and ΔR-H7, but not H7-tail, co-immunoprecipitates with gADI. Antibody against gADI was used to immunoprecipitate comparable amounts of protein from WB transgenic cells. Western blotting of original lysate was stained with anti-HA mAb labeled with alkaline phosphatase (Lysate, bottom panel). UT stands for untransfected cells. Mr is molecular mass expressed in kDa.
To confirm the interaction of gADI with the tail of VSPs, we have created WB transgenic trophozoites that stably express different VSPH7 versions (Fig. 1C) and performed immunoprecipitation assays (IPP). VSPH7 is not genetically codified nor expressed in the Giardia clone WB/1267, thereby allowing detection of the extracellular portion of VSPH7 using VSPH7-specific mAb G10/4 or the C-terminus by using anti-HA mAb, respectively. Our results showed that H7-HA and ΔR-H7, but not H7-tail, co-immunoprecipitates with gADI pAb (Fig. 1D). This suggests that the whole tail of the VSPs rather than one amino acid is necessary for gADI-VSP interaction as expected from an enzyme/substrate association.
gADI acts as a peptidyl-arginine deiminase
Using a C-terminus HA-tagged ADI and IFA analysis, we found that the subcellular localization of gADI-HA is cytoplasmic (Fig. 2A, ADI-HA). We next examined whether ADI localizes close to the VSPs' tail. The confocal imaging showed that gADI partially colocalizes with the VSP9B10 (VSP9B10) close to the plasma membrane (Fig. 2, Merge in top panel). Similar results were found when anti-gADI pAb, raised against recombinant gADI protein (Ringqvist et al., 2008) was used, thereby confirming the cytoplasmic localization as well as the partial colocalization of gADI with VSP9B10 (Fig. 2A, bottom panels).
Figure 2. VSP citrullination is likely mediated by the PAD activity of gADI.
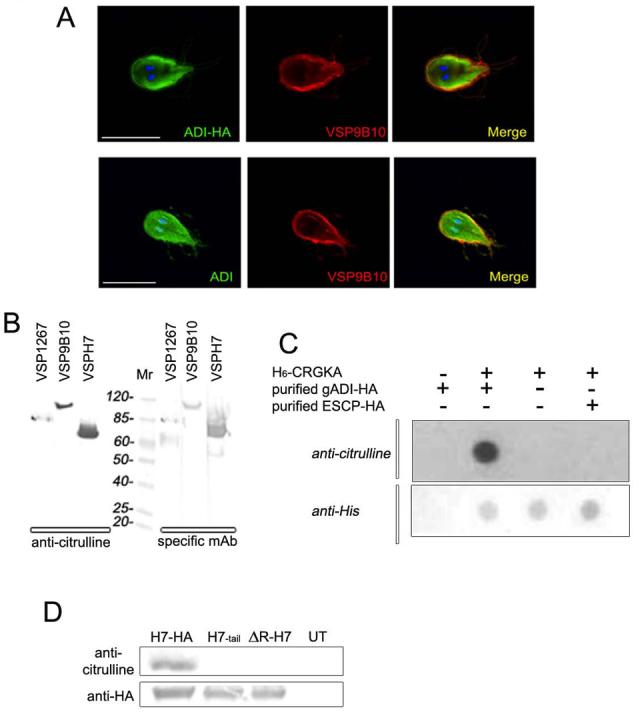
(A) Confocal direct IFA was performed on permeabilized cells, showing a cytoplasmic distribution of gADI-HA (green) by using FITC-labeled anti-HA mAb, and its partial colocalization (yellow in merge) with VSP9B10 (red) underneath the plasma membrane of the transgenic trophozoite (top panels). The same result was obtained by using Alexa 488-anti-gADI in wild-type cells (bottom panels). Texas Red-9B10 mAb was used to visualize VSP9B10. Nuclei are stained with DAPI (blue). Scale bar represents 10 μm.
(B) Western blotting of Giardia homogenates expressing different VSPs reacted with anti-citrulline pAb (left pannel). The same filter membranes were stripped, cut, and reacted with 5C1, 9B10, and G10/4 mAbs against VSP1267, VSP9B10, and VSPH7, respectively (right panel), indicating that these VSPs are citrullinated. Mr is molecular mass expressed in kDa.
(C) Dot-blotting to detect citrullination of the H6-CRGKA peptide after incubation with the purified recombinant ADI-HA. A non-related purified enzyme ESCP-HA was used as a negative control. Dot-blotting to detect H6-CRGKA was performed by using anti-H6mAb.
(D) Specific citrullination of the CRGKA tail is shown (top panel). Western blotting using anti-citrulline pAb performed after immunoprecipitation with anti-HA mAb of H7-transgenic trophozoites. The presence of H7-HA, H7-tail, and ΔR-H7 after immunoprecipitation was analyzed by using anti-HA mAb labeled with alkaline phosphatase (bottom panel).
Two observations suggested that gADI possesses peptidyl-arginine deiminase activity. First, by sequence analysis and 3D structure prediction, we found that gADI contains a “Cys-His-Glu” active site similar to the PADs (data not shown). Second, we discovered that it binds to the cytoplasmic tail of VSPs, a finding not expected of an exclusively energy pathway enzyme. To determine whether VSPs are citrullinated, Western blotting using extracts from cloned trophozoites expressing VSP1267, VSP9B10 (both from isolate WB), or VSPH7 (from isolate GS) was performed. Figure 2B showed that only the full-length VSP1267, VSP9B10, and VSPH7 is citrullinated. It was previously shown that VSPs are processed by deletion of the conserved C-terminus (Papanastasiou et al., 1997a; Touz et al., 2005). This event explains why the processed N-terminus (lower band of VSP1267 and VSPH7) that is also recognized by their specific mAbs, is not citrullinated.
To demonstrate that gADI possesses peptidyl-arginine deiminase activity, we mixed active gADI-HA, purified from transgenic Giardia trophozoites, and His6-CRGKA. After His6-CRGKA purification and Western blotting, we were able to demonstrate that gADI, but not a non-related enzyme, citrullinates the His6-CRGKA peptide (Fig. 2C).
The requirements for peptidyl-arginine deiminase activity of gADI to citrullinate the VSP tail were determined by analysis of transfected VSPH7 mutants for the presence of citrulline. Citrullination was found in H7-HA, but not in H7-tail or ΔR-H7, indicating that citrullination could not occur without the presence of the critical arginine in the CRGKA tail (Fig. 2D). As shown in the bottom panel of figure 2D, equal amounts of transgenic VSPs were detected after IPP.
Giardia arginine deiminase participates in the control of antigenic variation
Antibodies directed against VSPs either induce antigenic variation or inhibit grow or kill recognized trophozoites, allowing the repopulation of trophozoites expressing other VSPs. In vitro, results depends either on the concentration of the antibodies added to Giardia cultures or the exposure time. It has been observed that high concentration of the antibodies cause immediate immobilization as well as detachment and aggregation of the trophozoites. On the other hand, it was reported that when low concentration of anti-VSP antibodies is added to the culture or during a short time, no cytotoxicity is observed, but a rapid accumulation of parasites expressing VSPs that are different to the original expressed one arise (Aggarwal et al., 1989; Stager et al., 1997; Touz et al., 2005).
To determine whether citrullination of VSPs abrogates antibody mediated cytotoxicity, we utilized specific VSPH7 Abs and WB/1267 transfected trophozoites which expressed ∼100% VSPH7 mutants (Fig. 1C). Controls for cytotoxicity effects included a GS/H7 clone expressing ∼100% VSPH7 (+ control) and wild-type WB/1267 expressing VSP1267 and a non-specific antibody (8G8 mAb) (− controls). An equal surface expression of VSPH7-HA and ΔR-H7 was controlled by selecting trophozoites with the same level of surface fluorescence using a fluorescence-activated cell sorter (FACS) and anti-VSPH7 antibodies (data not shown). Figure 3A, showed that after addition of anti-VSPH7 monoclonal (G10/4) or polyclonal Abs (anti-GS/VSP pAb), immobilization, aggregation, detachment and complement-independent cytotoxicity was observed for the native GS/H7 and H7-HA transgenic trophozoites. These demonstrated cell survival rates lower than 5% after 24 hours. On the other hand, recovery of the non-citrullinated ΔR-H7 mutant expressing trophozoites exposed to the anti-VSPH7 Abs showed no cytotoxicity after 24 hours compared to controls. As expected, WB/1267 trophozoites exposed to anti-VSPH7 Abs and the samples exposed to 8G8 mAb showed no cytotoxicity (Fig. 3A).
Figure 3. gADI participates in the control of cell death, probably by altering VSP switching.
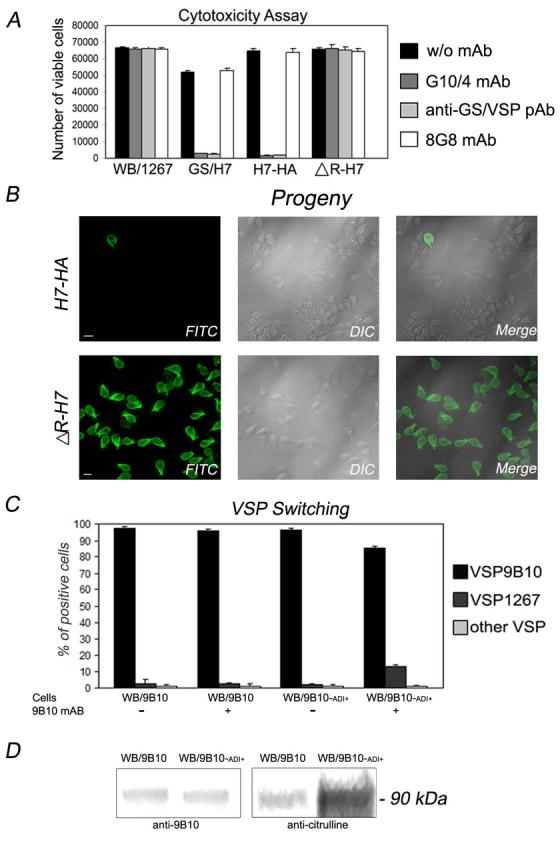
(A) Cytotoxicity assays of Giardia trophozoites WB/1267, GS/H7, and WB/1267 transfected with H7-HA or ΔH7 (X axis), is analyzed after 24 hours post-addition of anti-VSPH7 specific antibodies G10-4 and anti-GS-VSPs pAb by estimating the number of adherent viable parasites. Controls include cells without treatment (w/o mAb) and the use of a non-related mAb (8G8 mAb). Data represent the means ± s.d. for n=2 of three independent experiments.
(B) Progenies were analyzed by addition of goat anti-mouse FITC-conjugated antibody in IFA. Positive cells correspond to trophozoites expressing VSPH7. Nuclei are stained with DAPI (blue). DIC: Differential interference contrast. Scale bar represents 10 μm.
(C) VSP expression is established in WB/9B10 wild-type and WB/9B10-ADI+ transgenic trophozoites after a short time exposure to specific VSP9B10 mAb. Controls include no exposure to the mAb. Data represent the means ± s.d. for n=3 of two independent experiments.
(D) VSP9B10 citrullination is analyzed by Western blotting in WB/9B10 and WB/9B10-ADI+ trophozoites after a short time exposure to the anti-VSP9B10 mAb. The membrane labeled with anti-citrulline pAb, was stripped and reblotted with anti-VSP9B10 mAb. Similar amount of loaded VSP9B10 is observed. Molecular mass of VSP9B10 is indicated on the right.
Analysis of the progeny revealed that the surviving GS/H7 (not shown) and H7-HA trophozoites were nearly all VSPH7 negative, while the surviving ΔR-H7 trophozoites were almost 100% VSPH7 positive (Fig. 3B). These findings strongly suggest that, like palmitoylation, VSP citrullination play an essential role in Giardia survival (Touz et al., 2005); and this work).
Since an inability to citrullinate VSPs results in decreased antigenic switching under Ab pressure, then increased citrullination may result in enhanced antigenic switching in the same situation. To test this hypothesis, wild-type WB/9B10 trophozoites (WB/9B10) and WB/9B10 transgenic trophozoites over-expressing active gADI (WB/9B10-ADI+) were exposed to the specific anti-VSP9B10 mAb (but not for controls) for only 10 minutes, with the cytotoxicity as well the VSP switching being analyzed at 24 h. As expected, no cytotoxicity was observed. However, a significant increase of VSP switching was seen in the transfected trophozoites, with this being accompanied by enhanced citrullination (Fig. 3C, D). Similar results were also obtained when WB/1267wt and WB/1267-ADI+ were tested (not shown). The VSP switching is relatively frequent and even small populations of recently cloned organisms contain parasites expressing different VSPs. Related to this, we observed that trophozoites that highly expressed gADI switch faster than wild-type cells under antibody pressure. These results could not be explained by differences in growth rates since transfected and non transfected trophozoites multiply at the same speed.
Arginine deiminase is post-translationally modified
Giardia arginine deiminase break down products were reported previously (Palm et al., 2003). In this work, analysis of protein expression of either gADI-HA or the native gADI showed that this protein is highly unstable and/or post-translationally modified, since several bands were observed including a major 85 kDa band instead of the predicted 64 kDa (Fig. 4A). Analysis of gADI failed to find likely glycosylation, myristolation, prenylation, or GPI binding sites. However, phosphorylation was predicted in several positions and we also found that gADI possessed two motifs with a high probability of modification by the 11 kDa SUMO-1 protein (small ubiquitin-related modifier, also called Sentrin) at positions K101 (LKYE) and K387 (IKAD). Western blot assays using anti-SUMO mAb detect an 85 kDa protein corresponding to the gADI higher band (Fig. 4B). In addition, anti-SUMO mAb was able to immunoprecipitate the 85 kDa band of gADI, thus confirming the gADI-SUMO interaction (Fig. 4C). These results suggest that gSUMO-1 binds to gADI, probably to the K101 and K387 residues, thereby explaining why the gADI Mr increased from the predicted 64 kDa to 85 kDa. Nevertheless, the role of sumoylation on the modification of gADI and the biological function of this modification during Giardia growth and differentiation are the subject of on-going experiments.
Figure 4. Post-translational modifications of gADI.
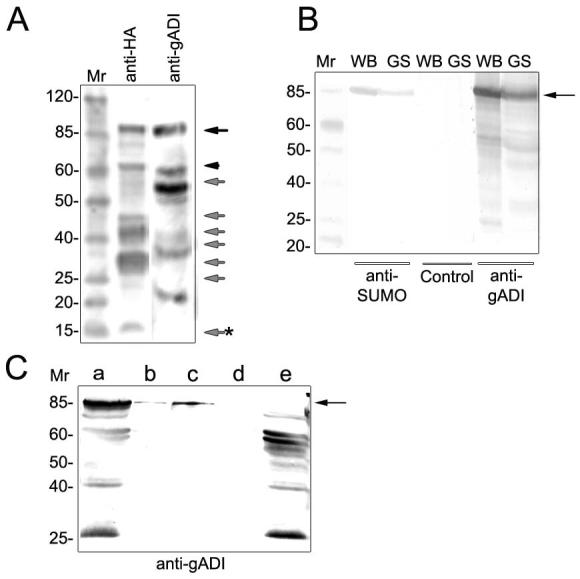
(A) Western blotting using anti-HA mAb shows gADI-HA in ADI-transgenic trophozoites. Multiple bands are also obtained using specific anti-gADI pAb in wild-type cells. The band of 64-66 kDa corresponding to the predicted protein sequences is observed in both cases (arrowhead) together with degradation products (gray arrows) including the low weight band (gray arrow with asterisk) found in the peptide pull-down (see Figure 1). Also, for both HA-tagged and native gADI, an increase in Mr from 64-66 kDa to 85 kDa band is shown (black arrow) suggesting that gADI undergoes posttranslational modification. Mr is molecular mass expressed in kDa.
(B) Western blot assays using anti-SUMO mAb recognize an ∼85 kDa band in both WB/1267 and GS/H7 Giardia clones. The same filter membrane was stripped and reblotted with gADI pAb, showing a perfect match with the higher band recognized by the gADI pAb. To confirm the lack of residual primary antibodies after stripping, only the secondary antibody was added to the stripped blots showing no signal (Control). Mr is molecular mass expressed in kDa.
(C) Western blotting using biotin conjugated anti-gADI pAb was performed to detect gADI bands after immunoprecipitation with anti-SUMO mAb. a) gADI in lysate before IPP, b) gADI after immunoprecipitation by using 0.1 μg of anti-SUMO mAb (arrow), c) gADI after immunoprecipitation by using 1 μg of anti-SUMO mAb (arrow), d) control using a non-related anti-HA mAb, and e) supernatant of sample c. Mr is molecular mass expressed in kDa.
Arginine deiminase may play an additional role during Giardia encystation
Post-translational modifications of proteins occur and are important in many cellular processes, including cell-cycle progression, apoptosis, cellular proliferation, development, and were more recently found in cell differentiation (Dohmen, 2004; Leight et al., 2005; Poulin et al., 2005; Shalizi et al., 2006). These findings prompted us to investigate whether gADI is involved in the process of encystation, the developmental process that a trophozoite goes through to turn it into a cyst. This process is initiated when trophozoites are deprived of cholesterol or challenged with high bile concentrations, resulting in the appearance of large secretory granules called encystation-specific secretory vesicles (ESVs). These vesicles transport cyst wall materials (e.g. CWP1 and CWP2) for the subsequent release and extracellular assembly of the rigid cyst wall that protects the parasite outside its host (Fig. 5A). First, we characterized gADI by analyzing the level of gADI mRNA and protein expression in both wild-type (WB/1267wt) and ADI-transgenic trophozoites (WB/1267-ADI+) at 0, 6, 24, and 48 hours of encystation. Slot-blot assays showed that there was a minor increase in gadi mRNA during encystation, compared to gdh and cwp2 in WB/1267wt (Fig. 5B, left panel). A higher expression of gadi and a lower expression of cwp2 were observed when the same probes were added to the membranes containing mRNA of WB/1267-ADI+, compared to gdh mRNA (Fig. 5B, right panel).
Figure 5. gADI increases during Giardia differentiation.
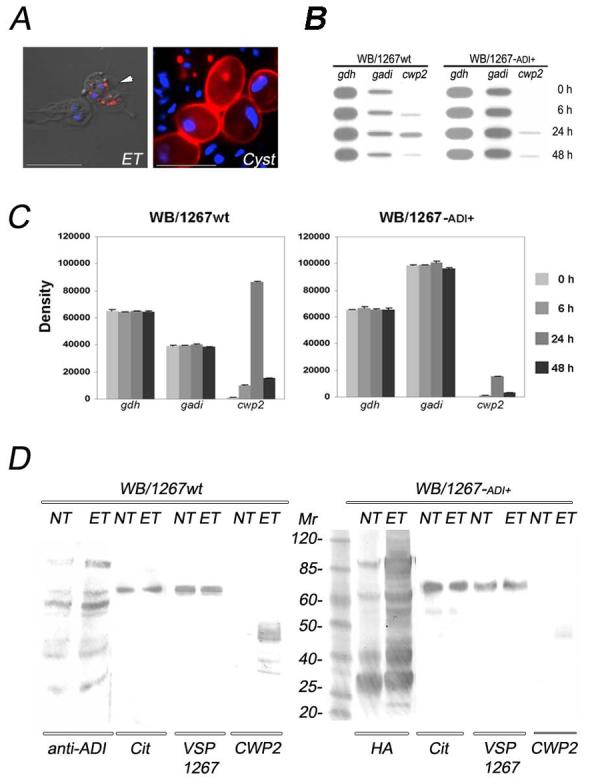
(A) IFA and confocal microscopy show the Giardia encystation process. CWP2 is synthesized and transported in ESVs (red) in encysting trophozoites (ET, arrowhead). At the end of the encystation process, CWP2 (red) is found in mature cyst walls (Cyst). Nuclei are stained with DAPI (blue). Scale bars represent 10 μm.
(B) Slot-blotting qualitatively shows gdh, gadi, and cwp2 gene expression at 0, 6, 24, and 48 hours of encystation in wild-type cells (WB/1267wt) and transgenic trophozoites (WB/1267-ADI+). The assay was performed in triplicate.
(C) Analysis of gdh, gadi, and cwp2 gene expression by RT-PCR and density quantification comparing both wild-type (WB/1267wt) and ADI-transgenic trophozoites (WB/1267-ADI+). Data represent the means ± s.d. for n=4 of two independent experiments.
(D) Western blotting using specific antibodies shows gADI, modified citrulline, VSP1267, and CWP2 protein expression in both wild-type (WB/1267wt) and ADI-transgenic trophozoites (WB/1267-ADI+). 10 μg of non-encysting (NT) and 24 h-encysting trophozoites (ET) were used for each sample. Mr is molecular mass expressed in kDa.
Semi-quantitative RT-PCR assays corroborated the data obtained by slot-blotting. gdh mRNA expression showed no variation during encystation for either WB/1267wt or WB/1267-ADI+ trophozoites. Furthermore, no variation in mRNA levels was detected for gadi during encystation in either case. However, its expression was found to be 2.5 times higher in transgenic cells. cwp2 mRNA dramatically increased at 24 h in wild-type cells, but not in ADI-transgenic trophozoites, suggesting that gADI inhibited cwp2 expression and therefore may participate in the regulation of the cwp2 gene (Fig. 5C).
Western blotting using specific antibodies showed that in WB/1267wt and WB/1267-ADI+ trophozoites, gADI increases after 24 h encystation. The expression of VSP1267 was equivalent in both cells types (VSP1267) but the amount of citrulline detected in WB/1267-ADI+ was higher than in WB/1267wt trophozoites (also demonstrated in Fig. 3D). Surprising, although gADI expression increased, VSP1267 citrullination was not modified during encystation (Cit). As expected, CWP2 rose in WB/1267wt during encystation (Fig. 5D, left panel). Conversely, there was a decrease in CWP expression to almost undetectable levels in WB/1267-ADI+ trophozoites, validating a role of gADI in the regulation of CWP2 (Fig. 5D, right panel). Generally, WB/1267 trophozoites differentiate into cysts in about 4-8 % of total cells after 48 h in encysting medium. Our results showed that over-expression of ADI reduced the number of cysts produced (approximately 0.1 %), compared to wild-types (not shown). Even though the amount of formed cyst is drastically reduced in transgenic trophozoites highly expressing ADI, the kinetics of differentiation is unaltered and the formed cysts are resistant to harsh environmental conditions in cells expressing gADI at low levels (Vranych and Rópolo, unpublished results).
Because Lys residues are also the target for ubiquitylation, it is possible that gADI sumoylation functions as an inhibitor of gADI proteolysis during differentiation. Another possibility is that the addition of SUMO proteins to gADI allows cytoplasm/nuclei translocation (Ishov et al., 1999). To test this last hypothesis, the presence of sumoylated gADI was analyzed in nuclear and cytoplasmic fractions both before and during encystation by Western blotting. In wild-type trophozoites, the gADI-85 kDa band increased in the nuclear fraction during encystation (Fig. 6A) To test for cytoplasmic contamination of nuclear fractions, the presence of VSP1267, a protein not found in the nucleus, was investigated being undetected in these fractions by Western blotting (Fig. 6A, VSP1267).
Figure 6. During encystation, gADI is translocated to the nuclei and inhibits CWP expression.
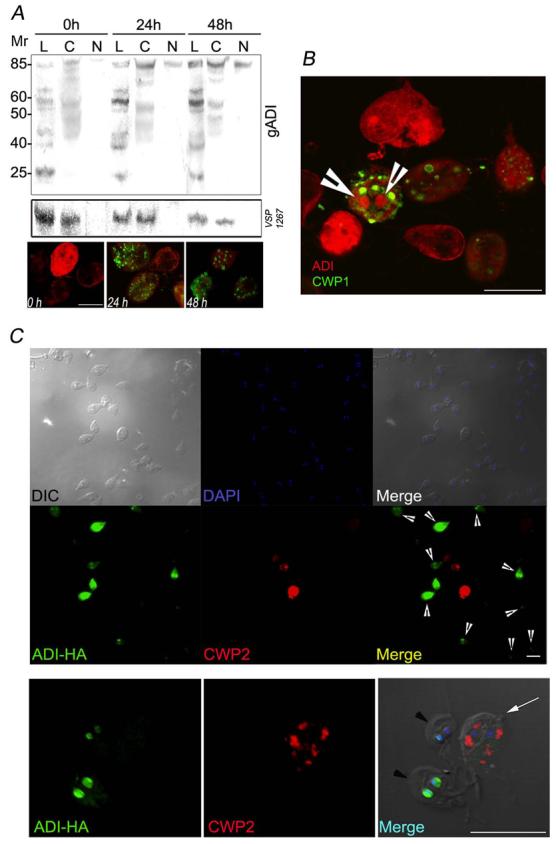
(A) Western blotting results after cytoplasm/nuclear fractionation assay using anti-gADI pAb at 24 and 48 hours post-encystation induction in wild-type (gADI) cells. (L): cell lysate previous fractionation, (C): cytoplasmic fraction, and (N) nuclear fraction (upper panel). Anti-VSP1267 mAb is used to detect cytoplasm/nuclear fraction contamination. Mr is molecular mass expressed in kDa (middle panel). IFA shows a representative time course gADI (red) distribution during encystation (ESVs in green, lower panel).
(B) Confocal microscopy and IFA using wild-type cells show that gADI (red) is translocated to the nuclei when the encysting cell is filled of ESVs (arrowheads). CWP1 is stained in green. Scale bar represents 10 μm.
(C) Top panels: Differential interference contrast (DIC) and DAPI staining show a representative image of an ADI-stable transgenic culture (Merge1). Middle panels: Direct IFA shows that trophozoites highly over-expressing gADI-HA in the nuclei (green) do not express CWP2 (red) during encystation (arrowheads in Merge2). Magnification 400 x. Bottom panel: Closer analysis of three ADI-transgenic cells demonstrated that the ones that show ADI-HA (green) on the nuclei (arrowheads) do not reveal CWP2 (red) expression after 24 h encystation while the low expressing ADI-HA does (arrow in Merge 3). Scale bar represents 10 μm.
Confirmation of the nuclear translocation of gADI was obtained by IFA in WB/1267 wild-type (Fig. 6A-B) and gADI-HA transfected trophozoites (Fig.6C), both demonstrating a shift of gADI to the nuclei during encystation. Wild-type organisms only showed the presence of nuclear gADI late during encystation (when the cells contained a large number of ESVs) (Fig. 6A-B). Interestingly, those transgenic organisms showing a clear translocation of gADI-HA to the nuclei had few if any ESVs (Fig. 6C). The failure of ESV development together with the decrease in cwp2 mRNA in transfected encysting cells suggest, that gADI has a role in the control of the encystation process.
DISCUSSION
Previous studies determined that arginine deiminase plays a role in the energy metabolism in Giardia. In the present work, we show an additional gADI action, which affects or controls essential biological activities of the organism. There are four major findings. First, we establish that gADI acts as a peptidyl-arginine deiminase in addition to its known function as a metabolic enzyme. Secondly, gADI influences important processes in Giardia. Specifically, by way of its ability to citrullinate VSPs gADI alters VSP biology, the response to cytotoxic antibodies, and antigenic variation. Moreover, gADI appears to play an important, if not controlling, role in encystation. Lastly, because arginine deiminase is sumoylated, it is likely that this modification alters its the activity, degradation and/or localization.
The evidence that VSPs are citrullinated by gADI is compelling. gADI binds specifically to the CRGKA tail and localizes close to the VSPs found on the plasma membrane of the trophozoite. Using specific antibodies to modify citrulline, VSPs were shown to be citrullinated specifically, at the arginine residue (R) of the CRGKA cytoplasmic tail. Also, purified gADI citrullinates the arginine in the conserved tail in vitro, verifying gADI's PAD activity. This modification has profound effects on the biology of the parasite.
The most commonly cited biological role of antigenic variation in pathogenic microorganisms is immunological escape, in which the host antibodies produced against a dominant antigen destroy those organisms bearing it, resulting in the organisms being replaced by ones that possess a variant form of the antigen. However, the function of antigenic variation among organisms differs and, in some cases, its analysis is complex. In Giardia, antibodies directed against VSPs, including VSP-specific surface reacting mAbs (Nash and Aggarwal, 1986), immune lactogenic IgA (Stager et al., 1998), and serum from infected humans (Nash et al., 1990a; Nash et al., 1990b) or animals, either inhibit growth or kill recognized trophozoites (Hemphill et al., 1996; Stager and Muller, 1997), thus allowing the repopulation of trophozoites expressing other VSPs. The in vitro effects range from little or no inhibition of growth to cytotoxicity, and the effects appear to depend on the concentration (direct correlation), affinity, and even perhaps the nature of the antibody and target epitopes (Hemphill et al., 1996; Nash and Aggarwal, 1986; Stager and Muller, 1997). In contrast, monovalent F(ab') of the same antibodies exhibited none of these cytological effects (Hemphill et al., 1996; Nash and Aggarwal, 1986), although the VSP switching was not analyzed in this case.
Our results support the findings that exposure of trophozoites to a high level of specific-VSP Abs results in cell death and the emergence of trophozoites expressing an antigenically different VSP. Most significantly, this event is strongly linked to deimination (citrullination) of the cytoplasmic tail of VSPs, since mutation of the amino acid R led to survival of targeted trophozoites and failure to switch. Conversely, we have now shown that an increase in the VSP citrullination by over-expression of gADI in the presence of the specific Ab causes deregulation of VSP switching, probably due to an amplification of a signal transduction event (Touz et al., 2005 and the present work). Therefore, it is now clear that post-translational modifications are important for the control of the antigenic variation in Giardia. These studies are consistent with the hypothesis that both immunological and biological factors act in concert to select which VSPs are expressed in a particular host.
The functional significance of gADI in cell survival appears not to be restricted to its role in energy production and antigenic variation. We found that the subcellular localization of gADI is cytoplasmic and significantly close to the plasma membrane but it is up-regulated and translocated to the nuclei when the trophozoites are induced to encyst. At least two possibilities arise from this event: 1) as an arginine deiminase, gADI may be sequestered from the cytoplasm to enter in a “stand-by” process during encystation, because the requirement of energy during this process is lower than that needed during active growth; 2) as a PAD, it may be directed into the nuclei for histone modification and transcription regulation (Wang et al., 2004). However, the results presented in this work suggest that the second possibility is more likely. Post-translational histone modifications, such as phosphorylation, acetylation, methylation, and citrullination, regulate a broad range of DNA and chromatin-templated nuclear events, including transcription (Jenuwein and Allis, 2001; Wang et al., 2004). Because acetylation was shown to be a Giardia histone post-translational modification (Kulakova et al., 2006), we propose that gADI modifies histones by citrullination, with this posttranslational modification being involved in the down-regulation of the encystation process. This hypothesis is based on the fact that translocation of the functionally over-expressed gADI to the nuclei avoids cyst formation. We also suggest that gADI causes the cwps genes to turn off as an essential requirement in order to successfully complete the encystation. This effect takes place early on in gADI-transgenic cells, where the high over-expression of gADI together with its nuclear translocation avoids CWP expression and cyst formation. Nevertheless, we can not exclude the possibility that gADI is also involved in the control of its major surface antigen when its life cycle is completed, as was demonstrated by Svard et al. (Svard et al., 1998). Differentiation-associated switching of surface antigens could be one reason for the common occurrence of repeated infections (Gilman et al., 1988), due to the down-regulation of the major VSP expressed during encystation and the appearance of new VSPs after excystation.
gADI possesses two predicted nuclear localization signals (PQRRREQ and RRGIVMGQFQAPQRRRE) (SignalIP program) (Le Panse et al., 1997), suggesting that these motifs are involved in the translocation of this protein to the nuclei during encystation. There is, however, no evidence of the participation of these motifs in the nuclear protein translocation of any protein in this parasite. Thus, further analyses are needed to show how this parasite utilizes the nuclear signaling motifs that are highly conserved during evolution. Another possibility is that gADI is translocated to the nuclei by means of SUMO protein binding. Recently, it was shown that conjugation of SUMO-1 could lead to protein stabilization and protection from degradation, but an increasing body of evidence implicates sumoylation in the targeting of certain proteins to the cell nucleus and to the subnuclear structures (Dohmen, 2004). The underlying mechanisms, however, are still largely unknown. In Giardia, the molecular biology of enzymes of the SUMO system has never been addressed. In fact, only three putative proteins involved in sumoylation have been posted in the Giardia DB: SUMO-1 (or Sentrin, GGD 7760), the dimmer of the SUMO-activating enzyme E1 (Uba2, GGD 6288), and the SUMO ligase (GGD 17386). Our results indicate that gADI is a sumoylated protein based on “in silico” data, Western blotting, and immunoprecipitation using anti-SUMO mAb. This protein modification may be essential in the nuclear translocation of gADI in order to accomplish its function during encystation, since the 85 kDa-gADI was mainly located in the nuclear subfractions. Further research into the possibility that gADI function could be regulated by sumoylation may provide insight into the biology of this important intestinal parasite and also contribute to the understanding of the evolution of protein modification in eukaryotic cells.
Taking earlier studies and this work together, we have been able to unravel the multiple functions of arginine deiminase in the survival of Giardia (Fig. 7). One of these concerns the ability to obtain energy by using free-arginine as the preferential fuel under anaerobic conditions. During the proliferative stages of growth, gADI converts arginine into citrulline, with ATP production occurring at the final enzymatic step of the ADH pathway (Schofield et al., 1992) (Fig. 7A). In addition to this function, it was reported that Giardia uses gADI as a competitor to NOS from the host cell for the free-arginine, thereby reducing the production of NO and the host defense mechanism against microbial infection (Eckmann et al., 2000). Supporting this finding, it was confirmed that gADI is released from Giardia to the extracellular space as a 66 kDa protein when the trophozoites are in contact with human colon epithelial cells (Ringqvist et al., 2008) (Fig. 7B). Also, during growth, gADI acts as a peptidyl-arginine deiminase on the cytoplasmic tail of VSPs, probably by functioning as a regulator of antigenic variation (Fig. 7C). During encystation, gADI is in the nuclei and cysts formation is reduced (Fig. 7D). This translocation event is most likely associated with gADI sumoylation, but the possibility of the participation of gADI nuclear localization signals can not been discarted.
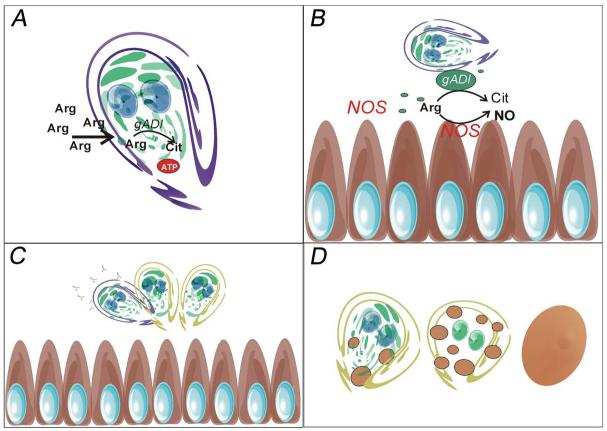
Figure 7. Schematic representation of gADI functions during growth and encystation.
A) During growth, the trophozoites acquire free arginine from the extracellular medium. Inside the cell, cytoplasmic gADI converts arginine into citrulline, with ATP production occurring at the final enzymatic step of the ADH pathway. B) gADI is released to the extracellular space when the trophozoites are in contact with human colon epithelial cells and compete with the host NOS for the free-arginine thereby reducing the production of NO. C) Under low exposure to specific antibodies, gADI acts as a peptidyl-arginine deiminase on the cytoplasmic tail of VSP inducing VSP switching. D) At the last step of encystation, gADI is translocated from the cytoplasm to the nuclei turning encystation specific genes off and ending the process.
Changes in the environment (anaerobiosis, presence of Abs, presence of the host cell, or depletion of cholesterol) define which functions are essential for gADI to be performed at one point in time. The underlying mechanisms, however, are still largely unknown. We believe that the analysis of the protein modifications associated with differentiation, along with the mediators of these activities reported here, represent an important contribution in our understanding of the control of gene expression in parasitic protozoa.
MATERIALS AND METHODS
Giardia lamblia Cultivation and Transfection
Trophozoites of the isolate WB, clone 1267, 9B10, and from the isolate GS, clone H7, were cultured in TYI-S-33 supplemented with 10% fetal bovine serum and bovine bile (Keister, 1983). Trophozoites of the WB/1267 clone were transfected by electroporation and selected with puromycin (Singer et al., 1998; Yee and Nash, 1995). The transfection of stable WB/1267 cells was 100%, as determined by IFA and flow cytometry. A two step encystation procedure was used by increasing the medium pH and by addition of porcine bile as reported by Boucher and Gillin (Boucher and Gillin, 1990).
Pull-Down Assays
Giardia clones GS/H7 and WB/1267 were grown, harvested, and suspended in 1 ml of lysis buffer (50 mM NaH2PO4, 300 mM, NaCl, 10 mM imidazol, pH 8.0, 1% Triton X-100, and protease inhibitors) for 3 hours at 4°C. After mild sonication using a Branson sonifier 250 (Branson, CT, USA) with an output control of 3 and a 50% duty cycle (sonication complex), the lysate was centrifuged at 7500 × g, for 30 minutes at 4°C. The supernatant was then mixed with 1 mg of His6-CRGKA peptide dissolved in 10 μl of DMSO overnight at 4°C. Controls of specific binding had no His6-CRGKA peptide. The lysate and peptide were mixed with 200 μl of Ni-agarose beads (QIAGEN, Valencia, CA) and incubated for 4 hours at 4°C. Beads were spun down at 700 × g and washed four times with wash buffer (50 mM NaH2PO4, 300 mM NaCl, pH 8.0, 0.1% Triton X-100, and protease inhibitors). Bound proteins were eluted four times with 100 μl of elution buffer (50 mM NaH2PO4, 300mM NaCl, 250 mM imidazol, pH 8.0, 0.1% Triton X-100, and protease inhibitors). Peptide bound proteins were analyzed by SDS-PAGE, stained with Coomassie G-250 (Fig. 1A). The detected bands were cut and submitted to the Research Technologies Branch for Protein Identification (NIAID, NIH) for LC-MS/MS analysis. After two independent experiments, one protein associated with the His6-CRGKA peptide was identified.
Yeast Two-Hybrid Assay
The MATCHMAKER Two-hybrid system was used following the protocol suggested by the company (Clontech, Palo Alto, CA). The two-hybrid pGBKT7(TRP1) vector (GAL4 DNA binding domain, BD) containing the genes for vsph7, vsp1267, or vsp1267 without the 3' (TGCAGAGGCAAGGCG) gene sequence were used as bait while the gadi gene was ligated to the pGADT7-Rec(LEU2) vector (GAL4 transcription activation domain, AD), yielding H7-BD, 1267-BD, 1267-tailBD, and ADI-AD vectors, respectively. AH109 transformants were cultured at 30°C for 4–5 days on plates with minimal medium lacking leucine and tryptophan (−L/−T) to test for positive transformation, or in the absence of leucine, tryptophan, and histidine (TDO – triple dropout medium), to study specific protein interactions as previously described (Touz et al., 2004). Controls of the methodology include ESCP-BD/gMuA-AD interaction (Touz et al., 2004) and the empty BD/gADI-AD vector.
Expression of VSPH7 variant and ADI-HA in WB/1267 trophozoites
The construction of VSPH7 and H7-tail was detailed previously (Touz et al., 2005). ΔR-H7 was prepared using a site-directed mutagenesis kit (QuikChange, Stratagene), and the presence of the mutation confirmed by using dye terminator cycle sequencing (Beckman Coulter). For ADI-HA construction, the vsph7 gene was replaced by the adi gene using the forward 5'GCCTCCATGGCTGACTTCTCCAAGGATAAAGAG3' and 5'GATGGTTAACCTTGATATCGACGCAGATGTCAGC3' oligonucleotides yielding the ADI-HA vector. ADI-HA, H7-HA, H7-tail, and ΔR-H7 vectors were used to transfect the WB/1267 clone in order to generate stable puromycin selected cells.
Antibodies
Anti-HA mAb (Sigma), anti-HA mAb labeled with alkaline phosphatase (SIGMA), anti-gADI pAb (Ringqvist et al., 2008), Anti-H6 mAb (Invitrogen), anti-CWP1 FITC labeled mAb (Warerborne, New Orleans, LA), and CWP2-Texas Red (Touz et al., 2003) were used. Citrullinated proteins were detected by using a polyclonal antibody against a chemically modified version of citrulline (anti-MC) (Senshu et al., 1992). To detect sumoylated proteins, mouse anti-GMP-1 mAb (ZYMED® Laboratories, Invitrogen) was used. The 5C1, G10/4, and 9B10 mAbs were used to detect VSP1267, VSPH7, and VSP9B10, respectively (Mowatt et al., 1991; Nash et al., 2001; Nash and Mowatt, 1992). Anti-ADI pAb was biotinylated by using the EZ-Link NHS-SS-Biotin (Pierce, Rockford, IL), and detected using alkaline phosphatase-labeled streptavidin in Western blotting. For direct IFA anti, Anti-HA mAb FITC labeled (SIGMA) VSP9B10-Texas Red and anti-CWP2-Texas Red (Touz et al., 2003) were used.
Western blotting and IFA
Western blot assays were performed as previously reported (Touz et al., 2005). 10 μg of total protein from parasite lysates were incubated with sample buffer, boiled for 10 min and separated on 10% Bistris gels. Samples were transferred to nitrocellulose membranes, blocked with 5% skimmed milk and, 0.1% Tween 20 in TBS, and incubated with primary antibody diluted in the same buffer. After incubation with conjugated secondary antibody, proteins recognized by antibodies were visualized with SuperSignal West Pico chemiluminescent substrate (Pierce) and autoradiography, or by using BCIP/NBT substrate (SIGMA). Controls included omission of the primary antibody, use of an unrelated antibody, or assays using non-transfected cells. In figure 3D, the AutoDeblur v9.3 program was used (AutoDeblur v9.3, AutoQuant Imaging Inc. NY).
For IFA of fixed cells, trophozoites cultured in growth medium were harvested and processed as described (Touz et al., 2005). Briefly, parasites were attached to slides for 30 min, and then fixed with fresh 4% formaldehyde for 40 min. The cells were incubated sequentially with blocking solution (10% Goat serum and 0.1% Triton X-100 in PBS) at 37°C for 30 min and antibodies conjugated with fluorescent dyes for 1 h. The cells were then washed three times with PBS before the addition of mounting medium (VECTASHIELD® Mounting Medium). Images were collected on a Leica TCS-NT/SP confocal microscope (Leica Microsystems, Exton, Pa.) using 40× or 63× oil immersion objectives (NA 1.32, zoom X). Fluorochromes were excited using an argon laser at 488 nm for FITC and a krypton laser at 568 nm for Texas Red 568. DAPI was excited using a 364 nm Argon laser. Detector slits were configured to minimize any cross-talk between the channels. Differential interference contrast images were collected simultaneously with the fluorescence images by use of a transmitted light detector. Images were processed using Leica TCS-NT/SP (version 1.6.587), Imaris 3.1.1 (Bitplane AG, Zurich, Switzerland), and Adobe Photoshop 5.5 (Adobe Systems) software.
Immunoprecipitation assay (IPP)
Cultured Giardia trophozoites were harvested and resuspended in 1 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 1% Triton X-100, and protease inhibitors) for 1 h at 4°C. The lysate was centrifuged at 10,000 × g for 10 min at 4°C and the supernatant mixed with anti-gADI, anti-HA (1 μg) or anti-SUMO (0.2 or 1 μg) and incubated overnight at 4°C. 50 μl of Protein A-agarose beads (Qiagen, Valencia, CA) were added to each sample and incubated for 4 h at 4°C. Beads were pelleted at 700 × g and washed four times with wash buffer (50 mM NaH2PO4, 300 mM NaCl, (pH 8.0), 0.1% Triton X-100, and protease inhibitors). Beads were suspended in sample buffer and boiled for 10 min before Western blot analysis.
Citrullination assay
Non-encysting and trophozoites encysted at different time points were collected, washed in mPBS, resuspended in 20 ul of lysis buffer without protease inhibitors (50 mM Tris pH 8.0, 120 nM NaCl, 1% Triton X-100), and incubated with 5 mM dithiothreitol for 30 minutes at 37°C prior to being subjected to SDS-PAGE and nitrocellulose blotting using the Anti-citrulline (modified) Detection Kit (Upstate) as was previously described (Senshu et al., 1995). Controls included omission of the anti-citrulline or absence of the chemically modifying citrulline step.
gADI activity assay
Giardia arginine deiminase activity was assayed by measuring the modification of His6-CRGKA peptide to His6-CcitGKA. First, trophozoites expressing gADI-HA or ESCP-HA (Touz et al., 2004) were cultured, collected, and sonicated in PBS buffer at 4°C until there were no intact cells before being centrifuged for 10 minutes at 700 × g. gADI-HA and ESCP-HA were purified using anti-HA mAb and protein A/G-agarose as previously described (Touz et al., 2004). 5 mM of soluble His6-CRGKA peptide (10 mg/ml of ADI activity buffer: 50 mM Tris-HCl pH 7.6, 2 mM DTT, and 5 mM CaCl2) was mixed with either purified gADI-HA or ESCP-HA for 16 hours at 50°C. After samples were boiled for 5 minutes, the peptide was purified by using Ni-agarose beads and subjected to dot-blot and citrullination assays.
Cytotoxicity and switching assays
Cytotoxicity was assayed as we previously reported (Touz et al., 2005). Briefly, Giardia trophozoites expressing native VSPH7 or transgenic VSPH7 at the same intensity of surface VSPH7 were selected and harvested using CELLQuest software and a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA). These clones were grown until confluence before a second IFA analysis using G10-4 mAb was performed to test for 100% expression of VSPH7. A WB/1267 clone expressing almost 100% VSP1267 was used as a negative control. After addition of G10-4 mAb (specific for VSPH7), mouse polyclonal antiserum (anti-GS/VSP pAb, raised against GS/H7 Giardia clone), or anti-CWP2 8G8 mAb (generated against ESVs) (Lujan et al., 1995) in 1:20 dilution, the immobilization, aggregation, detachment, and complement-independent cytotoxicity were analyzed. Cytotoxicity was determined in triplicate after 24 hours by estimating the number of adherent viable parasites. The progeny were analyzed by growing the assayed trophozoites for 24 hours and by labeling the remaining H7 positive viable trophozoites using goat anti-mouse FITC-conjugated antibody (Cappel). For VSP switching assay, Giardia ADI-transgenic and non-transgenic parasites expressing approximately 100% VSP9B10 or VSP1267 were maintained in vitro before the addition of anti-VSP9B10 or anti-VSP1267 (5C1) mAbs, respectively, for only 10 minutes in a 1:20 dilution. VSP expression was analyzed by FACS as previously described (Nash et al., 2001). Controls consisted of identical cultures not exposed to mAb.
Slot-Blot Assay
Two micrograms of total RNA extracted from growing and encysting trophozoites were immobilized onto a Nytran SuperCharge nylon membrane (Schleicher & Schuell, Keene, NH) by using the MINIFOLD II slot-blot system, according to the manufacturer's instructions. Membranes were first hybridized with antisense probes specific for gadi gene, and then stripped and reprobed by using an antisense specific for gdh or cwp2 as the controls. Oligonucleotides used (in 5'-3' orientation): GDH (forward ATGCCTGCCCAGACGATCGA / reverse GAGCCAGAAAGAAGGACGTT), ADI (forward CACTTGTGGAAATTACGTCT / reverse CTTGATATCGACGCAGATGT), ADI-HA (forward CACTTGTGGAAATTACGTCT / reverse CTATGCATAGTCTGGTACAT), and CWP2 (forward ATGATCGCAGCCCTTGTTCT / reverse ATCCATCTCTCTCGAGAGTT).
Semi-quantitative RT-PCR
The total RNA from wild-type and ADI-transgenic trophozoites was isolated using RNA STAT- 60 reagent (Tel-Test Inc., Friendswood, TX) followed by digestion with 50 U of DNAse (Roche Diagnostics, Indianapolis, IN) for 15 min at 25°C, and a final purification was performed by using SV Total RNA Isolation System (Promega, Madison, WI). For reverse transcription and PCR amplification a One-step RT-PCR kit (Qiagen, Valencia, CA) was used with dilutions of the total RNA from 0.2 ng to 20 ng per reaction in a final reaction volume of 50 μl. The reverse transcription reaction was done at 50°C for 30 min followed by inactivation of the reverse transcriptase at 95°C for 15 min. For PCR, 30 cycles (30 sec at 94°C, 30 sec at 50°C and 1 min at 72°C) were used followed by 10 min at 72°C for final extension. DNA-contamination control was carried out by adding ADI oligonucleotides at the PCR step of the RT-PCR reaction. Aliquots (5 μl) of the RT-PCR reaction were size-separated on 1.2% agarose gel in TAE (E-Gel, Invitrogen Corporation, Carlsbad, CA) prestained with ethidium bromide. Amplification products were directly quantified by densitometric scanning of the fluorescence intensity under UV light, using EagleSight® software for image acquisition, documentation and analysis (Stratagene, La Jolla, CA). Only three examples of RT-PCR using 0.2 ng of RNA are shown since all the results were similar. The oligonucleotides used were the same as the ones described for Slot-blotting.
Subcellular Fractionation
Non-encysting and encysting Giardia trophozoites were suspended in buffer A (20 mM Tris-HCl, pH 7.6, 5 mM MgCl2, 1.5 mM KCl, 1 mM phenylmethanesulfonyl fluoride, 2 mM dithiothreitol, and 0.1% Nonidet P-40) and disrupted with sonication. The homogenate was centrifuged at 760 × g at 4°C for 10 minutes to separate it into supernatant (cytoplasmic) and pellet (nuclear) fractions. The supernatant and pellet fractions were subjected to Western blotting.
ACKNOWLEDGMENTS
Dr. Adrian Hehl is acknowledged for his collaboration in the production of anti-ADI pAb, and Dr. Alfredo Caceres and laboratory members for providing numerous reagents. This work was partially supported by the Agencia Nacional para la Promoción de la Ciencia y Tecnología FONCyT Jóvenes PICT2004.
REFERENCES
- Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal A, Merritt JW, Jr., Nash TE. Cysteine-rich variant surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1989;32:39–47. doi: 10.1016/0166-6851(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Boucher SE, Gillin FD. Excystation of in vitro-derived Giardia lamblia cysts. Infect Immun. 1990;58:3516–22. doi: 10.1128/iai.58.11.3516-3522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen RJ. SUMO protein modification. Biochim Biophys Acta. 2004;1695:113–31. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J Immunol. 2000;164:1478–87. doi: 10.4049/jimmunol.164.3.1478. [DOI] [PubMed] [Google Scholar]
- Gilman RH, Marquis GS, Miranda E, Vestegui M, Martinez H. Rapid reinfection by Giardia lamblia after treatment in a hyperendemic Third World community. Lancet. 1988;1:343–5. doi: 10.1016/s0140-6736(88)91131-2. [DOI] [PubMed] [Google Scholar]
- Hemphill A, Stager S, Gottstein B, Muller N. Electron microscopical investigation of surface alterations on Giardia lamblia trophozoites after exposure to a cytotoxic monoclonal antibody. Parasitol Res. 1996;82:206–10. doi: 10.1007/s004360050096. [DOI] [PubMed] [Google Scholar]
- Hiltpold A, Frey M, Hulsmeier A, Kohler P. Glycosylation and palmitoylation are common modifications of giardia variant surface proteins. Mol Biochem Parasitol. 2000;109:61–5. doi: 10.1016/s0166-6851(00)00229-2. [DOI] [PubMed] [Google Scholar]
- Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, Yeh ET, Strauss JF, 3rd, Maul GG. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol. 1999;147:221–34. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–8. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Kulakova L, Singer SM, Conrad J, Nash TE. Epigenetic mechanisms are involved in the control of Giardia lamblia antigenic variation. Mol Microbiol. 2006;61:1533–42. doi: 10.1111/j.1365-2958.2006.05345.x. [DOI] [PubMed] [Google Scholar]
- Le Panse S, Ayani E, Nielsen S, Ronco P, Verroust P, Christensen EI. Internalization and recycling of glycoprotein 280 in epithelial cells of yolk sac. Eur J Cell Biol. 1997;72:257–67. [PubMed] [Google Scholar]
- Leight ER, Glossip D, Kornfeld K. Sumoylation of LIN-1 promotes transcriptional repression and inhibition of vulval cell fates. Development. 2005;132:1047–56. doi: 10.1242/dev.01664. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Conrad JT, Bowers B, Nash TE. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. Implications for secretory granule formation and protein assembly into the cyst wall. J Biol Chem. 1995;270:29307–13. doi: 10.1074/jbc.270.49.29307. [DOI] [PubMed] [Google Scholar]
- Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–6. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- Mowatt MR, Aggarwal A, Nash TE. Carboxy-terminal sequence conservation among variant-specific surface proteins of Giardia lamblia. Mol Biochem Parasitol. 1991;49:215–27. doi: 10.1016/0166-6851(91)90065-e. [DOI] [PubMed] [Google Scholar]
- Mowatt MR, Nguyen BY, Conrad JT, Adam RD, Nash TE. Size heterogeneity among antigenically related Giardia lamblia variant-specific surface proteins is due to differences in tandem repeat copy number. Infect Immun. 1994;62:1213–8. doi: 10.1128/iai.62.4.1213-1218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash TE. Surface antigenic variation in Giardia lamblia. Mol Microbiol. 2002;45:585–90. doi: 10.1046/j.1365-2958.2002.03029.x. [DOI] [PubMed] [Google Scholar]
- Nash TE, Aggarwal A. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol. 1986;136:2628–32. [PubMed] [Google Scholar]
- Nash TE, Conrad JT, Merritt JW., Jr. Variant specific epitopes of Giardia lamblia. Mol Biochem Parasitol. 1990a;42:125–32. doi: 10.1016/0166-6851(90)90120-b. [DOI] [PubMed] [Google Scholar]
- Nash TE, Herrington DA, Levine MM, Conrad JT, Merritt JW., Jr. Antigenic variation of Giardia lamblia in experimental human infections. J Immunol. 1990b;144:4362–9. [PubMed] [Google Scholar]
- Nash TE, Lujan HT, Mowatt MR, Conrad JT. Variant-specific surface protein switching in Giardia lamblia. Infect Immun. 2001;69:1922–3. doi: 10.1128/IAI.69.3.1922-1923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash TE, Mowatt MR. Characterization of a Giardia lamblia variant-specific surface protein (VSP) gene from isolate GS/M and estimation of the VSP gene repertoire size. Mol Biochem Parasitol. 1992;51:219–27. doi: 10.1016/0166-6851(92)90072-r. [DOI] [PubMed] [Google Scholar]
- Palm JE, Weiland ME, Griffiths WJ, Ljungstrom I, Svard SG. Identification of immunoreactive proteins during acute human giardiasis. J Infect Dis. 2003;187:1849–59. doi: 10.1086/375356. [DOI] [PubMed] [Google Scholar]
- Papanastasiou P, Bruderer T, Li Y, Bommeli C, Kohler P. Primary structure and biochemical properties of a variant-specific surface protein of Giardia. Mol Biochem Parasitol. 1997a;86:13–27. doi: 10.1016/s0166-6851(97)90002-5. [DOI] [PubMed] [Google Scholar]
- Papanastasiou P, McConville MJ, Ralton J, Kohler P. The variant-specific surface protein of Giardia, VSP4A1, is a glycosylated and palmitoylated protein. Biochem J. 1997b;322(Pt 1):49–56. doi: 10.1042/bj3220049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin G, Dong Y, Fraser AG, Hopper NA, Ahringer J. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. Embo J. 2005;24:2613–23. doi: 10.1038/sj.emboj.7600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringqvist E, Palm JE, Skarin H, Hehl AB, Weiland M, Davids BJ, Reiner DS, Griffiths WJ, Eckmann L, Gillin FD, et al. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol Biochem Parasitol. 2008 doi: 10.1016/j.molbiopara.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PJ, Costello M, Edwards MR, O'Sullivan WJ. The arginine dihydrolase pathway is present in Giardia intestinalis. Int J Parasitol. 1990;20:697–9. doi: 10.1016/0020-7519(90)90133-8. [DOI] [PubMed] [Google Scholar]
- Schofield PJ, Edwards MR, Matthews J, Wilson JR. The pathway of arginine catabolism in Giardia intestinalis. Mol Biochem Parasitol. 1992;51:29–36. doi: 10.1016/0166-6851(92)90197-r. [DOI] [PubMed] [Google Scholar]
- Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. Detection of deiminated proteins in rat skin: probing with a monospecific antibody after modification of citrulline residues. J Invest Dermatol. 1995;105:163–9. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- Senshu T, Sato T, Inoue T, Akiyama K, Asaga H. Detection of citrulline residues in deiminated proteins on polyvinylidene difluoride membrane. Anal Biochem. 1992;203:94–100. doi: 10.1016/0003-2697(92)90047-b. [DOI] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–7. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Singer SM, Yee J, Nash TE. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol Biochem Parasitol. 1998;92:59–69. doi: 10.1016/s0166-6851(97)00225-9. [DOI] [PubMed] [Google Scholar]
- Stager S, Felleisen R, Gottstein B, Muller N. Giardia lamblia variant surface protein H7 stimulates a heterogeneous repertoire of antibodies displaying differential cytological effects on the parasite. Mol Biochem Parasitol. 1997;85:113–24. doi: 10.1016/s0166-6851(96)02818-6. [DOI] [PubMed] [Google Scholar]
- Stager S, Gottstein B, Sager H, Jungi TW, Muller N. Influence of antibodies in mother's milk on antigenic variation of Giardia lamblia in the murine mother-offspring model of infection. Infect Immun. 1998;66:1287–92. doi: 10.1128/iai.66.4.1287-1292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stager S, Muller N. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect Immun. 1997;65:3944–6. doi: 10.1128/iai.65.9.3944-3946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svard SG, Meng TC, Hetsko ML, McCaffery JM, Gillin FD. Differentiation-associated surface antigen variation in the ancient eukaryote Giardia lamblia. Mol Microbiol. 1998;30:979–89. doi: 10.1046/j.1365-2958.1998.01125.x. [DOI] [PubMed] [Google Scholar]
- Touz MC, Conrad JT, Nash TE. A novel palmitoyl acyl transferase controls surface protein palmitoylation and cytotoxicity in Giardia lamblia. Mol Microbiol. 2005;58:999–1011. doi: 10.1111/j.1365-2958.2005.04891.x. [DOI] [PubMed] [Google Scholar]
- Touz MC, Kulakova L, Nash TE. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol Biol Cell. 2004;15:3053–60. doi: 10.1091/mbc.E03-10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touz MC, Lujan HD, Hayes SF, Nash TE. Sorting of encystation-specific cysteine protease to lysosome-like peripheral vacuoles in Giardia lamblia requires a conserved tyrosine-based motif. J Biol Chem. 2003;278:6420–6. doi: 10.1074/jbc.M208354200. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–18. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–83. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Yee J, Nash TE. Transient transfection and expression of firefly luciferase in Giardia lamblia. Proc Natl Acad Sci U S A. 1995;92:5615–9. doi: 10.1073/pnas.92.12.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]