Abstract
This paper postulates that all forms of the neurological movement disorder, dystonia, can be argued to reflect excessive function of one or more components of the brain postural system. This is based on four central arguments. First, because some forms of postural control are already known to be dynamic, rather than static, it is suggested that hyperkinetic dystonias reflect excessive function of dynamic postures, rather than abnormal movements. Second, the range of functional roles served by the postural system is hypothesized to include direct control of movement, suggesting a postural basis for task-specific dystonias. Third, by defining posture as a neural system that maintains body stabilization, it can be shown that the range of mechanical means of implementing stabilization, including co-contraction of antagonistic muscles, matches the range of presentations of dystonia. Fourth, it is shown that the above premises are able to account for previously unexplained observations in dystonia. Based on the inhibitory influence that stabilizing mechanisms exert on movement, it is suggested that the broad functional role that is here referred to as posture may be the function served by the indirect pathway of the basal ganglia. Specifically, it is proposed that this pathway centrally coordinates function of the distributed network of brain regions controlling posture and, in conjunction with the direct pathway, coordinates posture and movement.
Keywords: posture, dystonia, antagonistic muscles, basal ganglia, indirect pathway, dopamine, brainstem, cerebellum, movement disorders
I. Introduction
Motor control at the neural systems level, and particularly as it is modulated by the basal ganglia (BG), is traditionally thought to be achieved via a balance of excitation and inhibition of competing motor programs [1–3]. This model has been applied to explain function of the direct versus indirect pathways of the BG, which are thought to activate versus inhibit movement, respectively [4]. This model has also been used to guide hypotheses about motor control generally, and to describe the neural basis for some neurological movement disorders.
This paper presents an alternative view that motor control may be achieved by complementary, but opposing forces of two distinct neural systems: one for movement and another for posture. This is argued based on the hypothesis that our current view of what constitutes the brain postural system can be expanded in such a way as to build simpler models for motor control and movement disorders. This alternative view supports the notion that all forms of the movement disorder, dystonia, can be viewed as an abnormal amplification of one or more components of the brain postural control system. It also suggests that the indirect pathway of the BG modulates activation of postural programs, rather than directly inhibiting movement; it is just that postural function as defined here can have an inhibitory influence on movement.
Why are alternate/revised models needed?
While the concepts proposed here have implications for all aspects of our understanding of motor and postural control, they were specifically driven by the need for improved models for dystonia. Current models for dystonia fail to explain fundamental features of the disorder. For example, dystonia sometimes manifests as abnormal or amplified “movements” and current models suggest this may result from abnormal co-activation of competing motor programs, especially when the symptoms are triggered by a specific movement or motor skill [5]. However, dystonia can also present as static (i.e. hypokinetic) postures, often in the same individuals who present with dynamic (i.e. hyperkinetic) symptoms [6]. So what makes us call each of these dystonia and do they have neural mechanisms in common? The conceptual model proposed here suggests that the reason we classify hypo- and hyperkinetic dystonias as a single, stereotyped symptom is that they each reflect abnormalities within the same functional neural system. The kinetic heterogeneity of symptoms reflects the fact that this system exhibits a range of static to dynamic features.
Another puzzling feature of dystonia is that this symptom can be associated with abnormal function and/or lesions at multiple sites across the sensorimotor system, including the BG [7], the cerebellum [8], the brainstem [9] and sensorimotor cortex [10]. Based on this, it is now becoming accepted that dystonia is a heterogeneous disorder which can arise from a number of different genetic and anatomical brain abnormalities [11]. What is argued here, however, is that although there is clinical and mechanistic heterogeneity across dystonias, homogeneity of the disorder can, indeed, be found at the systems level.
Finally, dystonia is thought to sometimes arise from a disorganized brain sensorimotor system, as suggested by studies showing evidence for impaired sensorimotor integration [12] and disorganized somatotopy [10] in patients with the disorder. However, why would such a highly stereotyped symptom arise from disorganization? The conceptual model proposed here argues that it is not functional disorganization which causes dystonia but, instead, hyperactivity within an inherently complex functional neural system whose function is normally highly subtle and often automatic (i.e. non-volitional).
In sum, dystonia is stereotyped clinically, but mechanistically heterogeneous. This suggests that we have the intuition to classify all dystonias as the same type of symptom because they all reflect a “hit” to a single functional neural system: the postural control system. Below, the case is made that a distributed brain postural system that includes dynamic (i.e. motor-related), as well as static components, is one of the “missing links” in our understanding of what all dystonias have in common. In order to make this case it is necessary both to consider broadening the scope of what we classify as postural control, and to integrate these ideas and aspects of the existing postural literature with the movement disorders literature.
It is important to emphasize that the general concepts put forth in Section II are not dependent on the specific mechanisms suggested in Section III. The goal is to outline a flexible, conceptual, systems-level model for postural control and for dystonia, and then to show that there are indeed pieces of existing literature which can be brought together to hypothesize at least one way in which the conceptual model might be physiologically feasible.
II. Rationale for expanding the functional domain of postural control and how this gives us insights into motor control and dystonia
From existing literature about postural function: Posture stabilizes the body, and can be either static or dynamic in nature
Research investigating brain control of body posture has focused on postural function in relation to (a) body position and orientation in space and (b) maintaining balance, particularly during movement [13–15]. Each of these functions exerts a stabilizing effect on the body.
Posture can be dynamic, as well as static
Despite the fact that postural function stabilizes the body and that there are tonic components of postural control, particularly to maintain normal muscle tone at rest, it is recognized in the posture literature that postural control involves dynamic, as well as static components [13]. For example, the programs controlling compensatory adjustment of posture in relation to balance, both during quiet stance [16] and during movement, [14] must be dynamic and reactive. Taking into account the potential for postural control to be dynamic, it is argued here that one must consider that some disorders appearing to manifest as abnormal movements (e.g. hyperkinetic dystonias) might instead manifest as abnormal function of dynamic postures.
Neural control of posture
Regions known to be involved in postural control include the spinal cord, brainstem, cerebellum, and vestibular nuclei [17]. There is also evidence that sensorimotor cortex, premotor regions, and the BG participate in postural control. The role of the BG in postural control is of particular interest to movement disorders, given that a number of movement disorders exhibit postural abnormalities, including dystonia itself. It is also known that postural control is abnormal in Parkinson’s Disease (PD) [18], in rats with neonatal dopamine depletion [19] and in hemiparkinsonian monkeys with lesions of the substantia nigra pars reticulata [20]. Dopamine replacement therapy has been shown to improve background postural tone in PD patients, but to weaken force generation to resist external displacements [18]. Horak et al. suggested from this finding that the BG may contribute to each of these two aspects of postural control. The current article builds on this idea by hypothesizing that the BG, and specifically the indirect pathway, might provide a central “control station” for all forms of posture.
How does posture relate to movement?
There are currently two prevailing theories regarding the functional interaction of posture and movement. One is that movement itself can be achieved via a trajectory of postural equilibrium points [21], suggesting that movement is effectively an emergent property of shifts between static postures. Others have argued that movement and posture are controlled by distinct neural systems which implement separate functions (movement versus balance and body orientation, respectively) [22, 23]. Neither of these prevailing theories indicates that there might be two distinct functional systems (posture and movement) which each play an active role in controlling movement.
From a general mechanical/motor control perspective: Why posture might be a direct component of motor control and exert its effects via co-contraction of antagonistic muscles
As described above, posture is discussed in the literature as a stabilizing function which maintains body shape and compensates for gravity and movement to maintain balance. From a mechanical perspective, the best way to achieve stabilization of the body would be either (a) to simultaneously activate (i.e. co-activate) antagonistic muscles (e.g. stabilization around a joint or joints) [24], or (b) to maintain sustained contraction of one or more synergistic muscles (e.g. stabilization of a particular posture or body position). It is suggested here that all forms of postural stabilization may be achieved by one or both of these two basic mechanisms and reciprocally, that such functional/mechanical characteristics may be a hallmark of postural function. Note that for purposes of simplification the term “antagonistic muscles” is used in a general way here; this term will be used to refer both to muscles antagonistic to each other in the context of producing opposing movements (e.g. flexors/extensors), and to opposing left/right core postural fixators, such as the left/right paraspinal muscles, which must exert forces in opposite directions simultaneously to maintain stable trunk posture. Note, also, that the term “co-activation” in is used here to refer both to tonic activity occurring simultaneously in antagonistic muscles (i.e. resting muscle tone) and to active (phasic) co-contraction of these muscles. In other words, posture is referred to here from a mechanical perspective, rather than by the specific muscles involved or the type of activity (tonic or phasic) in these muscles.
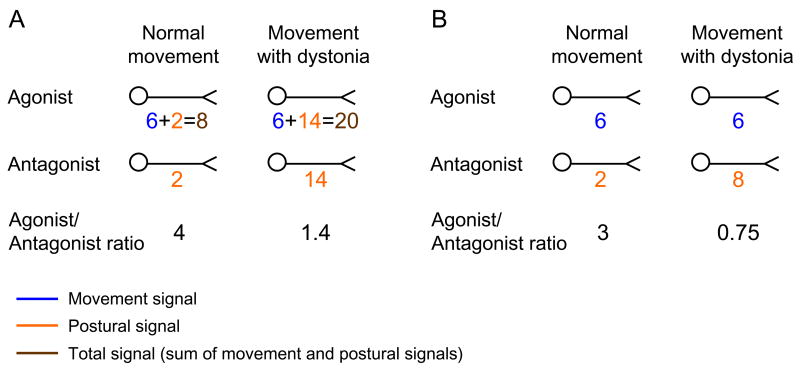
It is important to emphasize that when stabilization involves co-activation of opposing muscles, the postural system’s job is either to recruit both muscles, or to make the addition of antagonistic muscles when agonists are already active (see discussion of Fig. 1, below). Thus, while the postural signal itself need not always recruit both agonist and antagonist muscles, co-activation of opposing muscles is a sure sign that the postural system is functioning.
Figure 1.
Schematic diagrams of movement system versus postural system contributions to the total activity in agonist and antagonist muscles during movement as proposed by the conceptual model, during healthy movement and movement with dystonia. (A) and (B) show schematic representations of relative amounts (numbers are arbitrary and unit-less) of neural activity transmitted to agonist and antagonist muscles by the movement system (blue numbers) and the postural system (orange numbers) during “Normal movement” (left column) and “Movement with dystonia” (right column). In scenario (A) the postural system is depicted as recruiting both agonists and antagonists. In scenario (B) the postural system is depicted as recruiting only muscles antagonistic to the movement. In either scenario, an abnormally amplified postural signal (i.e. in dystonia) yields similar amounts of activity in both agonist and antagonist muscles (i.e. co-contraction).
Some level of postural control, and therefore antagonist muscle activation, is desired rather than completely inhibited in the local control of movement
Given the above definition of how posture might be mechanically implemented, might such stabilizing forces be applied in additional ways, suggesting a broader role for this functional system? A key element of the conceptual model proposed here is that the component of the postural system that mediates volitional (as opposed to default) control of body position and orientation in space also plays a crucial role in the local control of movement, i.e. control of the moving body part itself. It is currently thought that, given two functionally antagonistic muscles, when one muscle (the agonist) contracts during a movement, activity in the other muscle (the antagonist) is centrally inhibited so as not to interfere or “compete” with that movement [3]. While it is known that movements often involve activity in both agonists and antagonists in sequence (i.e. over time) [25], it is not thought that these muscles are “active” simultaneously. Although activity in muscles opposing the movement must be inhibited to some degree in order to move, it is argued here that this does not necessarily mean that these muscles must drop out of the picture entirely during movement. Specifically, because movements must be controlled or “sculpted” locally, and because the same movement can be controlled to varying degrees, it is proposed that the “control” aspect of motor control is accomplished by some component of the postural system dynamically (i.e. continually changing in relation to the trajectory of the movement) modulating activity in antagonist muscles to “guide” movements.
For example, individual finger movements must be controlled in order for the hand to write and to react to any unanticipated changes or obstacles of the movement that might not be hard-wired in the “master” writing program itself. This could conceivably be accomplished by counteracting the agonist muscle with subtle amounts of activity in the antagonist muscle, pulling the agonist in the desired direction and fine-tuning the trajectory of movement. Thus, although the tonic control of antagonistic muscles observed at rest may indeed be inhibited during movement, dynamic/phasic patterns in the same muscles would take over. In other words, it is suggested here that neural signals both to move and to mildly oppose the movement are activated during movement and work as complementary processes to produce what we call motor control.
In addition to making motor programs flexible, such a dynamic postural system might provide the equivalent of “training wheels” during motor learning. People tend to perform more “stiffly” when first learning a task, and then more fluidly once the task is learned; this is consistent with the idea that there might be a higher level of postural system activation while learning a task, with a gradual reduction of activity in this system as the task is learned.
Given the opposing effect that antagonist muscle activation exerts against agonist muscles, such a system might also play a role in controlling movement speed (i.e. velocity, acceleration, deceleration and stopping), with higher levels of postural function required locally, as well as globally, for slow and decelerating movements (a possible explanation for findings by DeLong [26]). Conversely, since such “movement-controlling” postural signals must work dynamically along the trajectory of movement, they may themselves appear as movements if they are abnormally amplified or occur in the absence of the opposing movement (agonist) signal itself, despite the fact that they are not actually caused by “movement” programs.
As noted above, posture involved in the local control of movement may be implemented by signals to co-activate agonists and antagonists, distinct from the “movement” signal to the agonist (Fig. 1A); alternatively, it may only implement muscles antagonistic to a movement while the movement implements agonists (Fig. 1B). A previous observation of abnormal motor unit synchronization of antagonistic muscles during dystonia [27] suggests that the former may be more likely than the latter. In the former case (Fig. 1A) there would be parallel and simultaneous recruitment of the same agonist muscle by both the “movement” and the “postural” systems. Because the activity from the two systems would be integrated peripherally, we would only observe the net effect, not the individual components of the signal. In either case, there would be a net co-activation of antagonistic muscles during movement, although normally, the postural muscle activity would be relatively much less than the movement muscle activity and not noticeable to the eye (Fig 1A, B; “Normal movement”). It is only when the postural signal is abnormally amplified (e.g. in dystonia) that antagonist muscle contraction (and thus co-contraction) becomes noticeable (Fig. 1A, B; “Movement with dystonia”).
Two complementary motor control variables (i.e. posture and movement) are more efficient than one variable in producing an unlimited range of movements
It would be inefficient to create a different motor program for each of the infinite number of movements the body can make, necessitating a “grandmother cell hypothesis” for all combinations of movements ever made. Instead, it would be much more elegant from a design perspective to start with fewer master programs which can then be modulated by a distinct, but parallel, functional system according to ongoing modulatory variables (obstacles, size and shape of objects manipulated, etc.) which are conveyed to the brain by sensory and vestibular feedback.
The concept of parallel functional systems for motor control is highly consistent with models for movement in the kinematics literature, with proposed open- versus closed-loop components of movement. The open loop component of movement is dependent on centrally generated, feedforward motor programs, while the closed loop component consists of positional adjustments made in response to sensory feedback during the movement [28]. Here, it is proposed that at least some elements of this closed loop component of movement might be considered part of the closed loop postural system rather than the movement system per se. The complexity and flexibility of movement would then reside not in the patterns of specific motor programs themselves, but in the balance and interaction between the movement and the postural control systems.
The conceptual model therefore argues that elements of both theories of posture/movement interaction discussed above are correct: The postural system plays a major role in controlling movements (among other things), but as a dynamic process (rather than shifting of static equilibrium points) which functions simultaneously and in parallel with a distinct movement system.
From the dystonia perspective: Co-contraction of antagonistic muscles and excessive, sustained agonist activity are salient clues that dystonia reflects amplified postural function
One of the most fundamental features of dystonia is that it often manifests as a co-contraction of antagonistic muscles [29]. While this could be viewed as the result of a co-activation of competing motor programs, such co-contraction is precisely what is proposed above as a fundamental mechanism used to achieve postural stabilization. Other features of dystonia include excessive and/or sustained contraction of agonist muscles or postural fixators [29]. While these could be viewed as the result of excessive activity in movement programs, such sustained contraction is the other mechanism proposed above for postural stabilization. Thus, the range of mechanisms for postural stabilization qualitatively matches the range of symptoms we call dystonia.
The clinical presentation of dystonia might then be viewed as an over-activation or disproportionate amplification of the signal in one or more components of the postural control system. Thus, rather than an inappropriate activation of a movement program, dystonia may reflect an inappropriate level of activation of an appropriate postural program. The net effect of this is to block movement, contort posture, or generate what looks like movement, depending on the specific component of posture affected (see Section III). Thus, it is proposed here that the systems-level similarity of all dystonias (what makes us call them dystonia) lies in the abnormal and disproportionate amplification of a discrete functional neural system.
Based on (a) the fact that we already know from the postural control literature that posture can be dynamic as well as static, and (b) the hypothesis that postural control occurs within a moving body part, this defines postural components which might underlie hyperkinetic dystonias and task-specific dystonias, respectively. Each of these types of symptoms has previously been considered in the movement disorders literature to reflect abnormal movement, rather than amplified postural function.
Synthesis of the information discussed above
Logic path 1
The postural system recruits neural programs required for stabilization.
Mechanically, the most effective stabilization could be achieved via concurrent activity in antagonistic muscles (i.e. co-contraction) or sustained contraction of a set of agonist (synergistic) muscles.
If (1a) and (1b) are true, then it is possible that:
HYPOTHESIS 1: Postural stabilization is implemented by an appropriate level of co-contraction in antagonistic muscles or by sustained contraction of agonist (synergistic) muscles.
Logic path 2
Dystonia manifests as a high level of co-contraction in antagonistic muscles and/or excessive and sustained contraction of agonist muscles.
If (1c) and (2a) are true, then it is possible that:
HYPOTHESIS 2: Dystonia is a disorder of amplified postural function
Logic path 3
Hyperkinetic dystonias look like “movements”
Although stabilizing in the right context and “dose”, some postural programs are dynamic, rather than static
If (3a) and (3b) are true, then it is possible that:
HYPOTHESIS 3: Hyperkinetic dystonias reflect amplification of dynamic postural programs, rather than “movements”
An important remaining question not answered by the above logic is how, mechanistically, static/tonic versus dynamic/phasic components of posture might be implemented and coordinated. This question is addressed in Section III.
III. Potential mechanisms for coordinating tonic and phasic postural programs
Section II discussed the potential range of functional roles for postural control. These might be organized into at least three general types of postural function:
Posture component 1
Ongoing maintenance of resting muscle tone and body shape. These are automatic (i.e. non-volitional), tonic postural programs that maintain baseline muscle tone and body shape at rest and background muscle tone during other states. For any given muscle, these programs may be inhibited when movement-related (i.e. components 2 and 3) postural programs are activated. The brainstem may contribute to control of this component. Excessive function of this component may underlie hypokinetic and/or fixed dystonias.
Posture component 2
Phasic postural function for maintaining balance both during static states (e.g. standing) and during movement. Although these programs may disengage when an individual is completely at rest and mechanically supported, they are active, at least intermittently, whenever the body must counteract gravity in any way, such as when standing, sitting, moving, or even when lying supine for body regions which do not have complete support (e.g. side-to-side head movements). This component likely exhibits both feedforward (anticipatory, open-loop) and feedback (reactive, closed loop) control of balance, which might be controlled by the supplementary motor area and the cerebellum, respectively. Excessive function of this component may underlie hyperkinetic axial dystonias.
Posture component 3
Phasic postural function involved in actively orienting the body in space, including local control of movement trajectory and speed, anchoring limbs at the base during skilled motor tasks (e.g. stabilizing the shoulder while writing), and voluntary maintenance of static postures. Muscle activity triggered by this component is normally superimposed over and smoothly integrated with muscle activity related to movement. Primary sensorimotor cortex may contribute to control of this component. Excessive function of this component may underlie hyperkinetic dystonias observed within body regions executing a motor skill (e.g. task- or motor skill-specific dystonias).
Hypo-versus hyperkinetic dystonias
It should be noted that although excessive function of the tonic postural component would be most likely to cause hypokinetic dystonias, and excessive function of phasic components would be most likely to cause hyperkinetic dystonias, the kinetic characteristics of any given dystonia might deviate from this general rule depending on the specific sub-component of posture involved and/or the physiology of the malfunction.
The current Section describes potential mechanisms for implementing and coordinating the range of functional roles described above for the brain postural system.
Potential role of the BG indirect pathway in coordinating multiple postural components
Given that the BG are connected with other brain regions controlling posture [30–34], this set of nuclei is well positioned to coordinate different anatomical and functional components of the brain postural system. The paragraphs below discuss evidence that the indirect pathway of the BG, specifically, may serve this role.
First, there is evidence that the subthalamic nucleus, which is part of the indirect pathway of the BG, plays a role in postural control since focal inhibition of this nucleus leads to postural asymmetries in rodents [35].
Second, dopamine D2 receptor antagonists (e.g. neuroleptics and antiemetics) can cause both acute [36] and tardive [37] dystonias. If dystonia is a disorder of amplified activity in the postural control system, then it is not unreasonable to suggest, based on these observations, that the indirect pathway (which is mediated by D2 receptors) plays a role in controlling posture.
Third, the indirect pathway is thought to be the component of the BG circuitry that inhibits movement [4]. From a functional perspective, it is suggested that the mechanisms described above for stabilization have the potential to work against or prevent movement, although the inhibition is implemented peripherally and mechanically, rather than via direct central inhibition of movement programs. If the postural system’s job is to recruit neural programs required for stabilization, it is possible that this is actually what the indirect pathway does that makes it appear to inhibit movement. This reasoning leads to a fourth, slightly more speculative, path of logic:
Logic path 4 (also refers back to logic paths 1–3 in Section II)
Co-contraction of antagonistic muscles and sustained contraction of an agonist muscle can inhibit or slow movement
The indirect pathway of the BG is thought to inhibit movement
- If (4a) and (4b) are true, then it is possible that:HYPOTHESIS 4: The indirect pathway’s job is to generate signals leading to co-contraction of antagonistic muscles and sustained contraction of agonist muscles
- If (1c) and (4c) are true, then it is possible that:HYPOTHESIS 5: The indirect pathway mediates postural function
- If (2b) and (4d) are true, then it is possible that:HYPOTHESIS 6: Malfunction of the indirect pathway can cause dystonia.
Although activity in the indirect pathway may be sufficient to trigger postural function, it is may not always be necessary for postural function: given the distribution of this functional system, postural function (and malfunction) could be generated by regions independent of the BG. It therefore follows that certain functional abnormalities in any of the brain regions controlling posture could potentially lead to dystonia; the type of dystonia would reflect which region and/or postural component was malfunctioning. However, if there were pathophysiology in other posture-controlling regions, these functional abnormalities might, in at least some cases, be perpetuated through the BG as part of the etiology of the resulting abnormal motor output.
The direct pathway of the BG, in contrast, might encode programmed movements and activate the muscles required to execute specific learned motor skills. Since the direct and indirect pathways of the BG are extensively interconnected [38], they are well suited for efficient and continuous communication between two parallel, but interdependent control systems (i.e. movement and posture).
Alternative hypotheses for the relative roles of the direct and indirect pathways should also be considered. For example, fibers in the indirect pathway might control tonic forms of posture while fibers in the direct pathway control movement and phasic forms of posture. Alternatively, fibers in the indirect pathway might control closed-loop forms of posture while fibers in the direct pathway control movement and open-loop forms of posture. The general functional concept is the same across each of the suggested scenarios, however, and the organizational principles discussed below could be applied in a similar way to each.
A potential neural mechanism for coordinating tonic and phasic postural programs: Collateral projections from pallidal output fibers
How, mechanistically, might the indirect pathway activate muscles controlling postural function both at rest and during movement, given that efferents of the internal pallidal segment (GPi) are inhibitory, and are thus thought to inhibit movement at rest? It is proposed here that some of the inhibitory GPi output neurons themselves synapse on inhibitory neurons (or interneurons) in their target nuclei and thereby exert a net excitatory effect on postural output during tonic/resting states. Thus, the key to the proposed system from a mechanistic perspective is the hypothesis that GPi output neurons can activate brain postural programs either via inhibition of neurons in projection regions or via the removal of such inhibition. Sanger [39] has previously proposed, in the context of dystonia, that the indirect pathway may have the potential to both inhibit and facilitate motor-related activity, although in relation to movement rather than postural function, and via different mechanisms than suggested in the current model.
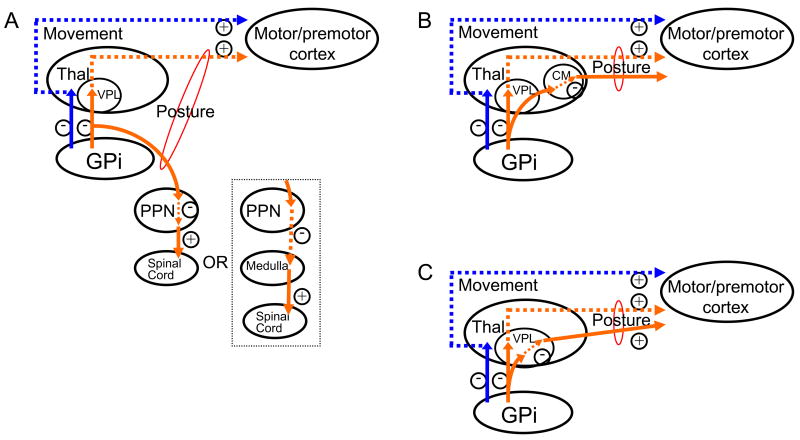
How might such a mechanism be achieved? Axonal tracing studies in monkeys have detected arborization patterns of GPi output fibers which seem ideally suited for such a control system. Parent and colleagues [33] have shown that some of the output neurons of the GPi send collateral projections to the pedunculopontine nucleus and/or the centromedian nucleus, in addition to their projection to the motor nucleus of the thalamus (ventral anterior nucleus in monkey, which corresponds to the ventral tier motor nuclei in humans). Ilinsky and colleagues [40] have shown that there are axon collaterals which project to different neurons within the motor nucleus. This included pairs of fibers in which one fiber synapsed on an excitatory neuron and the other on an inhibitory interneuron. Through the use of such dual (or multiple) projections, GPi output fibers could exert dual mode (tonic/phasic) control of posture, either within a nucleus or across nuclei. Note that the BG neural activation patterns themselves need not be tonic or phasic; this region would only need to act as a gate to activate or inhibit tonic or phasic programs in other regions controlling posture.
Specifically, it is proposed that, for a given GPi output neuron controlling posture, one collateral synapses directly on an excitatory neuron that controls phasic, movement-related posture, while the other collateral synapses on an inhibitory neuron that controls a neuron exerting tonic control of muscle tone (Fig. 2). The latter might be accomplished either by an inhibitory neuron that projects to a region that maintains tonic muscle activity [e.g. the pedunculopontine nucleus to the medulla [17]; Fig. 2A] or by an inhibitory interneuron within a region whose output maintains tonic muscle activity (Fig. 2A–C). During low/tonic levels of dopamine receptor activity in the putamen, GPi inhibitory output neurons would be active and inhibit phasic postural programs via the first collateral, while simultaneously inducing a net activation (i.e. disinhibition) of tonic postural programs in the same muscle(s) via the second collateral. Conversely, when dopamine is released in the putamen, the resulting removal of GPi inhibition of target nuclei would activate phasic postural programs via the first collateral, while simultaneously inducing a net inhibition of tonic postural programs in the same muscle(s) via the second collateral. It is not possible at this time to ascertain whether such a dual effect would be exerted within a projection nucleus (Fig. 2C) or across nuclei (Fig. 2A, B). However, given the expected level of complexity required to control multiple components of posture, it seems likely that both types of mechanisms may contribute.
Figure 2.
Pallidal output fiber arborization patterns in monkeys could support a system that activates (or disinhibits) postural system function whether or not these inhibitory output neurons are active. Some of the output neurons from the internal segment of the pallidum (GPi) in monkeys have been shown to send off collaterals that project to regions other than the motor thalamus (ventral posterolateral nucleus [VPL] shown here for humans; data is from ventral anterior nucleus in monkeys). These regions include (A) the pedunclopontine nucleus (PPN) and (B) the centromedian nucleus of the thalamus (CM) [33]. Collaterals are also sent off (C) within the motor nucleus of the thalamus [40]. Orange lines indicate pathways controlling posture; blue lines indicate pathways controlling movement. Solid arrows indicate neurons which are active/disinhibited when GPi inhibition is tonically active (i.e. in the absence of dopamine-induced activation of putamen output neurons) and inhibited when this inhibition is removed (during dopamine-induced activation of putamen output neurons). Dotted arrows indicate neurons that are inhibited when GPi inhibition is tonically active and active/disinhibitied when this inhibition is removed.
No matter what the specific “micro-organization” of the system, the GPi output neurons that send off collaterals provide an ideal mechanism for a dual-mode (tonic/phasic) postural control system. The suggested mechanism would be efficient since posture both at rest and during movement would be controlled by toggle switch of a single neuron, meaning there would be no lag time or additional circuitry required for communication between tonic and phasic postural systems. Such a set-up would also leave no confusion as to which component of the system should be active at any given time.
Potential physiological mechanisms for neural “hyperactivity” in dystonia
There are several potential mechanisms for the excessive neural activity proposed to underlie excessive postural function in dystonia, either within the BG or in other regions controlling posture. These include a reduction or loss of tonic neural inhibition (e.g. [41], see also Fig. 2), receptor “supersensitivity” (potentially the basis for findings by Schicatano and colleagues [42]), long term potentiation, and abnormal increases in synaptic connectivity. It is likely that the mechanism differs across dystonias, and influences whether a dystonia has the potential to “remit” or improve cumulatively with treatment.
Because supersensitivity is known to develop in response to reduced innervation of a brain region [43], such a mechanism provides a potential explanation for patients who experience both hypokinetic and hyperkinetic dystonic symptoms: If innervation of a region is reduced or a neurotransmitter system resides at lower than normal baseline tone, leading to hypokinetic dystonias, this system is also more likely also to have developed receptor supersensitivity and be hyperactive during a “bolus” of receptor activation, leading to hyperkinetic dystonias. If supersensitivity is not uniform somatotopically or across hemispheres, hyperkinetic and hypokinetic symptoms might even manifest simultaneously.
The idea of dopamine receptor supersensitivity is also consistent with the frequent lag-time (several days to a few months) to maximum effectiveness of L-Dopa treatment in dopa-responsive dystonia (DRD) [44]. L-Dopa has been shown to reverse receptor upregulation over weeks after substantia nigra lesions in rodents [45], suggesting that this may account for some of the effectiveness of L-Dopa in DRD, as well as in other dystonias that respond to L-Dopa.
IV. How the concepts proposed here offer explanations for seemingly paradoxical or puzzling observations in dystonia
This Section summarizes how the concepts and potential mechanisms proposed in this paper offer explanations for clinical or neurobiological observations in dystonia which have previously appeared paradoxical and/or cannot be accounted for by other models for the disorder.
The clinical heterogeneity of dystonia
Despite the fact that dystonia can be hyper- or hypokinetic, occur during movement or at rest, involve co-contraction of antagonistic muscles or excessive contraction of a single agonist muscle or group of synergistic muscles, the proposed conceptual model argues that each of these appears to be the same symptom (i.e. dystonia) because each involves excessive function of some component of the brain postural system. Rather than describing dystonia as a disorder which sometimes manifests as abnormal movements and other times as abnormal postures, it might instead be described as a disorder which manifests as excessive recruitment of dynamic or static postural programs. The precise presentation of any given dystonic symptom will depend on the product of all of the factors involved, including which postural component(s) is(are) affected and the anatomy, somatotopy, laterality, and pathophysiology of the abnormality.
The heterogeneity of the genetic and/or pathophysiologic basis for dystonia
The conceptual model described here puts forth, most centrally, that dystonia manifests as abnormal activity specific to a single, but anatomically distributed, functional neural system. Given the complexity and distribution of any functional neural system, it is not difficult to imagine how the system could potentially malfunction due to any number of alterations in this system at the genetic, anatomic, or pharmacologic levels. A similar concept has been put forth for Major Depressive Disorder [46].
Varied effects of dopaminergic agents and Deep Brain Stimulation (DBS) on dystonia
Because the proposed conceptual model postulates that postural control programs can be active during both resting/tonic and active/phasic dopamine states, it suggests that dystonia could manifest as the result of either abnormally low or abnormally high levels of dopamine release or postsynaptic activation in neurons controlling posture. This is consistent with the observation that dystonia can occur after exposure either to dopamine agonists [47] or to dopamine antagonists [36] and why dopamine antagonists can cause dystonia in some, while improving dystonia [48] in others. This also suggests why dystonia in PD can be observed both at peak and at end-dose of L-dopa treatment [49], even within the same individual.
Because the proposed conceptual model predicts that dystonia can manifest either as the result of too little or too much GPi inhibitory output, this also suggests why DBS is more effective and/or becomes effective more rapidly in some patients than in others [50]. It also suggests that stimulation in other brain regions [e.g. putamen, external segment of the GPi, or motor cortex [51] for “fixed” dystonias] may be more effective for some patients than GPi stimulation. Finally, it suggests why in some cases stimulation of the GPi can lead to worsening of dystonia (i.e. if too much GPi output causes the dystonia in the first place).
Hemispheric asymmetries and control of posture versus movement
The kinematics literature has suggested the open loop component of movement is left-hemisphere dominant, while the closed loop component is right-hemisphere dominant [28]. If closed loop movement is really a component of postural control, this raises the intriguing possibility that motor and postural control exhibit opposite hemispheric dominance. A lateralization of postural control might offer potential explanations for the hemispheric asymmetry in dopamine D2 receptor upregulation observed in animals with 6-hydroxydopamine lesions [52], the asymmetry of eyelid blink onset and amplitude observed in healthy humans [53], and the abnormal microstructural asymmetries previously observed medial to the GPi in hand and cervical dystonia patients [54].
Sensory tricks and the role of the somatosensory system in dystonia
One of the most exciting implications of the proposed conceptual model is that it provides a very logical explanation for the efficacy of “sensory tricks” in improving dystonia. Although often quite effective in reducing or eliminating dystonic symptoms [55], the mechanism for such maneuvers has remained largely elusive.
The postural system must rely on sensory feedback from the periphery to constantly update calculations of required postural support [23]. This includes taking into account sources of external support, in addition to the position of the body itself [56]. The less external stabilization available, the more the brain must recruit postural programs to stabilize the body. A sensory trick may “trick” the brain into believing that there is an increase in external stabilization and thereby reduce the level of recruitment of hyperactive postural programs. The effects may be so dramatic because the relevant neurons require a certain threshold of input before they become hyperactive, so that a sensory trick need only reduce input just below this threshold to be effective.
A sensory trick may alter the brain’s perception of required postural support in potentially two ways. First, the application of an upward force against some part of the body (e.g. the head) or even a simple tactile sensation can transiently “trick” the brain into believing that external support for the body has increased and that activity in postural programs supporting that region against gravity or movement can therefore decrease. However, since one can’t actually hold up one’s own body, the trick only lasts until the brain realizes there is not any real external support. In contrast, an external support device, such as an orthosis [57], may indeed have persistent beneficial effects if it is anchored outside the dystonic body region. Second, the very act of applying one’s own sensory trick with another part of the body (e.g. touching the hand to the chin) leads to a change in body position. If that new position itself reduces load on the hyperactive postural muscles, then demand for activity in and subsequent recruitment of these muscles is also likely to decrease. This component of a trick may lead to more persistent efficacy because it truly reduces postural load on the affected muscles; it also suggests why sensory tricks are often more effective when the patient applies them him/herself.
Certain voluntary movements may also reduce dystonic symptoms by reducing demand on the specific postural program causing dystonia: if the body position or motor task is changed, the required postural control and compensation will also change. Moving also reduces the persistence with which any given set of muscles is required to exert postural control and thus may reduce the recruitment level of affected programs below the threshold for dystonia.
Based on the fact that sensory feedback is more critical for modulating dynamic than static aspects of postural control, it is likely that dystonias involving tonic aspects of the postural system (e.g. “fixed” dystonias) would be less likely than phasic aspects to respond to sensory or motor tricks. However, hyperkinetic dystonias may also be unresponsive to sensory tricks if the threshold for dystonia is very low and neuronal hyperactivity is severe and persistent.
Impaired sensorimotor integration in dystonia
Previous studies have suggested that sensorimotor integration may be impaired in some forms of dystonia [12] According to the conceptual model presented here, sensorimotor (or to be consistent with the model, “sensoripostural”) integration need not be impaired in dystonia in the sense that there is a lack of communication or wrong information exchanged between the two systems. However, if the postural system were hyperactive in response to normal sensory input, the ratio of sensory to postural activity would be distorted. Given that the predicted impairment is in activation magnitude, it is also possible that a phasic postural program might be activated instead of a tonic one, or vice versa. In this case, the postural response would be qualitatively as well as quantitatively incorrect. Such a phenomenon might lead to the loss of cortical center-surround inhibition previously observed in focal hand dystonia [58].
Impaired motor learning in dystonia
There is also evidence for abnormal motor learning in dystonia [59, 60]. The conceptual model proposed here suggests that motor learning may be impaired at least in part because excessive postural function interferes with the learning process and/or task performance itself. If, as suggested in Section II, motor learning is accompanied by increased levels of postural function, this may amplify or unmask sub-clinically elevated levels of postural function throughout the body in these individuals, sufficiently to interfere with motor task performance. While interference may take place primarily at a mechanical level (e.g. making patients more “clumsy” if a tonic component is overactive, or misguiding movement if the motor control component is overactive), it may also take place at an attentional/cognitive level if mechanical interference increases task difficulty.
Visibility and stability of dystonic symptoms
In many patients there may be dystonic activity in muscles which do not appear dystonic if there is no net deviation from a normal posture (e.g. if opposing axial muscles exhibit similar levels of dystonia). If “masked” dystonic muscles are overlooked when other muscles are injected with botulinum toxin, they may continue to cause the patient significant discomfort. This may be one source of the frequent differences between a clinician’s and patient’s rating of symptom improvement after injections. Dystonia in these muscles may also be “unmasked” after such injections in conjunction with the altered balance of muscle activity across the patient’s body.
In addition, given that the proposal here that the muscles affected by dystonia are muscles used for stabilization, identifying muscles for botulinum toxin injection based on the muscles known to move the body in the direction that the dystonia moves the body may be limiting and/or misleading. The conceptual model predicts that the ability of a muscle to stabilize, in addition to move, a given body region should be taken into account when considering which muscles might benefit from botulinum toxin injections.
Dystonias after injury
If dystonia is a manifestation of excessive postural function, and postural function has a stabilizing effect, this suggests one potential reason that dystonias sometimes develop after an injury. Injury may signal the brain to generate greater stabilization of the body part itself or even of the body overall as a protective mechanism, such as after back injuries when many individuals require muscle relaxants in addition to analgesic medications. In some cases, however, this increased demand on the postural system may trigger an over-facilitation of this system which then persists in the form of dystonia. The higher frequency of “fixed” dystonias after injury than hyperkinetic dystonias [61] suggests that such stabilization may be implemented by the tonic postural component, which seems logical given that it would maintain the most constant level of stabilization.
V. Potential relevance of the conceptual model to other movement disorders
The range of function described here for the brain postural system also offers potential explanations for the broad spectrum of symptoms across movement disorders more generally. For example, ballism may be the opposite of a “fixed” dystonia, representing a reduction or loss of resting postural tone, while tremor may reflect an “unstable” postural program within a single component of posture. Since tremor usually is observed during only one type of kinetic or postural state, and disappears when the individual switches to another state [32], this symptom provides evidence for the mutually opposing mechanisms of tonic versus phasic components of posture hypothesized here, i.e., that these components cannot function simultaneously in any given muscle. The range of symptoms in PD also likely reflects malfunction of postural, in addition to movement, systems. For example, PD patients may have difficulty initiating movement not only because of reduced function in movement programs, but also because they must first counteract abnormally high levels of tonic/resting postural function.
Tics and chorea, in contrast, may reflect amplified function of “movement” programs. However, the sensory urge that usually precedes tics, and its reduction with botulinum toxin injections (which reduce muscle tone) [62], suggests that the tic itself (i.e. the movement) might be driven by an attempt to inhibit and thereby relieve abnormal focal increases in resting postural tone.
VI. Conclusions and future implications of the conceptual model
The conceptual model presented here proposes organizational principles of postural and motor control which aim to integrate existing knowledge about these systems at the mechanical, clinical, and neurobiological levels. Most specifically, the model aims to define what all dystonias may have in common, while offering explanations how and why the causes and manifestations of this set of disorders can be so heterogeneous. The ideas presented here are intended to establish a framework which will drive future hypothesis testing and refinement of the model. It is hoped that the resulting knowledge will improve our understanding of basic mechanisms for postural and motor control and guide development of new treatments for disorders affecting these systems.
Acknowledgments
The ideas for this conceptual model came together as the author was working on research projects funded by NINDS (R21 NS46348; A.J.B.) and a Dystonia Medical Research Foundation (DMRF) Grant (A.J.B.). Ideas were developed further during preparation of NINDS R01 NS052368 (A.J.B.) and a second DMRF grant (A.J.B.). The general infrastructure of the Martinos Center for Biomedical Imaging in which research on these grants was conducted, was supported by NCRR (P41 RR14075, B.R.R.), and the Mental Illness and Neuroscience Discovery (MIND) Institute (B.R.R.).
The author would like to thank Hans C. Breiter, M.D. for valuable discussion of the proposed conceptual model and feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Hallett M. Physiology of basal ganglia disorders: an overview. Can J Neurol Sci. 1993;20(3):177–83. doi: 10.1017/s0317167100047909. [DOI] [PubMed] [Google Scholar]
- 3.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt WJ. Balance of transmitter activities in the basal ganglia loops. J Neural Transm Suppl. 1995;46:67–76. [PubMed] [Google Scholar]
- 5.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60(10):1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 6.Fahn S. The varied clinical expressions of dystonia. Neurol Clin. 1984;2(3):541–54. [PubMed] [Google Scholar]
- 7.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117(Pt 4):859–76. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 8.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22(17):7825–33. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan EK, Chan LL, Auchus AP. Hemidystonia precipitated by acute pontine infarct. J Neurol Sci. 2005;234(1–2):109–11. doi: 10.1016/j.jns.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Bara-Jimenez W, Catalan MJ, Hallett M, Gerloff C. Abnormal somatosensory homunculus in dystonia of the hand. Ann Neurol. 1998;44(5):828–31. doi: 10.1002/ana.410440520. [DOI] [PubMed] [Google Scholar]
- 11.Jinnah HA, Hess EJ, Ledoux MS, Sharma N, Baxter MG, Delong MR. Rodent models for dystonia research: characteristics, evaluation, and utility. Mov Disord. 2005;20(3):283–92. doi: 10.1002/mds.20364. [DOI] [PubMed] [Google Scholar]
- 12.Rosenkranz K, Altenmuller E, Siggelkow S, Dengler R. Alteration of sensorimotor integration in musician’s cramp: impaired focusing of proprioception. Clin Neurophysiol. 2000;111(11):2040–5. doi: 10.1016/s1388-2457(00)00460-0. [DOI] [PubMed] [Google Scholar]
- 13.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 14.Gahery Y, Massion J. Co-ordination between posture and movement. Trends Neurosci. 1981;4:199–202. [Google Scholar]
- 15.Deliagina TG, Zelenin PV, Beloozerova IN, Orlovsky GN. Nervous mechanisms controlling body posture. Physiol Behav. 2007;92(1–2):148–54. doi: 10.1016/j.physbeh.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96(5):2678–87. doi: 10.1152/jn.00406.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ghez C. In: Principles of neural science. Posture SJ, Kandel ER, Jessel TM, editors. New York: Elsevier; 1991. pp. 567–607. [Google Scholar]
- 18.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75(6):2380–96. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 19.Whishaw IQ, Gorny B, Tran-Nguyen LT, Castaneda E, Miklyaeva EI, Pellis SM. Making two movements at once: impairments of movement, posture, and their integration underlie the adult skilled reaching deficit of neonatally dopamine-depleted rats. Behav Brain Res. 1994;61(1):65–77. doi: 10.1016/0166-4328(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 20.Henderson JM, Stanic D, Tomas D, et al. Postural changes after lesions of the substantia nigra pars reticulata in hemiparkinsonian monkeys. Behav Brain Res. 2005;160(2):267–76. doi: 10.1016/j.bbr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Shadmehr R, Mussa-Ivaldi FA, Bizzi E. Postural force fields of the human arm and their role in generating multijoint movements. J Neurosci. 1993;13(1):45–62. doi: 10.1523/JNEUROSCI.13-01-00045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci. 2005;8(4):498–504. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- 23.Massion J, Alexandrov A, Frolov A. Why and how are posture and movement coordinated? Prog Brain Res. 2004;143:13–27. doi: 10.1016/S0079-6123(03)43002-1. [DOI] [PubMed] [Google Scholar]
- 24.Lestienne F, Polit A, Bizzi E. Functional organization of the motor process underlying the transition from movement to posture. Brain Res. 1981;230(1–2):121–31. doi: 10.1016/0006-8993(81)90396-6. [DOI] [PubMed] [Google Scholar]
- 25.Cooke JD, Brown SH. Movement-related phasic muscle activation. II Generation and functional role of the triphasic pattern. J Neurophysiol. 1990;63(3):465–72. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- 26.DeLong MR. Putamen: activity of single units during slow and rapid arm movements. Science. 1973;179(79):1240–2. doi: 10.1126/science.179.4079.1240. [DOI] [PubMed] [Google Scholar]
- 27.Farmer SF, Sheean GL, Mayston MJ, et al. Abnormal motor unit synchronization of antagonist muscles underlies pathological co-contraction in upper limb dystonia. Brain. 1998;121(Pt 5):801–14. doi: 10.1093/brain/121.5.801. [DOI] [PubMed] [Google Scholar]
- 28.Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127(Pt 5):1145–58. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- 29.Marsden CD, Rothwell JC. The physiology of idiopathic dystonia. Can J Neurol Sci. 1987;14(3 Suppl):521–7. doi: 10.1017/s031716710003804x. [DOI] [PubMed] [Google Scholar]
- 30.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491–3. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 31.Lai H, Tsumori T, Shiroyama T, Yokota S, Nakano K, Yasui Y. Morphological evidence for a vestibulo-thalamo-striatal pathway via the parafascicular nucleus in the rat. Brain Res. 2000;872(1–2):208–14. doi: 10.1016/s0006-8993(00)02457-4. [DOI] [PubMed] [Google Scholar]
- 32.Parent A. In: Carpenter’s Human Neuroanatomy; Rev ed of: Human neuroanatomy. 8th ed. C1983. 9. Carpenter Malcolm B, Sutin Jerome., editors. Lippincott, Williams, and Wilkins; 1996. [Google Scholar]
- 33.Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol. 2001;439(2):162–75. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- 34.Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119(1):293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 35.Dybdal D, Gale K. Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus. J Neurosci. 2000;20(17):6728–33. doi: 10.1523/JNEUROSCI.20-17-06728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rupniak NM, Jenner P, Marsden CD. Acute dystonia induced by neuroleptic drugs. Psychopharmacology (Berl) 1986;88(4):403–19. doi: 10.1007/BF00178501. [DOI] [PubMed] [Google Scholar]
- 37.Chiu HF, Lee S. Tardive dystonia. Aust N Z J Psychiatry. 1989;23(4):566–70. doi: 10.3109/00048678909062626. [DOI] [PubMed] [Google Scholar]
- 38.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20(1):128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 39.Sanger TD. Childhood onset generalised dystonia can be modelled by increased gain in the indirect basal ganglia pathway. J Neurol Neurosurg Psychiatry. 2003;74(11):1509–15. doi: 10.1136/jnnp.74.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilinsky IA, Yi H, Kultas-Ilinsky K. Mode of termination of pallidal afferents to the thalamus: a light and electron microscopic study with anterograde tracers and immunocytochemistry in Macaca mulatta. J Comp Neurol. 1997;386(4):601–12. doi: 10.1002/(sici)1096-9861(19971006)386:4<601::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Pisani A, Martella G, Tscherter A, et al. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol Dis. 2006;24(2):318–25. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Schicatano EJ, Basso MA, Evinger C. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. J Neurophysiol. 1997;77(5):2842–6. doi: 10.1152/jn.1997.77.5.2842. [DOI] [PubMed] [Google Scholar]
- 43.Kostrzewa RM. Dopamine receptor supersensitivity. Neurosci Biobehav Rev. 1995;19(1):1–17. doi: 10.1016/0149-7634(94)00019-w. [DOI] [PubMed] [Google Scholar]
- 44.Nygaard TG, Marsden CD, Fahn S. Dopa-responsive dystonia: long-term treatment response and prognosis. Neurology. 1991;41(2 Pt 1):174–81. doi: 10.1212/wnl.41.2_part_1.174. [DOI] [PubMed] [Google Scholar]
- 45.Schneider MB, Murrin LC, Pfeiffer RF, Deupree JD. Dopamine receptors: effects of chronic L-dopa and bromocriptine treatment in an animal model of Parkinson’s disease. Clin Neuropharmacol. 1984;7(3):247–57. [PubMed] [Google Scholar]
- 46.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 47.Fountoulakis KN, Siamouli M, Kantartzis S, Panagiotidis P, Iacovides A, Kaprinis GS. Acute dystonia with low-dosage aripiprazole in Tourette’s disorder. Ann Pharmacother. 2006;40(4):775–7. doi: 10.1345/aph.1G331. [DOI] [PubMed] [Google Scholar]
- 48.Wohrle JC, Weigel R, Grips E, Blahak C, Capelle HH, Krauss JK. Risperidone-responsive segmental dystonia and pallidal deep brain stimulation. Neurology. 2003;61(4):546–8. doi: 10.1212/01.wnl.0000078032.71703.44. [DOI] [PubMed] [Google Scholar]
- 49.Kidron D, Melamed E. Forms of dystonia in patients with Parkinson’s disease. Neurology. 1987;37(6):1009–11. doi: 10.1212/wnl.37.6.1009. [DOI] [PubMed] [Google Scholar]
- 50.Alterman RL, Snyder BJ. Deep brain stimulation for torsion dystonia. Acta Neurochir Suppl. 2007;97(Pt 2):191–9. doi: 10.1007/978-3-211-33081-4_21. [DOI] [PubMed] [Google Scholar]
- 51.Romito LM, Franzini A, Perani D, et al. Fixed dystonia unresponsive to pallidal stimulation improved by motor cortex stimulation. Neurology. 2007;68(11):875–6. doi: 10.1212/01.wnl.0000256816.83036.c9. [DOI] [PubMed] [Google Scholar]
- 52.Xu ZC, Ling G, Sahr RN, Neal-Beliveau BS. Asymmetrical changes of dopamine receptors in the striatum after unilateral dopamine depletion. Brain Res. 2005;1038(2):163–70. doi: 10.1016/j.brainres.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 53.Kassem IS, Evinger C. Asymmetry of blinking. Invest Ophthalmol Vis Sci. 2006;47(1):195–201. doi: 10.1167/iovs.04-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blood AJ, Tuch DS, Makris N, Makhlouf ML, Sudarsky LR, Sharma N. White matter abnormalities in dystonia normalize after botulinum toxin treatment. Neuroreport. 2006;17(12):1251–55. doi: 10.1097/01.wnr.0000230500.03330.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Filipovic SR, Jahanshahi M, Viswanathan R, Heywood P, Rogers D, Bhatia KP. Clinical features of the geste antagoniste in cervical dystonia. Adv Neurol. 2004;94:191–201. [PubMed] [Google Scholar]
- 56.Jeka JJ, Lackner JR. The role of haptic cues from rough and slippery surfaces in human postural control. Exp Brain Res. 1995;103(2):267–76. doi: 10.1007/BF00231713. [DOI] [PubMed] [Google Scholar]
- 57.Blood A, Flaherty A, Sudarsky L, et al. Evaluation of the lerman minerva cervical orthosis for treatment of cervical and upper truncal dystonias. Mov Disord. 2005 [Google Scholar]
- 58.Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004;56(4):595–9. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- 59.Ghilardi MF, Carbon M, Silvestri G, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54(1):102–9. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- 60.Sharma N, Baxter MG, Petravicz J, et al. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J Neurosci. 2005;25(22):5351–5. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain. 2004;127(Pt 10):2360–72. doi: 10.1093/brain/awh262. [DOI] [PubMed] [Google Scholar]
- 62.Kwak CH, Hanna PA, Jankovic J. Botulinum toxin in the treatment of tics. Arch Neurol. 2000;57(8):1190–3. doi: 10.1001/archneur.57.8.1190. [DOI] [PubMed] [Google Scholar]