Abstract
A series of 4-alkoxycarbonyl-1,5-diaryl-1,2,3-triazoles were synthesized regioselectively using click chemistry and evaluated at CB1 cannabinoid receptors. The n-propyl ester 11 (Ki = 4.6 nM) and phenyl ester 14 Ki = 11 nM) exhibited the most potent affinity of the series.
The wide range of pharmacological effects of cannabinoid and endogenous cannabinoid ligands, are mediated by two subtypes of transmembrane G-protein coupled receptors: CB1 and CB2.1–4 CB1 receptors are expressed in the central nervous system (CNS) with high density in the cerebellum, hippocampus and striatum.5 CB1 receptors are found in some peripheral tissues (urinary bladder, testis, and ileum) as well. CB2 receptors are predominantly located in the immune system (tonsils, spleen and immune cells) with very low concentration in the CNS.6 CB1 agonists have potential therapeutic applications in developing drugs for pain, nausea, glaucoma, stroke, cancer and neurological disorders such as multiple sclerosis and Parkinson’s disease.7,8 The potential applications of CB1 antagonists include the therapeutic treatment of obesity and related metabolic disorders as well as medications for drug addiction.7–9 The role of CB1 receptors in these disease states and disorders, the nature of the receptors active sites, and the molecular interactions between the receptors and ligands are not fully understood and are under intensive investigation.
Our interest in developing new effective medications for drug abuse has focused our attention on the CB1 receptors because of their selective presence within CNS and their mediation of the effects of psychostimulants on brain circuitry.9 Pharmacological studies have shown that CB1 receptor antagonists or inverse agonists have the ability to attenuate the elevation of dopamine levels that occurs with psychostimulant use, suggesting their potential applications in the treatment of drug abuse disorders.9–11
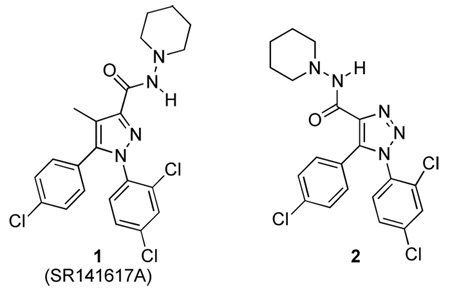
The pyrazole derivative SR141716 (1) was the first compound reported to be a potent and selective antagonist for the CB1 receptor.12 However, further characterization of SR1411716 has shown it to possess an inverse agonist pharmacological profile.13 This prototypical CB1 receptor antagonist/inverse agonist has been studied as a potential therapeutic for the treatment of obesity, smoking cessation and a variety of other CB1 receptor mediated pathological conditions.14 However, inverse agonists typically elicit pharmacological responses opposite to agonists and thus are not ideally suited for the treatment of drug addiction due to potential dysphoric side effects associated with these drugs. These include increased nociceptive sensitivity, decreased food intake and body weight, disruption of operant behavior and potential nausea in humans.15–19 Such side effects undoubtedly would lead to low compliance and relapse among addicts. Moreover, recent reports that describe increased levels of anxiety and depression in patients, along with a higher incidence of suicide (3 to 1 over placebo) in clinical trials with the diet drug Rimonabant (SR141716) strongly disfavors its development as a drug abuse medication.20
Based upon the structure of 1,5-diarylpyrazole core template in SR141716 (1), bioisosteric replacement has been an important approach to discover new lead compounds of potent CB1 ligands and a variety of bioisosteric analogues of SR14716 have been synthesized and identified as potent ligands.14,21 In reviewing the literature, the absence of 1,2,3-triazole analogues was significant. To explore this deficiency, our aim was to synthesize and characterize a series of 1,2,3-triazole analogues of 1, and explore further the binding motifs of CB1 receptor ligands.
Our design rationale was to incorporate the 1,2,3-triazole ring system into the vicinal diaryl group which is presumed to interact with a unique region of the CB1 receptors.22–24 While it was envisaged that the 1,2,3-triazole moiety in target molecule 2 could replace the pyrazole ring and provide good overlap of the vicinal diaryl system, it was not clear at that time how the juxtaposition of the carboxamide moieties of the triazole analogue 2 relative to 1 would affect molecular recognition at cannabinoid receptors. However, the reduced lipophilicity of the 1,2,3-triazole 2 (ClogP 5.33) relative to 1 (ClogP 6.26) and potentially enhanced bioavailability made 2 an attractive target for investigation.25, 26
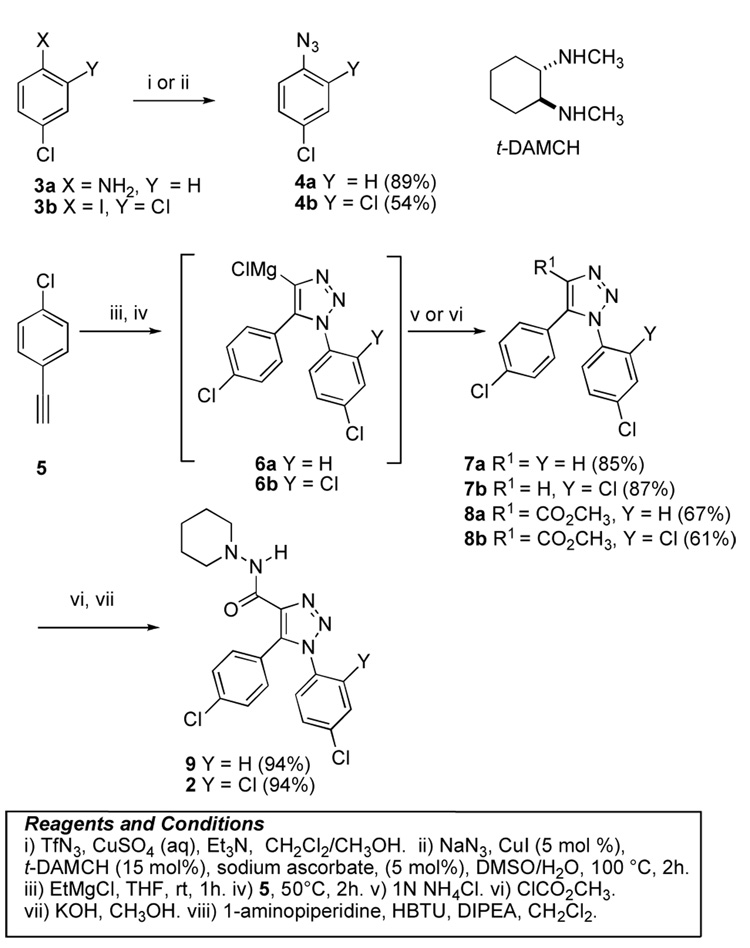
The construction of the 1,2,3-triazole ring system was envisaged to exploit the 1,3-dipolar cycloaddition of an azide and a terminal alkyne. To this end, the click chemistry developed by Sharpless and coworkers27 would allow rapid regioselective ring construction as well as provide suitable intermediates for parallel synthesis of potential analogues. To explore the potential of this approach, a model 1,2,3-triazole ring system was prepared (Scheme 1). The 4-chloroaniline (3a) was conveniently converted into the azide 4a with triflyl azide in 89% yield.28 The subsequent reaction of 4a with 4-chloroacetylene (5) under the Sharpless click chemistry conditions resulted in the formation of the intermediate 1,5-diaryl-1,2,3-triazole-4-magesium chloride (6a). Subsequent quench of the reaction mixture with aqueous NH4Cl furnished the 1,2,3-triazole 7a in 85% yield. Alternatively, the 4-methoxycarbonyl-1,5-diaryl-1,2,3-triazole 8a was obtained in 67% yield by treatment of 6a with methyl chloroformate. It was noteworthy that only one triazole regioisomer was isolated from these reactions.
Scheme 1.
Upon successful generation of the 1,5-diaryl-1,2,3-triazole ring system, our attention focused on the construction of the 1,2,3-triazole analogue 2. The synthesis of 2 required the initial preparation of 2,4-dichlorophenylazide (4b). The conditions used to prepare 4a did not furnish useful quantities of the azide 4b, Fortunately, the copper catalyzed procedure [CuI, trans-1,2-di(aminomethyl)-cyclohexane (t-DAMCH)] for the conversion of iodobenzenes into the corresponding azides29 provided 4b in 54% yield from 2,4-dichloriodobenzene (3b).
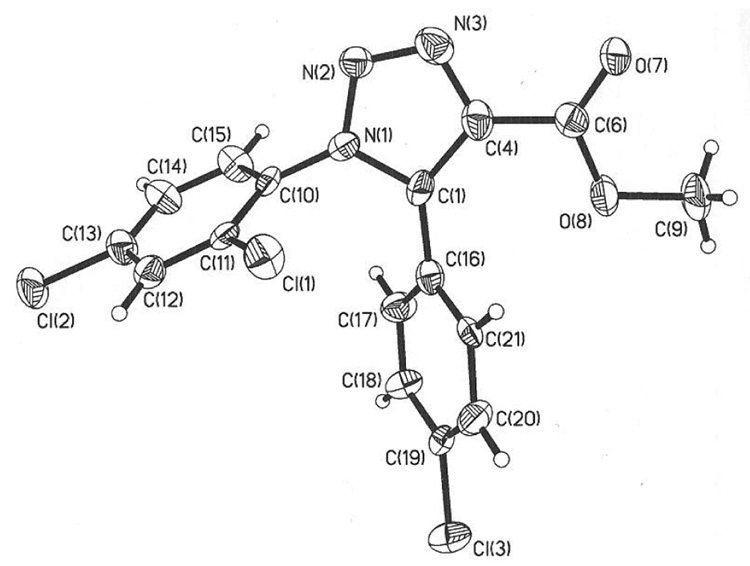
The syntheses of the corresponding substituted 1,5-diaryltriazole derivatives 7b and 8b were achieved via the one-pot three-step transformation described above (Scheme 1) in 87% and 61% yield, respectively. As shown in Figure 1, X-ray crystallographic analysis of 4-methoxycarbonyl-1,5-diaryl-1,2,3-triazole 8b, served to confirm the regioselectivity of the click chemistry and unequivocally establish the regiochemistry of the triazole ring system.30
Figure 1.
ORTEP Drawing of 4-methoxycarbonyl-1-(4-chlorophenyl)-5-(2,4-dichlorophenyl)-1,2,3triazole 8b.
The esters 8a and 8b were readily converted into the desired SR141716 analogues 9 and 2 by hydrolysis and concomitant amidation with 1-aminopiperidine (Scheme 1).
The binding affinities of the triazole derivatives (Table 1) were determined by the inhibition of [3H]SR141716 binding in homogenates of rat cerebellum.31 For direct comparison of ligand affinity at CB1 receptors with 1, [3H]SR141716 was used as the radioligand,
Table 1.
Inhibition of [3H]SR141716A at CB1 Receptors
| Cmpda | Code | ClogPb | Ki (nM)c |
|---|---|---|---|
| 2 | HS69 | 5.33 | 590 ± 170 |
| 7a | HS53-2 | 4.46 | 6,900 ± 1,300 |
| 7b | HS57-2 | 5.11 | 1,420 ± 266 |
| 8a | HS53-1 | 4.68 | 4,400 ± 760 |
| 8b | HS57-1 | 5.32 | 66 ± 7.0 |
| 9 | HS60 | 4.69 | 54% d |
| 10 | HS57-3 | 5.68 | 180 ± 27 |
| 11 | HS57-4 | 6.21 | 4.6 ± 0.012 |
| 12 | HS57-5 | 6.70 | NAe |
| 13 | HS57-6 | 7.62 | NAe |
| 14 | HS57-8 | 6.83 | 11 ± 3.4 |
| 15 | HS57-9 | 6.85 | 97 ± 55 |
| 16 | HS57-7 | 7.23 | 240 ± 79 |
All compounds were tested as the freebase.
See Ref. 25.
All values are the mean ± SEM of three experiments performed in triplicate.
Percent inhibition at 100µM.
NA: not available
For each compound, each concentration was tested in triplicate and each experiment was replicated three times. The binding data were analyzed using the non-linear regression analysis program of GraphPad Prism®.
The Ki values summarized in Table 1 indicated that the SR141716 analogue 2 exhibited only modest affinity (Ki = 590 nM) for CB1 receptors. In addition, the mono-chloro-substituted 5-aryl derivatives 9, and the unfunctionalized 1,5-diaryl triazoles 7a and 7b exhibited low affinity for cannabinoid receptors. However, it was serendipitous to find that the methyl ester 8b (Ki = 66 nM) was nearly an order of magnitude more potent than 2. This then provided a new lead compound for investigation.
As illustrated in Scheme 2, a series of 1,2,3-triazole analogues with varying ester groups were prepared using the click chemistry described earlier. The esters 10–15 were prepared efficiently in 50–76 % yield by trapping the 1,5-diaryl-1,2,3-triazole-4-magnesium chloride intermediate 6b (Scheme 1) with the corresponding commercially available chloroformates. The cyclohexyl ester derivative 16 was prepared by a simple transesterification of the methyl ester 8b.
Scheme 2.
The esters 10–16 were evaluated for binding affinity at CB1 receptors as described above (Table 1). The n-propyl ester 11 (Ki = 4.6 nM) was the most potent derivative of the series and was slightly more potent than SR141716A (11.5 nM)21. It is worth noting that the potent triazole ester 11, exhibited similar lipophilicity to the that of SR141716A (ClogP 6.26).25 The phenyl ester 14 (Ki = 11 nM) also exhibited high affinity for CB1 receptors while the affinity of the benzyl ester 15 was somewhat diminished. The larger alkyl ester congeners 12 and 13 exhibited high lipophilicity (Table 1, ClogP 6.70, 7.23) and were difficult to handle in the binding assay leading to inconsistent results. However, the binding data generally indicated that 12 and 13 exhibited diminished binding affinity relative to 11 and thus were not pursued. In general, analogues with either decreased or increased lipophilicity relative to 11 exhibited diminished affinity. This seems to suggest that a narrow window of lipophilic character may exist for binding of these triazoles at CB1 receptors.
In summary, we have reported the discovery and regioselective synthesis of a series of 4-alkoxy-carbonyl-1,5-diaryl-1,2,3-triazoles as a novel class of potent cannabinoid receptor ligands. The further in vitro and in vivo evaluation of the potent analogues 11 and 14 is currently under investigation and will be reported elsewhere.
Acknowledgements
We thank Prof. Gregory C. Fu at M. I. T. for the assistance provided to Ms. Hong Shu after Hurricane Katrina. This research was funded by the National Institute on Drug Abuse (DA023916).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Mol. Pharmacol. 1988;34:605. [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Nature. 1990;346:561. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abu-Shaar M. Nature. 1993:61. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Gerard CM, Mollereau C, Vassart G, Parmentier M. Biochem. J. 1991;279:129. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1932. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Herkenham M, Lynn AB, de Costa BR, Richfield EK. Brain Res. 1991;547:267. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]; (c) Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. J. Neurosci. 1991;11:563. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mailleux P, Vanderhaeghen JJ. Neuroscience. 1992;48:655. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]; (e) Howlett AC, Qualy JM, Khachatrian LL. Mol. Pharmacol. 1986;29:307. [PubMed] [Google Scholar]
- 6.Herkenham M. In: Cannabinoid Receptors. Pertwee RG, editor. New York: Academic Press; 1995. p. 145. [Google Scholar]; (b) Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA. Pharmacol. Rev. 2002;54:161. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]; (c) Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Ann. N.Y. Acad. Sci. 2006;1074:514. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 7.DiMarzo V, Bifulco M, De Petrocellis L. Nat. Rev. Drug Discov. 2004;3:771. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 8.Salo OMH, Savinainen JR, Parkkari T, Nevalainen T, Lahtela-Kakkonen M, Gynther J, Laitinen JT, Järvinen T, Poso A. J. Med. Chem. 2006;49:554. doi: 10.1021/jm0505157. [DOI] [PubMed] [Google Scholar]
- 9.Beardsley PM, Thomas BF. Behav. Pharmacol. 2005;16:275. doi: 10.1097/00008877-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 10.LeFoll B, Goldberg SR. J. Pharmacol. Exp. Ther. 2005;312:875. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 11.Tucci SA, Halford JCG, Harrold JA, Kirkham TC. Curr. Med. Chem. 2006;13:2669. doi: 10.2174/092986706778201512. [DOI] [PubMed] [Google Scholar]
- 12.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D. FEBS Lett. 1994;350:240. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 13.Pertwee R. Life Sci. 2005;76:1307. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Barth F. Annu. Rep. Med. Chem. 2005;40:103. [Google Scholar]
- 15.Tanda G, Goldberg SR. Psychopharmacology. 2003;169:115. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 16.Mclaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. Behav. Pharmacol. 2003;14:583. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Jarbe TU, DiPatrizio NV, Li C, Makriyannis A. Pharmacol., Biochem. Behav. 2003;75:809. doi: 10.1016/s0091-3057(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 18.Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. J. Pharmacol. Exp. Ther. 1998;286:697. [PubMed] [Google Scholar]
- 19.Winsauer PJ, Lambert P, Moerschbaecher JM. Behav. Pharmacol. 1999;10:497. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Christensen R, Kristensen PK, Bartels EM, Biddal H, Astrup A. Lancet. 2007;370:1671. [Google Scholar]
- 21.Thakur GA, Nikas SP, Li C, Makriyannis A. Handb. Exp. Pharmacol. 2005;768:209. doi: 10.1007/3-540-26573-2_7. [DOI] [PubMed] [Google Scholar]
- 22.Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HHJ. Pharmacol. Exp. Ther. 1998;285:285. [PubMed] [Google Scholar]
- 23.Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, Song Z-H, Nie J, Lewis D, Reggio PH. Mol. Pharmacol. 2002;62:1274. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- 24.Shim JY, Welsch WJ, Cartier E, Edwards JL, Howlett AC. J. Med. Chem. 2002;45:1447. doi: 10.1021/jm010267o. [DOI] [PubMed] [Google Scholar]
- 25.ClogP values were calculated using software from Collaborative Drug Discovery, Inc. at www.collabora-tivedrug.com
- 26.(a) Lipinski CA, Lombardo F, Dominy BW, Feeny PJ. Adv. Drug Deliv. Rev. 1997;23:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]; (b) Lipinski CA. J. Pharmacol. Toxicol. Methods. 2000;44:235. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 27.Krasinski A, Fokin VV, Sharpless KB. Org. Lett. 2004;6:1237. doi: 10.1021/ol0499203. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Tor Y. Org. Lett. 2003;5:2571. doi: 10.1021/ol034919+. [DOI] [PubMed] [Google Scholar]
- 29.Andersen J, Madsen U, Bjorkling F, Liang X. Synlett. 2005;14:2209. [Google Scholar]
- 30.The authors have deposited the crystallographic data for structure 8b with the Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/); Deposition number: CCDC 710290.
- 31.Breivogel C, Sim L, Childers S. J. Pharmacol. Exp. Ther. 1997;282:1632. [PubMed] [Google Scholar]