Abstract
Several studies [1–3] have reported an association of Alzheimer disease (AD) with polymorphic markers in SORL1. Data from a recently published genome wide association study in AD [4] have been made publically available. We tested the association of AD with SORL1 in this dataset (TGEN), which included 31 SORL1 SNPs, 8 of which overlapped the original study [1]. Six SNPs, near the 3′ region of SORL1 containing SNPs which were strongly associated with AD in previous studies, showed significant association in the TGEN dataset. These results provide an independent replication of the association between AD and SORL1.
Keywords: Alzheimer disease, SORL1, association, genome-wide association study
Introduction
Rogaeva et al.[1] reported an association between Alzheimer disease (AD) and several SNPs in the gene encoding the sortilin-related receptor, low-density lipoprotein receptor class A repeat–containing protein - SORL1 (11q23–q24) in four different ethnic groups (Caucasians, African Americans, Israeli-Arabs and Hispanics) analyzed separately. Although the precise identity of the genetic effectors in SORL1 remains to be determined, this initial study pointed to two clusters of SNPs in distinct regions of the gene, implying the existence of multiple allelic variants associated with AD in different populations. Two positive replications have been reported [2,3]. Recently, results of a genome wide association study in AD was published [4] with a focus on a novel association of SNPs in the GAB2 gene with AD. This report however did not mention any results for SORL1. We were therefore interested to test whether the association of AD with SORL1 is replicated in this dataset (TGEN) which is publicly available.
Methods
Subjects
The TGEN dataset [4] was obtained from from the website http://www.tgen.org/neurogenomics/data. Although both the original study by Rogaeva et al [1] and the study by Reiman et al describing the TGEN dataset [4] included subjects ascertained at the Mayo Clinic in Rochester, Minnesota, the Mayo subjects in the TGEN data set and the Mayo subjects included in Rogaeva et al. study are independent. Consequently, we analyzed SORL1 data for 1,408 subjects in the TGEN database which included 1,044 autopsied individuals (641 cases, 403 controls) and 364 clinically examined subjects from the Mayo Clinic (218 cases and 146 controls).
Statistical analyses
SNP marker data were assessed for deviations from Hardy-Weinberg equilibrium using Haploview [5] software. Single point allelic and genotypic tests were performed using PLINK [6]. Marker genotype distributions in cases and controls were compared in several ways: (1) a genotypic test with two degrees of freedom, models assuming (2) dominant and (3) recessive inheritance, and (4) the Cochran-Armitage trend test.
Linkage disequilibrium
The LD structure among the SORL1 SNPs was examined using Haploview[5]. Haplotype blocks were defined using the confidence intervals algorithm. The default settings were used in these analyses, which create 95% confidence bounds on D′ to delineate SNP pairs in strong LD.
Results
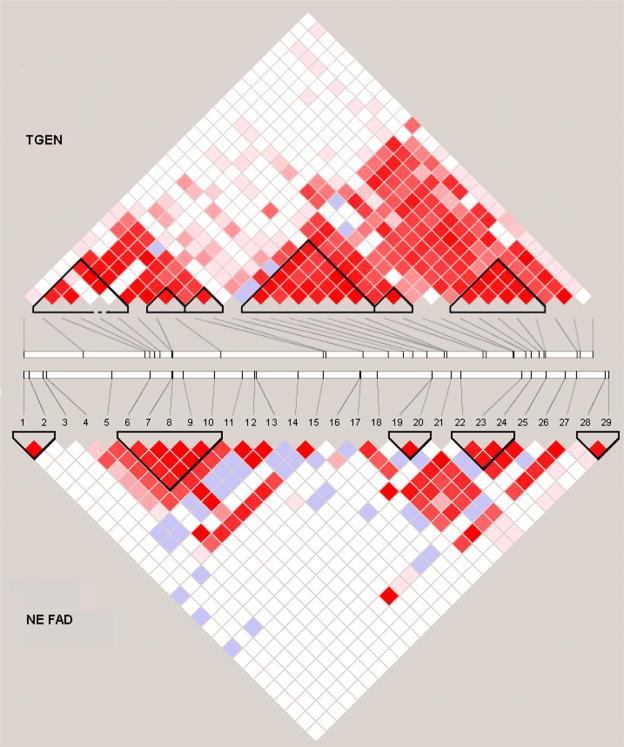
There were 31 SORL1 SNPs in the TGEN database, and eight of those overlapped the 29 SNPs in the by Rogaeva et al study [1]. These 31 SNPs are referred to by their sequential order on the physical map in TGEN database, i.e. T.1, T.2, T.n, T.31. Therefore, a total of 52 unique SNPs were analyzed in these two studies (Table 1). All SNPs are in Hardy-Weinberg equlibrium in control samples. The LD structures of SNPs in the 5′ and 3′ regions are similar in the north European family data [1] and TGEN data [2] (Figure 1).
Table 1.
SORL1 SNP information in the original study and in the TGEN dataset.
| SNP order | dbSNP rs Number | Physical Map Location (bp) | Distance (bp) from Previous Marker | SNP Type | Orig order | TGEN order | NE FAD | Israeli Arab | TGEN |
|---|---|---|---|---|---|---|---|---|---|
| 1 | rs4935774 | 120,826,964 | Upstream of 5′ UTR | 1 | T.1 | 0.20 | 0.18 | 0.39 | |
| 2 | rs578506 | 120,828,687 | 1,723 | intron | 2 | 0.07 | ND | ||
| 3 | rs582446 | 120,833,069 | 4,382 | intron | 3 | 0.32 | 0.08 | ||
| 4 | rs661057 | 120,834,164 | 1,095 | intron | 4 | 0.12 | 0.07 | ||
| 5 | rs676160 | 120,845,443 | 11,279 | intron | T.2 | 0.49 | |||
| 6 | rs11218304 | 120,854,321 | 8,878 | intron | 5 | 0.27 | 0.046 | ||
| 7 | rs676759 | 120,864,475 | 10,154 | intron | T.3 | 0.57 | |||
| 8 | rs560573 | 120,866,094 | 1,619 | intron | 6 | T.4 | 0.64 | 0.29 | 0.55 |
| 9 | rs985421 | 120,867,526 | 1,432 | intron | T.5 | 0.28 | |||
| 10 | rs593769 | 120,869,213 | 1,687 | intron | T.6 | 0.57 | |||
| 11 | rs12364988 | 120,872,836 | 3,623 | H269H | 7 | T.7 | 0.15 | 0.041 | 0.33 |
| 12 | rs668387 | 120,873,131 | 295 | intron | 8 | T.8 | 0.52 | 0.021 | 0.32 |
| 13 | rs689021 | 120,876,330 | 3,199 | intron | 9 | 0.71 | 0.040 | ||
| 14 | rs641120 | 120,886,175 | 9,845 | intron | 10 | 0.57 | 0.07 | ||
| 15 | rs11218313 | 120,888,081 | 1,906 | intron | T.9 | 0.83 | |||
| 16 | rs4935775 | 120,894,712 | 6,631 | intron | 11 | 0.22 | 0.030 | ||
| 17 | rs12285364 | 120,898,436 | 3,724 | intron | 12 | 0.014 | 0.56 | ||
| 18 | rs2298813 | 120,898,894 | 458 | T528A | 13 | 0.012 | 0.43 | ||
| 19 | rs11600231 | 120,911,918 | 13,024 | intron | 14 | 0.69 | 0.12 | ||
| 20 | rs2276346 | 120,919,686 | 7,768 | intron | 15 | T.10 | 0.61 | 0.36 | 0.25 |
| 21 | rs10502262 | 120,920,522 | 836 | intron | T.11 | 0.17 | |||
| 22 | SORL1-T833T | 120,931,165 | 10,643 | T833T | 16 | 0.41 | 0.0026 | ||
| 23 | rs556349 | 120,931,417 | 252 | intron | 17 | 0.0057 | 0.20 | ||
| 24 | rs7131432 | 120,932,080 | 663 | intron | T.12 | 0.14 | |||
| 25 | rs11218340 | 120,936,564 | 4,484 | intron | 18 | 0.08 | 0.14 | ||
| 26 | rs10892756 | 120,939,766 | 3,202 | intron | T.13 | 0.49 | |||
| 27 | rs11218346 | 120,944,391 | 4,625 | intron | T.14 | 0.48 | |||
| 28 | rs1790213 | 120,947,399 | 3,008 | intron | T.15 | 0.33 | |||
| 29 | rs11218347 | 120,951,758 | 4,359 | intron | T.16 | 0.78 | |||
| 30 | rs2070045 | 120,953,300 | 1,542 | S1187S | 19 | 0.031 | 0.00082 | ||
| 31 | rs3824966 | 120,953,393 | 93 | intron | 20 | 0.15 | 0.15 | ||
| 32 | rs1699103 | 120,957,136 | 3,743 | intron | T.17 | 0.045 | |||
| 33 | rs7116734 | 120,957,150 | 14 | intron | T.18 | 0.30 | |||
| 34 | rs11218350 | 120,957,861 | 711 | intron | T.19 | 0.040 | |||
| 35 | SORL1-18ex26 | 120,959,359 | 1,498 | (−18) 5′ of exon 26 | 21 | 0.41 | 0.0091 | ||
| 36 | rs1699102 | 120,962,172 | 2,813 | N1246N | 22 | 0.06 | 0.91 | ||
| 37 | rs10892759 | 120,969,298 | 7,126 | intron | T.20 | 0.021 | |||
| 38 | rs1792113 | 120,970,156 | 858 | intron | T.21 | 0.20 | |||
| 39 | rs11218360 | 120,978,601 | 8,445 | intron | T.22 | 0.42 | |||
| 40 | rs7128608 | 120,978,808 | 207 | intron | T.23 | 0.44 | |||
| 41 | rs3824968 | 120,981,132 | 2,324 | A1584A | 23 | 0.0031 | 0.00073 | ||
| 42 | rs1629493 | 120,982,306 | 1,174 | intron | T.24 | 0.09 | |||
| 43 | rs2282649 | 120,984,168 | 1,862 | intron | 24 | T.25 | 0.020 | 0.64 | 0.26 |
| 44 | rs726601 | 120,986,617 | 2,449 | intron | T.26 | 0.07 | |||
| 45 | rs1784931 | 120,988,148 | 1,531 | intron | T.27 | 0.049 | |||
| 46 | rs1010159 | 120,988,611 | 463 | intron | 25 | T.28 | 0.09 | 0.91 | 0.14 |
| 47 | rs1784933 | 120,994,626 | 6,015 | intron | 26 | 0.07 | 0.80 | ||
| 48 | rs1614735 | 120,998,211 | 3,585 | intron | 27 | T.29 | 0.010 | 0.96 | 0.19 |
| 49 | rs17125558 | 120,999,237 | 1,026 | intron | T.30 | 0.70 | |||
| 50 | rs10892761 | 121,003,094 | 3,857 | intron | T.31 | 0.56 | |||
| 51 | rs1133174 | 121,006,965 | 3,871 | downstream of 3′UTR | 28 | 0.07 | 0.09 | ||
| 52 | rs1131497 | 121,007,955 | 990 | downstream of 3′UTR | 29 | 0.20 | 0.06 |
Notes: The nominal P values for allelic association with AD are shown for each dataset. NE FAD -- north European familial AD dataset; Israeli-Arab -- Israeli-Arab case-control dataset, and TGEN – TGEN dataset. Boldface rs number indicates that the SNP was genotyped in both Rogaeva et al. [1] and Reiman et al. [4] studies. Bold face p-value indicates that the SNP is nominally associated with AD in the original study. Bold face p-value with white font, black background refer to significant results from analyses of the TGEN data.
Figure 1.
Linkage disequilibrium (LD) structure of SORL1. Relative locations of SNPs included in each dataset are shown on two parallel stick diagrams, with LD maps for the TGEN dataset located above and the north European familial AD dataset studied by Rogaeva et al. [1] below the gene structure. The measure of LD (D′) among all possible pairs of SNPs is shown graphically according to the shade of red where white represents very low D′ and dark red represents very high D′. High D′ estimates associated with a large confidence interval (most likely due to one of the alleles being rare) are denoted by blue squares.
Six SNPs (T.17, T.19, T.20, T.21, T.26, T.27) showed nominally significant association (0.01 ≤ p <0.05) with AD under at least one model (Table 2). These six SNPs span a region of approximately 35 kb including SNPs 21–25 near the 3′ end of SORL1 which were strongly associated with AD in the Rogaeva et al. study. TGEN SNPs T.17 and T.19 are located between SNPs 20 and 21, TGEN SNPs T.20 and T.21 are between SNPs 22 and 23, and TGEN SNPs T.26 and T.27 are between SNPs 24 and 25 (Table 2). Haplotype analysis strengthened the association signal in the region including the two SNPs between SNPs 22 and 23 (global p-value = 0.005, data not shown).
Table 2.
Single-SNP association results in the TGEN dataset.
| order | SNP | interval of SNPs in the original paper | Minor Allele | F_A | F_U | OR | Allele.P | Geno.P | DOM.P | REC.P | TREND.P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T.1 | rs4935774 | 1 | 1 | 0.215 | 0.201 | 1.09 | 0.39 | 0.54 | 0.54 | 0.29 | 0.38 |
| T.2 | rs676160 | 4~5 | 2 | 0.100 | 0.092 | 1.10 | 0.49 | 0.78 | 0.50 | 0.73 | 0.48 |
| T.3 | rs676759 | 5~6 | 2 | 0.389 | 0.378 | 1.05 | 0.57 | 0.35 | 0.88 | 0.19 | 0.57 |
| T.4 | rs560573 | 6 | 2 | 0.386 | 0.374 | 1.05 | 0.55 | 0.40 | 0.95 | 0.21 | 0.55 |
| T.5 | rs985421 | 6~7 | 2 | 0.016 | 0.022 | 0.74 | 0.28 | NA | NA | NA | 0.27 |
| T.6 | rs593769 | 6~7 | 1 | 0.385 | 0.374 | 1.05 | 0.57 | 0.48 | 0.98 | 0.25 | 0.57 |
| T.7 | rs12364988 | 7 | 2 | 0.496 | 0.477 | 1.08 | 0.33 | 0.56 | 0.61 | 0.28 | 0.34 |
| T.8 | rs668387 | 8 | 2 | 0.430 | 0.411 | 1.08 | 0.32 | 0.57 | 0.52 | 0.31 | 0.32 |
| T.9 | rs11218313 | 10~11 | 2 | 0.088 | 0.086 | 1.03 | 0.83 | 0.92 | 0.78 | 0.82 | 0.83 |
| T.10 | rs2276346 | 15 | 1 | 0.351 | 0.372 | 0.91 | 0.25 | 0.22 | 0.10 | 0.95 | 0.24 |
| T.11 | rs10502262 | 15~16 | 1 | 0.288 | 0.312 | 0.89 | 0.17 | 0.33 | 0.14 | 0.60 | 0.17 |
| T.12 | rs7131432 | 17~18 | 2 | 0.016 | 0.024 | 0.67 | 0.14 | NA | NA | NA | 0.14 |
| T.13 | rs10892756 | 18~19 | 1 | 0.075 | 0.068 | 1.11 | 0.49 | 0.40 | 0.65 | 0.18 | 0.49 |
| T.14 | rs11218346 | 18~19 | 1 | 0.085 | 0.078 | 1.11 | 0.48 | 0.60 | 0.55 | 0.37 | 0.47 |
| T.15 | rs1790213 | 18~19 | 2 | 0.375 | 0.393 | 0.93 | 0.33 | 0.34 | 0.17 | 0.99 | 0.33 |
| T.16 | rs11218347 | 18~19 | 1 | 0.076 | 0.073 | 1.04 | 0.78 | 0.94 | 0.81 | 0.78 | 0.78 |
| T.17 | rs1699103 | 20~21 | 1 | 0.404 | 0.442 | 0.85 | 0.045 | 0.13 | 0.07 | 0.14 | 0.043 |
| T.18 | rs7116734 | 20~21 | 1 | 0.399 | 0.419 | 0.92 | 0.30 | 0.42 | 0.61 | 0.19 | 0.29 |
| T.19 | rs11218350 | 20~21 | 2 | 0.215 | 0.248 | 0.83 | 0.040 | 0.11 | 0.11 | 0.07 | 0.045 |
| T.20 | rs10892759 | 22~23 | 1 | 0.325 | 0.368 | 0.83 | 0.021 | 0.040 | 0.011 | 0.35 | 0.020 |
| T.21 | rs1792113 | 22~23 | 1 | 0.351 | 0.375 | 0.90 | 0.20 | 0.06 | 0.041 | 0.68 | 0.21 |
| T.22 | rs11218360 | 22~23 | 1 | 0.027 | 0.032 | 0.83 | 0.42 | NA | NA | NA | 0.41 |
| T.23 | rs7128608 | 22~23 | 1 | 0.044 | 0.038 | 1.17 | 0.44 | 0.75 | 0.45 | 0.84 | 0.45 |
| T.24 | rs1629493 | 23~24 | 2 | 0.356 | 0.388 | 0.87 | 0.09 | 0.20 | 0.15 | 0.14 | 0.08 |
| T.25 | rs2282649 | 24 | 2 | 0.289 | 0.309 | 0.91 | 0.26 | 0.52 | 0.31 | 0.43 | 0.26 |
| T.26 | rs726601 | 24~25 | 2 | 0.296 | 0.329 | 0.86 | 0.07 | 0.12 | 0.038 | 0.56 | 0.06 |
| T.27 | rs1784931 | 24~25 | 1 | 0.377 | 0.415 | 0.86 | 0.049 | 0.12 | 0.049 | 0.24 | 0.046 |
| T.28 | rs1010159 | 25 | 1 | 0.336 | 0.363 | 0.89 | 0.14 | 0.32 | 0.21 | 0.24 | 0.14 |
| T.29 | rs1614735 | 27 | 2 | 0.479 | 0.505 | 0.90 | 0.19 | 0.27 | 0.11 | 0.58 | 0.18 |
| T.30 | rs17125558 | 27~28 | 2 | 0.020 | 0.022 | 0.90 | 0.70 | NA | NA | NA | 0.70 |
| T.31 | rs10892761 | 27~28 | 2 | 0.411 | 0.422 | 0.96 | 0.56 | 0.07 | 0.10 | 0.32 | 0.56 |
Notes: F_A: Minor allele frequency in cases; F_U: Minor allele frequency in controls; OR: estimated odds ratio for AD of minor allele; Allele.P: p-value for allelic association; Geno.P: p-value for genotypic (2 df) test; DOM.P: p-value for genotypic (1 df) test with dominant model; REC.P: p-value for genotypic (1 df) test with recessive model; TREND.P: Cochran-Armitage trend test; NA: Not available (allelic, genotypic and dominant/recessive tests require that each cell has a frequency of 1 or greater). SNPs studied by both Rogaeva et al. [1] and Reiman et al. [4] are indicated in boldface.
Discussion
The results from analysis of the TGEN data provide an independent replication of the association between AD and SORL1. We recognize that the magnitude of the significance of these results would not survive correction for multiple testing in a hypothesis-generating study (e.g., genome-wide association study); however a p-value of less than 0.05 is sufficient to confirm a previously reported association between variants within a specific gene and disease. Furthermore, although the particular SNP associations in this study are novel, a Bonferroni or similar correction is not appropriate or justified because all of the associated SNPs are within the region of and in LD with SNPs implicated in previous investigations[1–3], and therefore these are not truly independent tests.
Conclusion
While the precise identity of genetic effectors in SORL1 remains unknown, the TGEN data support the hypothesis that at least one disease risk enhancing allele is located near the 3′ end of SORL1.
Acknowledgments
This work was supported by grants from the US National Institute on Aging: R01-AG09029, R01-AG25259, R01-AG17173, and P30-AG13846 (LAF); R37-AG15473 and P01-AG07232 (RM); the Canadian Institutes of Health Research, Howard Hughes Medical Institute, Alzheimer Society of Ontario, Canada Foundation for Innovation, Ontario Research and Development Challenge Fund, Ontario Mental Health Foundation, Genome Canada (PSGH); the Blanchett Hooker Rockefeller Foundation and Charles S. Robertson Gift from the Banbury Fund (RM); and Fonds de la Recherche en Santé (YM). This research was conducted using the Boston University Linux Cluster for Genetic Analysis funded by an NCRR Shared Instrumentation grant (RR163736).
Footnotes
The authors declare that there are no conflicts of interest.
References
- 1.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Cheng R, Schupf N, Manly J, Lantigua R, Stern Y, et al. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, community-based cohort. Arch Neurol. 2007;64(4):501–506. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Cheng R, Schupf N, Honig LS, Vonsattel GJ, Clark L, et al. The Association Between Genetic Variants in SORL1 and Autopsy-Confirmed Alzheimer’s Disease. Neurology. 2007 doi: 10.1212/01.wnl.0000280581.39755.89. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, Zismann VL, et al. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007;54(5):713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 6.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: A tools set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007:81. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]