Abstract
Objectives
(1) To estimate incremental effects of patients' dependence and function on costs of care during early stages of AD; (2) To compare strengths of their relationships with different cost components.
Design
Multicenter, cross-sectional, observational study.
Setting
Three university hospitals in the US.
Participants
179 community-living patients with probable AD, with modified Mini-Mental State examination score ≥30.
Measurements
Patients' dependence was measured by Dependence Scale (DS). Functional capacity was measured by Blessed Dementia Rating Scale (BDRS). Total cost was measured by summing direct medical costs and informal costs. Direct medical costs included costs of hospitalization, outpatient treatment/procedures, assistive devices, and medications. Informal costs were estimated from time spent helping with basic and instrumental activities of daily living for up to three caregivers per patient using national average hourly earnings as wage rate.
Results
Both DS and BDRS were associated with higher total cost: a one-point increase in DS was associated with a $1,832 increase in total cost and a one-point increase in BDRS was associated with a $3,333 increase. Examining component costs separately identified potential differences between DS and BDRS. A one-point increase in BDRS was associated with a $1,406 increase in direct medical cost. A one-point increase in DS was associated with a $1,690 increase in informal cost.
Conclusion
Patients' dependence and function related differently to direct medical and informal cost, suggesting that measures of function and dependence provided unique information for explaining variations in cost of care for AD patients, highlighting the value in measuring both constructs.
Keywords: Alzheimer's disease, cost, function, dependence
Introduction
A defining feature of Alzheimer's disease (AD) is patients' loss of function. Instruments used in rating functional deficits in AD typically focus on patients' ability to perform self-care tasks.1-4 One of the most frequently used instruments is the Blessed Dementia Rating Scale (BDRS).1 However, BDRS and other measures of functional deficits do not fully assess patients' dependence on other individuals due to deteriorations in cognition and function. To address this issue, the Dependence Scale (DS) has been developed to directly measure the required amount of assistance AD patients need.5 Earlier studies have demonstrated that compared with function, patients' dependence on others measures related but distinct aspects of disability in AD.6-8
The tremendous impact of loss of function on costs of caring for patients with AD has been clearly established.9-14 In earlier works from the Predictors Study, a large, multi-center study of patients with probable AD followed from early stages of the disease, we examined the association between costs of direct medical care and informal caregiving and patients' functional status, as measured by BDRS.13, 14 To date, few studies have examined the effect of dependence on costs of care for patients with dementia.15 Whether dependence has an incremental effect on costs of care for patients with AD, beyond that from loss of function, is yet to be determined. Therefore, the goals of this study are (1) to examine how patients' dependence on others relates to costs of care and components of costs, (2) to estimate the incremental effect of dependence on costs during early stages of AD, independent of loss of function, and (3) to compare the strengths of the relationships between patients' function and dependence and the different components of costs of care.
Methods
Sample
The sample was drawn from the Predictors 2 cohort, and consisted of 204 patients with probable AD recruited between 1998-2004 from three sites: Columbia University Medical Center, Johns Hopkins School of Medicine, and Massachusetts General Hospital.16, 17 Specifically, at the Columbia site, patients were recruited from the Memory Disorder Center and from physician's private practices through the Alzheimer's Disease Research Center (ADRC). At the Johns Hopkins site, patients were recruited from the ADRC and from several university clinics treating elderly, cognitively impaired patients. At the Massachusetts General Hospital site, patients were recruited from the Geriatric Neurobehavioral Clinic. The inclusion and exclusion criteria are fully described elsewhere.16, 17 Briefly, subjects met DSM-III-R criteria for primary degenerative dementia of the Alzheimer type and NINDS-ADRDA criteria for probable AD. Enrollment required a modified Mini-Mental State examination (mMMS) score ≥30, equivalent to a score of approximately ≥16 on the Folstein Mini-Mental State Examination (MMSE).18, 19 The study was approved by the appropriate local Institutional Review Boards. Because patients were followed at academic AD centers, they were well characterized, with high degrees of certainty in their AD diagnosis. To date, 109 patients have had brain autopsies. Postmortem diagnoses have been completed for 96 patients, 96% of whom had AD-type pathological changes based on CERAD and NIA-Reagan Criteria.20, 21
Following our earlier work,13, 14 we excluded 15 patients (7.3%) living in nursing homes because patterns of care utilization and costs differ substantially for nursing home patients.22 We further excluded nine patients (4.4%) with missing cost data and one patient with missing Dependence Scale data from our analysis sample. The final analysis sample consisted of baseline data from 179 patients.
Measures
Patient characteristics and cost outcomes used in this study are briefly described below. Details of the measures and the costing methods used were reported in earlier studies.13, 14
Cost Outcomes
Patients and informants reported utilization of four domains of medical care in the previous year, including hospitalization, outpatient treatment/procedures, assistive devices, and medications. Prices were obtained using public databases as described in detail in earlier reports. All costs values were adjusted to constant 2005 dollars using the medical care component of the Consumer Price Index. Informal caregiving time for basic and instrumental activities of daily living (BADL and IADL) and for supervision was obtained from up to three caregivers (primary and two secondary caregivers) for each patient. BADLs included eating, dressing, and personal care. IADLs included shopping, chores, personal business, and transportation. Hours of informal care provided per day for each caregiving task were asked in the following categories: 0, up to 3 hours, 3-6 hours, 6-9 hours, 9-12 hours, and more than 12 hours. We transformed the categories into continuous values using the mean of each category as the estimated hours of care provided. For subjects who reported more than 12 hours per day for a particular type of task, we top coded the values to 12 hours. We followed the literature and top coded total hours of care provision for IADL and BADL tasks at 16 hours to provide 8 hours of sleep for the caregivers.23 No caregiver provided more than 16 hours of care per day at baseline. We summed the hours reported for each task to obtain an estimate of total caregiving hours each patient received. We used the national average hourly earning for all private industries for each year as the hourly wage rate to estimate unpaid caregiving costs.24 Total costs of care were estimated by summing costs of direct medical care and informal caregiving costs.
Dependence Scale
The Dependence Scale (DS) consists of 13 items, representing a wide range of levels of care required by a patient, from relatively subtle items such as needing reminders or advice to more gross forms such as needing to be fed.5 All items deal with patients' needs. In some cases, the need is only for supervision, without any specific tasks linked to the need. The instrument is designed to be administered to a reliable informant who lives with the patient or one who is well informed about the patient's daily activities and needs. With the exception of the first two items (needs reminders to manage chores, needs help to remember important things such as appointments) which are coded as 0 (no), 1 (occasionally, at least once a month), and 2 (frequently, at least once a week), responses to the rest of the items are coded dichotomously and indicate whether the patient requires assistance in a particular item (0=no, 1=yes). The total DS score is the sum of scores on all 13 items (range=0-15), and provides a continuous index of progressively greater dependence on others. Reliability and validity of the scale have been established in earlier studies, with reliability coefficients ranging between 0.66 and 0.93.5 For ease of presenting descriptive results, we stratified the sample into quartiles based on the total DS score, with the first quartile representing the lowest level of dependence and the highest quartile the most severe level of dependence.
Functional Assessment
Functional capacity was measured by the Blessed Dementia Rating Scale (BDRS) Parts I (Instrumental Activities of Daily living, IADLs) and II (Basic Activities of Daily living, BADLs).1 The following IADL items are included in the BDRS: difficulty with doing chores around the house (e.g., cleaning), handling money, remembering short lists (e.g., shopping), walking across a room, walking several blocks, recognizing one's whereabouts, and remembering things that happened recently. The response options for these items were none (0), some difficulty (0.5), and a lot of difficulty (1). The following three BADL items are included: eating, dressing, and bladder and bowel control. The response options for these items ranged from 0-3, with higher score indicating more difficulty. For example, for the item on eating, the response options were eat cleanly (0), messily or only with a spoon (1), only able to eat simple solids such as pudding (2), and need to be fed (3). The total BDRS score is the sum of scores on all 10 items (range=0-17), with higher scores indicating worse functional status. Reliability and validity of the scale have been established in earlier studies, with reliability coefficients between 0.60 and 0.80.1
Other Clinical and Demographic Characteristics
In addition to measures of dependence and function, several other clinical and demographic characteristics were obtained in the study. Disease progression was characterized by transition from milder stages of dementia to more severe stages, measured by MMSE.18 Lower MMSE scores indicate worse cognitive status. Columbia University Scale for Psychopathology in Alzheimer's Disease (CUSPAD), a semi-structured interview administered by a physician or a trained research technician, was used to measure patients' psychotic, behavioral, and depressive symptoms.25, 26 We used the Unified Parkinson's Disease Rating Scale (UPDRS) to measure extrapyramidal signs (EPS).26-28 Patients' medical histories were used to construct a modified version of the Charlson index of comorbidity.29 Comorbid conditions included myocardial infarct, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal diseases, mild liver disease, diabetes, chronic renal disease, and systemic malignancy. No patients reported clinical strokes, metastatic tumors, or AIDS at baseline. Patients' age, ethnicity, sex, highest level of education, and marital status were also recorded.
Analysis
We compared patient characteristics across the DS score quartiles. Comparisons of categorical variables were performed using χ2 tests and comparisons of continuous variables were performed using ANOVAs. Following our previous work, we estimated separate equations for total costs, direct medical costs, and informal caregiving costs using generalized linear models (GLM).13, 14
Our independent variables fall into three groups: main independent variables (DS and BDRS scores), other clinical variables (e.g., MMSE), and demographic variables. We were concerned with possible collinearity between our main independent variables and other clinical variables. We therefore estimated two sets of models for each equation: a full model, which controlled for all clinical and demographic variables in addition to our main independent variables, and a trimmed model, which only controlled for the demographic variables in addition to our main independent variables. We chose to present results from the trimmed models over the full models for the following reasons: (1) Aside from depressive symptoms and comorbidity index, the excluded clinical variables were highly correlated with DS and BDRS (correlations between the excluded clinical variables with DS ranged from r=-0.28 for MMSE to r=0.20 for EPS, and with BDRS ranged from 0.15 for behavioral problems to 0.25 for EPS); (2) The individual clinical variables were not statistically significant in the estimating models and coefficient estimates for DS and BDRS in the full models were not substantially different from those in the trimmed models; (3) Comparison of the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) suggest that the trimmed models performed as well as the full models. Results of the full models are available upon request. We also examined the effect of an interaction term for DS and BDRS. Results showed that the interaction term was not statistically significant in any of the models and therefore was dropped from the final specification. All analyses were performed using Stata 9.0.30
Results
Baseline demographic and clinical characteristics of the patients are summarized in Table 1 and compared across DS quartiles. Because of the study inclusion criteria, patients were at early stages of AD. The typical patient was female (58%), 76 years old, white, and had over 14 years of education. Average DS score was 5.2 (sd=2.2), indicating a mild level of dependence. Almost a quarter of the patients (23.5%) had DS scores of 3 or lower; another 31.2% had DS scores 4 and 5; the modal DS score was 6 (24.0%), and the rest had DS scores between 7 and 12 (21.2%). At baseline, no patient had DS scores above 12. Average MMSE in this sample was 22.1 (sd=3.6) and mean BDRS score was 3.5 (sd=2.1). While almost all patients had some IADL limitations (98.8%), two-thirds (64%) were not limited in BADLs. Behavioral problems (41.6%) were common. About a third (30.2%) had psychotic symptoms, 20.5% had depressive symptoms, and 14.5% had EPS. On average, patients had fewer than one comorbid condition (mean=0.8, sd=0.9); almost half of the patients (47.8%) did not have any comorbid conditions. The most prevalent comorbid conditions included hypertension (36.9%), diabetes (9.6%), and myocardial infarction (6.2%). During the previous year, patients averaged fewer than one hospitalization (mean=0.3, sd=0.6), two outpatient treatment/procedures (mean=2.0, sd=2.1), one assistive device (mean=1.2, sd=1.1), and four medications (mean=3.8, sd=1.5). Although patients were at early stages of AD, they received an average of 20.7 hours of informal care a week (sd=24.0).
Table 1. Demographic and Clinical Characteristics by Quartile of Dependence Scale.
| Quartile of Dependence Scale | |||||
|---|---|---|---|---|---|
| All Sample | 1 | 2 | 3 | 4 | |
| N | 179 | 42 | 56 | 43 | 38 |
| Range of DS Score | 0-15 | 0-3 | 4-5 | 6 | 7-15 |
| Female | 58.1 (104) | 66.7 (28) | 53.6 (30) | 53.5 (23) | 60.5 (23) |
| Age in years, mean (SD)*** | 76.03 (8.0) | 72.83 (7.7) | 75.41 (7.3) | 76.77 (8.8) | 79.63 (7.1) |
| Younger than 65, % (n)*** | 8.9 (16) | 14.3 (6) | 7.1 (4) | 11.6 (5) | 2.6 (1) |
| 65-74, % (n) | 28.5 (51) | 42.9 (18) | 37.5 (21) | 14.0 (6) | 15.8 (6) |
| 75-84, % (n) | 49.2 (88) | 40.5 (17) | 44.6 (25) | 55.8 (24) | 57.9 (22) |
| 85 or older, % (n) | 13.4 (24) | 2.4 (1) | 10.7 (6) | 18.6 (8) | 23.7 (9) |
| Race, % (n) | |||||
| White | 95.5 (171) | 95.2 (40) | 96.4 (54) | 93.0 (40) | 97.4 (37) |
| Other | 4.5 (8) | 4.8 (2) | 3.6 (2) | 7.0 (3) | 2.6 (1) |
| Years of schooling completed, mean (SD) | 14.37 (3.1) | 14.83 (3.5) | 14.64 (3.3) | 14.40 (2.8) | 13.42 (2.7) |
| <12, % (n) | 9.5 (17) | 9.5 (4) | 12.5 (7) | 7.0 (3) | 7.9 (3) |
| 12, % (n) | 33.5 (60) | 31.0 (13) | 26.8 (15) | 32.6 (14) | 47.4 (18) |
| 13-15, % (n) | 15.6 (28) | 9.5 (4) | 14.3 (8) | 23.3 (10) | 15.8 (6) |
| >=16, % (n) | 41.3 (74) | 50.0 (21) | 46.4 (26) | 37.2 (16) | 28.9 (11) |
| BDRS, mean (SD)*** | 3.50 (2.1) | 1.69 (1.1) | 2.91 (1.1) | 4.05 (1.5) | 5.75 (2.3) |
| MMSE, mean (SD)*** | 22.12 (3.6) | 23.55 (3.2) | 22.64 (4.0) | 21.33 (3.6) | 20.68 (2.6) |
| Behavioral problems, % (n)** | 41.6 (74) | 21.4 (9) | 43.6 (24) | 48.8 (21) | 52.6 (20) |
| EPS, % (n)*** | 14.5 (25) | 10.0 (4) | 3.6 (2) | 9.8 (4) | 40.5 (15) |
| Depressive symptoms, % (n) | 20.5 (36) | 11.9 (5) | 27.8 (15) | 16.3 (7) | 24.3 (9) |
| Psychotic symptoms, % (n)** | 30.2 (54) | 11.9 (5) | 30.4 (17) | 32.6 (14) | 47.4 (18) |
| Number of comorbidities, mean (SD)** | 0.78 (0.9) | 0.48 (0.8) | 0.71 (0.9) | 0.95 (1.0) | 1.03 (0.9) |
| 0, % (n)*** | 47.8 (85) | 69.0 (29) | 53.6 (30) | 34.9 (15) | 29.7 (11) |
| 1, % (n) | 34.3 (61) | 19.0 (8) | 26.8 (15) | 48.8 (21) | 45.9 (17) |
| 2+, % (n) | 18.0 (32) | 11.9 (5) | 19.6 (11) | 16.3 (7) | 24.3 (9) |
| Utilization of direct medical care | |||||
| Number of hospitalizations, mean (SD) | 0.32 (0.6) | 0.19 (0.5) | 0.32 (0.6) | 0.42 (0.5) | 0.34 (0.6) |
| Number of outpatient treatments/procedures, mean (SD) ** | 2.02 (2.1) | 1.31 (1.4) | 1.88 (2.0) | 2.40 (2.2) | 2.61 (2.4) |
| Number of assistive devices, mean (SD) *** | 1.19 (1.1) | 0.93 (0.7) | 0.86 (0.6) | 1.51 (1.3) | 1.61 (1.4) |
| Number of medications, mean (SD) | 3.79 (1.5) | 3.50 (1.5) | 3.98 (1.3) | 3.70 (1.4) | 3.92 (1.9) |
| Informal caregiving hours per week, mean (SD) ** | 20.70 (24.0) | 14.09 (25.6) | 17.11 (21.6) | 27.75 (24.2) | 25.38 (23.3) |
: Differences between quartiles of Dependence Scale significant at p<0.10,
:Differences between quartiles of Dependence Scale significant at p<0.05,
: Differences between quartiles of Dependence Scale significant at p<0.01.
DS=Dependence Scale (range=0-15); BDRS=Blessed Dementia Rating Scale (range=0-17); MMSE=Mini-Mental State Examination (range=0-30); EPS=Extrapyramidal signs.
Data in Table 1 show that DS was related to other clinical characteristics in expected ways and suggest that DS captures global severity and various aspects of the disease. Aside from depressive symptoms, DS was strongly associated with all other clinical characteristics included in the analysis. Specifically, patients with more severe levels of dependence were older (p=0.001), had more functional limitations (p<0.001), worse MMSE scores (p<0.001), more comorbidities (p=0.006), and were more likely to exhibit behavioral problems (p=0.02), EPS (p=0.005), and psychotic symptoms (p=0.007). In addition, patients with more severe levels of dependence had more outpatient treatment/procedures (p=0.02), more assistive devices (p<0.001), and received more informal caregiving time (p=0.02).
Unadjusted Costs
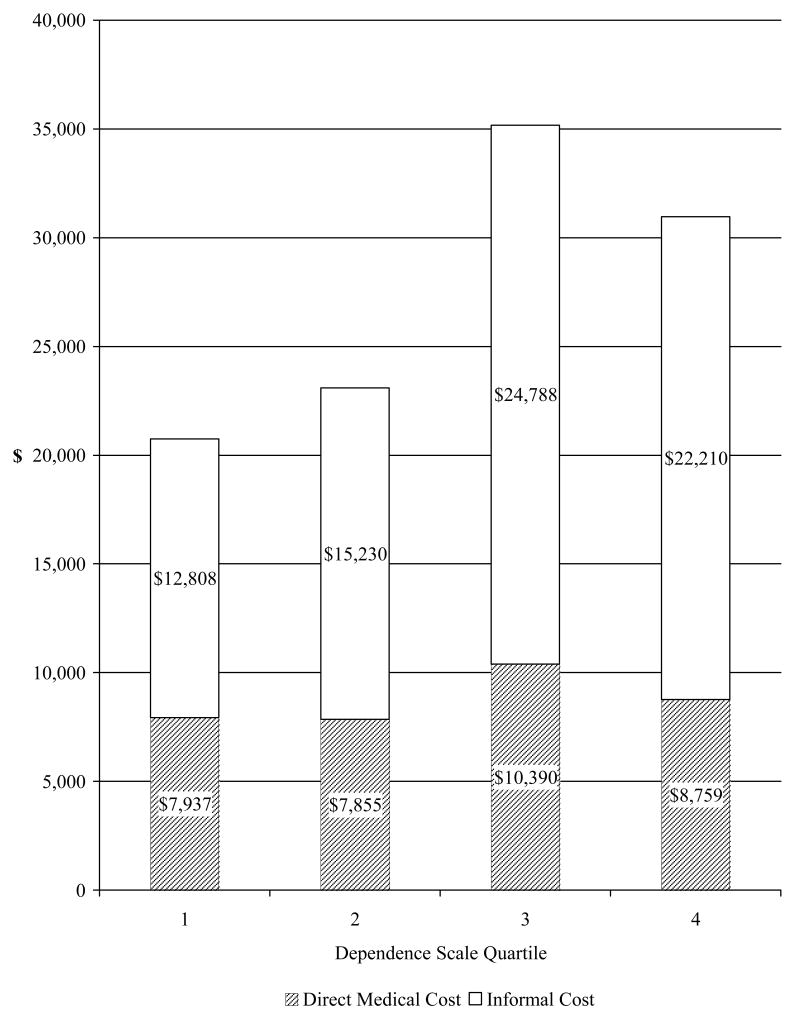
At baseline, almost all patients used some medical care (98.3%, n=176) and informal care (93.9%, n=168). Average annual direct medical costs were estimated at $8,675 and informal costs at $18,440 (∼19 hours per week). Figure 1 plots reported average annual direct medical costs and informal costs by DS score quartiles. Total costs and informal costs were significantly higher among patients in higher DS score quartiles (p=0.03 and p=0.04).
Figure 1. Direct Medical and Informal Costs by Dependence Scale Quartile.
Direct medical costs included costs of hospitalization, outpatient treatment/procedures, assistive devices, and medications, with prices obtained from public databases. Informal costs were estimated by costs of informal caregiving time for basic and instrumental activities of daily living obtained from up to three caregivers for each patient. National average hourly earning for all private industries was used as the hourly wage rate to estimate informal caregiving costs.
Adjusted Costs
Table 2 presents multivariate regression results of patient characteristics on total costs, direct medical costs, and informal costs. Both the DS and BDRS were entered in the model as continuous variables and were significantly associated with higher total costs: A one-point increase in DS was associated with $1,832 increase in total costs and a one-point increase in BDRS was associated with $3,333 increase in total costs. Examining direct medical costs and informal costs separately identified potential differences between DS and BDRS. Direct medical costs were significantly associated with BDRS: a one-point increase in BDRS was associated with a $1,406 increase in direct medical costs. On the other hand, informal costs were significantly associated with DS: a one-point increase in DS was associated with a $1,690 increase in informal costs.
Table 2. Generalized Linear Models of Direct Medical Costs and Informal Costs at Baseline.
| Total Costs | Medical Costsa | Informal Costsb | |
|---|---|---|---|
| Dependence Scale (DS) | 1,832 ** | 298 | 1,690 ** |
| (916) | (408) | (749) | |
| Blessed Dementia Rating Scale (BDRS) | 3,333 ** | 1,406 ** | 1,941 |
| (1,698) | (712) | (1,458) | |
| Age younger then 65 (1=yes, 0=no) | -5,751 | -5,323 ** | -1,322 |
| (6,055) | (2,641) | (5,058) | |
| Women (1=yes, 0=no) | 887 | -1,214 | 1,965 |
| (3,794) | (1,613) | (3,197) | |
| Site (reference=Columbia) | |||
| Johns Hopkins | -3,611 | -2,276 | -1,784 |
| (4,551) | (1,927) | (3,835) | |
| Massachusetts General | -12,902 ** | -1,316 | -12,074 *** |
| (4,471) | (1,959) | (3,754) | |
| Constant | 50,305 *** | 8,942 *** | 23,515 *** |
| (5,457) | (2,368) | (4,519) | |
| Log likelihood | -2118.66 | -1907.99 | -2038.94 |
| Akaike Information Criterion (AIC) | 22.38 | 20.19 | 21.54 |
| Bayesian Information Criterion (BIC) | -809.44 | -795.75 | -870.14 |
Direct medical costs included costs of hospitalization, outpatient treatment/procedures, assistive devices, and medications, with prices obtained from public databases.
Informal costs were estimated by costs of informal caregiving time for basic and instrumental activities of daily living obtained from up to three caregivers for each patient. National average hourly earning for all private industries was used as the hourly wage rate to estimate informal caregiving costs.
: p<0.10,
: p<0.05,
: p<0.01
Discussion
In this study we estimated in a sample of patients in early stages of AD the incremental effect of patients' dependence on total costs of care and on two main component costs, direct medical care costs and informal caregiving costs, controlling for patient's function. We measured patients' dependence on others by the Dependence Scale (DS) and patients' function by the Blessed Dementia Rating Scale (BDRS). As expected, as patients' dependence increased, all aspects of costs substantially increased. Similar to previous studies, patients' function was independently associated with costs. We found that both function and dependence were significantly associated with total costs, yet related differently to direct medical care costs and informal caregiving costs. Poorer function was associated with higher direct medical care costs, while more severe levels of dependence were associated with higher informal caregiving costs. These results confirm that BDRS and DS represent distinct components of disability in AD, and suggest that measures of patients' function and dependence provide unique information for explaining variations in costs of care for patients with AD, and highlight the value of measuring both constructs in economics and outcomes research.
These results have substantial policy implications. They provide information for deriving estimates of potential cost savings if interventions are developed that aim to improve patients' function and lessen their dependence on others. Earlier studies have estimated that BDRS and DS scores worsen by, respectively, 1.5 points and 1 point per year.6, 31 Results in this study suggest that small differences in patients' function and dependence may be associated with large differences in medical care costs and informal caregiving costs. For example, an intervention that delays the worsening of BDRS score by 1 point among AD patients could be expected to yield average savings of $1,406 per year in direct medical costs. An intervention that delays the worsening of DS score by 1 point among AD patients could be expected to yield average savings of $1,690 per year in informal caregiving costs. Thus, the choice of interventions that aim to delay a patient moving to higher levels of either functional impairment or dependence on others have the potential to yield substantial economic benefit. Comparison of the strengths of the effects of BDRS and DS on different cost components suggests that success of the interventions to control costs and improve patient outcome depend on the cost component targeted.
It is notable that the potential cost savings we estimated are generated from a sample of mildly demented patients. Although most cost savings may not be realized immediately, a delay in disease progression for patients at early stages of the disease may yield greater cost savings than the same delay experienced by patients at later disease stages. Because subjects have been followed closely in our study, our future work will address issues of lifetime cost savings more appropriately by using longitudinal analyses. Longitudinal analyses also will confirm whether the relationships of BDRS and DS with different cost components are consistent over time.
In this study, we focused on direct medical costs and informal costs. An important component of costs that is not included in this analysis is non-medical costs, which include, among others, costs for home health aides, respite care, and adult day care. Previous studies have shown that, compared to direct medical costs and informal costs, the proportion of total costs attributable to non-medical costs is relatively small.32 Therefore, the effects of excluding non-medical costs from our total cost estimations should be minimal. Results from secondary analyses including use of non-medical care as an explanatory variable showed that it was not significantly associated with direct medical care costs or informal costs. Indeed, few patients in this sample (12.3%, n=22) reported using non-medical care, precluding detailed analysis of its relationship to patients' dependence. Bivariate analysis of the relationship between utilization (and costs) of non-medical care and patients' dependence showed that there was minimal use (and costs) of non-medical care for patients at mild levels of dependence, and that costs did not begin to rise until moderate levels of dependence were reached. This suggests that over time, as patients' dependence increases, utilization and costs of non-medical care will likely increase. While the magnitude of these costs may continue to be relatively small compared to direct medical care and informal care costs, they are nevertheless important for patients and families. Future longitudinal analyses will examine utilization and costs of non-medical care in more detail.
Several limitations of this study should be noted. First, data reported here are cross-sectional; therefore results can only be interpreted as associations. While poorer function and dependence may lead to higher costs, it also is possible that low spending on healthcare indicates insufficient medical care and results in poor health. In this sample of patients with relatively high education levels, however, the latter explanation is less likely. Second, aside from the patient characteristics included in the model, other variables may be associated with higher costs, however, the focus of this study was to examine whether the dependence scale could explain variations in costs, and identifying predictors of informal care was beyond the scope of the paper. Third, data on patients' healthcare costs from this study were reported by patients and informants, most of whom were the patients' primary caregivers. Studies have shown that caregivers are able to accurately report medical information of their care recipients.33, 34 There is no reason to believe our sample is systematically different, although it is possible that there are additional costs important to patients and families beyond the resource items collected. Our cost estimates were from a society's perspective, as all costs, regardless of the payer, were collected. Fourth, patients were selected from tertiary care university hospitals and specialized diagnostic and treatment centers and thus represent a nonrandom sample of those affected by AD in the population. The patients in our sample also were predominantly white and highly educated. Caution is needed in generalizing the results of this study to patients with lower levels of education and income and to non-white patients. Future research will need to examine the relationship between costs and the potential variables in samples that are more representative of the general population. However, because patients were drawn from multiple locations, generalizability of our findings is enhanced. We found substantial cost differences across sites. This result is consistent with regional differences in health services utilization and costs documented in the literature35 and more specifically a recent study on service utilization and costs among patients with AD.36 Because different sites were included in these studies, our results are not directly comparable with these studies. Further investigations are needed to examine whether variations in utilization and costs reflect differences in regional preferences, availability or access of services, ethnic and cultural differences, or socioeconomic factors.
Acknowledgments
Financial Disclosures: The Predictors Study is supported by Federal grants AG07370, RR00645, and U01AG010483. Drs. Zhu and Sano also are supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors all certify that they have no relevant financial interests in this manuscript and report no conflict of interest.
Footnotes
Author Contributions: Carolyn W. Zhu: conception and design, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content. Nikolaos Scarmeas and Yaakov Stern: conception and design, acquisition of data, critical revision of manuscript for important intellectual content. Christopher Leibman and Trent McLaughlin: critical revision of manuscript for important intellectual content. Marilyn Albert, Jason Brandt, Deborah Blacker, and Mary Sano: acquisition of patients and data, critical revision of manuscript for important intellectual content.
Sponsor's Role: The sponsors had no role in executing or publishing this project.
References
- 1.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 2.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 3.Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14:116–118. doi: 10.1097/00005650-197605001-00018. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y, Hesdorffer D, Sano M, et al. Measurement and prediction of functional capacity in Alzheimer's disease. Neurology. 1990;40:8–14. doi: 10.1212/wnl.40.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer's disease. J Gerontol. 1994;49:M216–222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 6.Brickman AM, Riba A, Bell K, et al. Longitudinal assessment of patient dependence in Alzheimer disease. Arch Neurol. 2002;59:1304–1308. doi: 10.1001/archneur.59.8.1304. [DOI] [PubMed] [Google Scholar]
- 7.Holtzer R, Wegesin DJ, Albert SM, et al. The rate of cognitive decline and risk of reaching clinical milestones in Alzheimer disease. Arch Neurol. 2003;60:1137–1142. doi: 10.1001/archneur.60.8.1137. [DOI] [PubMed] [Google Scholar]
- 8.Sarazin M, Stern Y, Berr C, et al. Neuropsychological predictors of dependency in patients with Alzheimer disease. Neurology. 2005;64:1027–1031. doi: 10.1212/01.WNL.0000154529.53488.30. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DH, Jr, Schenkman M, Zhou J, et al. The relative effect of Alzheimer's disease and related dementias, disability, and comorbidities on cost of care for elderly persons. J Gerontol B Psychol Sci Soc Sci. 2001;56:S285–293. doi: 10.1093/geronb/56.5.s285. [DOI] [PubMed] [Google Scholar]
- 10.Small GW, McDonnell DD, Brooks RL, et al. The impact of symptom severity on the cost of Alzheimer's disease. J Am Geriatr Soc. 2002;50:321–327. doi: 10.1046/j.1532-5415.2002.50065.x. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson L, Eriksdotter Jonhagen M, Kilander L, et al. Determinants of costs of care for patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:449–459. doi: 10.1002/gps.1489. [DOI] [PubMed] [Google Scholar]
- 12.Hill J, Fillit H, Thomas SK, et al. Functional impairment, healthcare costs and the prevalence of institutionalisation in patients with Alzheimer's disease and other dementias. Pharmacoeconomics. 2006;24:265–280. doi: 10.2165/00019053-200624030-00006. [DOI] [PubMed] [Google Scholar]
- 13.Zhu CW, Scarmeas N, Torgan R, et al. Clinical characteristics and longitudinal changes of informal cost of Alzheimer's disease in the community. J Am Geriatr Soc. 2006;54:1596–1602. doi: 10.1111/j.1532-5415.2006.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu CW, Scarmeas N, Torgan R, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67:998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]
- 15.Murman DL, Von Eye A, Sherwood PR, et al. Evaluated need, costs of care, and payer perspective in degenerative dementia patients cared for in the United States. Alzheimer Dis Assoc Disord. 2007;21:39–48. doi: 10.1097/WAD.0b013e31802f2426. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7:3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Richards M, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). II. Neurological, psychiatric, and demographic influences on baseline measures of disease severity. Alzheimer Dis Assoc Disord. 1993;7:22–32. doi: 10.1097/00002093-199307010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Stern Y, S M, Paulson J, et al. Modified mini-mental state examination: validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- 20.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 21.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 22.Menzin J, Lang K, Friedman M, et al. The economic cost of Alzheimer's disease and related dementias to the California Medicaid program (“Medi-Cal”) in 1995. Am J Geriatr Psychiatry. 1999;7:300–308. [PubMed] [Google Scholar]
- 23.Penrod JD, Kane RL, Finch MD, et al. Effects of post hospital Medicare home health and informal care on patient functional status. Health Services Research. 1998;33:513–529. [PMC free article] [PubMed] [Google Scholar]
- 24.Council of Economic Advisers. Economic Report of the President. Washington, DC: 2006. [Google Scholar]
- 25.Devanand DP, Miller L, Richards M, et al. The Columbia University Scale for Psychopathology in Alzheimer's disease. Arch Neurol. 1992;49:371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- 26.Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. Jama. 1997;277:806–812. [PubMed] [Google Scholar]
- 27.Stern MB, Hurting HI. The Comprehensive Management of Parkinson's Disease. PMA Corp; New York: 1978. The clinical characteristics of Parkinson's Disease and parkinsonian syndromes: diagnosis and assessment; pp. 3–50. [Google Scholar]
- 28.Richards M, Marder K, Bell K, et al. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol. 1991;48:1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Statacorp, Stata: College Station, Texas.
- 31.Stern Y, Liu X, Albert M, et al. Application of a growth curve approach to modeling the progression of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 1996;51:M179–184. doi: 10.1093/gerona/51a.4.m179. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C, Scarmeas N, Torgan R, et al. Home Health and Informal Care Utilization and Costs Over Time in Alzheimer's Disease. Home Health Care Services Quarterly. 2008;27:1–20. doi: 10.1300/J027v27n01_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corder LS, Woodbury MA, Manton KG. Proxy response patterns among the aged: effects on estimates of health status and medical care utilization from the 1982-1984 long-term care surveys. J Clin Epidemiol. 1996;49:173–182. doi: 10.1016/0895-4356(95)00507-2. [DOI] [PubMed] [Google Scholar]
- 34.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: lessons, challenges, and opportunities. Journal of the American Geriatrics Society. 2000;48:1646–1654. doi: 10.1111/j.1532-5415.2000.tb03877.x. [DOI] [PubMed] [Google Scholar]
- 35.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 36.Harrow BS, Mahoney DF, Mendelsohn AB, et al. Variation in cost of informal caregiving and formal-service use for people with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2004;19:299–308. doi: 10.1177/153331750401900507. [DOI] [PMC free article] [PubMed] [Google Scholar]