Abstract
Plasma C-reactive protein (CRP) is an inflammatory biomarker that predicts cardiovascular disease. We investigated whether vitamins C or E could reduce CRP. Healthy nonsmokers (n=396) were randomized to three groups:1000 mg/day vitamin C, 800 IU/day vitamin E, or placebo, for two months. Median baseline CRP was low, 0.85 mg/L. No treatment effect was seen when all participants are included. However, significant interaction was found, indicating that treatment effect depends on baseline CRP concentration. Among participants with CRP indicative of elevated cardiovascular risk (≥1.0 mg/L), vitamin C reduced median CRP by 25.3% vs. Placebo (p=0.02), (median reduction in the vitamin C group, 0.25 mg/L, 16.7%). These effects are similar to those of statins. The vitamin E effect was not significant. In summary, treatment with vitamin C but not E significantly reduced CRP among individuals with CRP ≥ 1.0 mg/L. Among the obese, 75% had CRP ≥ 1.0 mg/L. These data extend previous results in smokers, and identify CRP levels susceptible to reductions. Research is needed to determine whether reducing this inflammatory biomarker with vitamin C could reduce diseases associated with obesity. But research on clinical benefits of antioxidants should limit participants to persons with elevations in the target biomarkers.
Keywords: C-reactive protein, inflammation, vitamin C, vitamin E, obesity, cardiovascular risk, antioxidants, oxidation, randomized controlled trial
INTRODUCTION
Multiple avenues of research have led to the recognition that cardiovascular disease involves a systemic inflammatory process [1]. Prospective studies and primary prevention trials have demonstrated that high-sensitivity C-reactive protein (CRP), an acute phase protein and marker of inflammation, predicts overall cardiovascular disease risk in those without prior disease, and predicts multiple types of cardiovascular disease including myocardial infarction, stroke, and peripheral arterial disease [2]. Further evidence of a link between inflammation, CRP, and atherosclerosis comes from studies of aspirin and statins. Such anti-inflammatory therapies have been shown to lower CRP, while also reducing cardiovascular events and slowing the progression of atherosclerosis. Morever, the magnitude of the clinical benefit of these agents appears to be greatest in individuals with elevated CRP [3–8].
CRP may directly contribute to the atherosclerotic process. It has been found to play a role in early monocyte recruitment [9], adhesion molecule expression [10], and reduced production of the endothelial dilator, nitric oxide [11], among other activities. However, use of purified preparations [12–13], and transgenic mouse models [14–16], have yielded mixed results. Thus, while the extent of the direct role of CRP in atherosclerosis has not yet been clearly elucidated, at the least CRP represents a surrogate marker by which the anti-inflammatory effectiveness of therapies can be evaluated, and it may itself be a potential target for reducing cardiovascular disease risk.
Based on prospective studies and primary prevention trials, the American Heart Association Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases and the Centers for Disease Control and Prevention (CDC/AHA) have designated a CRP concentration of < 1.0 mg/L as representing “low risk” for cardiovascular disease, while concentrations of 1.0 to 3.0 mg/L and > 3.0 mg/L have been designated as representing “average risk” and “high risk,” respectively [17]. Recent research has confirmed that concentrations in the intermediate range of 1.0 to 3.0 mg/L do in fact confer excess risk of cardiovascular disease [18]. CDC/AHA also note that CRP values > 10 mg/L represent acute infection or inflammatory disease, and should not be used in evaluation of cardiovascular disease risk.
We have previously shown CRP to be significantly associated with the biomarkers of oxidative stress, malondialdehyde and F2-isoprostanes [19]. F2-isoprostanes appear to have a direct effect on inflammation, inducing the expression of interleukin-8 and intracellular inflammatory signals [20]. We recently demonstrated that treatment with vitamin C significantly lowers F2-isoprostanes [21]. Our earlier research found a significant reduction in CRP as a result of treatment with 500 mg/day vitamin C among active and passive smokers [22]. The present study was designed to follow up on these observations, in nonsmokers. In this randomized, placebo-controlled parallel-design study, we examined the separate effects of 1000 mg/day vitamin C and 800 IU/day vitamin E for two months on plasma CRP among healthy nonsmokers. Based on our previous work, we hypothesized a greater CRP-lowering effect of vitamin C as compared to vitamin E. In addition, we sought to confirm an earlier observation [23] that antioxidant treatment effect depended on baseline CRP level and was limited to persons with baseline CRP concentrations indicative of elevated cardiovascular risk, CRP ≥ 1.0 mg/L.
MATERIALS AND METHODS
Participants
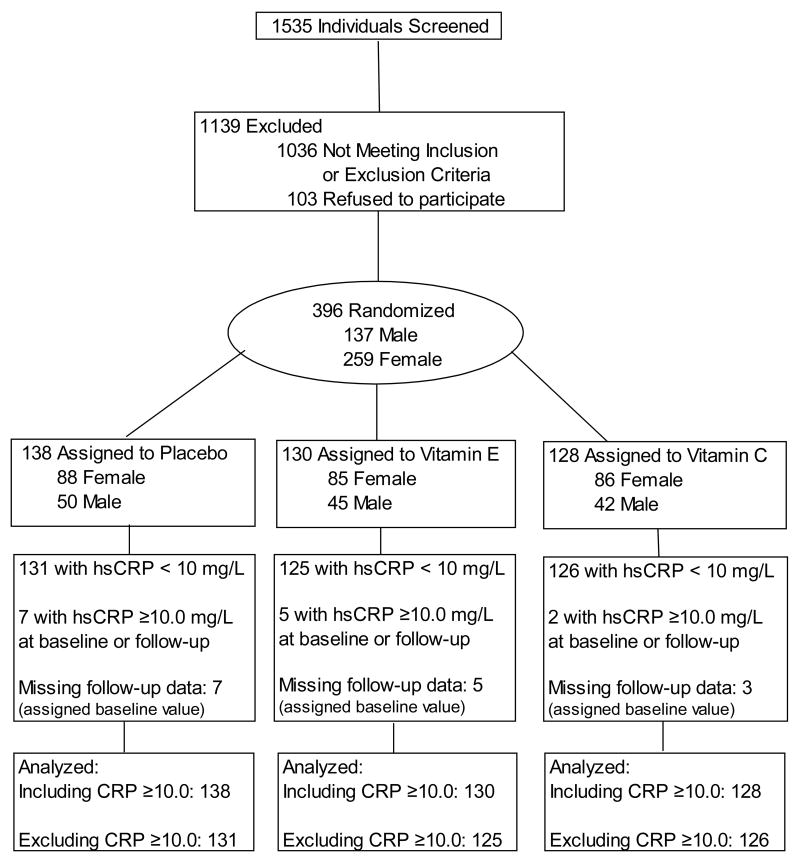
Healthy nonsmokers were recruited between January 2005 and March 2006 from the communities of San Francisco, Berkeley and Oakland, CA. Eligibility was not limited to persons with elevated CRP. Exclusion criteria included age <18 years, smoking, passive smoke exposure (exposed indoors ≥ 5 days/week), alcohol consumption (≥ 2 drinks/day), pregnancy or breast feeding, disease conditions (hemochromatosis, history of kidney stones or other kidney diseases, cancer, stroke, diabetes mellitus, human immunodeficiency virus infection), use of certain prescription medications (anti-inflammatory, statin, lipid-lowering, blood-thinning, hormone replacement therapy, or steroid medications), consumption of single iron supplements or vitamin E supplements in amounts greater than 400 IU/day, and body weight ≥ 300 pounds or height > 75 inches (due to equipment constraints). Those taking multivitamin supplements, vitamin C, lower-dose vitamin E supplements, or over-the-counter anti-inflammatory medications were given the option of participating if they discontinued use of such agents for 30 days prior to their baseline visit and throughout the 60-day intervention. Of 1535 subjects assessed for eligibility, 1139 were ineligible or declined to participate, 396 were enrolled, and 385 completed the study (Figure 1). Of the 11 who terminated early, 3 withdrew due to inconvenience or unwillingness to refrain from taking supplements, 4 were lost to follow-up, 1 died in an automobile accident, and 3 were withdrawn administratively. The study design was approved by the institutional review boards of the University of California at Berkeley and Children’s Hospital & Research Center of Oakland, CA. Signed informed consent was obtained from all participants.
Figure 1.
Flow.diagram of participant progress through the study
Study supplements and allocation
Participants were assigned to one of three different treatment groups using blocked, stratified randomization, and a randomization sequence of letters of the alphabet representing treatment groups was generated by computer program. Randomization was stratified by gender, weight (men: < 68 kg, 68–82 kg, and > 82 kg; women: < 54 kg, 54–68 kg, > 68 kg), and either menopausal status (pre-, peri-, or post-menopausal) for women, or age (18–44 years, 45–59 years, or ≥ 60) for men. Lettered bottles containing treatment components were provided to participants by clinical staff unaware of treatment allocation. The three treatment arms were 1) vitamin C tablet (1000 mg/day ascorbic acid) and placebo capsule; 2) vitamin E capsule (800 IU/day all-natural mixture of RRR d-alpha tocopherol) and placebo tablet; and 3) placebo tablet and placebo capsule. Placebo tablets contained lactose, microcrystalline cellulose, and small amounts of stearic acid, magnesium stearate, and croscarmellose sodium. Placebo capsules contained medium consisting of lecithin and soybean oil. Vitamin C tablets and vitamin E capsules were indistinguishable from placebo tablets and capsules. Study supplements and placebo pills were provided by Pharmavite, LLC (Mission Hills, CA). Investigators, clinic and laboratory staff and participants were masked to treatment assignment. Separate study staff administered the intervention and analyzed data.
Procedures
Pre- and peri-menopausal women were tested for pregnancy and excluded if pregnant (Quidel QuickVue Semi-Q hCG-Combo Test). Anthropometric measurements were obtained by nurses and technicians who underwent extensive initial training and mid-study refreshers. Body weight (Health-O-Meter digital electronic) was measured without shoes, jackets, or heavy sweaters. Height was measured without shoes, using a wall mounted stadiometer (Perspective Enterprise). Height and weight measurements were repeated until two measurements in succession were within 0.5 cm and 0.3 kg respectively. Waist and hip circumference (Fiberglass, Gulick II) were measured using the National Heart, Lung, and Blood Institute’s published guidelines [24]. Sagittal abdominal diameter was measured with abdominal calipers (Holtain-Kahn) at the location of maximal abdominal diameter (directly above the iliac crest), with participants wearing sweatshirt or hospital gown and in the supine position. These latter three measurements were repeated until two consecutive measurements were within 1.0 cm. Body fat was assessed by a whole body scan, at baseline only, using dual energy x-ray absorptiometry (DXA) (Hologic, Delphi A, Model QDR 4500A (S/N 4500); Serial No: 70580; Software: QDR system software version 11.2).
Fasting venous blood was drawn into Vacutainer tubes (Beckton Dickenson, Rutherford, NJ), protected from light, maintained at <15°C, and p rocessed within 6 hours. Vacutainers were centrifuged at 5°C for 10 minutes at 1,200 x g and aliquoted. Plasma aliquots for ascorbic acid were mixed 1:1 with freshly-prepared 10% (w/v) meta-phosphoric acid to stabilize the ascorbic acid. All aliquots were protected from light and stored at −80°C. All sample batches included masked dupli cates and replicated internal control samples.
Participants provided demographic information and completed self-administered diet and physical activity questionnaires (NutritionQuest, formerly Block Dietary Data Systems, www.nutritionquest.com). At both the baseline and follow-up visits, participants completed a questionnaire related to their health and symptoms. At the follow-up clinic visit, unused capsules were counted to assess adherence, and participants completed a questionnaire related to the adequacy of masking.
Laboratory methods
Pre- and post-treatment aliquots were assayed in the same batch to reduce laboratory artifact. High-sensitivity C-reactive protein concentration was measured by latex-enhanced nephelometry using a Hitachi 917 Analyzer [25]. Serum lipids were measured using timed-endpoint, coupled enzymatic methodology [26]. Serum alpha tocopherol and serum carotenoids were measured by reversed-phase HPLC with UV/Vis detection [27]. Serum ascorbic acid was measured spectrophotometrically using 2,4 dinitrophenylhydrazine as chromogen [28], a method that has been shown to correlate highly with HPLC analysis [28–29].
Statistical analysis
Differences in baseline measurements by treatment group were assessed using chi-square tests for categorical variables and analysis of variance tests for continuous measures. Treatment effect was assessed using both nonparametric and parametric multiple regression techniques. In nonparametric analyses, median concentrations at baseline and at study completion were computed, and the significance of the changes within and between groups was assessed by the Wilcoxon rank sum test. In parametric analyses, change in CRP was the dependent variable and CRP was log-transformed to reduce skewness. For the intent-to-treat analysis, participants who did not complete the study or were withdrawn administratively (7, 3, and 5 participants in the placebo, vitamin C, and vitamin E groups, respectively) were given a follow-up CRP value equal to their baseline value.
The examination of effect modification by baseline CRP level was begun by identifying three subgroups of baseline CRP based on previous data [23] and on pre-existing recommendations by CDC/AHA [17]. Interaction was assessed by including the relevant cross-product term in the model. Age, sex, and body mass index (BMI), a variable strongly associated with CRP [30], were also examined for interaction.
Potential covariates were examined, including demographic factors (education, income, and poverty index ratio) and plasma measures at baseline (total, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol. Gender and BMI (associated with change in CRP) and LDL-cholesterol (associated with both exposure and outcome) were included in further models. All models included adjustment for baseline CRP concentration. Significance was defined as p<0.05 (two-tailed) for treatment effect, and at p<0.2 for interaction terms.
RESULTS
Participants had a mean age of 44 years, and 34.6% were male (Table 1). The treatment groups did not differ with respect to gender, ethnicity, age, BMI, or plasma vitamin C or E at baseline, but did differ with respect to LDL-cholesterol (p=0.047). Plasma vitamin C changes were 3.8%, −1.7% and +49.3% in Placebo, Vitamin E and Vitamin C groups respectively, and changes in plasma vitamin E were 11.9%, 103.3% and 5.2% in the respective groups. Adherence, defined as having taken 90% of prescribed pills, was 87% overall and did not differ by treatment group (p=0.41).
TABLE 1.
Participant characteristics.
| Variable | Total | Placebo | Vitamin C | Vitamin E | P* |
|---|---|---|---|---|---|
| N | 396 | 138 | 128 | 130 | |
| Male (n,%) | 137 (34.6) | 50 (36.2) | 42 (32.8) | 45 (34.6) | 0.84 |
| Ethnicity (n,%) | |||||
| White | 284 (71.7) | 96 (69.6) | 92 (71.9) | 96 (73.9) | 0.90 |
| African American | 38 (9.6) | 15 (10.9) | 13 (10.2) | 10 (7.7) | |
| Other | 74 (18.7) | 27 (19.6) | 23 (18.0) | 24 (18.5) | |
| Age (mean, SD) | 44.0 (15.1) | 43.8 (15.7) | 44.2 (14.7) | 44.0 (14.9) | 0.98 |
| BMI (mean, SD) | |||||
| Total | 26.6 (5.5) | 26.3 (5.4) | 26.5 (5.1) | 26.9 (5.9) | 0.67 |
| Male | 25.1 (3.7) | 24.8 (3.6) | 24.6 (3.6) | 25.7 (3.9) | 0.32 |
| Female | 27.4 (6.1) | 27.2 (6.2) | 27.5 (5.5) | 27.5 (6.6) | 0.91 |
| BMI ≥25 (n,%) | |||||
| Total | 223 (56.3) | 72 (52.2) | 74 (57.8) | 77 (59.2) | 0.47 |
| Male | 63 (46.0) | 22 (44.0) | 17 (40.5) | 24 (53.3) | 0.46 |
| Female | 160 (61.8) | 50 (56.8) | 57 (66.3) | 53 (52.4) | 0.43 |
| C-reactive protein (mg/L) | |||||
| Including Ss with CRP≥ 10 | 1.83 (3.07)† | 1.96 (3.14) | 1.43 (2.25) | 2.07 (3.64) | 0.21 |
| 0.87 [0.36,1.73]‡ | 0.81 [0.36, 1.92] | 0.81 [0.36, 1.47] | 0.95 [0.38, 2.02] | ||
| Excluding Ss with CRP≥ 10 | 1.41 (1.64) | 1.46 (1.68) | 1.29 (1.51) | 1.49 (1.72) | 0.48 |
| 0.84 [0.36, 1.61] | 0.81 [0.37, 1.70] | 0.72 [0.35, 1.47] | 0.93 [0.36, 1.78] | ||
| 1.0 ≤CRP ≤10.0 mg/L (n, %)# | 173 (44.7) | 60 (44.8) | 53 (41.7) | 60 (47.6) | 0.64 |
| Serum Vitamin C (reduced, μM/L) (mean, SD) | 57.77 (17.4) | 57.32 (18.0) | 56.06 (17.5) | 59.93 (16.6) | 0.19 |
| Serum Vitamin E (μM/L) (mean, SD) | 28.32 (6.9) | 28.16 (7.2) | 28.67 (6.2) | 28.15 (7.4) | 0.79 |
| LDL cholesterol (mmol/L) (mean, SD) | 3.16 (0.8) | 3.10 (0.8) | 3.31 (0.8) | 3.07 (0.8) | 0.047 |
Significance of the difference across treatment groups.
among those without missing data.
mean (SD)
median [25th, 75th percentile]
The mean and median CRP in the sample was low, 1.83 and 0.87 mg/L respectively overall (1.43 mg/L and 0.85 mg/L among those who did not have evidence of acute inflammation) (Table 1). Among persons with CRP < 1.0 mg/L, the mean and median levels were 0.46 mg/L and 0.39 mg/L, while in those with CRP ≥ 1.0 and < 10.0 mg/L, mean and median levels were 2.70 mg/L and 1.94 mg/L, respectively (data not shown). In the nonparametric and parametric strict intent to treat analysis including all 396 participants, there was no overall treatment effect (p=0.11 and 0.42 for vitamin C and vitamin E respectively in parametric comparison with change in placebo (Table 2).
Table 2.
Change in CRP, n=396. Includes subjects with CRP>10 mg/L.
| Placebo (n=138) | Vitamin C (n=128) | Vitamin E (n=130) | |
|---|---|---|---|
| Pre-treatment, unadjusted (median, mg/L) ‡ | 0.81 | 0.81 | 0.95 |
| Post-treatment, unadjusted (median, mg/L) ‡ | 0.97 | 0.84 | 0.99 |
| Unadjusted median change, mg/L (IQR) † | +0.05 (−0.17 – +0.65) | −0.01 (−0.28 – +0.38) | 0.00 (−0.20 – +0.39) |
| Unadjusted median % change (%, IQR)† | + 8.33 (−25.00 – +58.33) | − 1.10 (−33.02 – +40.00) | 0.00 (−20.00 – +40.77) |
| P (Is within-group median change different from zero?) (Wilcoxon rank sum, 2-sided) * | 0.36 | 0.59 | 0.69 |
| P (Is change different from change in Placebo?) (Wilcoxon rank sum, 2-sided) ** | -- | 0.16 | 0.28 |
| Parametric: Adjusted change (%) (mean, CL) ± | +19% (+2 – +37) | − 1% (−14 – +16) | + 9% (−7 – + 27) |
| Parametric: P (significance of contrast with change in placebo) ± | -- | 0.11 | 0.42 |
Unadjusted median values, subjects without missing values.
IQR: 25th and 75th percentiles.
Wilcoxon rank sum test, nonparametric, significance of within-group changes
Wilcoxon rank sum test, nonparametric, significance of contrast with change in Placebo.
Parametric analysis, intention to treat, all 396 subjects, including those with missing data (assigned zero change) or with CRP>10 mg/L. Based on least squares mean and standard error, from model with the dependent variable, change in CRP, on the log scale. Adjusted for baseline CRP, gender, BMI and LDL. CL: 95% confidence limits.
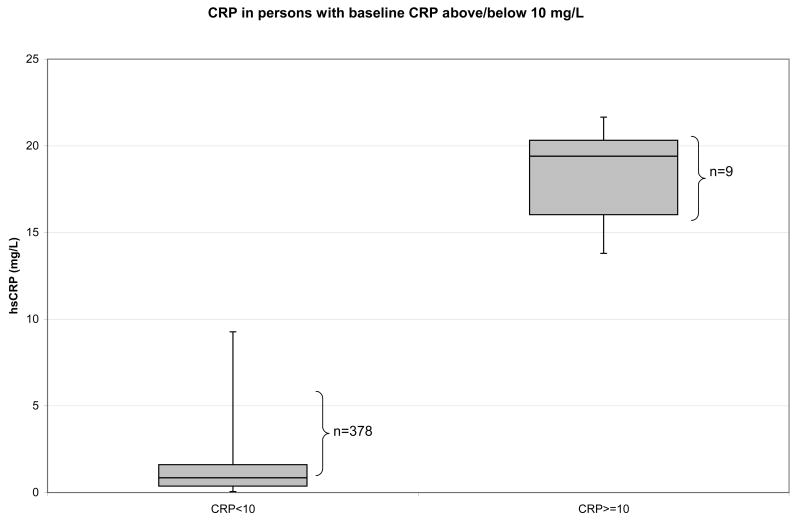
We tested for the presence of interaction between treatment effect and baseline CRP concentration, based on values specified by the CDC/AHA. The first such value was CRP > 10 mg/L, found in nine persons at the baseline visit. CDC/AHA specifies that CRP concentrations > 10 mg/L are not informative regarding cardiovascular risk and should not be used: “If a level of >10 mg/L is identified, there should be a search initiated for an obvious source of infection or inflammation, which could obscure any prediction of coronary risk that might be attributed to the elevated level. That result of >10 mg/L should then be discarded….” [17]. The interaction between treatment group and having CRP > 10 mg/L was highly significant, p<0.0001. As can be seen in Figure 2, persons with CRP > 10 mg/L represent a distinctly different population, with no overlap with those with lower CRP values. There was no evidence of an effect of either antioxidant on persons with acute elevations (n=14 at either baseline or follow-up) (data not shown.) Because of this interaction, and the fact that the study was intended to examine the potential benefit of antioxidants on chronic cardiovascular-relevant inflammation, further analysis focused on participants with CRP<10.0 mg/L.
Figure 2.
Vertical axis, CRP mg/L. Two plots are shown, CRP distribution in those with CRP < 10 mg/L, and in those with CRP ≥ 10 mg/L. The boxes indicate 25th, 50th and 75th percentile, and the tails on the boxes represent minimum and maximum.
Among the 382 subjects with CRP concentrations < 10 mg/L, indicative of chronic inflammation, a significant interaction between baseline CRP level (above/below 1.0 mg/L) and treatment group remained. The a priori stratification was again based on the CDC/AHA criteria. The interaction between treatment group and CVD risk category was significant at p = 0.03, indicating that treatment effect differed depending on baseline CRP level.
Among those with CRP < 1.0 mg/L, there was no significant treatment effect (p=0.91 for the comparison of change in either active treatment group with change in Placebo). Indeed, both placebo and active treatment groups had increases in CRP (Table 3, top). Among those with increased cardiovascular risk as represented by CRP ≥ 1.0 mg/L, treatment with vitamin C reduced CRP significantly in both nonparametric and parametric analyses (Table 3, bottom). In the vitamin C group with baseline CRP > 1 mg/L, the unadjusted median change was −0.25 mg/L, a 16.70% reduction. This within-group change was significant by Wilcoxon two-sided rank sum test, p=0.049. The Placebo group had an 8.57% increase in CRP. Thus, compared with subjects assigned to Placebo, allocation to the vitamin C treatment group was associated with a 25.27% reduction in median CRP levels (p=0.02 by Wilcoxon rank sum test). Vitamin E treatment effects were lower and were not significant. In parametric intention-to-treat analyses, the change in CRP in the vitamin C group was significantly different from change in Placebo, p=0.01, adjusted for baseline CRP level, gender, BMI and LDL. Again, vitamin E effects were weaker and not significant.
Table 3.
Change in CRP, n=382. Excludes subjects with CRP>10 mg/L;,by baseline CRP level
| CRP < 1.0 mg/L at baseline | |||
|---|---|---|---|
| Placebo (n= 76) | Vitamin C (n= 75) | Vitamin E (n= 69) | |
| Pre-treatment, unadjusted (median, mg/L) ‡ | 0.46 | 0.39 | 0.38 |
| Post-treatment, unadjusted (median, mg/L) ‡ | 0.54 | 0.47 | 0.38 |
| Unadjusted median change, mg/L (IQR) † | + 0.03 (−0.08 – +0.31) | + 0.01 (−0.08 – +0.43) | + 0.02 (−0.04 – +0.29) |
| Unadjusted median % change (%, IQR) † | + 6.06 (−21.28 – +62.86) | +5.88 (−25.00 – +105.56) | +8.33 (−11.36 – +62.50) |
| P (Is within-group median change different from zero?) (Wilcoxon rank sum, 2-sided) * | 0.15 | 0.13 | 0.33 |
| P (Is change different from change in Placebo?) (Wilcoxon rank sum, 2-sided) ** | -- | 0.83 | 0.92 |
| Parametric: Adjusted change (%) (mean, CL) ± | + 31.0% (+9 – +57) | + 29% (+8 – +54) | + 29% (+7 – +56) |
| Parametric: P (significance of contrast with change in placebo) ± | -- | 0.91 | 0.91 |
| CRP ≥1.0 mg/L at baseline | |||
| Placebo (n= 55) | Vitamin C (n= 51) | Vitamin E (n=56 ) | |
| Pre-treatment, unadjusted (median, mg/L) ‡ | 1.92 | 1.68 | 2.02 |
| Post-treatment, unadjusted (median, mg/L) ‡ | 2.19 | 1.34 | 1.83 |
| Unadjusted median change, mg/L (IQR) † | + 0.12 (−0.56 – +1.09)) | − 0.25 (−01.23 – +0.14) | − 0.10 (−0.78 – +0.40) |
| Unadjusted median % change (%, IQR) † | + 8.57% (−21.82 – +40.12) | − 16.70% (−48..49 – +11.57) | − 6.80% (−47.83 - +24.84) |
| P (Is within-group median change different from zero?) (Wilcoxon rank sum, 2-sided) * | 0.85 | 0.049 | 0.35 |
| P (Is change different from change in Placebo?) (Wilcoxon rank sum, 2-sided) ** | -- | 0.02 | 0.15 |
| Parametric: Adjusted change (%) (mean, CL) ± | − 7% (−23 – +12) | − 33% (−55 - −19) | − 20% (−34 - +4) |
| Parametric: P (significance of contrast with change in placebo) ± | -- | 0.01 | 0.24 |
Unadjusted median values, subjects without missing values.
IQR: 25th and 75th percentiles.
Wilcoxon rank sum test, nonparametric, significance of within-group changes
Wilcoxon rank sum test, nonparametric, significance of contrast with change in Placebo.
Parametric analysis, intention to treat, all 382 subjects with CRP<10 mg/L, including those with missing data (assigned zero change). Based on least squares mean and standard error, from model with the dependent variable, change in CRP, on the log scale. Adjusted for baseline CRP, gender, BMI and LDL. CL: 95% confidence limits.
Other analyses
There was no evidence of interaction between ethnic group and treatment group. Tests of interaction between gender and treatment group, and between BMI and treatment group, were not statistically significant. However, the point estimates suggested a considerably stronger treatment effect among women and among persons with elevated BMI. This is likely due to the substantially higher mean baseline CRP in women than in men, and in those with higher BMI.
In the small subgroup with baseline CRP >3 mg/L, there was a suggestion of a substantial effect of vitamin E, but neither treatment group effect reached statistical significance.
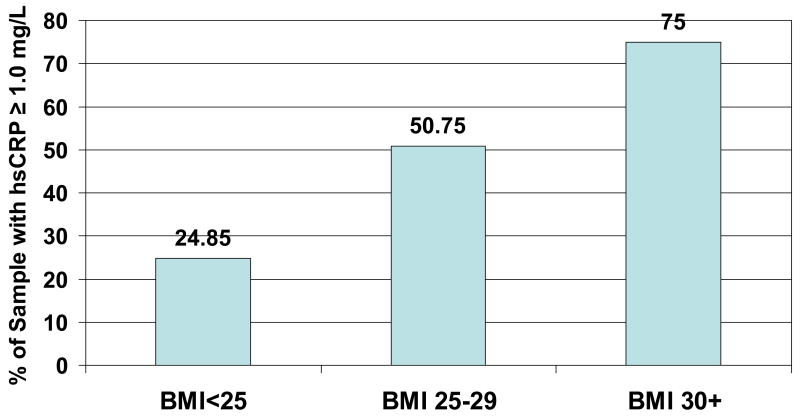
We examined the proportion with baseline CRP above 1.0 mg/L in BMI groups, and found a strong association of elevated CRP with BMI category (Figure 3). The percent with CRP ≥1.0 mg/L increased from 24.9% to 50.8% to 75% as BMI categories increased from Normal to Overweight to Obese.
Figure 3.
Percent with CRP ≥ 1.0 mg/L at baseline, by BMI status. P=0.0001 for difference in groups.
Baseline CRP was weakly associated with LDL-cholesterol (Spearman r=0.23). The changes in these two variables were not associated (Spearman r=0.02), and control for the change in LDL-cholesterol did not alter the conclusions. In addition, there were no significant differences between treatment groups in the proportion who reported taking new over-the-counter or prescription medications during the study.
Safety, adverse events, and adequacy of masking
One death, in the vitamin E group, occurred due to an automobile accident. No other serious adverse events were reported. Other adverse event reports were obtained by checklist for the following symptoms: health problems, upset stomach, diarrhea, nausea or vomiting, constipation, bleeding gums, poor night vision, nosebleeds, fatigue, tiredness, headache, irritability, dizziness, skin rash, bruising easily, any other symptoms. Post-intervention, the vitamin E group reported significantly more dizziness than the placebo group, p=0.008 (data not shown), whereas the vitamin C group reported a significantly lower prevalence of fatigue (p=0.005). There were no other significant differences across treatment groups.
A post-intervention questionnaire revealed that approximately 15%, 20% and 17% of those in the vitamin E, vitamin C and placebo groups respectively guessed their treatment correctly.
DISCUSSION
This analysis has found that antioxidants have no effect on inflammation marker C-reactive protein when all randomized subjects are included, but that one of the antioxidants, vitamin C, does have a statistically significant effect when analysis is restricted to those deemed by CDC/AHA [17] to be at elevated cardiovascular risk. Among persons with baseline CRP ≥ 1.0 mg/L, treatment with 1000 mg/day vitamin C for two months was associated with a 16.7% within-group change, p<0.05. Compared to subjects in Placebo, allocation to vitamin C was associated with a 25.27% reduction in median CRP concentrations, p=0.02. Significant differences between the vitamin C and Placebo group was also seen in parametric analysis, despite the very conservative approach of assuming that those with missing data had zero change. Treatment with 800 IU/day vitamin E was also associated with some reduction in CRP, but the treatment effect did not achieve statistical significance.
We have also reported a similar influence of baseline level on the treatment effect of antioxidants on the oxidative stress biomarker F2-isoprostanes [21]. In that analysis, treatment with vitamin C reduced F2-isoprostanes by 22%, p=0.01, only among those with baseline concentrations ≥ 50 μg/mL, a subgroup representing 45% of the sample. The effect of vitamin E in that analysis was weaker and not significant. It is also perhaps worth noting that there was a significant (p=0.008) association between having F2-isoprostanes ≥ 50 μg/mL and having CRP ≥ 1.0 mg/L.
Common sense suggests that biomarkers are only likely to be reduced if they are not already low. Indeed, in this study concentrations initially low tended to increase, even in the active intervention groups, reflecting the effect of regression to the mean. Studies investigating the effect of antioxidant treatment should attempt to limit intervention to subjects who are susceptible to having a reduction in the baseline values. Often, such a cut-point is not known a priori. Inflammation research is fortunate in having such values defined, and in having their prognostic value confirmed [17, 18]. We have suggested such a cut-point for studies of F2-isoprostanes [21].
These observations may shed light on the mixed results found in large “antioxidant” trials with clinical endpoints. No such trials have limited participants to persons with elevated CRP or oxidative stress, and most have not characterized participants with respect to those factors [31]. Clinical trial methodology demands that the primary result represents analysis that includes all randomized participants. Such an analysis protects against unexpected biases. However, such an analysis also risks obscuring a potentially important result. As Meagher et al. have noted [32], inclusion of subjects without evidence of an increase in the target biomarker in trials of antioxidants would tend to dilute the population susceptible to benefit and undermine the sample size and power calculations, leaving such studies open to type II error and outcomes reflecting random variation. If one mechanism of potential antioxidant treatment is reduction in CRP, inclusion of subjects without elevations in CRP would tend to have weakened such studies, particularly those involving healthy volunteers. In the present study of healthy volunteers, 58% of participants had CRP values < 1.0 mg/L.
We found, as others have [30], that CRP is strongly associated with BMI. We also found that a BMI reflective of obesity (BMI ≥30) is a strong marker of having CRP ≥ 1.0 mg/L, the level indicative of increased risk of cardiovascular events. In our study, 75% of the obese had CRP ≥1.0 mg/L (Figure 3). This at-risk group is an appropriate target for efforts to reduce inflammation, although elevations in CRP are found in all BMI categories.
It is notable that our sample was quite well-nourished with respect to vitamin C, with a baseline mean plasma reduced ascorbic acid level of 57.8 μmol/L, whereas the average plasma vitamin C level in the U.S. adult population is 42.6 μmol/L (G. Block, unpublished data from the Third National Health and Nutrition Examination Survey). Only 1.6% of our participants had ascorbic acid levels < 22.7 μmol/L (0.4 mg/dL), whereas in national data 25.7% have levels below 22.7 μmol/L. It is likely that the treatment effect would be more substantial in a population more representative of the U.S. with respect to plasma ascorbic acid status. Vitamin E status was comparable to national levels. In our sample, mean α-tocopherol was 28.33 μmol/L, and 0.79% had deficient levels. In national data among adults, mean serum vitamin E was 29.23 μmol/L, and 1.02% had deficient plasma levels (G. Block, unpublished data from the National Health and Nutrition Examination Survey 1999–2000).
The impact of antioxidants on plasma CRP may be mediated by effects on upstream cytokines, in particular interleukin-1 (IL-1), tumor necrosis factor-alpha (TNFα), and interleukin-6 (IL-6) which are the main inducers of the acute phase response [33]. Hartel et al. found that vitamin C inhibited the lipopolysaccharide-induced IL-6 and TNFα production, as well as IL-2 production after phorbol 12-myristate 13-acetate/ionomycin stimulation [34]. Those authors suggest several potential mechanisms, including both oxidative and non-oxidative processes [34]. Oxidative damage leads to an inappropriate activation of the transcription factor nuclear factor kappa beta (NFκB) and subsequently to an overexpression of inflammatory proteins [35]. Vitamin C has been shown to inhibit NFκB activation [36–39]. This potential oxidative mechanism is consistent with our observation in the present dataset that plasma F2-isoprostane, a biomarker of oxidative stress, was significantly reduced by vitamin C treatment [21]. We have also reported in earlier research that treatment with 500 mg/day vitamin C significantly reduced plasma F2-Isoprostanes among active and passive smokers [40].
Five studies have examined the effect of vitamin E on CRP [41–45], of which two found statistically significant treatment effects. All studies were small, n=5 to 25 per treatment group, and several were in diabetics or hemodialysis patients. The factor that appears to distinguish studies that found or failed to find an effect of vitamin E is the presence of elevated CRP at baseline. The two that found significant CRP reductions with vitamin E treatment had subjects with very high initial CRP, > 5 mg/L in both cases [41, 42]. Both also had subjects with high mean BMI, 27.5 and 31.5 kg/m2 respectively. In contrast, two of the three finding no effect had mean baseline CRP of 1.0 mg/L, and mean BMI of only 24 kg/m2. This is consistent with our results, in which vitamin E had no effect among persons with baseline CRP < 1.0 mg/L, but in the group with CRP > 1.0 mg/L (mean CRP 2.7 mg/L) there was a 6.8% reduction in the vitamin E group, although this did not reach significance. These studies suggest that vitamin E may be effective in reducing CRP, in persons with substantial CRP elevations, higher than what we observed in our healthy volunteers.
Three small earlier studies (n=12 to 17 per treatment group) found no effect of vitamin C on CRP [41, 46, 47]. The two studies that did find an effect had adequate sample size (n≥49 [22] and 43 [23] in active treatment group), and were the only studies of either vitamin C or E to exclude persons with CRP > 10 mg/L and to adjust for baseline CRP. Both studies utilized non-diseased participants with BMI approximately 27 kg/m2, similar to that of the US population. Both had a mean baseline CRP ≥ 2.4 mg/L. These two studies are consistent with our finding of a significant CRP-lowering effect of vitamin C in persons with elevated baseline CRP concentrations.
Church et al. [23] also conducted an analysis stratified by baseline CRP above or below 1.0 mg/L. Their results were identical to ours: there was no treatment effect among persons with initial CRP levels < 1.0 mg/L, but a significant treatment effect in persons initially above that concentration.
Two studies examined a combination of vitamin C and E with adequate sample size (n≥49 in the active treatment group), and neither found a significant reduction in CRP [22, 48]. One had a sample with low initial CRP (median 1.0 mg/L) [48]. Both used a fairly low dose,182 mg α-tocopherol and 500 mg vitamin C [48] or 371 mg α-tocopherol and 515 mg vitamin C [22]. The failure of the combination dosage in the latter study is unexplained, and warrants further study.
A number of studies have shown that statins reduce CRP concentrations [49–51]. In an analysis of myocardial infarction patients enrolled in the Cholesterol and Recurrent Events trial, median percent change in CRP in the pravastatin group was 17.4% (p = 0.004) after five years of treatment [49]. In the Air Force/Texas Coronary Atherosclerosis Prevention Study, median change in CRP in the lovastatin group after one year was 0.2 mg/L, 14.8% (p<0.001) [50]. In the Pravastatin Inflammation/CRP Evaluation trial, median CRP concentration in the Pravastatin group was reduced by 0.2 mg/L, 14.2% (p < 0.001) [51]. In that study the baseline median CRP levels in the placebo and pravastatin groups were 2.1 mg/L and 2.0 mg/L respectively, similar to the 1.9 mg/L found in our sample among participants with CRP ≥ 1.0 mg/L. Thus, the 0.25 mg/L, 16.7% within-group reduction in mean CRP in the vitamin C group in the present study, in a group of healthy persons with baseline CRP levels above 1.0 mg/L, suggests an effect of vitamin C on CRP that is similar to that observed with statins.
In the ongoing JUPITER trial, rosuvastatin therapy is being evaluated among healthy individuals, to investigate the effects of statins in the primary prevention of cardiovascular disease [52]. A positive finding would suggest that elevated CRP levels should be treated aggressively to lower the risk of a first major cardiovascular event. Therefore, the CRP-lowering effects of vitamin C are of potential clinical importance and warrant further investigation.
Given the continuing epidemics of obesity and diabetes in the US [53], and the demonstrated difficulty in achieving population-wide weight loss, strategies are needed to reduce the sequellae of obesity. Thus, future studies to determine whether vitamin C can reduce some of the inflammation-related adverse consequences of obesity should be considered. Such trials should focus on individuals with elevations (≥ 1.0 mg/L) in CRP, since studies with low-risk persons are less likely to show an effect, resulting in misleading outcomes. If persons with lower CRP levels must be included, separate randomization of those with CRP ≥ 1.0 mg/L would justify separate examination of this subgroup, assuming adequate power in this stratum. In addition, if the potential independent effect of vitamin C is to be determined, it would be necessary to exclude persons who are taking other anti-inflammatory drugs (except low-dose aspirin for heart disease prevention), and to exclude users of multiple vitamins (something which has not been done in most large antioxidant trials), since multiple vitamins alone can raise plasma ascorbic acid levels substantially and make the control group insufficiently different from the active treatment group. Finally, it may be prudent to evaluate vitamin C alone, unpaired with vitamin E, as we found a weaker CRP-lowering effect with the combination than with vitamin C alone, in our previous trial [22].
In conclusion, this study has found a significant effect of treatment for two months with 1000 mg/day vitamin C on plasma CRP, in nondiseased moderately overweight nonsmokers with baseline CRP ≥ 1.0 mg/L. The magnitude of the effect was similar to that of statins. There was no significant effect of vitamin E. These data represent the largest study to date on the effects of vitamins C and E on CRP, and extend our previous findings in overweight active and passive smokers. They indicate that vitamin C should be further investigated for its potential for reducing chronic inflammation and its consequences. And they identify a threshold concentration above which there is a potential for reduction in CRP.
Acknowledgments
The authors gratefully acknowledge the contributions of the study staff and nurses, including JoAnn Johnson, Sean Brennan, Nishat Shaikh, Lisa Lavrisha, Nancy Sweeters, Betty Flores, Bridget Canty, Ellen Butensky; the mparticipants; and the Data Safety and Monitoring Board members, Drs. Patricia A. Buffler and Ronald M. Krauss.
Acknowledgements and funding sources: The project described was supported by Grant number R01DK062378 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. This study was also made possible by the PCRC Grant Number MO1-RR01271 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and NIH Roadmap for Medical Research. The trial is listed in ClinicalTrials.gov, NCT00079963, http://www.clinicaltrials.gov/ct/show/NCT00079963?order=1. The authors gratefully acknowledge the contributions of the study staff and nurses, including JoAnn Johnson, Sean Brennan, Nishat Shaikh, Lisa Lavrisha, Nancy Sweeters, Betty Flores, Bridget Canty, Ellen Butensky; the participants; and the Data Safety and Monitoring Board members, Drs. Patricia A. Buffler and Ronald M. Krauss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.de Ferranti SD, Rifai N. C-reactive protein: a nontraditional serum marker of cardiovascular risk. Cardiovasc Pathol. 2007;16:14–21. doi: 10.1016/j.carpath.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 6.Packard CJ, O’reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GDO. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 8.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O’Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 9.Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, Koenig W, Schmitz G, Hombach V, Torzewski J. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–2099. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- 10.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Wang S, Deb A, Nath KA, Katusic ZS, McConnell JP, Caplice NM. Proapoptotic, antimigratory, antiproliferative, and antiangiogenic effects of commercial C-reactive protein on various human endothelial cell types in vitro: implications of contaminating presence of sodium azide in commercial preparation. Circ Res. 2005;97:135–143. doi: 10.1161/01.RES.0000174612.90094.fd. [DOI] [PubMed] [Google Scholar]
- 13.Singh U, Devaraj S, Jialal I. C-reactive protein decreases tissue plasminogen activator activity in human aortic endothelial cells: evidence that C-reactive protein is a procoagulant. Arterioscler Thromb Vasc Biol. 2005;25:2216–2221. doi: 10.1161/01.ATV.0000183718.62409.ea. [DOI] [PubMed] [Google Scholar]
- 14.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 15.Trion A, de Maat MP, Jukema JW, van der Laarse A, Maas MC, Offerman EH, Havekes LM, Szalai AJ, Princen HMG, Emeis JJ. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-Leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:1635–1640. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- 16.Reifenberg K, Lehr HA, Baskal D, Wiese E, Schaefer SC, Black S, Samols D, Torzewski M, Lackner KJ, Husmann M, Blettner M, Bhakdi S. Role of C-reactive protein in atherogenesis: can the apolipoprotein E knockout mouse provide the answer? Arterioscler Thromb Vasc Biol. 2005;25:1641–1646. doi: 10.1161/01.ATV.0000171983.95612.90. [DOI] [PubMed] [Google Scholar]
- 17.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon ROIII, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 18.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E PEACE Investigators. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 20.Scholz H, Yndestad A, Damas JK, Waehre T, Tonstad S, Aukrust P, Halvorsen B. 8-isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. Cardiovasc Res. 2003;59:945–954. doi: 10.1016/s0008-6363(03)00538-8. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GE, Hudes M, Dalvi TB, Crawford PB, Fung EB, Schumacher L, Harmatz P. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med. 2008;45:377–384. doi: 10.1016/j.freeradbiomed.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block G, Jensen C, Dietrich M, Norkus EP, Hudes M, Packer L. Plasma C-reactive protein concentrations in active and passive smokers: influence of antioxidant supplementation. J Am Coll Nutr. 2004;23:141–7. doi: 10.1080/07315724.2004.10719354. [DOI] [PubMed] [Google Scholar]
- 23.Church TS, Earnest CP, Wood KA, Kampert JB. Reduction of C-reactive protein levels through use of a multivitamin. Am J Med. 2003;115:702–707. doi: 10.1016/j.amjmed.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 24.The practical guide to the identification, evaluation, and treatment of overweight and obesity in adults. National Heart, Lung, and Blood Institute, no. 00–4084, 2000. (http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_b.pdf).
- 25.National Center for Health Statistics. Hyattsville, MD: 2007. Laboratory Methods of the National Health and Nutrition Examination Survey, 2001–2002. ( http://www.cdc.gov/nchs/about/major/nhanes/lab_methods01_02.htm) [Google Scholar]
- 26.Bachorik PS, Kwiterovich PO. The measurement of plasma cholesterol, low density lipoprotein- and high density lipoprotein-cholesterol. In: Hommes FA, editor. Techniques in diagnostic human biochemical genetics: A laboratory manual. New York, NY: Wiley-Liss, Inc; 1991. pp. 425–439. [Google Scholar]
- 27.Sowell AL, Huff DL, Yeager PR, Caudill SP, Gunter EW. Retinol, α-tocopherol, lutein/zeaxanthin, β-cryptoxanthin, lycopene, α-carotene, trans β-carotene and four retinyl esters in serum determined simultaneously by reversed-phase HPLC with multiwavelength detection. Clin Chem. 1994;40:411–416. [PubMed] [Google Scholar]
- 28.Gunter EW, Turner WE, Neese JW, Bayse DD. Laboratory procedures used by the Clinical Chemistry Division, Centers for Disease Control, for the Second Health and Nutrition Examination Survey (NHANES II) 1976–1980. Centers for Disease Control; 1981. [Google Scholar]
- 29.Sauberlich HE, Kretsch MJ, Taylor PC, Johnson HL, Skala JH. Ascorbic acid and erythorbic acid metabolism in nonpregnant women. Am J Clin Nutr. 1989;50:1039–1049. doi: 10.1093/ajcn/50.5.1039. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 31.Blumberg JB, Frei B. Why clinical trials of vitamin E and cardiovascular diseases may be fatally flawed. Commentary on “The relationship between dose of vitamin E and suppression of oxidative stress in humans”. Free Radic Biol Med. 2007;43:1374–1376. doi: 10.1016/j.freeradbiomed.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 33.Baumann H, Goldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 34.Hartel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Baeuerle PA, Henkel T. Function and activation of NFκB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 36.Bowie AG, O’Neill LAJ. Vitamin C inhibits NF-κB activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165:7180–7188. doi: 10.4049/jimmunol.165.12.7180. [DOI] [PubMed] [Google Scholar]
- 37.Carcamo M, Pedraza A, Borquez-Ojeda O, Golde DW. Vitamin C suppresses TNFα-induced NFκB activation by inhibiting IκBα phosphorylation. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 38.Campbell JD, Cole M, Bunditrutavorn B, Vella AT. Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis. Cell Immunol. 1999;194:1–5. doi: 10.1006/cimm.1999.1485. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Cruz I, Carcamo JM, Golde DW. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood. 2003;102:336–343. doi: 10.1182/blood-2002-11-3559. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich M, Block G, Hudes M, Morrow JD, Norkus EP, Traber MG, Cross CE, Packer L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:7–13. [PubMed] [Google Scholar]
- 41.Upritchard JE, Sutherland WHF, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–738. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- 42.Devaraj S, Jialal I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 2000;29:790–792. doi: 10.1016/s0891-5849(00)00420-2. [DOI] [PubMed] [Google Scholar]
- 43.Kaul N, Devaraj S, Grundy SM, Jialal I. Failure to demonstrate a major anti-inflammatory effect with alpha tocopherol supplementation (400 IU/day) in normal subjects. Am J Cardiol. 2001;87:1320–1323. doi: 10.1016/s0002-9149(01)01534-x. [DOI] [PubMed] [Google Scholar]
- 44.Vega-Lopez S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with w3 polyunstaurated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–240. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney International. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 46.Lu Q, Bjorkhem I, Wretlind B, Diczfalusy U, Henriksson P, Freyschuss A. Effect of ascorbic acid on microcirculation in patients with type II diabetes: a randomized placebo-controlled cross-over study. Clin Sci (Lond) 2005;108:507–513. doi: 10.1042/CS20040291. [DOI] [PubMed] [Google Scholar]
- 47.Fumeron C, Nguyen-Khoam T, Saltiel C, Kebede M, Buisson C, Drueke TB, Lacour B, Massy ZA. Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol Dial Transplant. 2005;20:1874–9. doi: 10.1093/ndt/gfh928. [DOI] [PubMed] [Google Scholar]
- 48.Bruunsgaard H, Poulsen HE, Pedersen BY, Nyyssonen K, Kaikkonen J, Salonen JT. Long-term combined supplementation with α-tocopherol and vitamin C have no detectable anti-inflammatory effects in healthy men. J Nutr. 2003;133:1170–1173. doi: 10.1093/jn/133.4.1170. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive Protein. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Rifai N, Clearfield M, Downs JR, Weise SE, Miles JS, Gotto AM. Meaurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 51.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 52.Mora S, Ridker PM. Justification of the use of statins in primary prevention: an intervention trial evaluating rosuvastatin (JUPITER) – can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97(suppl):33A–41A. doi: 10.1016/j.amjcard.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 53.National Center for Health Statistics. Health, United States, 2004 Chartbook on Trends in the Health of Americans. Hyattsville, MD: Department of Health and Human Services; 2004. [PubMed] [Google Scholar]