Abstract
Osteoprotegerin (OPG) serves as a soluble decoy receptor for RANKL to inhibit osteoclast formation and activity. Hormones such as PTH and glucocorticoids have been reported to decrease OPG concentrations, while estrogens, transforming growth factor b, related bone morphogenic factor and thrombopoietin reportedly enhance the OPG production in the osteoblastic and bone stromal cells. Since bone turnover shows a prominent circadian rhythm in laboratory animals and humans, with bone resorption increasing at night, we investigated the time structure of circulating OPG concentrations in a group of nine healthy subjects (six women and three men; in the age range of 26–49 years). Blood samples for OPG determination were collected every 4 h for 24 h on the same day, starting at 08:00 in the morning. Data were analyzed by inferential statistical procedures, including the single and population-mean cosinor. A 12-h component was found to characterize serum OPG concentrations (P = 0.038) with peak concentrations around noon and midnight. No statistically significant circadian rhythm of OPG concentrations could be found by cosinor in our study population. The mean 24-h OPG concentration was higher in women than in men (mean ± S.E.: 3.13 ± 0.44 vs. 1.94 ± 0.26 pmol/1, Student t = 2.325, P = 0.053). Since PTH concentrations also exhibit a bimodal pattern along the 24-h scale, PTH may be tested as a putative determinant of the observed changes in serum concentrations of osteoprotegerin.
Keywords: Osteoprotegerin, Circadian, Circasemidian, Bone turnover
1. Introduction
Bone turnover in laboratory animals and humans reportedly undergoes a circadian rhythm, with bone resorption and, to a lesser extent, bone formation increasing at night [1-3]. Such rhythms have clinical implications for the timing of sample collection and for the assessment of a given therapeutic intervention. Recently, osteoprotegerin (OPG), a secreted member of the tumor necrosis factor receptor (TNFR) super-family, has been identified as an osteoblast-derived coordinator of bone resorption and bone mass and has been implicated in the pathogenesis of postmenopausal osteoporosis and other metabolic bone diseases [4,5]. OPG acts by neutralizing the receptor activator of nuclear-kB ligand (RANKL), an essential cytokine required for osteoclast formation and action [6]. OPG is synthesized as a propeptide of which the signal peptide is cleaved, thus generating the mature peptide. In contrast to all other TNFR super-family members, OPG lacks transmembrane and cytoplasmatic domains and is secreted as a soluble protein into the blood stream, where it can be detected. We here investigate any variation in serum OPG concentrations in clinically healthy subjects maintained in a carefully standardized environment. We do so time-microscopically to ask whether this potent inhibitor of osteoclast formation is secreted according to a periodic pattern, and, if so, to determine what its pattern may be.
2. Subjects and methods
Our study population included nine subjects (six women and three men; mean age: 32.5 ± 7.8 years; range: 26–49 years). All subjects were hospitalized in our Internal Medicine Unit where the blood samples were obtained under standardized conditions. Domestic lights were turned off from 22:00 to 07:00. Meals were served at 08:00, 13:00 and 19:30 and were consumed within 30 min. Subjects were free to ambulate (sitting or walking) from 07:00 to 22:00 and were recumbent from 22:00 to 07:00 (sleeping hours), except to urinate. The medical history, physical examination and a general laboratory screening, including hematological and biochemical tests gave no indication of specific organ dysfunction. Subjects were not under the influence of bone active medication, including estro-progestin in any form. None of the subjects was a nightshift worker and/or recently traveled across several time zones. On April 28, 2004, blood samples for OPG determination were drawn every 4 h for 24 h starting at 08:00 in the morning, just before breakfast. Thus, each subject provided data at six timepoints.
Sera were separated through centrifugation at 3000 rpm for 15 min. and frozen at −20 °C until determination. OPG serum concentrations were determined using a highly sensitive commercial sandwich enzyme immunoassay provided by Immunodiagnostic (Bensheim, Germany). Measurements were performed in undiluted samples according to the manufacturer's instructions. The lower limit of detection of this assay is 0.14 pmol/1. The intra-assay (n = 16) coefficient of variation is <10% and the inter-assay (n = 16) is < 10%. All subjects gave written informed consent prior to inclusion in the study.
Each data series, expressed in original units or as a percentage of the arithmetic mean, was analyzed by single cosinor [7,8], using 24 and 12 h as trial periods. Estimates were thus obtained for the Midline Estimating Statistic Of Rhythm (MESOR), a chronome-adjusted mean value, and for each component for its double amplitude, a measure of the predictable extent of change within a cycle, and for its acrophase, a measure of the timing of overall high values recurring in each cycle. The acrophase was expressed in negative degrees, with 360 ° equated to the period length (24 or 12 h) and 0 ° (the reference time) set to local midnight.
Circadian and circasemidian rhythm parameters were further summarized by population-mean cosinor [7,8]. Statistical significance was determined by the zero-amplitude (no-rhythm) test. Statistical significance was considered at a value of P < 0.05. The Student's t-test was used to compare results from men and women. One-way analyses of variance (ANOVA) were used to test the equality of timepoint means.
3. Results
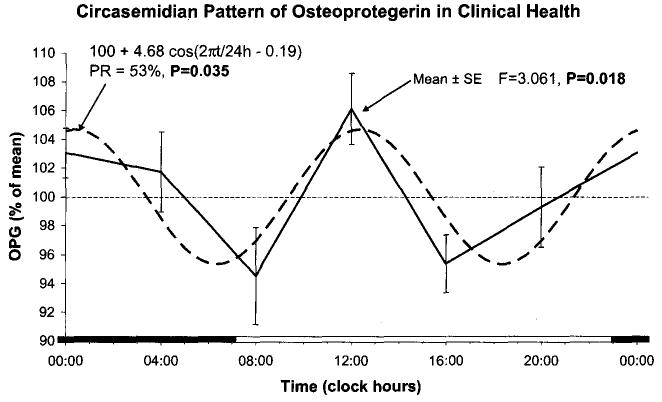
On an individual basis, with only six samples, a 24 or 12-h component cannot be detected with statistical significance. On a group basis, analysis of the data expressed in original units fails to detect a circadian rhythm, either by one-way ANOVA testing the equality of timepoint means (F = 0.104, P = 0.991) or by cosinor (P = 0.704). Cosinor analysis, however, detects a statistically significant 12-h component (P = 0.038). Because large inter-individual variation characterizes OPG concentrations, analyses were repeated after expressing the data as a percentage of each time series' mean value. A time effect is thereby detected by one-way ANOVA (F = 3.061, P = 0.018). Cosinor analysis confirms the presence of a 12-h component (P = 0.035) accounting for 53% of the total variation, on the average. The extent of predictable change averages 9.35% of the mean, with peaks around noon and midnight, Fig. 1.
Fig. 1.
Circasemidian pattern of serum osteoprotegerin concentration expressed as a percentage of each series' mean value. Means and standard errors from nine healthy subjects at each time point, 4 h apart for 24 h are shown along with the 12-h cosine curve derived by population-mean cosinor. The rest and/or sleep span is shown on the horizontal scale by the dark bars.
On the average, the MESOR of serum OPG was 2.74 ± 0.65 pmol/1. Part of the inter-individual variation is accounted for by a difference between men and women, women having higher OPG concentrations than men (MESOR: 3.13 ± 0.04 vs. 1.94 ± 0.26pmol/1; Student t = 2.325, P = 0.053 two-tailed). Although the sample size is too small to rigorously assess the circadian variation of circulating OPG separately for men and women, such analysis confirms the circasemidian variation in women (original units: P = 0.021; % of mean: P = 0.016), but finds the circadian component more prominent than the circasemidian one in men, without reaching statistical significance, however (original units: P = 0.054; % of mean: P = 0.113).
4. Discussion
A Medline search did not find any publication examining the circadian pattern of serum OPG concentrations in clinical health. Only one abstract [9] reported a circadian rhythm in OPG and PTH by cosinor (P < 0.001) in eight volunteers with a mean age of 56 ± 7 years, older than the subjects examined herein. On the basis of hourly peripheral blood sampling, these investigators reported the circadian rhythms of OPG and PTH to be out of phase, concluding that a decrease in OPG in response to increasing PTH may result in increased bone resorption by osteoclasts [9]. Our results based on sparser sampling show a bimodal variation along the 24-h scale instead. Further work is indicated with groups of different ages in order to understand the reasons for the discrepancy and to obtain information about specific features of the distribution volume and clearance of OPG that might account for the observed changes in its serum concentrations.
Regarding the production of OPG, hormones, cytokines and humoral factors produced in distant organs can also influence the RANKL/OPG expression within bone cells. In particular, hormones such as PTH [10] and glucocorticoids [11,12] have been reported to decrease OPG concentrations while anabolic agents such as estrogens, transforming growth factor β, related bone morphogenic factor and thrombopoietin [13-16] have been shown to enhance the OPG production in the osteoblastic and bone stromal cells. Recent studies [17-19] have described a bimodal pattern for the variation along the 24-h scale of circulating PTH, with a major peak during the night and a secondary peak in the late afternoon. Because of the negative influence of PTH on OPG production, it seems likely that this secretory pattern could contribute to the observed nadirs of OPG concentrations, respectively, during the late afternoon and the hours before awakening. As noted earlier, however, the majority of studies have revealed that the biochemical markers of bone resorption and bone formation show a circadian rhythm with a peak during the night and a nadir during the afternoon, but without any statistically significant ultradian variation, suggesting that the bimodal pattern of serum OPG concentrations observed herein could also be related to different stimuli.
When considering circulating OPG measurements, it should kept in mind that this receptor is produced by many tissues including the lung, kidney, heart, liver, stomach, brain, spinal cord and thyroid gland. Circulating concentrations may reflect the production by a number of different tissues [4,20]. Thus, it would appear likely that the observed circasemidian change in serum OPG observed herein may be related to the different contributions by different tissues to its circulating concentrations. Finally, because of the extra skeletal production of OPG, it seems likely that our measurements of OPG serum concentrations may have under- or over-estimated the changes in local OPG production within the confines of bone. These limitations, however, are inherent to all studies that would assess circulating concentrations of paracrine agents.
Other investigators report a steady increase in OPG with age in both genders without a gender difference [21]. The difference between men and women observed herein may have been a chance finding related to the small sample size. From a methodologic viewpoint, the inter-individual variation, whether related in part to a gender difference or not, likely obscured the circadian/circasemidian pattern of circulating OPG. Rhythmicity was uncovered by expressing the data as a percentage of each subject's mean value. Such a simple data transformation to enhance variability patterns within the normal range had been advocated as early as 1953 [22].
References
- 1.Eastel R, Simmons PS, Colwell A, Assir AMA, Burrit MF, Russel RGG, et al. Nyctohemeral changes in bone turnover assessed by serum gla-protein concentration and urinary deoxypyridinoline excretion: effects of growth and ageing. Clin Sci. 1992;83:375–82. doi: 10.1042/cs0830375. [DOI] [PubMed] [Google Scholar]
- 2.Eastel R, Calvo MS, Burrit ME, Offord KP, Russel RGG, Riggs BL. Abnormality in circadian pattern of bone resorption and renal calcium conservation in type 1 osteoporosis. J Clin Endocrinol Metab. 1992;74:487–94. doi: 10.1210/jcem.74.3.1740481. [DOI] [PubMed] [Google Scholar]
- 3.Hasseger C, Risteli J, Risteli L, Jensen Sb, Christiansen C. Diurnal variation in serum markers of type I collagen synthesis and degradation in healthy premenopausal women. J Bone Miner Res. 1992;11:1307–11. doi: 10.1002/jbmr.5650071110. [DOI] [PubMed] [Google Scholar]
- 4.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 5.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 6.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;17:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 7.Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- 8.Cornélissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. Vol. 1. John Wiley & Sons Ltd.; Chichester, UK: 1998. pp. 642–9. [Google Scholar]
- 9.Joseph F, Chan BY, Corlett P, Durham BH, Ahmad AM, White HD, et al. The circadian rhythm of osteoprotegerin and its association with parathyroid hormone secretion. doi: 10.1210/jc.2006-1832. http://www.endocrine-abstracts.org/ea/0009/ea0009p66.htm. [DOI] [PubMed]
- 10.Fu Q, Jilka RL, Manolagas SC, O'Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002;277:48868–75. doi: 10.1074/jbc.M208494200. [DOI] [PubMed] [Google Scholar]
- 11.Vidal NO, Brandstrom H, Jonsson KB, Ohlsson C. Osteoprotegerin mRNA is expressed in primary human osteoblast-like cells: down-regulation by glucocorticoids. J Endocrinol. 1998;159:191–5. doi: 10.1677/joe.0.1590191. [DOI] [PubMed] [Google Scholar]
- 12.Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoids-induced osteoporosis. Endocrinology. 1999;140:4382–9. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- 13.Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, et al. Osteoprotegerin produced by osteoblast is an important regulator in osteoclast development and function. Endocrinology. 2000;141:3478–84. doi: 10.1210/endo.141.9.7634. [DOI] [PubMed] [Google Scholar]
- 14.Schoppet M, Preissner KT, Hofbauer LC. RANKL ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22:549–53. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 15.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 16.Chagraoui H, Tulliez M, Smayra T, Komura E, Giraudier S, Yun T, et al. Stimulation of osteoprotegerin production is responsible for osteosclerosis in mice overexpressing TPO. Blood. 2003;101:2983–89. doi: 10.1182/blood-2002-09-2839. [DOI] [PubMed] [Google Scholar]
- 17.Fuleihan GEH, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA. The parathyroid hormone circadian rhythm is truly endogenous—a General Clinical Research Center Study. J Clin Endocrinol Metab. 1997;82:281–6. doi: 10.1210/jcem.82.1.3683. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen HK, Brixen K, Kassem M, Christensen SE, Mosekilde L. Diurnal rhythm in serum osteocalcin: relation with sleep, growth hormone, and PTH (1–84) Calcif Tissue Int. 1991;49:373–7. doi: 10.1007/BF02555845. [DOI] [PubMed] [Google Scholar]
- 19.Fraser WD, Ahmad AM, Vora JP. The physiology of the circadian rhythm of parathyroid hormone and its potential as a treatment for osteoporosis. Curr Opin Nephrol Hypertens. 2004;13:437–44. doi: 10.1097/01.mnh.0000133985.29880.34. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–37. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 21.Indridason OS, Franzson L, Sigurdsson G. Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporosis Int. 2005;16:417–23. doi: 10.1007/s00198-004-1699-x. [DOI] [PubMed] [Google Scholar]
- 22.Halberg F, Engel R, Treloar AE, Gully RJ. Endogenous eosinopenia in institutionalized patients with mental deficiency—A.M.A. Arch Neurol Psychiatr. 1953;69:462–9. doi: 10.1001/archneurpsyc.1953.02320280050005. [DOI] [PubMed] [Google Scholar]