Figure 1.
The Quaternary Organization of RNase E Is Flexible
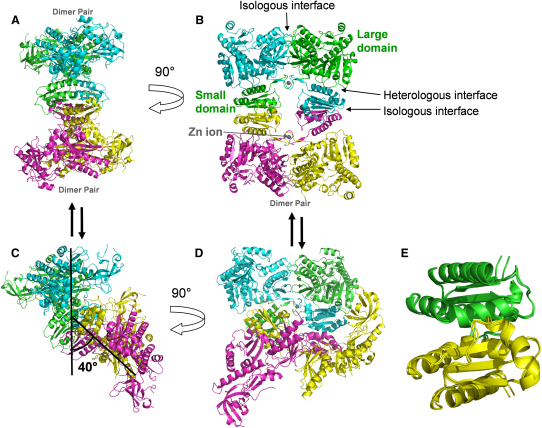
The protomers of the RNase E tetramer are colored pink, yellow, green, or cyan. The pink and yellow protomers form a dimer pair, as do the green and cyan protomers (A). The RNase E tetramer is observed to have D2 symmetry in the previously reported holoprotein configuration (A and B) and here, a large kink of about 40° was observed in the apoprotein structure (C and D). The isologous and heterologous domain-domain interfaces are indicated (B). The zinc ions are shown in gray. Quaternary structural changes are restricted to the heterologous interfaces. The bend in each tetramer was calculated by superimposing the RNase H and DNase I regions of a single dimer from each model and calculating the resulting angle between zinc ions. The dimer-dimer interaction mediated by the small domains is most easily viewed as in panel B, and we have labeled the large and small domains of an individual protomer (green in [B]) for clarity. It is clear that the dimer-dimer interaction is virtually identical in each structure when the small domains from each are superimposed (E). We found that the observed change in quaternary organization is due to a change in the orientation of the small domains relative to the large, but that in each case the region coordinating a zinc ion is unaltered.