Abstract
Pregna-5,7-dienes and their hydroxylated derivatives can be formed in vivo when there is a deficiency in 7-dehydrocholesterol (7-DHC) Δ-reductase function, e.g., Smith-Lemli-Opitz syndrome (SLOS). Ultraviolet B (UVB) radiation induces photoconversion of 7-DHC to vitamin D3, lumisterol3 and tachysterol3. Two epimers (20R and 20S) of pregna-5,7-diene-3β,17α,20-triol (4R and 4S, respectively) were synthesized and their UVB photo-conversion products identified as corresponding 9,10-secosteroids with vitamin D-like and tachysterol-like structures, and 5,7-dienes with inverted configuration at C-9 and C-10 (lumisterol-like). The number and character of the products and the dynamics of the process were dependent on the UVB dose. At high UVB doses, the formation of multiple oxidized derivatives of the primary products, and the formation of 5,7,9(11)-triene, were observed. The production of vitamin D-like, tachysterol-like and luminosterol-like derivatives was also observed in human skin treated with 4R and 4S, and subjected to UV irradiation, as shown by RP-HPLC. Newly synthesized compounds inhibited melanoma growth in dose dependent manner, and some of them showed equal or higher potency than 1,25(OH)2D3. In summary, we have characterized for the first time the products of UV induced conversion of pregna-5,7-diene-3β,17α,20-triols and documented that the newly synthesized compounds have antiproliferative properties against melanoma cells.
Keywords: secosteroids, UV radiation, lumisterol, tachysterol, SLOS, melanoma
Introduction
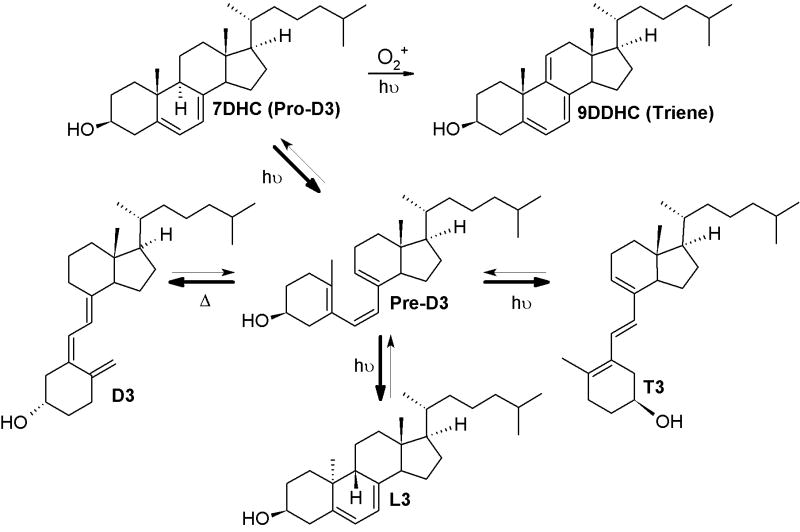
The UVB-driven isomerization of cholesta-5,7-diene-3β-ol (7-dehydrocholesterol, 7DHC) to (3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol (cholecalciferol, vitamin D3) is one of the most fundamental reactions in the photobiology of the skin.[1-3] This conversion is initiated by photolysis of the unsaturated B ring, with production of pre-D3 intermediate, and is followed by its slow isomerization to three main products: vitamin D3, tachysterol3 (6E-9,10-secocholesta-5(10),6,8-trien-3β-ol, T3) and lumisterol3 (9β,10α-cholesta-5,7-diene-3β-ol, L3) (Scheme 1). The relative ratio and the structure of products depend on a number of variables, including the ultraviolet B (UVB) dose (absorbed energy), temperature, wavelength, the presence of biological membranes or when the reaction is carried out in the skin.[3-5] Higher doses of UV or the presence of trace amounts of acid trigger further isomerization of T3 to Isotachysterol3 (isoT3) products, and is followed by autoxidation.[4,6] Another pathway of degradation/isomerization of vitamin D3 with production of 5,6-transvitamin D3, suprasterols I and II in response to high doses of UVB was also demonstrated in the skin.[4] It was shown that 7DHC in biological systems can also produce corresponding 5,7,9(11)-trienes. Although the detailed mechanism is not fully understood; it has been suggested that singlet oxygen and photosensitizers may play a role in this reaction.[7-11]
Scheme 1.
Photolysis of cholesta-5,7-diene-3β-ol.
Accumulation of 5,7-dienes and 5,7,9(11)-trienes is important in the etiology of a 7Δ-reductase deficiency syndrome (Smith-Lemli-Opitz syndrome – SLOS). [9,10,12-15] The lack of the key enzyme converting 7DHC to cholesterol results in multiple metabolic defects with accumulation of 7DHC and its 5,7-diene steroidal derivatives, including cholesta-5,7,9(11)-trien-3β-ol (9DDHC)[10] and pregna-5,7-diene-3β,17α,20-triol.[12-14] Recently, it was proposed that oxidation of 7DHC and 9DDHC leads to severe photosensitivity in SLOS patients.[7,9]
Vitamin D3 is not only a master regulator of calcium metabolism but also a powerful oncostatic agent affecting proliferation, differentiation and apoptosis of normal and malignant cells.[2,16] Although its potential therapeutic use against cancer or hyperproliferative disorders is largely limited due to toxicity (hypercalcemia) at pharmacological concentrations, this adversity can be reduced or attenuated by shortening or removal of the side chain.[17,18] Thus, the generation of vitamin D-like compounds with pregnane side chains may have a potential utility in treatment of cancers or other pathological disorders.
Here were report on the UVB photolysis of two epimers (20R and 20S) of pregna-5,7-diene-3β,17α,20-triols synthesized from series of 3-acetylated 5-diene precursors.[13,19] The dynamics of the photolytic reactions and identification of novel 9,10-secosteroids with D- T- and L-like structures, and corresponding 5,7,9(11)-trienes. In addition, we studied the formation of secosteroidal derivatives in human skin exposed to UVB and tested their biological activity on the human melanoma SKMEL-188 cell line.
Experimental
Chemical synthesis
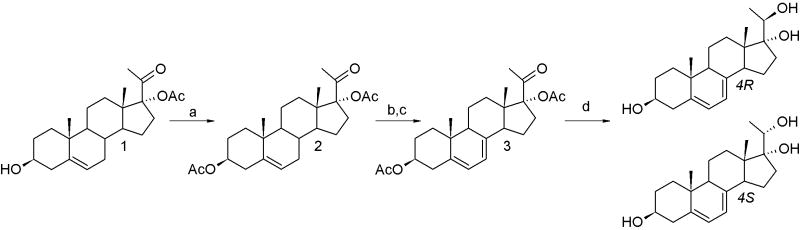
The synthesis of compounds 4R and 4S is shown in Scheme 1.
Synthesis of 2
The acetylation of steroid 1 was carried out following a known procedure [20]. Yield: 95%. 1H NMR (500MHz, CDCl3) for compound 2a: δ 5.39 (d, J = 5 Hz, 1H), 4.58-4.64 (m, 1H), 2.92-2.96 (m, 1H), 2.30-2.36 (m, 2H), 2.12 (s, 3H), 2.04 (s, 3H), 2.05 (s, 3H), 1.98-2.02 (m, 2H), 1.86-1.90 (m, 2H), 1.46-1.80 (m, 9H), 1.26-1.32 (m, 1H), 1.14-1.18 (m, 1H), 1.06-1.08 (m, 1H), 1.03 (s, 3H), 0.64 (s, 3H). ESI-MS: calculated for C25H36O5, 416.26, found 439.3 [M+Na]+.
General procedure for the synthesis 3
Compounds 3 were synthesized according to a known procedure [13]. Yield: 40-50%. 1H NMR (500MHz, CDCl3) for compound 3: δ 5.57-5.59 (m, 1H), 5.44-5.46 (m, 1H), 4.68-4.74 (m, 1H), 2.96-2.90 (m, 1H), 2.59-2.63 (m, 1H), 2.49-2.54 (m, 1H), 2.36 (t, J = 15 Hz, 1H), 2.11 (s, 3H), 2.08 (s, 3H), 2.05 (s, 3H), 2.02-2.04 (m, 1H), 1.82-1.94 (m, 4H), 1.56-1.73 (m, 6H), 1.38 (dt, J = 15 Hz, 5 Hz, 1H), 0.95 (s, 3H), 0.57 (s, 3H). ESI-MS: calculated for C25H34O5, 414.24, found 437.3 [M+Na]+.
General procedure for the synthesis of 4 (4R and 4S)
Compounds 4 (4R and 4S) were synthesized according to a known procedure [13]. Reaction resulted in a crude, white, solid mixture of compounds 4R and 4S: 40-50% yield. The mixture was subjected to flash chromatography (column eluted with hexane–ethyl acetate 20:1, 10:1, 5:1, 1:1 in order), but only the isomer with a faster retention time (Peak 1, 4R), was purified in this manner. The separation of the second isomer (Peak 2, 4S) was accomplished using RP-HPLC.
1H NMR (500MHz, CD3OD) for compound 4S: δ 5.54 (dd, J = 7.5Hz, 3.2Hz, 1H), 5.38-5.40 (m, 1H), 3.94 (q, J = 18Hz, 1H), 3.47-3.55 (m, 1H), 2.57 (t, J = 10Hz, 1H), 2.38-2.43 (m, 1H), 2.2-2.28 (m, 1H), 1.88 –1.98 (m, 3H), 1.62 – 1.88 (m, 8H), 1.42-1.58 (m, 3H), 1.26-1.34 (m, 2H), 1.15 (d, J = 6Hz, 3H), 0.96 (s, 3H), 0.77 (s, 3H). ESI-MS: calculated for C21H32O3, 332.24, found 355.3 [M+Na]+; 4R: δ 5.54 (dd, J = 8.3Hz, 2.5Hz, 1H), 5.41-5.44 (m, 1H), 3.79 (q, J = 18Hz, 1H), 3.47-3.56 (m, 1H), 2.60 (t, J = 10Hz, 1H), 2.38-2.43 (m, 1H), 2.20-2.28 (m, 1H), 2.07-2.14 (m, 1H), 1.89 –1.97 (m, 2H), 1.62 –1.89 (m, 8H), 1.42-1.53 (m, 2H), 1.26-1.34 (m, 2H), 1.12 (d, J = 6Hz, 3H), 0.95 (s, 3H), 0.7 (s, 3H). ESI-MS: calculated for C21H32O3, 332.24, found 355.3 [M+Na]+.
General procedure for UVB irradiation of 4R and 4S
The UVB irradiation was performed as described previously[21] with some modifications. Briefly, a methanol solution of 4R or 4S at 1 mg/mL was subjected to UV irradiation for various times in a quartz cuvette (or glass HPLC insert) using Biorad UV Transilluminator 2000 (Biorad, Hercules, CA). Spectral characteristics of the UVB (280-320 nm) source were published previously [22] and it's strength (4.8±0.2 mW cm-2) was routinely measured with digital UVB Meter Model 6.0 (Solartech Inc., Harrison Twp, MI). Irradiation was followed by 14 hours incubation at room temperature and selected products were purified by RP-HPLC chromatography as described.[21] The major products were identified on the basis of their retention time and characteristic UV absorption. Initial identification was confirmed by means of MS and NMR measurements. The quantities of products varied and were predominantly dependent on the UVB radiation dose. Fifteen minutes reaction resulted in 30-35% of pre-D-like, 20% T-like, 10% of substrate and lower quantity of other products.
UVB irradiation of 4R and 4S incorporated into human skin
Human neonatal foreskin from African-American donors was collected after routine circumcision, fat was removed, and the skin was washed with DMEM medium and cut into approximately 5×5 mm pieces. Skin fragments (epidermis facing up), were placed into 24 well plates containing 100 μL of DMEM medium, leaving the epidermis above the surface of the medium. The compounds 4R or 4S (2 μL of 10 mM solution) were dropped on the epidermis and the samples were incubated for 1-2 hours in DMEM with dermis down (liquid phase) and epidermis exposed to air a room temperature. Next, the samples were washed two times for 5 minutes with PBS to remove not absorbed steroids. Plates containing skin fragments were placed upside down on Biorad UV Transilluminator 2000 (Biorad, Hercules, CA) and irradiated with 100, 500, 1000 and 2500 mJ/cm2. After irradiation tissue was shredded and steroids were extracted with excess of methylene chloride (twice). The samples were dried under nitrogen and analyzed by RP-HPLC. Untreated skin (irradiated and non-irradiated), skin treated with 4R or 4S without irradiation and compounds 4R or 4S irradiated in ethanol served as controls.
Colony forming assay
To measure colony formation and growth of melanoma SKMEL-188, cells were plated at a colony-forming density (200 cells per well on six well plates). Cells were grown in F10 medium (GIBCO, Invitrogen Corp., Carlsbad, CA) supplemented with charcoal-stripped fetal bovine serum (HyClone, Logan, UT) and an antibiotic-antimycotic solution (Sigma, St. Louis, MO) at 37°C in an atmosphere of 95% air and 5% CO2.[23] Cells were treated with compounds: 4R, 4R-pD, 4R-pL, 4S, 4S-pD, 4S-pL and 1,25-dihydroxyvitamin D3 as a positive control at different concentrations (0.1, 10 and 100 nM). Compounds were added to the media from 1 mM ethanol stock solutions, and corresponding dilution of methanol was used as negative (vehicle) control. Cells were grown for 6-10 days and colonies were fixed with 4% paraformaldehyde in PBS at 4°C. The fixed cells were washed with distilled water, stained with 0.1% crystalline blue for 30 min and rinsed with distilled water to remove excess of the dye. Plates were photographed and the number and the size of colonies were measured by ImageJ software (NIH, Bethesda, MD). Colony forming efficiency (CFU) was determined by dividing the number of colonies formed by the number of cells plated, and multiplied by 100.
Statistical analyses
Data were tested for distribution and homogeneity of variances, and for statistical significance with one-way analysis of variance (Anova) with Tuckey post-hoc test (for more than two groups) using Prism 4.00 (GraphPad Software, San Diego, CA). Data is presented as the mean ± SEM; for n=4-6. *P<0.05, **P<0.01 and ***P<0.001.
Other procedures
All RP-HPLC analyses were performed using a Waters' HPLC-system equipped with a diode-array detector, auto-sampler and fraction collector (Waters Associates, Milford, MA) as was described elsewhere.[21] Samples were injected into an Atlantis C18 column running on 70/30 methanol/water at a flow rate of 1.5 mL/min. Collected fractions (15 sec) containing above 95% of pure compound (for 240 nm and 280 nm spectra) were pooled together and used for further characterization. The procedure was optimized to achieve the best separation of each selected product.
Mass spectra were recorded using a Bruker Esquire-LC/MS Spectrometer equipped with an electrospray ionization (ESI) source, as described previously [21].
NMR measurements were performed on a Varian Unity Inova-500 MHz spectrometer (Variannmr Inc., Palo Alto, CA) using either a 3 mm probe or a 5 mm probe. Deuterated methanol was used instead of CDCl3 to avoid observed decomposition due to traces of acid that are present in commercial chloroform.
Results
Synthesis of pregna-5,7-diene-3β,17α,20-triols
The synthesis was performed essentially as described previously [21] (See Scheme 2 and Material and Methods for details).
Scheme 2. Synthesis of 4R and 4S.a.
a Reagents and conditions: (a) Ac2O, microwave, p-toluenesulfonic acid monohydrate; (b) dibromantin, 2,2′-azobisisobutyronitrile, benzene/hexane (1:1), 100°C, reflux; (c) Bu4NBr, Bu4NF, THF, room temperature; (d) LiAlH4, THF, 0°C.
The synthesis of pregna-5,7-diene-3β,17α,20-triols (4) was carried out from 17α-acetylated 5-en precursor (1) by acetylation, bromination-dehydrobromination and reduction, following the known procedure.[13] Deprotection was performed simultaneously with reduction of the carbonyl group. The procedure resulted in formation of two diastereomers: 4R and 4S (20R and 20S) with a ratio of 50:50. The mixture was effectively separated by reverse phase HPLC (RP-HPLC). The presence of 4,6-dienes was not detected, as was reported during the synthesis of 3β-hydroxypregna-5,7-dien-20-one and androsta-5,7-diene-3β,17β-diol [21]. Physico-chemical properties of pregna-5,7-diene-3β,17α,20-triols are summarized in Table 1. The detailed NMR data are presented on Table 2. NMR chemical shifts for 4R and 4S are in agreement with those presented previously,[13] with small differences related to a solvent effect due to the use of CD3OD instead of CDCl3 for NMR experiments.
Table 1. UV and MS spectra data date for pregna-5,7-diene-3β,17α,20-triols and its derivatives.
| No | Parental compound | Structure type | UV max (nm) | Predicted MW | MS+ |
|---|---|---|---|---|---|
| 4R | 1 | 5,7-diene | 261,270,281,290 | 332.25 | 355.25 [M+Na]+ |
| 4R1 | 4R | preD-like | 260 | 332.25 | ND |
| 4R-D | 4R | D-like | 265 | 332.25 | 355.25 [M+Na]+ |
| 4R-L | 4R | L-like | 262,271,281 | 332.25 | 355.25 [M+Na]+ |
| 4R-T | 4R | T-like | 270,279,291 | 332.25 | 355.25 [M+Na]+ |
| 4R-iT | 4R | isoT-like (oxide) | 240,248, 257 | 364.25 | 387.15 [M+Na]+ |
| 4R7 | 4R | 5,7,9(11) triene | 312,323,340 | 330.22 | ND |
| 4S | 1 | 5,7-diene | 263,271,282,292 | 332.25 | 355.25 [M+Na]+ |
| 4S1 | 4S | preD-like | 260 | 332.25 | ND |
| 4S-D | 4S | D-like | 265 | 332.25 | 355.25 [M+Na]+ |
| 4S-L | 4S | L-like | 262,271,282 | 332.25 | 355.25 [M+Na]+ |
| 4S-T | 4S | T-like | 272,282,291 | 332.25 | 355.25 [M+Na]+ND |
| 4S-iT1 | 4S | isoT-like | 241,248, 257 | 332.25 | 355.25 [M+Na]+ |
| 4S-iT2 | 4S | isoT-like (oxide) | 241,248, 257 | 332.25 | 387.15 [M+Na]+ |
| 4S7 | 4S | 5,7,9(11) triene | 312,323,340 | 330.22 | ND |
Bold – purified and characterized (NMR), italics – characterized by UV spectra, ND – not determined
Table 2. 1H NMR chemical shifts of pregna-5,7-diene-3β,17α,20-triols, D-like, L-like and 5,7,9(11)-triene.
| 4R | 4S | 4R-D | 4S-D | 4R-L | 4S-L | 5S | |
|---|---|---|---|---|---|---|---|
| 1 CH2 | α 1.31 | α 1.29 | α 2.12 | α2.12 | α 1.31 | α 1.31 | 1.48 |
| β 1.90 | β 1.90 | β 2.42 | β2.41 | β 1.90 | β 1.90 | 1.70 | |
| 2 CH2 | α 1.92 | α 1.93 | α 1.97 | α1.97 | α 1.92 | α 1.92 | 1.89 |
| β 1.45 | β 1.54 | β 1.68 | β 1.68 | β 1.54 | β 1.54 | 1.68 | |
| 3 CH | 3.51 | 3.51 | 3.76 | 3.76 | 4.03 | 4.03 | 3.47 |
| 4 CH2 | α 2.41 | α 2.40 | α 2.55 | α 2.54 | α 2.41 | α 2.44 | 2.34 |
| β 2.23 | β 2.23 | β 2.19 | β 2.19 | β 2.29 | β 2.35 | 2.42 | |
| 6 CH | 5.54 | 5.54 | 6.23 | 6.24 | 5.59 | 5.54 | 5.65 |
| 7 CH | 5.42 | 5.39 | 6.09 | 6.09 | 5.47 | 5.39 | 5.40 |
| 9 CH | 2.11 | 2.02 | 2.85 | 2.85 | 2.02 | 2.02 | |
| 1.54 | 1.56 | ||||||
| 11 CH2 | α 1.67 | α 1.6-1.8 | α 1.77 | α 1.77 | α 1.7-1.8 | α 1.7-1.8 | 5.61 |
| β 1.75 | β 1.6-1.8 | β 1.77 | β 1.77 | β 1.7-1.8 | β 1.7-1.8 | ||
| 12 CH2 | α 1.49 | α 1.49 | α 1.54 | α 1.56 | α 1.49 | α 1.49 | 2.61 |
| β 1.80 | β 2.12 | β 2.05 | β 2.04 | β 2.18 | β 2.18 | 2.14 | |
| 14 CH | 2.62 | 2.57 | 2.12 | 2.12 | 2.05 | 2.05 | 2.78 |
| 15 CH2 | α 1.83 | α 1.84 | α 1.54 | α 1.61 | α 1.82 | α 1.82 | 1.85 |
| β 1.63 | β 1.56 | β 2.53 | β 1.61 | β 1.51 | β 1.51 | 1.50 | |
| 16 CH2 | α 1.56 | α 1.7-1.8? | α 1.87 | α 1.7 | α 1.76 | α 1.76 | 1.60 |
| β 1.74 | β 1.9? | β 2.7 | β 2.66 | β 2.22 | β 2.22 | 1.75 | |
| 18 CH3 | 0.7 | 0.77 | 0.6 | 0.67 | 0.76 | 0.76 | 0.67 |
| 19 CH3 | 0.94 | 0.95 | α 4.76 | α 4.74 | 0.67 | 0.75 | 1.24 |
| β 5.05 | β 5.05 | ||||||
| 20 C | 3.78 CH | 3.94 CH | 3.76 | 3.61 | 3.72 CH | 3.65 CH | 3.9 |
| 21 CH3 | 1.19 | 1.15 | 1.18 | 1.14 | 1.14 | 1.15 | 1.16 |
Italics - not fully determined presumably similar to parental compound
UVB irradiation of pregna-5,7-diene-3β,17α,20-triols (4) and identification of products by combination of RP-HPLC, UV and mass spectrometry
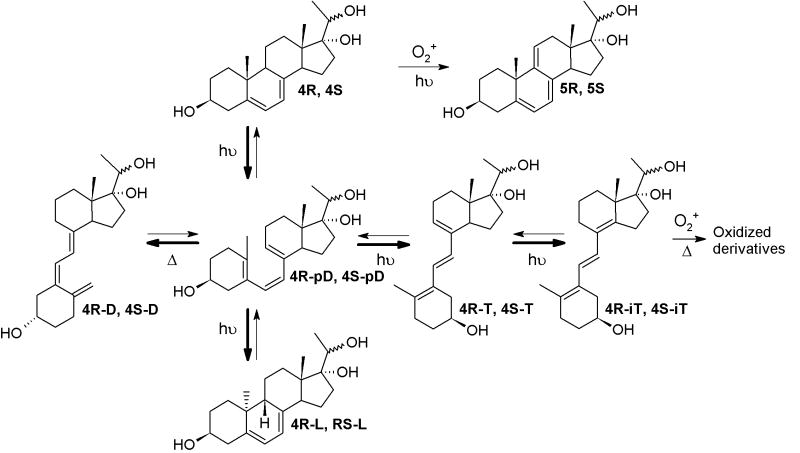
The UV conversion of 4R and 4S were performed using a UVB light source (4.8 ± 0.2 mW cm-2) with maximum emission spectrum in a range of 290–320 nm[22] as described previously.[21] The photo-conversion and subsequent time dependent structural rearrangements were monitored by an HPLC equipped with a diode array that enabled fast detection of products by characteristic UV spectra.[24] Similarly to cholesta-5,7-dien-3β-ol (7DHC)[24], four main products (Scheme 3) were formed in time-dependent fashion, namely pro-D-like, T-like, L-like and D-like. All products were separated by HPLC and identified based on their unique UV absorption spectra (Figure 1).
Scheme 3.
Photolysis of pregna-5,7-diene-3β,17α,20-triols.
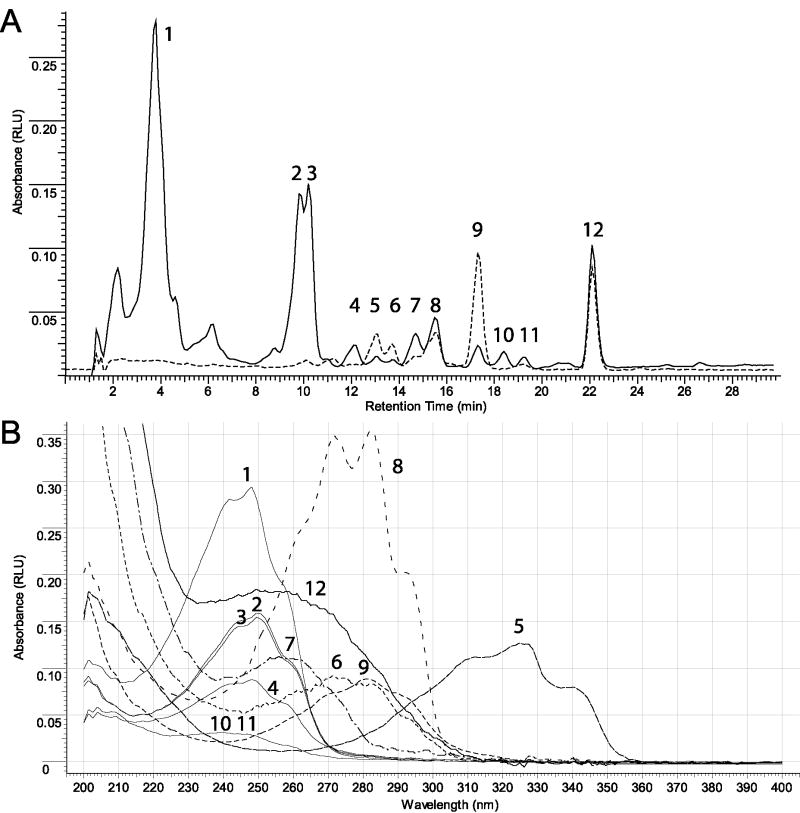
Figure 1. UVB- driven photolysis of 4S (as an example).
(A) RP-HPLC separation of 4S irradiation products after treatment with UVB for 15 minutes. (B) Representative UV spectra of irradiated samples. Peak number (Panel A) corresponds to predicted structure based on UV spectra (Panel B). Peaks were assigned as follows: 1-4, 10, 11 – isoT-like and oxidized isoT-like; 5 – 5S; 6 – 4S-L; 7 – 4S-D; 8 – 4S; 9 – 4S-T; 12 – 4S-pD. The UV spectra for RP-HPLC (Panel A) were measured at 280 nm.
Representative UV spectra of irradiated samples of 4R (c) and 4S (d). (1 – 5c, 2-5cD, 3 – unknown product, 4 – 5cT, 5 – 5c1, 6 – unknown product).
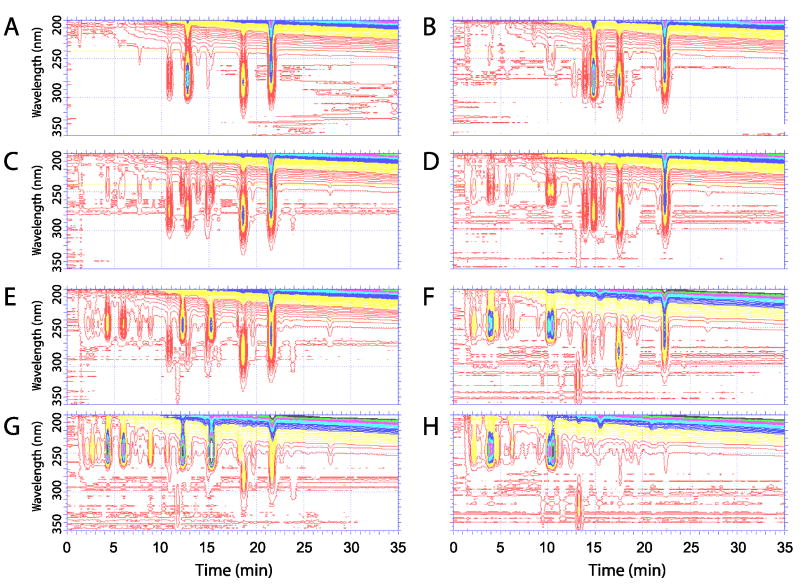
The irradiation of 4R and 4S with increasing UV doses resulted in the formation of diverse products, as shown in Figure 2. Short irradiation (5 min.; Figure 2A, B) of both 4R and 4S resulted in the formation of pre-D-like (λmax at 260 nm; 4R-pD and 4S-pD), T-like (λmax at 274, 281, 290 nm 4R-T and 4S-T) and L-like (262, 271, 281; 4R-L and 4S-L) products. The products 4R-pD and 4S-pD underwent subsequent slow isomerization into vitamin D-like compounds (4R-D and 4S-D) with maximum UV absorption at 265 nm. Longer UVB irradiation (15 minutes; Figure 2C, D) resulted in conversion of T-like products to isoT-like compounds with characteristic UV absorption for a conjugated double bond (λmax at 248 nm). Irradiation for 30 and 60 minutes (Figure 2E-H) resulted in further conversion into numerous iso-T-like products with a corresponding decrease in pre-D-like and T-like products, indicating further metabolism (Scheme 2) with the potential formation of suprasterols [4] with a λmax below 250 nm.
Figure 2.
Dose (time of irradiation) dependence of pregna-5,7-diene-3β,17α,20-triols (4R and 4S) photo-conversion. The compound 4R (Panels A, C, E and G) or 4S (Panels B, D, F and H) was irradiated for 5 (A,B), 15 (C,D), 30 (E,F) or 60 (G,H) minutes and products were separated by using RP-HPLC. The absorption profiles of products were recorded by using diode-array detector. The samples were separated after incubation for 2 hours at room temperature.
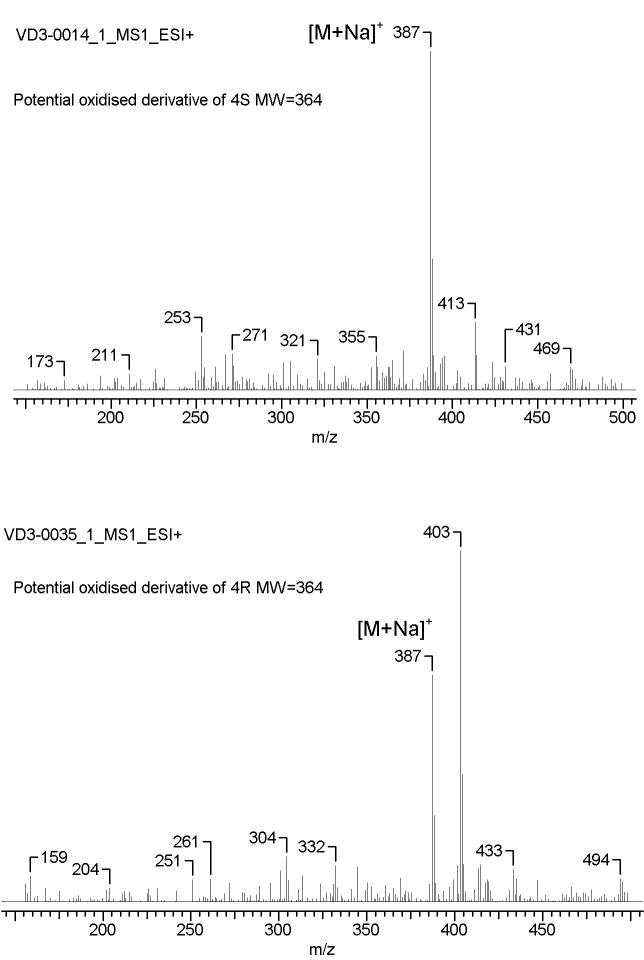
Further identification was performed after purification by RP-HPLC (see Materials and Methods for details) and the corresponding fractions were analyzed by MS. As expected, all D-like, L-like and T-like products had identical mass (m/z = 355.25 [M+Na]+) with the parental compounds (Table 1). In addition to a molecular ion at m/z = 355.25 [M+Na]+ the majority of iso-T-like products of irradiation had an additional ion at m/z = 387.15 (Figure 3). This indicates either the addition of O2 with formation of peroxide or hydroxyperoxide derivatives similar to those shown for iso-T3 (6E-9,10-secocholesta-5(10),6,8(14)-trien-3β-ol)[6], or oxidation of 4S and 4R without photolysis of the B ring, with production of of endoperoxide and hydroperoxide, as was shown for 7-DHC.[8,10] The small scale of reaction and instability of isoT-like compounds prevented their more complete characterization.
Figure 3.
Identity of oxidized derivatives of 4R (A) and 4S by mass spectrometry. Sample were purified after UV treatment using RP-HPLC and analyzed with LC-MS.
Identification of novel L-like, D-like, and T-like compounds by NMR
The D-, L- or T-like irradiation products of 4R and 4S of defined UV and mass spectra were subjected to NMR analysis. Elucidation of the structures was based on 1H -NMR data and selected 2D experiments (COSY, TOCSY). The detailed list of chemical shifts with an assignment of signals is shown in Table 2. The D-like (4R-D and 4S-D), and L-like (4R-L and 4S-L) compounds were assigned based on expected chemical shifts for vinylic and methyl protons with the characteristic pattern. The main difference between NMR data for L-like compounds (4R-L and 4S-L) and their respective parental compounds is a downfield shift of the methyl group at C19 (∼0.20 ppm).
Although we were able to detect and initially characterize T-like and isoT-like compounds derived from 4R and 4S, these were not stable, which prevented their in-depth characterization.
Detection and characterization of novel triene-like products of UVB irradiation of 4R and 4S
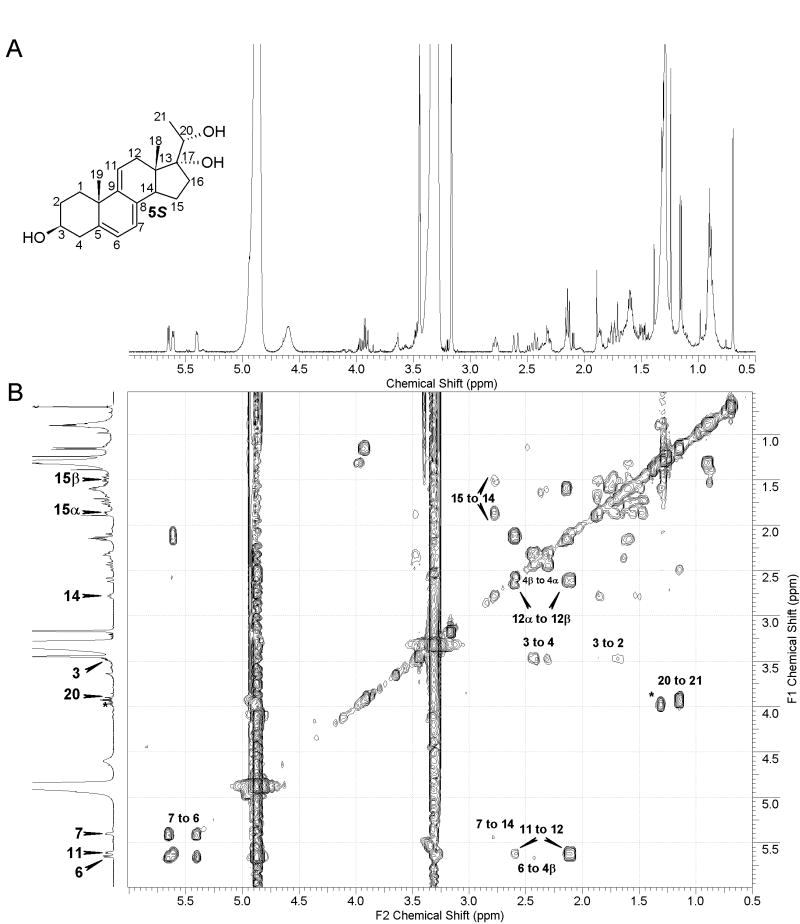
In addition to well-characterized products of 5,7-diene irradiation (D-like, L-like, T-like and isoT-like compounds), we detected other products with UV absorption (λmax) UV absorption suggests the presence of a triene system, presumable similar to cholesta-5,7,9(11)-trien-3β-ol (9-DDHC), with reported λmax at 324 nm.[9,10] Although irradiation of both 4R and 4S resulted in the formation of compounds with λmax max above 300 nm, the process was more efficient from 4S precursor (Figure 2). NMR analysis of this product confirmed the presence of 5,7,9(11)-triene system, and the product was identified as pregna-5,7,9(11)-triene-3β,17α,20S-triol (Figure 4), a compound that was previously reported.13 The chemical shifts we observe for 5S (Table 2) are essentially identical (with small changes due to different solvent) to these earlier data.
Figure 4.
Identification of pregna-5,7,9(11)-triene-3β,17α,20S-triol (A) 500 MHz proton NMR (B) COSY showing correlation from 11-CH to 12a-CH2, 20-H to 21-CH3 and 3-CH to 4-CH2 and 2CH2. Additionally, correlation from 7-CH to C-14 and from 14-CH to 15CH2. Impurities are marked with asterisk.
Production of pregna-5,7-diene-3β,17α,20-triols (4) derivatives in human skin subjected to UV radiation (UVR)
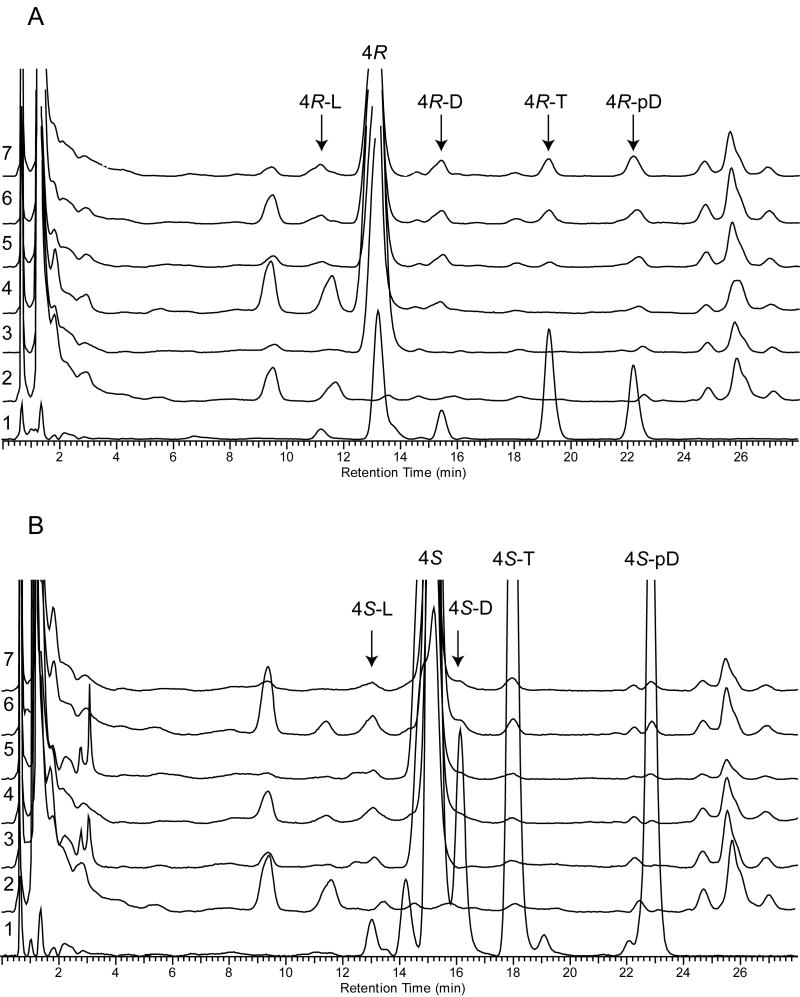
Fragments of neonatal human skin (approximately, 5×5 mm) were treated with 4R and 4S, incubated in culture medium for 1-2 hours, washed and subjected to UV-irradiation as described in Experimental section. The products of irradiation were separated using RP-HPLC (Figure 5). Previously identified products of UV radiation of 4R and 4S in methanol were used as standards. Based on identical retention times we detected UVR induced production of L-like, D-like, T-like and preD-like compounds in human skin from 4R (Figure 5A) and 4S (Figure 5B). The formation of 4R and 4S secoderivatives was UVR dose dependent and the maximum production was observed at 2500 mJ/cm2 (Figure 5; chromatograms 7 on panels A and B). These secosteroids were absent in the untreated human skin (Figure 5, chromatograms 2) or human skin exposed only to pregna-5,7-diene-3β,17α,20-triols without following UVR (Figure 5, chromatograms 3 on panels A and B). We also noted appearance of products corresponding to isoT-like derivatives with characteristic UV absorption (λmax at 250 nm) and retention time around 2 minutes (not shown). Further identification of these products was not possible due to limited absorption of steroidal precursors by skin and subsequently relatively low production of secosteroids.
Figure 5. Ex vivo production of pregna-5,7-diene-3β,17α,20-triols (4R and 4S) derivatives in human neonatal skin.
Skin fragments were treated with 2μL of 10 mM of 4R (Panel A) or 4S (Panel B) and subjected to UV irradiation. The products of photolysis in skin were subjected to RP-HPLC chromatography (chromatograms 2-7) and their retention times were compared with previously identified products of UV irradiation of 4R and 4S in methanol (chromatogram 1). Different UV doses were used to show dynamics of the process. The UVB doses per cm2 were as follows: chromatograms 1 and 7 – 2500 mJ/cm2, 3 – 0 mJ/cm2, 4 – 100 mJ/cm2, 5 – 500 mJ/cm2, 6 – 1000 mJ/cm2. Chromatograms 2 show normal profile of non-treated and non-irradiated skin.
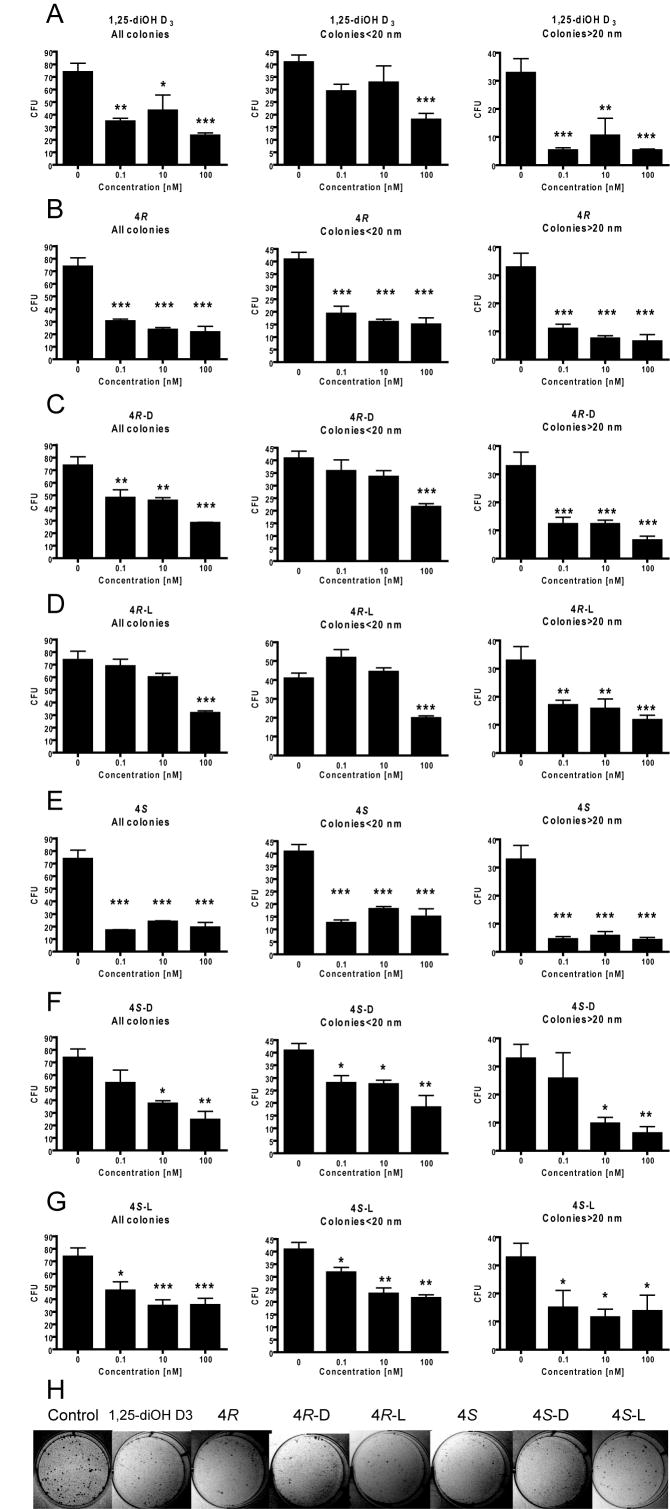
Inhibition of melanoma SKMEL-188 colony formation by pregna-5,7-diene-3β,17α,20-triols (4R and 4S) and their D-like and L-like derivatives
The inhibition of melanoma SKMEL-188 colony formation by 4R or 4S and their D-like and L-like derivatives was used to assay their biological activity as described in the Experimental section. All studied compounds had antiproliferative properties as showed by inhibition of colony formation (Figure 6). When all colonies were counted, the average inhibition for the highest concentrations of tested compounds (100 nM) was 50-70%. The inhibitory effect was relatively lower (≤50%) for small colonies with diameter below 20 nm and higher for colonies above 20 nm (50-80%) at 100 nM concentrations. Interestingly, the pregna-5,7-diene-3β,17α,20-triols precursors (4R and 4S) were more efficient in growth inhibition than their photoderivative products (Figure 6 B and E). When comparing to 1,25(OH)2D3, (Fig. 6 A) we found that the precursor 4S (Fig. E) was the most potent compound, while L-like compound 4R-L showed the lowest activity (Fig. 6 D). Furthermore other compounds including 4S-D, 4S-L and 4R-D had similar effects as 1,25-(OH)2D3.
Figure 6.
Inhibition of colony formation of melanoma SKMEL-188 treated with pregna-5,7-diene-3β,17α,20-triols 4R (B) and 4S (E) and their D-like and L-like derivatives: 4R-D (C), 4R-L (D), 4S-D (F) and 4S-L (G).
An active form of vitamin D3 1,25(OH)2D3 was used as positive control (A). The inhibition of melanoma SKMEL-188 colony formation was used to assay biological activity of newly synthesized compounds at 0.1, 10 and 100 nM concentrations. The average value (n=6) for ethanol treated cells was used as a 0 nM on all graphs. The total number of colonies and their diameters with cutoff below and above 20 nm was measured and expressed in colony forming units (CFU). Data are presented as mean±SEM for colony-forming assay (n=4 to 6); *P<0.05, **P<0.005, and ***P<0.001. Representative pictures of SKMEL treated with studied compounds and the vehicle control are shown (H).
Discussion
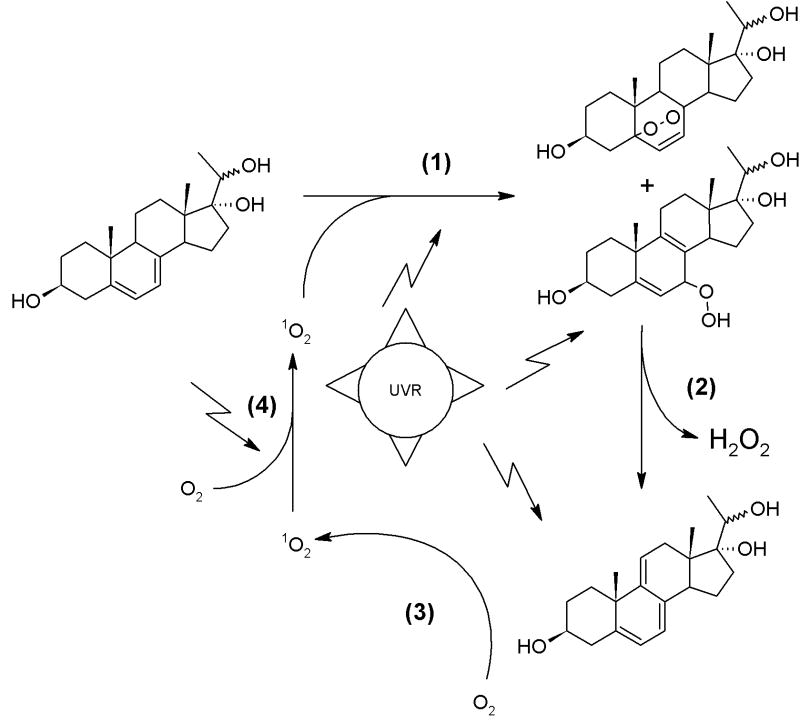
We are reporting for the first time an efficient, reproducible and detailed method for the UV-driven derivatization of two epimers (20R and 20S) of pregna-5,7-diene-3β,17α,20-triol (4R and 4S). To our knowledge, this is the first description of vitamin D-like (4S-D, 4R-D), L-like (4S-L and 4R-L), T-like (4S-T and 4R-T) derivatives of 4R and 4S. Careful observation of the dynamics of conversion reveal a rapid, UVB dose-dependent conversion of T-like compounds to iso-T, and their further oxidation. Longer UVB irradiation results in the synthesis of pregna-5,7,9(11)-triene-3β,17α,20S-triol (5S), its structure being confirmed by NMR. Although, chemical synthesis of 5S was previously reported,[13] the role of UV in its formation has not been described. Interestingly, it was postulated that pregna-5,7,9(11)-triene-3β,17α,20S-triol is produced in humans because 21-deoxypregnane is almost exclusively reduced to 20S-hydroxyls.[14] In our hands, compound 4S was more efficiently converted to triene by UVB irradiation, despite the reduction of compound 3 resulting in an almost 50-50 mixture of 4R and 4S. It was also shown that the synthesis of steroidal trienes has two potential intermediates of endoperoxide and hydroperoxide nature, and could be triggered by singlet oxygen in the presence of photosensitizing agents.[8,9,11] The 9-DDHC can also act as chromatophore, stimulating the production of singlet oxygen and subsequent oxidation of 7DHC to 7-hydroxy-5,8(9)-cholestatriene, which undergoes decomposition to give 9-DDHC and hydrogen peroxide.[9] Such a self regenerating system producing singlet oxygen was also shown for ergosterol and its triene derivative.[25] Our data not only support such a model but also indicate for the first time that UV irradiation is sufficient for the auto-catalytic conversion of pregna-5,7-diene-3β,17α,20S-triol (4S) to pregna-5,7,9(11)-triene-3β,17α,20S-triol (5S), as is presented in Scheme 3; the presence of oxidized derivatives was confirmed by MS (Figure 3). Interestingly, formation of triene for 7DHC was observed only in the presence of photosensitizers [8-11,25] and extensive irradiation of 3β-hydroxypregna-5,7-dien-20-one resulted in the production of oxidized iT-like compounds.[21] This suggests a correlation between the presence of two hydroxyls at C17 and C20 and UVR induced formation of the triene.
Since 4S and 4R and other androsta- and pregna-5,7-dienes derivatives were detected in living organisms under physiological and pathological conditions [10,13,14,26-30] our current data suggest (by inference) that they may be converted into corresponding secosteroids in the skin if exposed to solar radiation [31]. To test this hypothesis we performed ex vivo experiments with photolytic reactions conducted in human skin. Thus, using a physiologically relevant topical application of pregna-5,7-diene-3β,17α,20-triols with following ex-vivo incubation, washing of unincorporated products and UVR exposure, we were able to detect peaks with retention times corresponding to L-like, D-like, T-like and preD-like standards that were absent in negative controls. This indicates that under physiological conditions (skin) steroidal dienes without the side chain (pregna-5,7-diene-3β,17α,20-triols) undergo similar pathway of photochemical transformations to their corresponding secosteroidal products as in the model UVR induced transformation of 7-dehydrocholestrol to secosteroids containing the full side chain (vitamin D3, tachysterol3 and luminosterol3) described by the Holick group [1, 3]. These findings also substantiate future effort to study photolytic transformation of pregna-5,7-diene-3β,17α,20-triols in SLOS patients, since these were detected in body fluids of those patients [13,14].
Analogs of vitamin D3 without cholesterol-type side-chain have low calcemic activity [18,32] and vitamin D3 and its derivatives have broad spectrum of biological functions [2,16,33], including anti-carcinogenic properties in the skin (reviewed in [34,35]) and melanomas (reviewed in [36]). In the present studies we have shown that pregna-5,7-diene-3β,17α,20-triols and its D-like and L-like derivatives have clear tumorostatic activity as defined by their inhibition of colonies formation by human melanoma cells. Interestingly, 5,7-diene-3β,17α,20-triols precursors showed the highest activity with 4S being the most active, even more active than 1,25(OH)2D3, while L-like compound 4R-L was the least potent. The other compounds showed similar activity to 1,25(OH)2D3. Thus, these studies may help to identify efficient drugs for adjuvant melanoma therapy including the described secosteroids and, interestingly, their precursor 5,7-diene-3β,17α,20-triols. Thus, newly generated dihydroxy derivatives, as described in this paper, may serve as promising candidates for the treatment of various pathological processes, including malignant melanoma, which is resistant to any type of therapy once metastatic process has started.[37]
In conclusion, we have characterized in details an in vitro UVB induced conversion of two epimers (20R and 20S) of pregna-5,7-diene-3β,17α,20-triol, identified and defined the structures of new products of this process, demonstrated that similar transformation can occur in the skin and demonstrated anti-melanoma activity of the substrates and secosteroidal products of the photoconversion.
Scheme 4. Putative model of UVR induced generation of pregna-5,7,9(11)-triene-3β,17α,20S-triol.
(1) Singlet oxygen initiates photooxidation of 4S or 4R, with formation of endoperoxide and hydroperoxide.
(2) UV stimulates H2O2 and triene formation.
(3) Triene acts as a photosensitizer for singlet oxygen generation.
(4) The source of initial singlet oxygen is uncertain, but possibly it is formed from O2 during irradiation.
Acknowledgments
Supported by grant # AR052190 from NIH/NIAMS to AS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF, Clark MB. The photobiogenesis and metabolism of vitamin D. Fed Proc. 1978;37:2567–74. [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc Natl Acad Sci U S A. 1995;92:3124–6. doi: 10.1073/pnas.92.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–7. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- 5.Tian XQ, Holick MF. A liposomal model that mimics the cutaneous production of vitamin D3. Studies of the mechanism of the membrane-enhanced thermal isomerization of previtamin D3 to vitamin D3. J Biol Chem. 1999;274:4174–9. doi: 10.1074/jbc.274.7.4174. [DOI] [PubMed] [Google Scholar]
- 6.Jin X, Yang X, Yang L, Liu ZL, Zhang F. Autoxidation of isotachysterol. Tetrahedron. 2004;60:2881–2888. [Google Scholar]
- 7.Valencia A, Kochevar IE. Ultraviolet A induces apoptosis via reactive oxygen species in a model for Smith-Lemli-Opitz syndrome. Free Radic Biol Med. 2006;40:641–50. doi: 10.1016/j.freeradbiomed.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Feng K, Zhang RY, Wu LZ, Tu B, Peng ML, Zhang LP, Zhao D, Tung CH. Photooxidation of olefins under oxygen in platinum(II) complex-loaded mesoporous molecular sieves. J Am Chem Soc. 2006;128:14685–90. doi: 10.1021/ja0648256. [DOI] [PubMed] [Google Scholar]
- 9.Chignell CF, Kukielczak BM, Sik RH, Bilski PJ, He YY. Ultraviolet A sensitivity in Smith-Lemli-Opitz syndrome: Possible involvement of cholesta-5,7,9(11)-trien-3 beta-ol. Free Radic Biol Med. 2006;41:339–46. doi: 10.1016/j.freeradbiomed.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 10.De Fabiani E, Caruso D, Cavaleri M, Galli Kienle M, Galli G. Cholesta-5,7,9(11)-trien-3 beta-ol found in plasma of patients with Smith-Lemli-Opitz syndrome indicates formation of sterol hydroperoxide. J Lipid Res. 1996;37:2280–7. [PubMed] [Google Scholar]
- 11.Albro PW, Corbett JT, Schroeder JL. Doubly allylic hydroperoxide formed in the reaction between sterol 5,7-dienes and singlet oxygen. Photochem Photobiol. 1994;60:310–5. doi: 10.1111/j.1751-1097.1994.tb05109.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruan B, Wilson WK, Pang J, Gerst N, Pinkerton FD, Tsai J, Kelley RI, Whitby FG, Milewicz DM, Garbern J, Schroepfer GJ., Jr Sterols in blood of normal and Smith-Lemli-Opitz subjects. J Lipid Res. 2001;42:799–812. [PubMed] [Google Scholar]
- 13.Guo LW, Wilson WK, Pang J, Shackleton CH. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids. 2003;68:31–42. doi: 10.1016/s0039-128x(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 14.Shackleton CH, Roitman E, Kelley R. Neonatal urinary steroids in Smith-Lemli-Opitz syndrome associated with 7-dehydrocholesterol reductase deficiency. Steroids. 1999;64:481–90. doi: 10.1016/s0039-128x(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 15.Smith DW, Lemli L, Opitz JM. A Newly Recognized Syndrome of Multiple Congenital Anomalies. J Pediatr. 1964;64:210–7. doi: 10.1016/s0022-3476(64)80264-x. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 17.Plum LA, Prahl JM, Ma X, Sicinski RR, Gowlugari S, Clagett-Dame M, DeLuca HF. Biologically active noncalcemic analogs of 1alpha,25-dihydroxyvitamin D with an abbreviated side chain containing no hydroxyl. Proc Natl Acad Sci U S A. 2004;101:6900–4. doi: 10.1073/pnas.0401656101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murari MP, Londowski JM, Bollman S, Kumar R. Synthesis and biological activity of 3 beta-hydroxy-9,10-secopregna-5,7,10[19]-triene-20-one: a side chain analogue of vitamin D3. J Steroid Biochem. 1982;17:615–9. doi: 10.1016/0022-4731(82)90562-3. [DOI] [PubMed] [Google Scholar]
- 19.Bridgewater AJ, Cheung HTA, Vadasz A, Watson TR. Ring C aromatic steroids. Part 2. Rearrangement of 16a,17a-epoxy- and 17a-hydroxy-5,7-dienes. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry. 1980;2:556–62. [Google Scholar]
- 20.Marwah P, Marwah A, Lardy HA. Microwave induced selective enolization of steroidal ketones efficient acetylation of sterols in semisolid state. Tetrahedron. 2003;59:2273–2287. [Google Scholar]
- 21.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008 doi: 10.1039/b809005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. Faseb J. 2006;20:1564–6. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 23.Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511:102–6. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- 24.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–3. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 25.Albro PW, Bilski P, Corbett JT, Schroeder JL, Chignell CF. Photochemical reactions and phototoxicity of sterols: novel self-perpetuating mechanisms for lipid photooxidation. Photochem Photobiol. 1997;66:316–25. doi: 10.1111/j.1751-1097.1997.tb03154.x. [DOI] [PubMed] [Google Scholar]
- 26.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330:107–13. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 27.Nowaczyk MJ, Waye JS. The Smith-Lemli-Opitz syndrome: a novel metabolic way of understanding developmental biology, embryogenesis, and dysmorphology. Clin Genet. 2001;59:375–86. doi: 10.1034/j.1399-0004.2001.590601.x. [DOI] [PubMed] [Google Scholar]
- 28.Tait AD, Hodge LC, Allen WR. Biosynthesis of 3 beta-hydroxy-5,7-pregnadien-20-one by the horse fetal gonad. FEBS Lett. 1983;153:161–4. doi: 10.1016/0014-5793(83)80139-2. [DOI] [PubMed] [Google Scholar]
- 29.Tait AD, Hodge LC, Allen WR. The biosynthesis of 3 beta-hydroxy-5,7-androstadien-17-one by the horse fetal gonad. FEBS Lett. 1985;182:107–10. doi: 10.1016/0014-5793(85)81164-9. [DOI] [PubMed] [Google Scholar]
- 30.Tait AD, Santikarn S, Allen WR. Identification of 3 beta-hydroxy-5,7-pregnadien-20-one and 3 beta-hydroxy-5,7-androstadien-17-one as endogenous steroids in the fetal horse gonad. J Endocrinol. 1983;99:87–92. doi: 10.1677/joe.0.0990087. [DOI] [PubMed] [Google Scholar]
- 31.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar CC, Tuckey R. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holick MF, Garabedian M, Schnoes HK, DeLuca HF. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J Biol Chem. 1975;250:226–30. [PubMed] [Google Scholar]
- 33.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–44. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 34.Kamradt J, Rafi L, Mitschele T, Meineke V, Gartner BC, Wolfgang T, Holick MF, Reichrath J. Analysis of the vitamin D system in cutaneous malignancies. Recent Results Cancer Res. 2003;164:259–69. doi: 10.1007/978-3-642-55580-0_19. [DOI] [PubMed] [Google Scholar]
- 35.Bikle DD, Oda Y, Xie Z. Vitamin D and skin cancer: a problem in gene regulation. J Steroid Biochem Mol Biol. 2005;97:83–91. doi: 10.1016/j.jsbmb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Brozyna A, Zbytek B, Granese J, Ross J, Carlson JA, Slominski A. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev Dermatol. 2007;2:451–469. doi: 10.1586/17469872.2.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlson JA, Ross JS, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M., Jr Molecular diagnostics in melanoma. J Am Acad Dermatol. 2005;52:743–75. doi: 10.1016/j.jaad.2004.08.034. quiz 775-8. [DOI] [PubMed] [Google Scholar]