Abstract
Neuroscientific research has shown that the hippocampus is important for binding or linking together the various components of a learning event into an integrated memory. In a prior study, we demonstrated that the anterior hippocampus is involved in memory for the relations among informational elements to a greater extent than it is involved in memory for individual elements (Giovanello, Schnyer, and Verfaellie, 2004). In the current study, we extend those findings by further specifying the role of anterior hippocampus during relational memory retrieval. Specifically, anterior hippocampal activity was observed during flexible retrieval of learned associations, whereas posterior hippocampal activity was detected during reinstatement of study episodes. These findings suggest a functional dissociation across the long axis of human hippocampus based on the nature of the mnemonic process rather than the stage of memory processing or type of stimulus.
Introduction
The hippocampal formation – a brain structure within the medial temporal lobe – is known for its importance in memory for facts and events (Scoville and Milner, 1957). Neuroscientific research shows that the hippocampus is important for binding or linking together the various components of a learning event into an integrated memory (Yonelinas et al., 2001; Davachi and Wagner, 2002; Preston et al., 2002; Chua et al., 2004; Pihlajamaki et al., 2003; Giovanello et al., 2004; Jackson III and Schacter, 2004; Achim and Lepage, 2005; Law et al., 2005). Previously, using functional magnetic resonance imaging (fMRI), we demonstrated that the anterior hippocampus is involved in memory for the relations among informational elements (i.e., relational memory) to a greater extent that it is involved in memory for individual elements (i.e., item memory)(Giovanello et al. 2004). In that study, however, the relational condition which elicited hippocampal activity was presented at test in precisely the same format as during study. It, therefore, remained unknown whether the hippocampus is involved in the ‘flexible retrieval’ of relational information or, alternatively, whether the hippocampus is sensitive only to ‘exact reinstatement’ of prior episodes. Here we illuminate this issue by demonstrating anterior hippocampal activity during flexible retrieval of learned associations, and posterior hippocampal activity during reinstatement of study episodes. Retrieval related activity in anterior hippocampus correlated significantly with accuracy of associative recognition, while retrieval related activity in posterior hippocampus showed no such correlation. Finally, hippocampal activation occurred regardless of whether participants were instructed to explicitly engage in relational encoding at study. These findings provide empirical support for a functional dissociation across the long axis of human hippocampus and, furthermore, document a significant correlation of retrieval-related neural activity with behavioral accuracy.
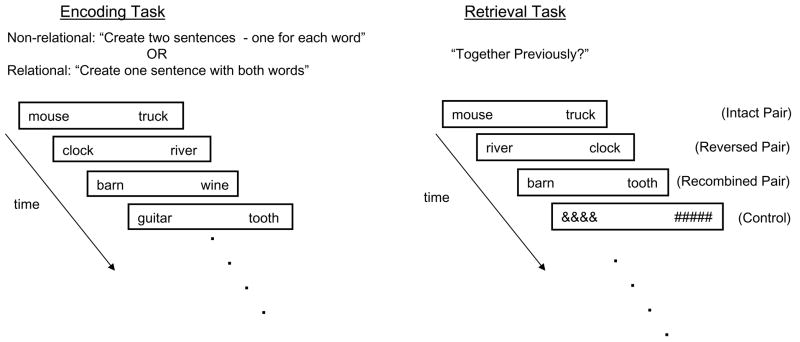
We contrasted the flexible retrieval and the exact reinstatement hypotheses by presenting subjects with unrelated word pairs (i.e., surgeon-arrow) and comparing neural activity during retrieval of previously shown stimuli (i.e., surgeon - arrow; Intact Pair [IP]) with neural activity during retrieval of previously shown, yet reversed stimuli (i.e., arrow - surgeon; Reversed Pair [RevP]) (Figure 1). We reasoned that if anterior hippocampal activation seen previously to be associated with relational memory is greater for IP than for RevP, then these results would support the notion that this region reflects primarily the exact reinstatement of the study episode. Alternatively, if anterior hippocampal activation is equivalent for IP and RevP, then these results would provide strong evidence that this regional is involved in flexible retrieval of learned associations.
Figure 1.
Experimental design. Subjects encountered word pairs and were instructed to covertly create sentences. During non-relational encoding, subjects were instructed to covertly generate two sentences, one for each word. During the subsequent test phase, participants encountered word pairs and were instructed to decide whether the words had be shown ‘together previously?’ (i.e., relational retrieval). During relational encoding, subjects were instructed to covertly generate one sentence that included both words. Similarly, participants were then instructed to decide whether the words had be shown ‘together previously?’ (i.e., relational retrieval). All four non-relational runs were completed before the four relational runs. Test lists consisted of pairs of words previously seen together (Intact Pairs [IP]), pairs of words previously seen together, but in the reversed presentation order (Reversed Pairs [RevP]), and pairs of words previously seen, but not together (Recombined Pairs [RecP]). Both encoding and retrieval tasks were performed in the scanner, but only the retrieval task was scanned.
Additionally, we introduced an encoding manipulation to determine whether hippocampal activation during relational retrieval is dependent on relational processing at encoding (Figure 1). To this end, half of the stimulus pairs were encoded non-relationally (i.e., covertly generate two sentences – one for each word), while the other half were learned in a relational manner (i.e., covertly generate one sentence that relates the two words). This manipulation allowed us to investigate the effect of encoding strategy on flexible and exact retrieval of study episodes. The retrieval conditions were identical across the 2 encoding manipulations.
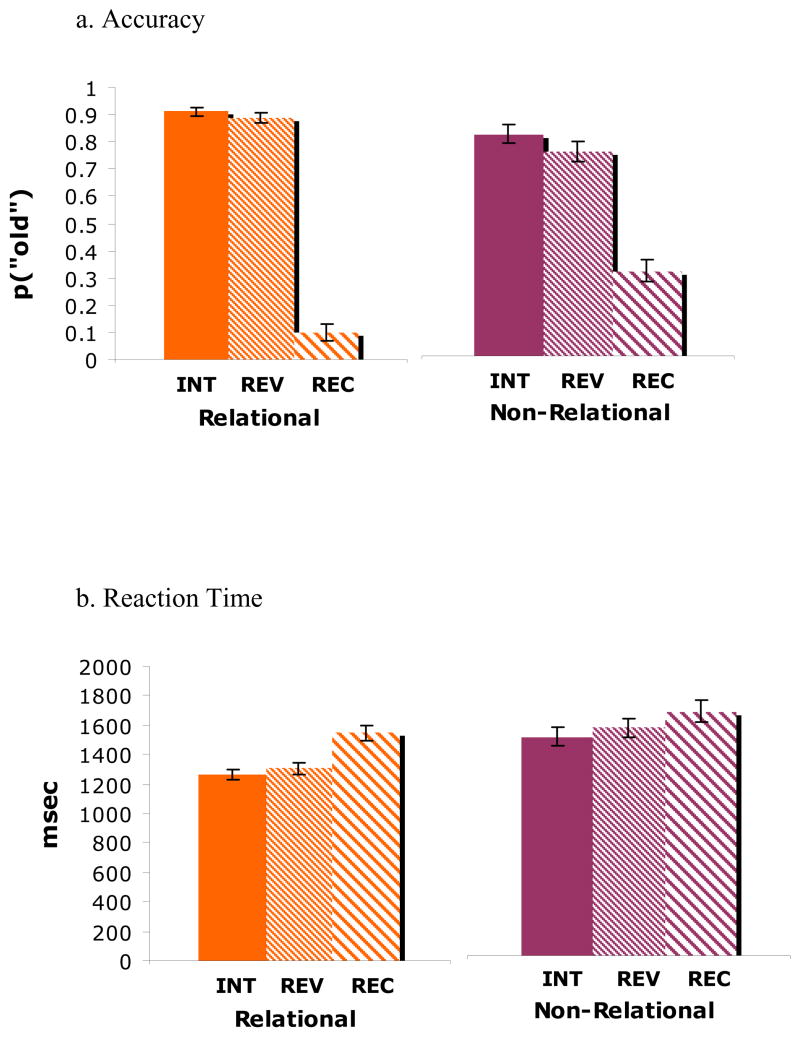
Behaviorally, associative recognition accuracy is calculated as the difference between “old” judgments to intact stimulus pairs and reversed pairs (hits) versus “old” judgments to recombined stimulus pairs (false alarms). Analysis of corrected recognition scores as a function of retrieval condition (intact, reversed) and type of encoding (relational, nonrelational) revealed a main effect of encoding type with higher associative recognition accuracy following relational encoding (Mean IP = .91; RevP = .89) than following non-relational encoding (Mean IP = .78; RevP = .72), F(1,12) = 20.39, p < .001 (Figure 2). Additionally, there was a main effect of retrieval condition, F (1,12) = 18.75, p < .001. There was no encoding type × retrieval condition interaction, F (1,12) = 2.39, p > .05.
Figure 2.
Behavioral accuracy and reaction time data for experimental participants.
Analysis of reaction times (RT) as a function of retrieval condition (intact, reversed) and type of encoding (relational, nonrelational) revealed a main effect of encoding type, as associative recognition trials following relational encoding (Mean IP = 1261.8; RevP = 1306.1) were faster overall than those following nonrelational encoding, (Mean IP = 1502.7; RevP = 1565.0) (Figure 2), F (1,12) = 57.73, p < .001 (Figure 2). In addition, a main effect of retrieval condition was observed, F (1,12) = 9.52, p < .01. There was no encoding type × retrieval condition interaction, F > 1.
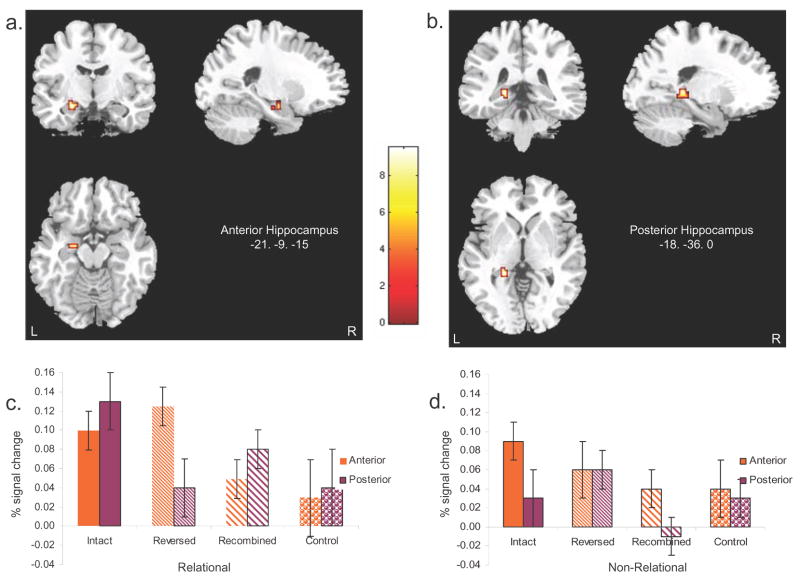
Event-related functional magnetic resonance imaging demonstrated greater activation for IP than for RecP (i.e., previously shown, yet recombined pairs) in left anterior hippocampus following relational encoding, replicating the finding in our previous fMRI experiment. Follow up region of interest (ROI) analysis of the mean activation level in left anterior hippocampal regions showed equivalent levels of activation for IP and RevP, with both conditions demonstrating greater activation than RecP (figure 3a). There was a significant positive correlation between relational memory accuracy and percent signal change in this region. That is, increased relational memory accuracy was associated with increased activity in anterior hippocampus (Table 1). Taken together, these findings provide support for the notion that anterior hippocampus mediates flexible retrieval of newly acquired associations.
Figure 3.
Statistical parametric map (SPM) results for the contrast showing activity greater in IP than RecP following relational encoding. The results are over laid on sagittal, coronal, and axial slices using MRIcro. Coordinates are in Montreal Neurological Institute space and appear in the lower right corner of the images. a. Anterior hippocampal region. b. Posterior hippocampal region. c. Mean percent signal change for each relational condition in anterior and posterior hippocampal regions. d. Mean percent signal change for each non-relational condition in anterior and posterior hippocampal regions. The black bars represent the standard error for each condition.
Table 1.
Regression results for predicting relational memory accuracy with hippocampal responses following relational encoding.
| Dependent variable | Region | Beta | Error | t-test value for β | P-value | R2 |
|---|---|---|---|---|---|---|
| IP - RecP | Anterior Hipp | 0.290 | 0.127 | 2.280 | 0.0436 | 0.321 |
| RevP - RecP | Anterior Hipp | 0.340 | 0.137 | 2.505 | 0.0293 | 0.363 |
| IP - RecP | Posterior Hipp | 0.127 | 0.548 | 0.233 | 0.8202 | |
| RevP - RecP | Posterior Hipp | 0.296 | 0.165 | 1.794 | 0.1004 |
The contrast of activity greater for IP than for RecP following relational encoding also revealed significant activity in a posterior region of left hippocampus. Follow up ROI analysis of the mean activation in this posterior hippocampal region showed greater levels of activation for IP than for RevP (Figure 3). Unlike activity in anterior hippocampus, however, activity in posterior hippocampus was not correlated with relational memory accuracyi (Table 1). These findings suggest that posterior hippocampus is sensitive to the exact reinstatement of learned associations, a process that may not have driven behavioral accuracy. To assess whether this pattern of activity in posterior hippocampus differed significantly from the pattern observed in anterior hippocampus, we conducted an ANOVA with region (anterior, posterior) and retrieval condition (intact and reversed, recombined) as factors. This analysis revealed a significant region × retrieval condition interaction, F(1,12) = 9.15, p<.01, suggesting that hippocampal activity during retrieval was greater in the anterior region (than the posterior region) for intact and rearranged pairs than for new pairs. This finding provides direct support for the notion that the observed patterns of activity in anterior and posterior hippocampus are functionally distinct.
The aforementioned results demonstrate that the hippocampus is involved in relational retrieval when stimuli are encoded relationally. To address whether hippocampal activity during relational retrieval depends upon relational encoding, we had subjects encode half of the stimuli in a non-relational manner. Following nonrelational encoding, we again observed hippocampal activity during recognition of intact pairs (IP) in anterior hippocampus suggesting that retrieval of relational information that was encoded incidentally when pairs were studied nonrelationally, also engaged the anterior hippocampus. Hippocampal activity was not observed during recognition of reversed pairs (RevP), but this may reflect a relatively low proportion of accurate responses and a weaker signal associated with non-relational encoding. An ANOVA directly comparing anterior hippocampal activation following relational and non-relational encoding revealed a main effect of retrieval condition, F(1,12) = 4.48, p<.05, indicating that activation was greater following retrieval of intact pairs (IP) and reversed pairs (RevP) than retrieval of recombined pairs (RecP). However, there was no main effect of encoding condition and no encoding × retrieval interaction, suggesting that the main effect of retrieval condition (IP = RevP > RecP) was similar for the two encoding conditionsii. These results are consistent with prior reports demonstrating hippocampal activity not only during intentional, but also during incidental (nonconscious) formation and reactivation of learned associations (Henke, Mondadori et al., 2003; Henke, Tryer et al., 2003; Degonda et al., 2005).
Our findings provide empirical support for a functional dissociation across the long axis of human hippocampus during relational retrieval. Although prior reports have documented regional differences in hippocampal functioning, such differences have been attributed to the type of information processed (e.g., objects versus spatial arrangements, Pihlajamaki et al., 2004; or items versus relations between items, Schacter and Wagner, 1999; Small et al., 2001), or the stage of memory processing (e.g., encoding versus retrieval, Lepage et al., 1998; Schacter and Wagner, 1999; Pihlajamaki et al., 2003; Zeineh et al., 2003; Eldridge et al., 2005; Prince et al., 2005). In our study, however, functional imaging data were acquired for one type of stimuli (i.e., unrelated word pairs) during the retrieval phase only. As such, the anterior-posterior distinction we observed could not reflect differences due to stimulus type or stage of memory. Instead, the anterior-posterior dissociation we observed likely arose from differences in the nature of the retrieval process.
During our experiment, participants encoded words pairs (relationally or non-relationally) while creating sentences, and subsequently decided whether or not pairs had been shown together previously. Significant retrieval-related activity in anterior hippocampus was associated with accurate recognition of relational information. This anterior hippocampal activity arose following retrieval of the relational links explicitly forged at encoding (i.e., generating sentences) or forged incidentally in the nonrelational encoding condition.
Additionally, relational retrieval mediated by anterior hippocampus appears to be flexible in nature, as it allowed participants to successfully make judgments about previously seen (IP) and previously seen, yet reversed (RevP) pairs following relational encoding. Anterior hippocampal activity was also observed for retrieval of IP following nonrelational encoding. Future studies will be needed to examine whether or not the anterior hippocampus is involved in flexible retrieval of incidentally encoded relational information.
Besides engaging in flexible retrieval, participants may have experienced the ecphory that sometimes accompanies the match between retrieval cues and stored information. This experience may have arisen because IP pairs have the additional property of being perceptually identical at study and test. The current findings suggest that posterior hippocampus is sensitive to the exact perceptual match between retrieval cues and learned information. These findings, coupled with those above, may suggest an anterior-posterior hippocampal dissociation based on the nature of the retrieval processes. Specifically, anterior hippocampus may mediate flexible retrieval of relational information, while posterior hippocampus may be sensitive to the reinstatement of learned associations.
A close inspection of relevant relational memory studies supports an anterior-posterior distinction based on retrieval processing differences. Preston et al. (2004) examined the flexible expression of declarative memories using a transitive inference paradigm. In their study, subjects received an initial set of face-house pairs consisting of pictures of faces and houses, a second set of face-house pairs consisting of the same houses paired with new faces, and a set of face-face pairs consisting of novel faces. During the scanned retrieval task, participants discriminated between learned face-house pairs, learned face-face pairs, and related face-face pairs. Related pairs were those for which the two faces had been associated with the same house on different trials, and thus required participants to flexibly associate information across trials. The comparison of activity during recognition of related face-face pairs with activity associated with recognition of explicitly learned pairs revealed greater activity in anterior hippocampal regions for the related face-face pair condition – providing support for the notion that flexible retrieval of associative information is mediated by the anterior hippocampus. In addition, a more posterior hippocampal region was activated for all pair types which were studied and then re-shown in the exact format at test.
Additional evidence for an anterior-posterior distinction in hippocampal processing during relational retrieval comes from a recent study by Addis and Schacter (2008) in which they examined the role of the hippocampus in relational processing during the elaboration of future events. Specifically, they had subjects remember past events and imagine future events, and then examined hippocampal contributions to the retrieval of event characteristics (e.g., the amount of detail generated and temporal distance), depending upon whether the event was in the past or imagined in the future. The results showed that left anterior hippocampus responded differentially to the amount of details comprising future events, while left posterior hippocampus was responsive to the amount of detail comprising both past and future events. The authors suggest that both past and future events require the retrieval of relational information (i.e., details of an autobiographical memory) and common engagement of left posterior hippocampus. In contrast, only future events require the novel (and flexible) use of such details, and thus recruit left anterior hippocampus.
Of note, a recent paper suggests that an anterior-posterior dissociation in hippocampal processing may also arise during relational encoding. Chua et al. (2007) reported increased activity in anterior hippocampus for subsequently remembered relational information (i.e., face-name pairs) compared with subsequently forgotten relational information. In contrast, posterior hippocampus showed significant activation above baseline during attempted encoding of face-name pairs, but no differential activation based on subsequent memory. Based on these findings, the authors suggested that the anterior hippocampus shows functional specialization for the successful formation of associations, while the posterior hippocampus may serve a more general role in relational encoding. In light of the present findings, another possibility is that the anterior hippocampus flexibly encodes and retrieves relational information, whereas the posterior hippocampal formation plays a role in establishing a fixed perceptual representation during relational memory processing.
The locus of posterior hippocampal activity in the current study closely matches that observed in an imaging study that manipulated perceptual aspects of stimuli (Schacter et al., 1997). Schacter et al., (1997) investigated the effects of size and orientation change on hippocampal activity during recognition of non-nameable objects. A comparison of activity for same objects relative to activity for reoriented objects revealed greater activity in posterior hippocampus when making recognition judgments about same objects than orientation-changed objects. Likewise, a comparison of activity for same objects and for size-changed objects revealed greater activity in posterior hippocampus when making recognition judgments about same objects than re-sized objects. These findings support the notion that posterior hippocampus may mediate perceptual matching or exact reinstatement of events between study and test phases. Delineating a more precise role for posterior hippocampus during relational retrieval will require further experimentation.
In summary, anterior hippocampal activity was observed during flexible retrieval of learned associations, whereas posterior hippocampal activity was detected during reinstatement of study episodes. These findings suggest a functional dissociation across the long axis of human hippocampus based on the nature of the mnemonic process rather than the stage of memory processing or type of stimulus. This dissociation was made possible by an experimental design that allowed us to distinguish between two retrieval processes: flexible retrieval and exact reinstatement. Finally, the current study demonstrates a significant correlation of retrieval-related neural activity with behavioral accuracy.
Methods and Materials
Participants
Fourteen (mean age = 22.6 years, 10 females) right-handed, native English speakers were paid $50 for their participation. Data from one subject was discarded due to poor behavioral performance (i.e., following relational encoding the subject’s hit rate of .77 and false alarm rate of .48 were more than two standard deviations outside the control mean). Informed consent was obtained, in compliance with the Boston University, the Boston VA Healthcare System and Massachusetts General Hospital Institutional Review Boards.
Stimulus Materials
Stimuli consisted of 768 nouns with a mean frequency = 107 occurrence/million (Francis and Kucera, 1982).
Task Procedure
Following extensive practice outside the magnet, participants received eight encoding/retrieval tests in the scanner. Only the retrieval phase was scanned. At encoding, participants simultaneously viewed two nouns on each of a total of 384 trials. During the initial 4 runs, participants were instructed to covertly generate a sentence for EACH of the two words (non-relational encoding). During the last 4 runs, participants were instructed to covertly generate a sentence that related the two words (relational encoding). At retrieval, which began immediately following the encoding phase, functional MR images were acquired for a total of 384 trials while participants performed an associative recognition task. For this task, participants saw pairs of words previously seen together (Intact Pairs [IP], N=128/16 per run), pairs of words previously seen together, but in the reversed presentation order (Reversed Pairs [RevP], N= 128/16 per run), and pairs of words previously seen, but not together (Recombined Pairs [RecP], N =128/16 per run). Participants were instructed to decide whether the two words were previously shown together. In addition, control trials (N=72/9per run) were included during which participants viewed ampersands and number signs and were instructed to indicate on which side of the screen the ampersands had appeared (See Figure 1). The retrieval task was self-paced and the control trials were jittered at varying durations (2, 4, 6 seconds). The order of the stimuli and timing of the control condition was determined using optseq (http://surfer.nmr.mgh.harvard.edu/optseq/).
Imaging Methods
Imaging was performed with a 3T whole-body MRI Scanner (Siemens Trio, Erlangen, Germany) using a gradient-echo echo-planar sequence designed to minimize susceptibility artifact in the anterior hippocampal regions and fully volume the long axis of the hippocampus (echo time = 23 ms, repetition time = 2500 ms, 37 oblique slices oriented along the long axis of the hippocampus, 3.125 × 3.125 × 3mm, 0 skip). High resolution T1-weighted (MP-RAGE) structural images were collected for anatomic visualization. Visual stimuli were back-projected onto a screen and viewed in a mirror mounted above the participant’s head. Responses were recorded using an MR-compatible response box. Head motion was restricted using a pillow and foam inserts.
Imaging Analysis
The fMRI data were preprocessed using SPM99 to correspond to our previous study (Wellcome Department of Neurology, UK). Slice acquisition timing was corrected by resampling all slices in time relative to the first slice, followed by rigid body motion correction across all runs. The functional data then were normalized spatially to the Montreal Neurological Institute template. Images were resampled into 2-mm cubic voxels and smoothed spatially with an 8-mm full-width half-maximum isotropic Gaussian kernel.
Statistical analysis was performed using the general linear model for event-related designs in SPM99. Trials from each condition were modeled by using a canonical hemodynamic response function. Effects for each condition were estimated by using a subject-specific fixed-effects model. These data were then entered into a second-level random-effects analysis. Retrieval analyses contrasted each trial type (i.e., IP, RecP, and RevP) to the control task and with each other. In light of our a prior prediction regarding the role of the anterior hippocampus in relational retrieval, as well as the established role of the hippocampus in relational memory, hippocampal activations of at least 5 contiguous voxels that exceeded a threshold of p < 0.01 were considered reliable (cfr. Dickerson, et al., 2004; Dickerson, et al., 2005). Analyses were done for each encoding task (i.e., relational and nonrelational) and all memory trials were modeled for correct decisions only. All incorrect trials were modeled as a single variable that was not examined.
Regions of interests were functionally defined in the following manner. First, based on our previous finding in which we observed greater anterior hippocampal activity for intact pairs as compared to recombined pairs, in the current study we directly contrasted intact pairs and recombined pairs following relational encoding. This contrast revealed the two hippocampal locations reported here. We then performed follow-up ROI analyses of the mean activation for each stimulus condition in each region.
Acknowledgments
This research was supported by NIH MH57681 and the Medical Research Service of the Dept. of Veterans Affairs.
Footnotes
The non-significant correlation between relational memory accuracy and activity in posterior hippocampus should be interpreted with caution, as the lack of correlation may reflect a threshold difference.
Unlike anterior hippocampal responses following relational encoding, however, hippocampal responses following non-relational encoding were not correlated with recognition performance. This finding may reflect a weak signal and a relatively lower proportion of accurate responses (for nonrelational encoding than for relational encoding) that were used in this comparison.
References
- Achim E, Lepage M. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition. NeuroImage. 2005;24:1113–1121. doi: 10.1016/j.neuroimage.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL. Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal activity. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Chua E, Rand-Giovennetti E, Schacter DL, Albert MS, Sperling RA. Dissociating confidence and accuracy: Functional magnetic resonance imaging shows origins of subjective memory experience. Journal of Cognitive Neuroscience. 2004;16:1131–1142. doi: 10.1162/0898929041920568. [DOI] [PubMed] [Google Scholar]
- Chua E, Schacter DL, Rand-Giovennetti E, Sperling RA. Evidence for a specific role of the anteior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Degonda N, Mondadori CRA, Bosshardt S, Schmidt CF, Boesiger P, Nitsch RM, Hock C, Henke K. Implicit associative learning engages the hippocampus and interacts with explicit associative learning. Neuron. 2005;46:505–520. doi: 10.1016/j.neuron.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation between encoding and retrieval processes in the human hippocampus. The Journal of Neuroscience. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency Analysis of English Usage: Lexicon and Grammar. Boston: Houghton Mifflin Co; 1982. [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Henke K, Mondadori CRA, Treyer V, Nitsch RM, Buck A, Hock C. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia. 2003;41:863–876. doi: 10.1016/s0028-3932(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Henke K, Tryer V, Nagy ET, Kneifel S, Dursteler M, Nitsch RM, Buck A. Active hippocampus during nonconscious memory. Consciousness and Cognition. 2003;12:31–48. doi: 10.1016/s1053-8100(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Jackson O, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Law JR, Flanery MA, Wirth S, Yanike M, Smith AC, Frank LM, Suzuki WA, Brown EN, Stark CEL. Functional magnetic resonance imaging during the gradual acquisition and expression of pair-associate memory. The Journal of Neuroscience. 2005;25:5720–5729. doi: 10.1523/JNEUROSCI.4935-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal activations of PET memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Mikkonen M, Jalkanen V, Partanen K, Aronen H, Soininen H. Encoding of novel picture pairs activates perirhinal cortex: An fMRI study. Hippocampus. 2003;13:67–80. doi: 10.1002/hipo.10049. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen H. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal subareas in humans. European Journal of Neuroscience. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to novel use of relational information in declarative memory. Hippocampus. 2002;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Uecker A, Reiman E, Yun LS, Bandy D, Chen K, Copper LA, Curran T. Effects of size and orientation change on hippocampal activation during episodic recognition: A PET study. NeuroReport. 1997;8:3993–3998. doi: 10.1097/00001756-199712220-00028. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nature Neuroscience. 2001;4:442–9. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NEA, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: An fMRI study. Neuroreport: For Rapid Communication of Neuroscience Research. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]