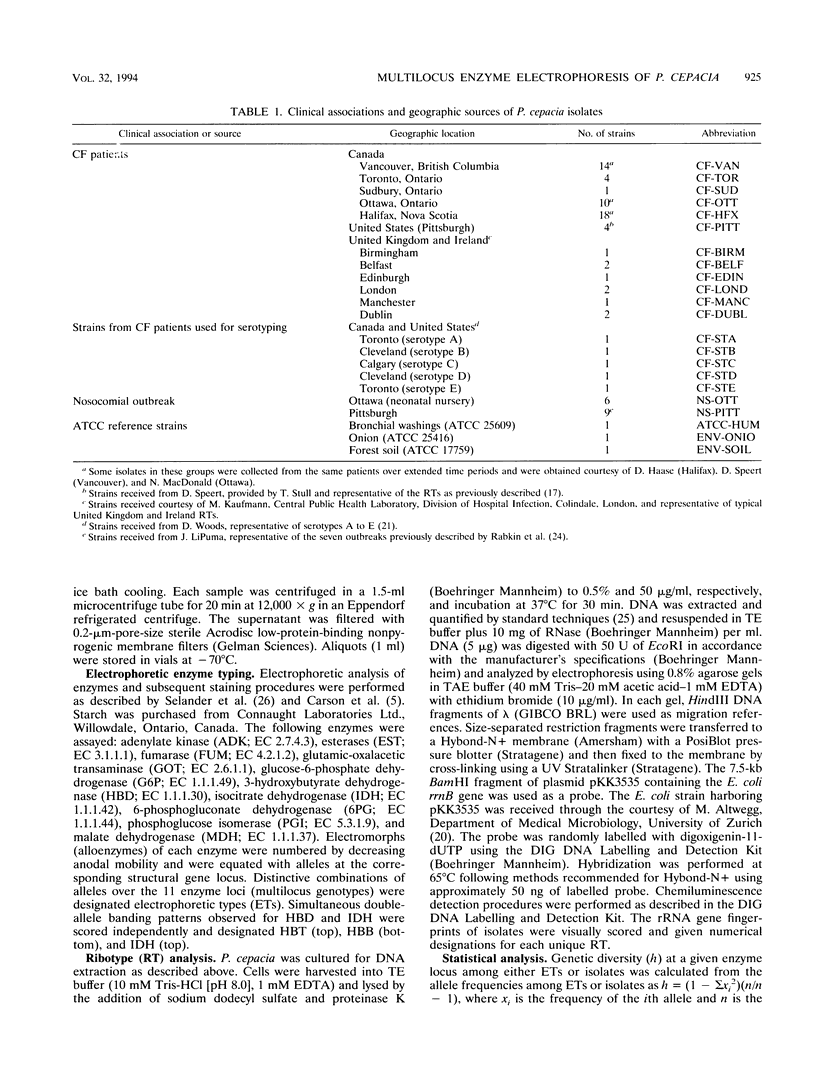
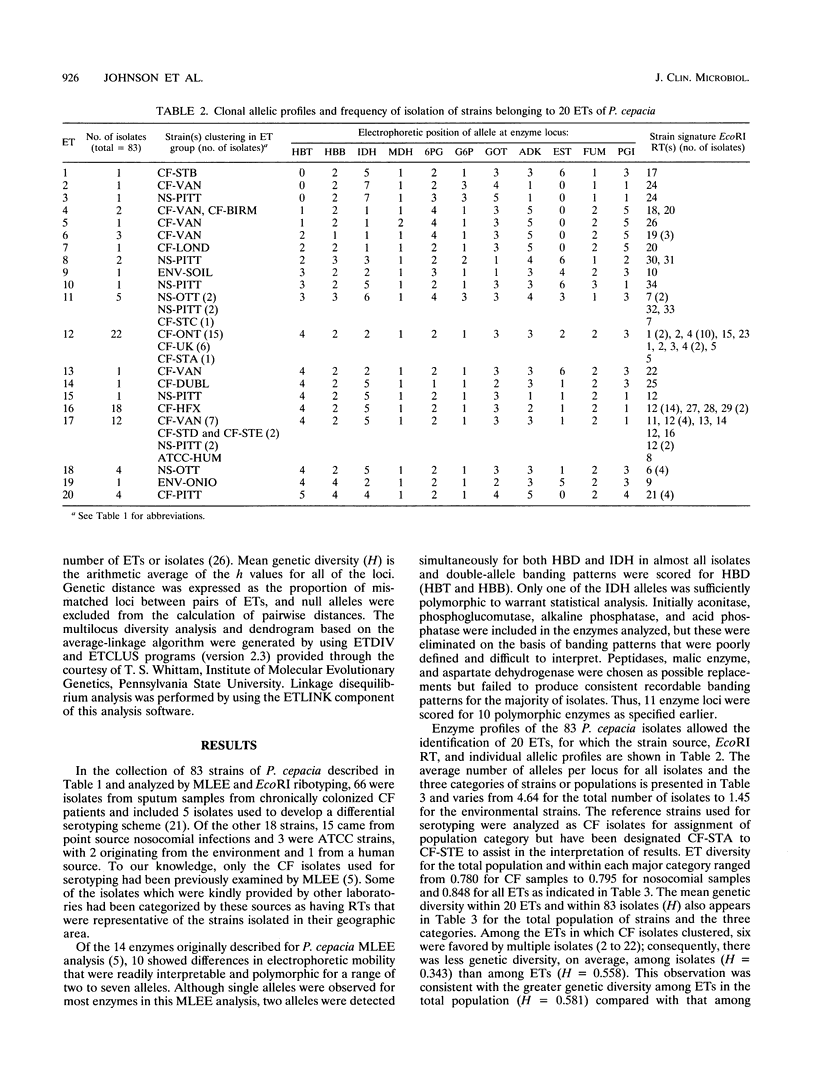
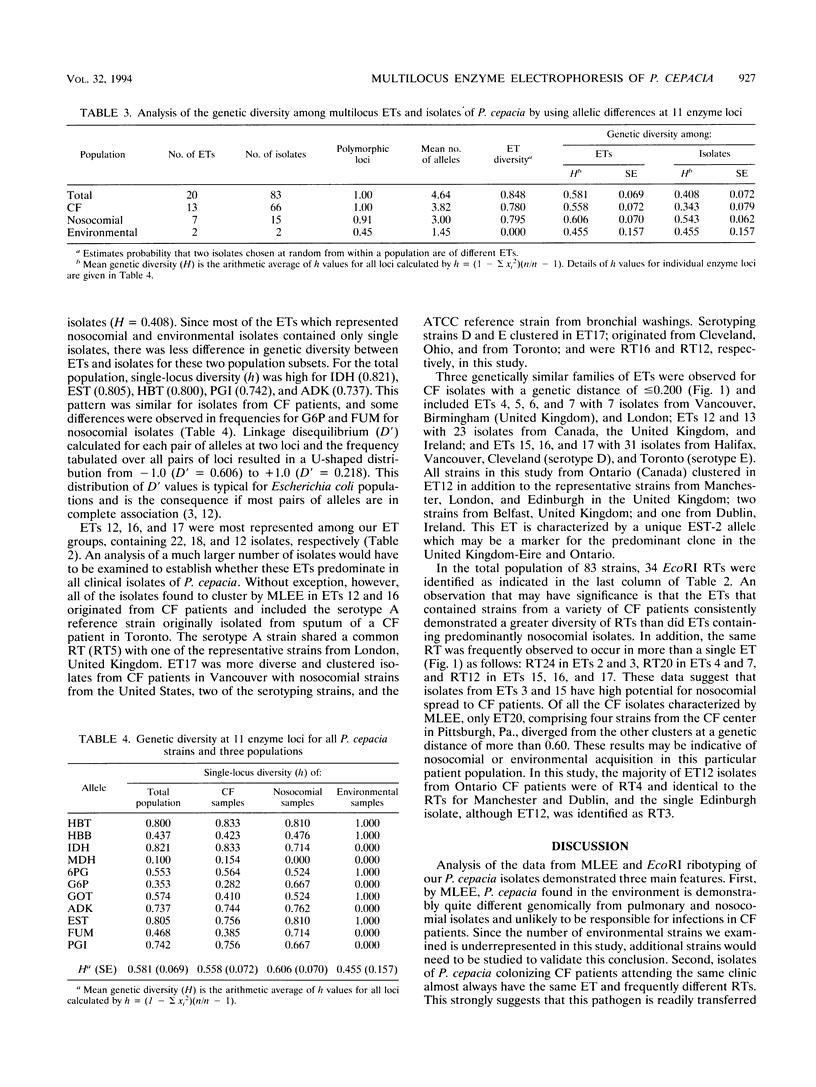
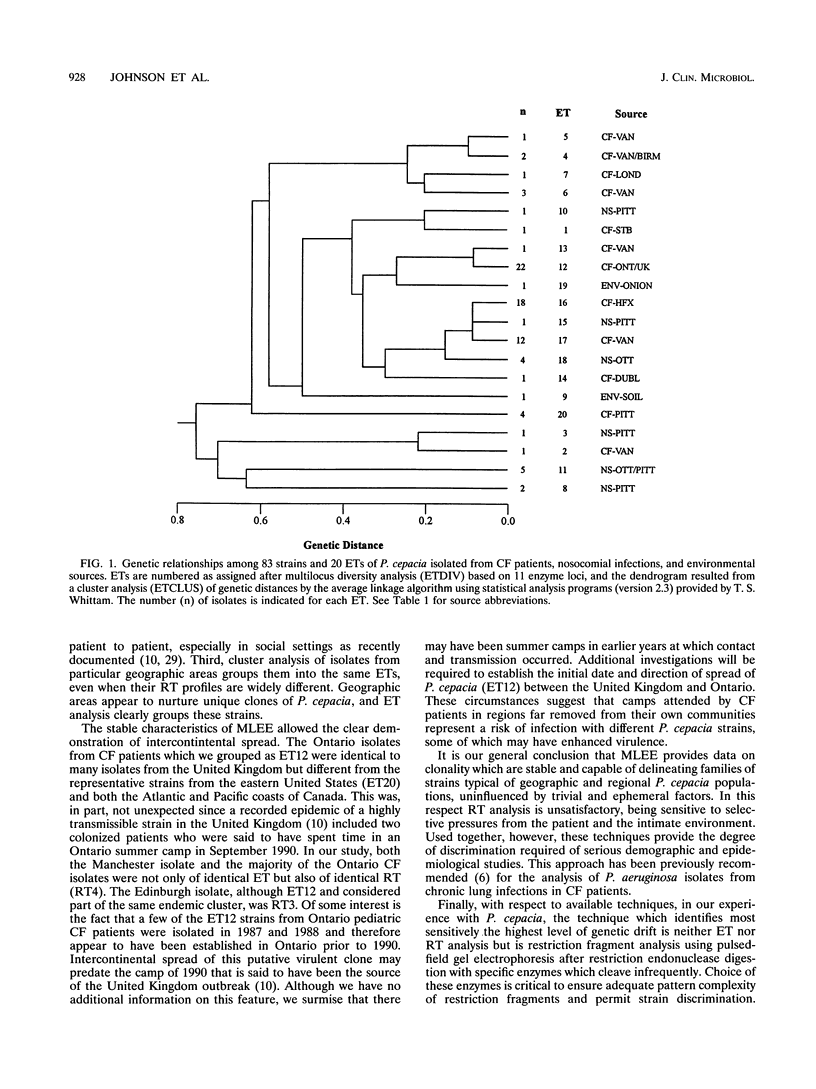
Abstract
Multilocus enzyme electrophoresis and ribotyping were used to characterize 83 strains of Pseudomonas cepacia, mostly isolated from cystic fibrosis (CF) patients, although a number of isolates from non-CF nosocomial infections and reference environmental strains were represented. Twenty enzyme electrophoretic types (ETs) were determined; of these, one clone (ET12) was associated with six of nine ribotypes (RTs) said to be geographically representative of the United Kingdom and all of the Ontario (Canada) isolates from CF patients. This clone was not associated with nosocomial infections or environmental strains and was never found in CF isolates from British Columbia or Nova Scotia, Canada, or a center in the eastern United States. Individual isolate EcoRI RT signatures did not cluster geographically as did the ET signatures by clonal analysis. Frequently RTs occurred in more than a single ET. Known point source focal nosocomial outbreaks were typified by single ETs and stable RTs. Dendrographic analysis of the strains grouped those strains from CF patients, nosocomial outbreaks, and environmental sources into separate ET families, and diversity analysis indicated that, with the exception of ET17, CF isolates clustered in unique and closely related ETs different from those from nosocomial and environmental sources. This study has also shown the potential of multilocus enzyme electrophoresis to monitor the intercontinental spread of P. cepacia strains in CF patients, and this may have a significant impact on plans for CF patient summer camps and design of infection control practices. Whether the intercontinental ET12 clone, which predominates in the United Kingdom and the province of Ontario, linked by summer camp acquisition, has increased virulence for CF patients remains to be established.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Kuhns J. S., Vasil M. L., Gerding D. N., Janoff E. N. DNA fingerprinting by pulsed field gel electrophoresis and ribotyping to distinguish Pseudomonas cepacia isolates from a nosocomial outbreak. J Clin Microbiol. 1991 Mar;29(3):648–649. doi: 10.1128/jcm.29.3.648-649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Weber M., Derelle J., Brahimi N., Lambert-Zechovsky N. Y., Vidailhet M., Navarro J., Elion J. Arbitrarily primed polymerase chain reaction as a rapid method to differentiate crossed from independent Pseudomonas cepacia infections in cystic fibrosis patients. J Clin Microbiol. 1993 Oct;31(10):2589–2593. doi: 10.1128/jcm.31.10.2589-2593.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. H., Feldman M. W., Nevo E. Multilocus Structure of Natural Populations of HORDEUM SPONTANEUM. Genetics. 1980 Oct;96(2):523–536. doi: 10.1093/genetics/96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge D. R., Nakielna E. M., Noble M. A. Case-control and vector studies of nosocomial acquisition of Pseudomonas cepacia in adult patients with cystic fibrosis. Infect Control Hosp Epidemiol. 1993 Mar;14(3):127–130. doi: 10.1086/646697. [DOI] [PubMed] [Google Scholar]
- Carson L. A., Anderson R. L., Panlilio A. L., Beck-Sague C. M., Miller J. M. Isoenzyme analysis of Pseudomonas cepacia as an epidemiologic tool. Am J Med. 1991 Sep 16;91(3B):252S–255S. doi: 10.1016/0002-9343(91)90377-a. [DOI] [PubMed] [Google Scholar]
- Denamur E., Picard B., Goullet P., Bingen E., Lambert N., Elion J. Complexity of Pseudomonas aeruginosa infection in cystic fibrosis: combined results from esterase electrophoresis and rDNA restriction fragment length polymorphism analysis. Epidemiol Infect. 1991 Jun;106(3):531–539. doi: 10.1017/s0950268800067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eerola E., Lehtonen O. P. Optimal data processing procedure for automatic bacterial identification by gas-liquid chromatography of cellular fatty acids. J Clin Microbiol. 1988 Sep;26(9):1745–1753. doi: 10.1128/jcm.26.9.1745-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S., Govan J. R. Pseudomonas cepacia--fatal pulmonary infection in a patient with cystic fibrosis. J Infect. 1986 Sep;13(2):157–158. doi: 10.1016/s0163-4453(86)92953-1. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Brown P. H., Maddison J., Doherty C. J., Nelson J. W., Dodd M., Greening A. P., Webb A. K. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993 Jul 3;342(8862):15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Nelson J. W. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992 Oct;48(4):912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- Hedrick P. W., Thomson G. A two-locus neutrality test: applications to humans, E. coli and lodgepole pine. Genetics. 1986 Jan;112(1):135–156. doi: 10.1093/genetics/112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Kostman J. R., Edlind T. D., LiPuma J. J., Stull T. L. Molecular epidemiology of Pseudomonas cepacia determined by polymerase chain reaction ribotyping. J Clin Microbiol. 1992 Aug;30(8):2084–2087. doi: 10.1128/jcm.30.8.2084-2087.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin L. O., Byard P. J., Davis P. B. Effect of Pseudomonas cepacia colonization on survival and pulmonary function of cystic fibrosis patients. J Clin Epidemiol. 1990;43(2):125–131. doi: 10.1016/0895-4356(90)90175-o. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Dasen S. E., Nielson D. W., Stern R. C., Stull T. L. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990 Nov 3;336(8723):1094–1096. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Fisher M. C., Dasen S. E., Mortensen J. E., Stull T. L. Ribotype stability of serial pulmonary isolates of Pseudomonas cepacia. J Infect Dis. 1991 Jul;164(1):133–136. doi: 10.1093/infdis/164.1.133. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Mortensen J. E., Dasen S. E., Edlind T. D., Schidlow D. V., Burns J. L., Stull T. L. Ribotype analysis of Pseudomonas cepacia from cystic fibrosis treatment centers. J Pediatr. 1988 Nov;113(5):859–862. doi: 10.1016/s0022-3476(88)80018-0. [DOI] [PubMed] [Google Scholar]
- Martinetti G., Altwegg M. rRNA gene restriction patterns and plasmid analysis as a tool for typing Salmonella enteritidis. Res Microbiol. 1990 Nov-Dec;141(9):1151–1162. doi: 10.1016/0923-2508(90)90088-8. [DOI] [PubMed] [Google Scholar]
- Mukwaya G. M., Welch D. F. Subgrouping of Pseudomonas cepacia by cellular fatty acid composition. J Clin Microbiol. 1989 Dec;27(12):2640–2646. doi: 10.1128/jcm.27.12.2640-2646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin C. S., Jarvis W. R., Anderson R. L., Govan J., Klinger J., LiPuma J., Martone W. J., Monteil H., Richard C., Shigeta S. Pseudomonas cepacia typing systems: collaborative study to assess their potential in epidemiologic investigations. Rev Infect Dis. 1989 Jul-Aug;11(4):600–607. doi: 10.1093/clinids/11.4.600. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Musser J. M., Caugant D. A., Gilmour M. N., Whittam T. S. Population genetics of pathogenic bacteria. Microb Pathog. 1987 Jul;3(1):1–7. doi: 10.1016/0882-4010(87)90032-5. [DOI] [PubMed] [Google Scholar]
- Simmonds E. J., Conway S. P., Ghoneim A. T., Ross H., Littlewood J. M. Pseudomonas cepacia: a new pathogen in patients with cystic fibrosis referred to a large centre in the United Kingdom. Arch Dis Child. 1990 Aug;65(8):874–877. doi: 10.1136/adc.65.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Gumery L. B., Smith E. G., Stableforth D. E., Kaufmann M. E., Pitt T. L. Epidemic of Pseudomonas cepacia in an adult cystic fibrosis unit: evidence of person-to-person transmission. J Clin Microbiol. 1993 Nov;31(11):3017–3022. doi: 10.1128/jcm.31.11.3017-3022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Smith E. G., Gumery L. B., Stableforth D. E. Pseudomonas cepacia infection in cystic fibrosis. Lancet. 1992 Jan 25;339(8787):252–252. doi: 10.1016/0140-6736(92)90063-9. [DOI] [PubMed] [Google Scholar]
- Tablan O. C., Chorba T. L., Schidlow D. V., White J. W., Hardy K. A., Gilligan P. H., Morgan W. M., Carson L. A., Martone W. J., Jason J. M. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985 Sep;107(3):382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]



