Abstract
Nitric oxide (NO) prodrugs such as O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K) are a growing class of promising NO-based therapeutics. Nitric oxide release from the anti-cancer lead compound, JS-K, is proposed to occur through a nucleophilic aromatic substitution by glutathione (GSH) catalyzed by glutathione S-transferase (GST) to form a diazeniumdiolate anion that spontaneously releases NO. In this study, a number of structural analogues of JS-K were synthesized and their chemical and biological properties were compared with those of JS-K. The homopiperazine analogue of JS-K showed anti-cancer activity that is comparable with that of JS-K but with a diminished reactivity towards both GSH and GSH/GST; both the aforementioned compounds displayed no cytotoxic activity towards normal renal epithelial cell line at concentrations where they significantly diminished the proliferation of a panel of renal cancer cell lines. These properties may prove advantageous in the further development of this class of nitric oxide prodrugs as cancer therapeutic agents.
1. Introduction
Nitric oxide (NO) mediates numerous and diverse physiological processes such as smooth muscle relaxation, platelet aggregation, vasodilation, neurotransmission, and immune response.1–3 Although the precise mechanisms of biological action of nitric oxide are not completely elucidated, the physiological effects of nitric oxide are dependent on its local concentration and duration of exposure.4,5 The relationship between nitric oxide and cancer is complex.6 Nitric oxide is reported to induce apoptosis and initiate differentiation in certain types of cancer cells, suggesting that NO is a potential cancer therapeutic agent with novel mechanisms of action.7–9 Nitric oxide as a cytotoxic agent is particularly relevant for drug discovery as drug resistance is emerging as an enoromous challenge in the treatment of highly invasive and potentially fatal cancers.10 The addition of therapeutic agents with unique mechanisms of action to the current crop of anti-cancer agents is highly desirable.
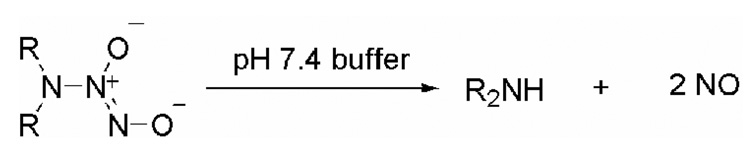
Exogenous nitric oxide donors that dissociate to form NO are frequently utilized tools in the elucidation of underlying mechanisms of NO-mediated biological processes.11 Diazeniumdiolate anions, which are complexes of nitric oxide and nucleophiles, are routinely used as reliable sources of nitric oxide.12–19 These nitric oxide prodrugs dissociate in aqueous buffer to release up to two moles of NO at predictable rates (Scheme 1).
Scheme 1.
Diazeniumdiolate ions as donors of nitric oxide
Diazeniumdiolate anions were found to be good inhibitors of human leukemia HL-60 cell proliferation in a schedule and dose-dependent manner.9 These anions can also be derivatized at the O2-position to produce potentially useful prodrug forms.20–26 The O2-protective groups can be designed to target a specific enzyme in a metabolic pathway to facilitate site-directed delivery of NO.19
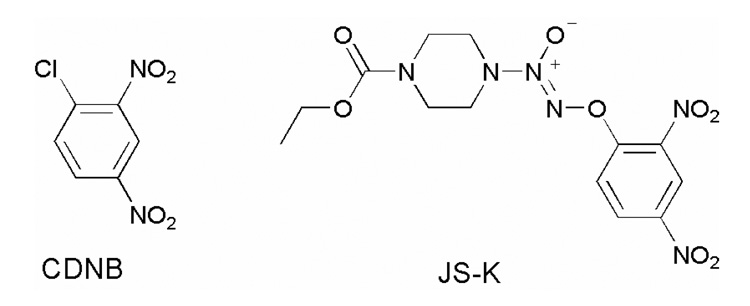
Glutathione S-transferases (GSTs), a class of phase II detoxification enzymes that are frequently over-expressed in cancers, catalyze the conjugation of xenobiotics with glutathione to facilitate their excretion.27–29 Due to its involvement in a tumor developing drug resistance, GST is an attractive molecular target for developing novel cancer therapeutics.30 Based on 1-chloro-2,4-dinitrobenzene (CDNB, Fig. 1),31 a known substrate of GST, our laboratory has developed a number of GSH/GST-activated nitric oxide prodrugs, several of which display potent anti-cancer activity against a number of cancer cell types.32–41 O2-(2,4-Dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate (JS-K, Fig. 1) is one such compound in this library that showed the highest in vitro inhibitory activity against the human leukemia HL-60 cell line (IC50 = 0.2–0.5 µM) that is comparable with that of Ara-C and superior to that of etoposide, both routinely used anti-leukemic drugs.32 Furthermore, it has shown promising anti-cancer activity in a number of in vivo xenograft studies in rodent models.32,36,39
Figure 1.
Structures of CDNB and JS-K
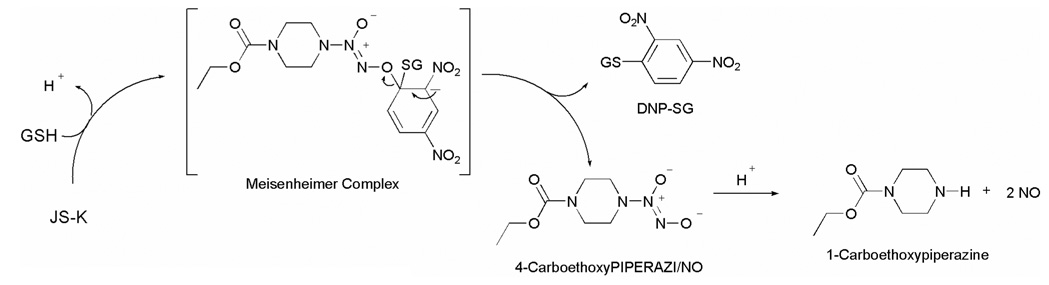
Nitric oxide release from JS-K is proposed to occur through a nucleophilic aromatic substitution by glutathione (GSH) to form a spontaneously NO-releasing diazeniumdiolate anion, a reaction that was found to be catalyzed by GST (Scheme 2). Despite a similarity in the proposed mechanism of NO release, a diverse anti-proliferative activity profile of these compounds was observed. Small structural perturbations to JS-K appear to significantly diminish the anti-proliferative activity, suggesting the involvement of discriminating cellular factors whose participation could be important as a nitric oxide-independent pathway for the observed anti-cancer activity.36 Recently, we reported that the rate of decomposition of the diazeniumdiolate anion was not a significant determinant of the anti-proliferative activity.40
Scheme 2.
Mechanism of nitric oxide release from JS-K
Due to its over-expression in cancer cells, a GSH/GST-mediated pathway would contribute to selective accumulation of NO in tumors. The origin of differences in the anti-proliferative activities among the various O2-(2,4-dinitrophenyl) diazeniumdiolates, however, remains to be fully detemined. In an attempt to address the structural dependence on anti-proliferative activity and to delineate the involvement of glutathione, glutathione S-transferase, and nitric oxide, we prepared a number of closely-related structural analogues, compared their reactivity towards GSH and three isoforms of GST, examined their anti-cancer activity against a variety of cancer cell lines and compared the results with those for the lead compound. Such a study should enable us to better understand the structural aspects of reactivity of these prodrugs towards GSH in the presence and absence of GST, and may help us elucidate the factors that contribute to the differing anti-proliferative effects among the members of this class of anti-cancer compounds.
2. Results and Discussion
2.1 Synthesis
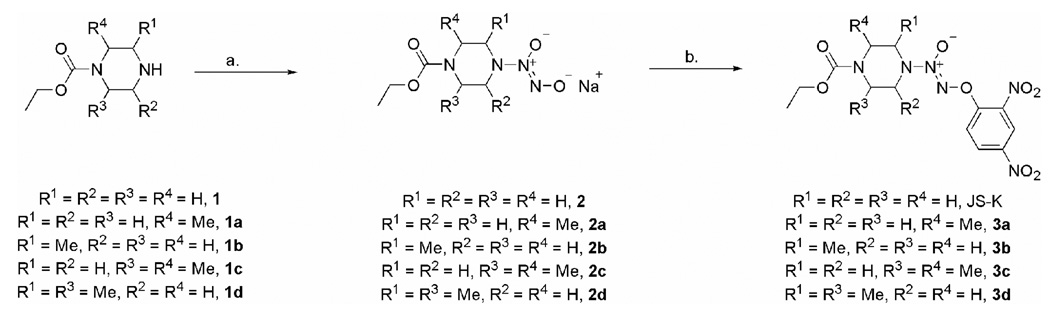
JS-K was prepared starting from 1-(ethoxycarbonyl)piperazine (Scheme 3).23 A range of differentially substituted piperazines 1–1d were either purchased or prepared using literature methods (See Experimental Section). These secondary amines were then diazeniumdiolated under standard conditions with 50 psi NO and the corresponding diazeniumdiolate anions 2a–2d were isolated as their sodium salts (See Experimental Section).
Scheme 3.
Synthesis of JS-K and compounds 3a–3d: a. NO (40-60 psi), NaOMe, MeOH; b. 1-Fluoro-2,4-dinitrobenzene, t-BuOH, 5 % aqueous NaHCO3.
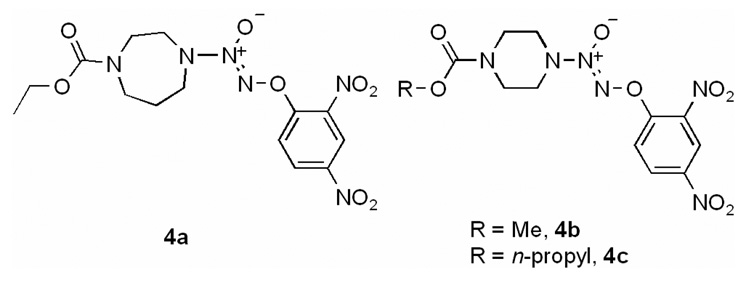
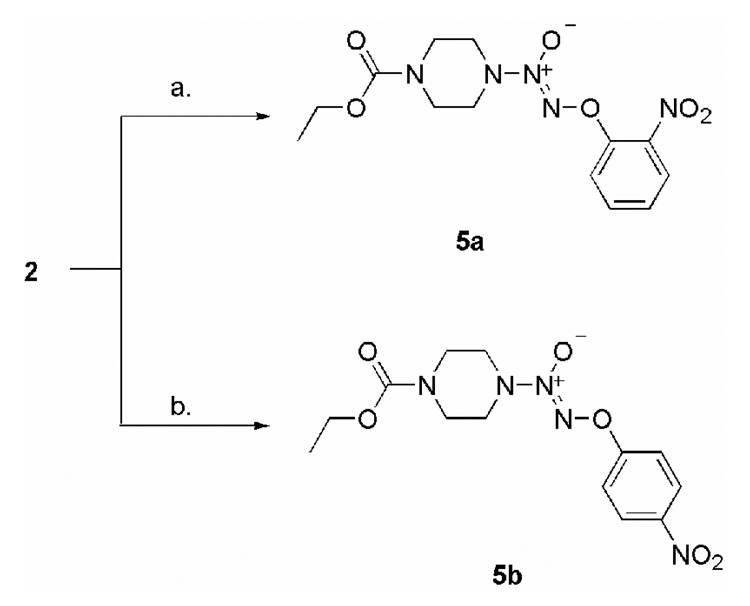
Treatment with 1-fluoro-2,4-dinitrobenzene afforded the corresponding O2-aryl derivatives 3a–3d. Compounds 4a–4c were prepared in a similar fashion using a reported method (Fig. 2).36 Finally, the JS-K analogues 5a and 5b with just one nitro group in the O2-aryl ring were synthesized by treating 2 with the corresponding fluoronitrobenzene (Scheme 4).
Figure 2.
Structures of compounds 4a, 4b, and 4c.
Scheme 4.
Synthesis of compounds 5a–5b; Conditions: a. 1-Fluoro-2-nitrobenzene, t-BuOH, 5 % aqueous NaHCO3; b. 1-Fluoro-4-nitrobenzene, t-BuOH, 5 % aqueous NaHCO3.
2.2 Decomposition studies and nitric oxide release
The rates of reaction of JS-K and its analogues with glutathione (GSH) in aqueous pH 7.4 phosphate buffered saline at 37 °C were determined (Table 1). The poor aqueous solubility of 5a prevented testing of this compound. Compound 5b was found to be unreactive to GSH under these conditions. The half-lives of JS-K, compounds 3a–3d, and 4a–4c were estimated from the rates of decomposition to be 26–52 min. The slowest among the reactive analogues was the homopiperazine derivative, 4a, while JS-K was found to be among the most labile under these conditions.
Table 1.
Decomposition of JS-K and its structural analogues in the presence of GSH and GSH/GST.
| GSTP1-1 | GSTA1-1 | GSTM1-1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Compd | moles of NO releaseda | half-life (min)b | specific activityc (µmol·mg−1min−1) | relative rate | specific activityc (µmol·mg−1min−1) | relative rate | specific activityc (µmol·mg−1min−1) | relative rate |
| JS-K | 1.7 | 30 | 1.36 | 1 | 66.3 | 1 | 126 | 1 |
| 3a | 1.0 | 44 | 1.15 | 0.85 | 55.5 | 0.84 | 112 | 0.89 |
| 3b | 1.3 | 29 | 0.88 | 0.65 | 47.0 | 0.71 | 110 | 0.87 |
| 3c | 1.75 | 26 | 1.17 | 0.86 | 70.3 | 1.06 | 172 | 1.36 |
| 3d | 1.7 | 26 | 0.14 | 0.10 | 17.6 | 0.27 | 49 | 0.39 |
| 4a | 2.0 | 52 | 0.16 | 0.12 | 16.8 | 0.25 | 38 | 0.30 |
| 4b | 1.35 | 30 | 0.98 | 0.72 | 45.1 | 0.68 | 102 | 0.81 |
| 4c | 2.0 | 27 | 1.2 | 0.88 | 59.0 | 0.89 | 126.3 | 1.0 |
Determined by measuring NO release from the compound (100–150 nM) in the presence of glutathione (1 mM) in 0.1 M pH 7.4 phosphate buffer at 37 °C by chemiluminescence analysis.
Determined by spectrophotometric analysis of the compound (33 µM) in the presence of glutathione (1 mM) in pH 7.4 phosphate buffered saline at 37 °C. Half-life is calculated by fitting the rate of decomposition to a first order process.
Determined by spectrophotometric analysis of the compound (50 µM) in the presence of GSH/GST at 37 °C
Under similar conditions, amount of nitric oxide released upon treatment with glutathione was measured for each of these analogues (Table 1). JS-K itself was found to release 1.7 moles of NO per mole (85 % yield). Quantitative yields of NO from 4a and 4c were observed, while other JS-K analogues released lower amounts of NO. Under similar conditions, when 5b, which possessed one nitro group, was treated with GSH, a negligible amount of NO was detected suggesting that one nitro group is not sufficiently activating towards nucleophilic aromatic substitution by GSH.
2.3 Enzymatic reaction of JS-K and its analogues with GSH/GST
JS-K and its analogues, 3a–3d and 4a–4c, were independently reacted with glutathione in the presence of three isoforms of human GST, P1-1, A1-1, and M1-1 (Table 1). Among the three GST isoforms tested, the M1-1 and A1-1 isoforms were found to be better catalysts for glutathione attack than the P1-1 isoform. The analogue 4a was found to be diminished in reactivity towards GSH in the presence of all the GST isoforms tested, in comparison with JS-K (Table 1). For example, the specific activity of GST P1-1 towards JS-K in the presence of GSH was 8-fold higher than that of 4a.
Of the several isoforms of GST that have been identified, the π isoform (GSTP) is by far the most interesting molecular target in cancer therapeutics due to its frequent over-expression in several tumors.27–29 None of these analogues displayed significant GSTπ selectivity but consistent activity across isoforms was observed. For example, specific activity of all isoforms of GST tested towards 3d was diminished relative to JS-K. The magnitude of the activity of 3d in comparison with that of JS-K ranged from a 10-fold diminution (GSTP1-1) to 2.5-fold (GSTM1-1). The half-life of 3d in 1 mM GSH solution, however, was 26 min, which is comparable with that of JS-K, suggesting that the half-life of the analogues was not a reliable predictor of the reactivity towards GST.
2.4 Anti-proliferative activity
The ability of JS-K and its analogues to inhibit proliferation of a number of cancer cell lines was determined. The study was initiated by testing the anti-proliferative activity of these compounds against human leukemia HL-60 and U 937 cell lines. Next, we determined the ability of these prodrugs to inhibit proliferation of: lung cancer cell lines A549 and H441; colorectal cancer cell lines HCT-116 and HCT-15; ovarian cancer cell line OVCAR-3; and prostate cancer cell line PC-3. Compound 5b was found to be inactive (IC50 >100 µM) in the U937 and HL-60 leukemia assays and was not tested further.
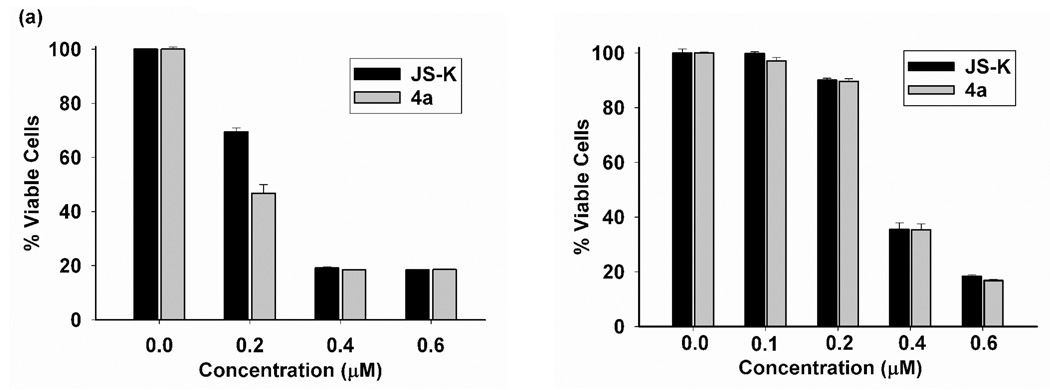
All other JS-K analogues were found to inhibit cancer cell proliferation to varying degrees (Table 2). In contrast to our earlier report,36 4a and 4b were found to be comparable in activity to JS-K against the HL-60 cell line, both IC50s being determined to be 0.2 µM. A representative comparative plot of the results of the cell viability assay shows that JS-K and 4a display nearly indentical anti-proliferative activity (Figure 3a). JS-K and 4a were also found to be especially effective at inhibiting the proliferation of human leukemia U 937 cells (Figure 3b).
Table 2.
In vitro anti-proliferative activity of JS-K and its structural analogues.
| cell line | cell type | JS-K IC50 (µM) |
3a IC50 (µM) |
3b IC50 (µM) |
3c IC50 (µM) |
3d IC50 (µM) |
4a IC50 (µM) |
4b IC50 (µM) |
4c IC50 (µM) |
|---|---|---|---|---|---|---|---|---|---|
| HL-60 | leukemia | 0.2–0.5 | 1.1 | 1.8 | 4.7 | 3.8 | 0.2 | 0.2 | 1.4 |
| U 937 | leukemia | 0.3 | 1.2 | 0.8 | 4.9 | 2.5 | 0.3 | 0.5 | 0.7 |
| H441 | lung | 1.0 | 5.5 | 4.7 | 8.1 | 7.5 | 1.0 | 1.2 | 2.4 |
| A549 | lung | 1.5 | 5.0 | 5.9 | >10 | >10 | 1.3 | 1.5 | 2.4 |
| HCT-116 | colon | 0.7 | 1.8 | 1.2 | 9.5 | 3.8 | 0.5 | 0.9 | 1.5 |
| HCT-15 | colon | 0.6 | 2.0 | 1.6 | 8.2 | 7.9 | 0.6 | 0.9 | 1.3 |
| OVCAR-3 | ovarian | 0.8 | 4.9 | 2.0 | 4.9 | 5.1 | 1.0 | 1.1 | 1.9 |
| PC-3 | prostate | 1.1 | 4.4 | 4.1 | >10 | 5.4 | 1.6 | 3.0 | 2.0 |
Figure 3.
Comparison of cell viability after treatment with JS-K and 4a as determined by MTT assay conducted on (a) HL-60 cells and (b) U937 cells.
In addition to JS-K, 4a was found to possess potent activity against lung and colorectal cancer cell lines, H441, HCT-116 and HCT-15, and the ovarian cancer cell line OVCAR-3, with 4b showing marginally lower activity. The IC50 values for JS-K and 4a were 1.1 and 1.6 µM, respectively, for the inhibition of proliferation of human prostate cancer PC-3 cells.
Despite their structural similarity, other O2-(2,4-dinitrophenyl) analogues were generally lower in potency in comparison with JS-K, 4a or 4b in all the cancer cell lines tested. The addition of two methyl groups at the piperazine ring as in 3c and 3d appears to significantly diminish the activity in comparison with one methyl group on the piperazine ring or altering the length of the carbamate group. No correlation between nitric oxide yields and anti-proliferative activity was observed. For example, the compounds that formed quantitative amounts of NO, 4a and 4c, both displayed excellent cytotoxicity against a number of cancer cell lines. However, 3c, 3d and JS-K formed comparable amounts of NO in the presence of GSH but showed different activities against the battery of cancer cell lines. The kinetics of reaction with both GSH and GSH/GST provided limited insight into the cytotoxicity of these compounds. For example, 3d and 4a were both similar in reactivity towards all isoforms of GST tested but in contrast, their anti-proliferative activity was quite different.
The prolonged half-life of 4a upon treatment with GSH in comparison with that of JS-K suggested a diminution in reactivity of the aromatic ring towards nucleophilic substitution. This notable feature may facilitate selective accumulation of the prodrug in cancer tissue by disfavoring reaction with free glutathione in the blood stream. Docking studies of these two compounds with GST (data not shown) do not predict large differences in binding energies, which is in agreement with comparative enzyme kinetics studies of these two compounds (Table 3). The kcat/Km of 4a was only marginally lower than that of JS-K, which may not be the major determinant for the difference in reactivity towards nucleophilic substitution by GSH/GST.
Table 3.
Enzyme kinetics of GSH/GST reaction with JS-K and 4a.
| GST isoform |
kcat/Km (mM−1s−1) JS-K |
kcat/Km (mM−1s−1) 4a |
|---|---|---|
| P1-1 | 1.5 | 1.3 |
| A1-1 | 20 | 12 |
| M1-1 | 62 | 42 |
The in vitro anti-proliferative activity of JS-K and 4a were further compared using additional cell lines: human lung cancer H1395 and H838; human colon cancer cell lines HT-29 and CaCo-2; human ovarian cancer OVCAR-5; and human renal cancer TK-10, 786-O, and UO-30 cell lines (Table 4). Both these compounds were found to exhibit almost identical cytotoxic activity against these cancer cell lines.
Table 4.
In vitro anti-proliferative activity of JS-K and 4a.
| Cell line | Type | IC50 (µM) JS-K |
IC50 (µM) 4a |
|---|---|---|---|
| H1395 | lung cancer | 0.8 | 0.7 |
| H838 | lung cancer | 4.3 | 5.1 |
| HT-29 | colon cancer | 0.8 | 0.8 |
| CaCo-2 | colon cancer | 2.6 | 1.0 |
| OVCAR-5 | ovarian cancer | 1.0 | 0.8 |
| TK-10 | renal cancer | 0.5 | 0.6 |
| 786-O | renal cancer | 0.8 | 1.0 |
| UO-30 | renal cancer | 0.9 | 1.0 |
| HREpC | normal renal epithelial | 11.3 | 13.5 |
| BJ-5ta | normal skin fibroblasts | 10.0 | 10.4 |
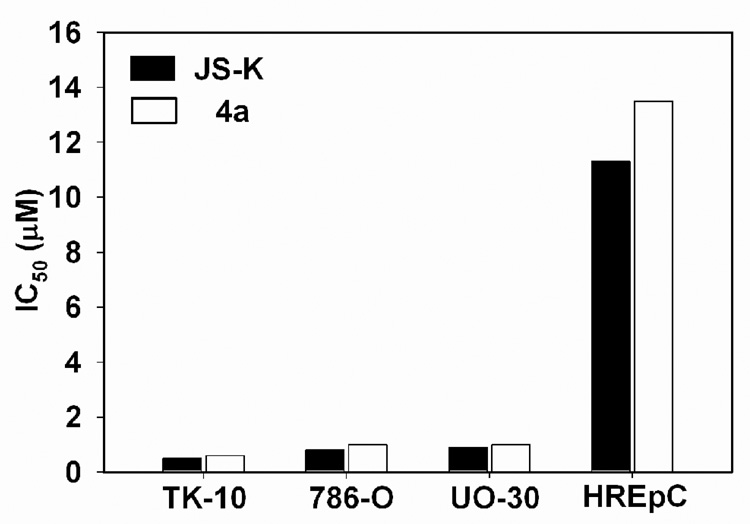
Finally, in order to determine if these compounds were cytotoxic towards normal cell lines, we determined the inhibitory activity against the human renal epithelial cell line, HREpC, and human skin fibroblasts, BJ-5ta. A therapeutic window of roughly an order of magnitude in concentration for the use of JS-K and 4a in the treatment of renal cancers is estimated (Figure 4). The IC50 of JS-K and 4a towards BJ-5ta was found to be 10.0 and 10.4 mM, respectively. These observations are consistent with a previous report where JS-K was found to have selective cytotoxicity towards multiple myeloma cells relative to normal bone marrow stromal cells.39
Figure 4.
Comparative study of the inhibitory activity of JS-K and 4a against a panel of renal cancer cell lines, TK-10, 786-O, UO-30, and a normal renal epithelial cell line, HREpC.
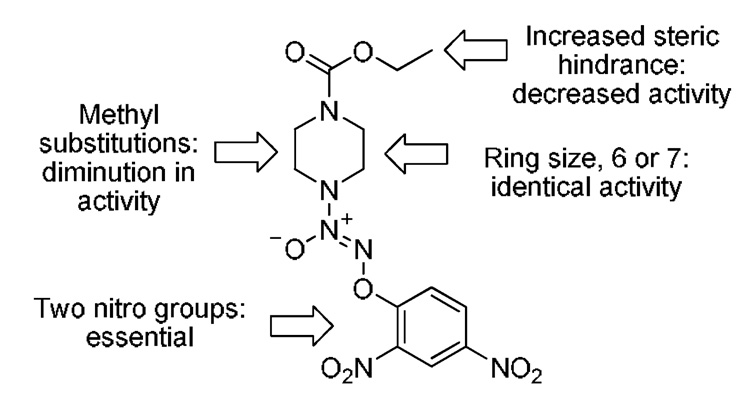
Based on this and previous studies, a summary of the effect of structural modifications on the anti-proliferative activity of this class of compounds is presented in Figure 5. First, two nitro groups in the aromatic ring are required for any observable anti-proliferative activity. Next, a piperazine or a homopiperazine ring with a carboethoxy group shows nearly identical activity. The addition of one or two methyl groups at the piperazine ring or any sterically hindered group on the carbamate resulted in diminished inhibitory potency as compared with JS-K.
Figure 5.
Effect of structural modifications on anti-proliferative activity of O2-(2,4-dinitrophenyl) diazeniumdiolates.
3. Conclusion
Despite the structural similarity of the anti-cancer lead compound JS-K and closely related O2-(2,4-dinitrophenyl) diazeniumdiolates, their anti-proliferative activities differ. In a majority of the cases, the reactivity of these analogues with GSH, their nitric oxide release profile and the specific activity of GSH/GST in catalyzing their dissociation do not appear to significantly affect their efficacy as inhibitors of cancer cell proliferation. Through this study we have identified a closely related analogue, 4a, that displays comparable anti-cancer activity to that of JS-K in a number of cancer cell lines but with a diminished reactivity towards glutathione and GSH/GST that may prove advantageous in the further development of this class of anti-cancer agents. Taken together, the involvement of cellular factors and pathways other than nitric oxide and GSH/GST in the mechanism of action, such as transfer of the dinitrophenyl ring to the attacking nucleophile (which may be the thiol group of a mechanistically important protein), is suggested. Finally, JS-K and 4a were selectively toxic towards renal cancer cell lines at concentrations that did not significantly affect the proliferation of normal renal epithelial cells.
4. Experimental Section
4.1 General
Nitric oxide gas was purchased from Matheson Gas Products (Montgomeryville, PA). Starting materials were purchased from Aldrich Chemical Co. (Milwaukee, WI) unless otherwise indicated. NMR spectra were recorded on a Varian UNITY INOVA spectrometer; chemical shifts (δ) are reported in parts per million (ppm) downfield from tetramethylsilane. Ultraviolet (UV) spectra were recorded on an Agilent Model 8453 or a Hewlett-Packard model 8451A diode array spectrophotometer. Elemental analyses were performed by Midwest Microlab (Indianapolis, IN). Nitric oxide measurements were performed using a Sievers Nitric Oxide Analyzer (NOA), model 280i (Instruments Business Group, Boulder, CO). Unless otherwise specified, compounds 1a,42 1b,43 1d,44 JS-K,23 4a,36 4b,36 and 4c36 were prepared by reported methods.
4.2 Synthesis and Characterization
General procedure for diazeniumdiolation
Unless otherwise specified, the following procedure was used to prepare the diazeniumdiolate anions. A solution of the amine in methanol (3 mL), was treated with an equimolar amount of 25 % methanolic sodium methoxide and ether (9 mL). The resulting solution was charged with 50 psi of NO and stirred overnight. A solid precipitate that resulted was collected by filtration, washed with ether and dried under vacuum to afford the desired product.
General procedure for arylation of diazeniumdiolate anions
Unless otherwise specified, the following procedure was followed to synthesize the O2-(2,4-dinitrophenyl)diazeniumdiolates and 5a and 5b. The diazeniumdiolate salt was dissolved in ice cold 5 % aqueous sodium bicarbonate solution (10 mL), and this mixture was treated with a slurry of fluoronitroarene in t-BuOH (5 mL). A yellow precipitate resulted, which was collected by filtration and aqueous washing. Recrystallization from ethanol afforded the desired product.
(2-Methyl-1-ethoxycarbonyl)piperazine (1a)
A solution of 2-methylpiperazine (15.7 g, 0.157 mol) in dichloromethane (250 mL) was cooled to 0 °C and triethylamine (55.7 mL, 0.4 mol) was added. A solution of ethyl chloroformate (30 mL, 0.314 mol) in dichloromethane (100 mL) was added dropwise; Triethylamine hydrochloride precipitated as the reagent was being added. After stirring overnight at room temperature, the mixture was filtered. The filtrate was extracted with 3 M HCl, and the organic layer was dried over sodium sulfate and evaporated to give methyl-1,4-bis(ethoxycarbonyl) piperazine (25.4 g, 66 % yield) as an oil, which was used in the next step to prepare 2-methyl-1-ethoxycarbonyl piperazine 1a. A solution of 2-methyl-1,4-bis-ethoxycarbonyl piperazine (9.2 g, 0.038 mol) and potassium hydroxide (35 g) in ethanol (100 mL) containing water (20 mL) was refluxed for 2 h. The solution was allowed to cool to room temperature, concentrated under vacuum and extracted with dichloromethane. The organic layer was extracted with 10 % hydrochloric acid. The resulting aqueous solution was made basic with sodium hydroxide and extracted with dichloromethane. The solution was dried over sodium sulfate, filtered through magnesium sulfate and evaporated to give a pale yellow oil. Vacuum distillation afforded 1a (3.7 g, 57 % yield) as a colorless liquid. The analytical data for this compound were consistent with the reported values for 1a, which was prepared using a different method.42
(3-Methyl-1-ethoxycarbonyl)piperazine (1b)
To a solution of 2-methylpiperazine (12.3 g, 0.123 mol), 12 N HCl (10 mL) was added and the mixture was cooled to 0 °C. To this, a solution of ethyl chloroformate (11.8 mL, 0.123 mol) in ethanol (25 mL) was added dropwise over 15 min. The reaction mixture was allowed to warm up to room temperature and stirred overnight. The resulting aqueous solution was washed with dichloromethane, made basic with 1 M sodium hydroxide solution and extracted with dichloromethane. The organic layer was separated and dried (sodium sulfate), filtered and the solvent was removed under reduced pressure to afford 1b (3.6 g) as an oil, whose analytical data matched those of a previous report that used a different method for synthesis of 1b.43 It was used in the preparation of 2b.
(2,6-Dimethyl-1-ethoxycarbonyl)piperazine (1c)
A solution of 2,6-dimethylpiperazine (5 g, 0.044 mol) in dichloromethane (50 mL) was cooled to 0 °C in an ice-bath and treated with triethylamine (21 mL, 0.15 mol). A solution of ethylchloroformate (8.64 mL, 0.09 mol) in dichloromethane (50 mL) was added dropwise under nitrogen. The mixture was allowed to warm up to room temperature and stirred for 2 h. The white precipitate was removed by filtration. The filtrate was washed with dilute hydrochloric acid followed by 5 % sodium bicarbonate. The solution was filtered through a layer of magnesium sulfate and concentrated under vacuum to give 4.5 g of an amber liquid that was purified by vacuum distillation to afford (2,6-dimethyl-1,4-bis-ethoxycarbonyl)piperazine (2.8 g, 25 % yield) as a colorless liquid: bp 124–125 °C (0.76 mmHg); 1H NMR (CDCl3) δ 1.23 (d, 6H, J = 6.9 Hz), 1.27 (t, 3H, J = 7.1 Hz), 1.28 (t, 3H, J = 7.1 Hz), 3.08 (broad, 2H), 3.92–3.99 (m, 2H), 4.14–4.19 (q, 4H, J = 7.1 Hz), 4.19–4.23 (m, 2H); 13C NMR δ 14.6, 20.1, 47.4, 47.8, 61.3, 61.5, 155.4, 155.3. Anal. Calcd for C12H22N2O4: C, 55.80, H, 8.58, N, 10.84. Found: C, 55.76, H, 8.29, N, 10.93.
A solution of (2,6-dimethyl-1,4-bisethoxycarbonyl) piperazine (4.5 g, 0.0174 mol) in ethanol (50 mL) and water (10 mL) containing potassium hydroxide (16.2 g, 0.3 mol) was refluxed for 2 h. After the solution cooled to room temperature, it was diluted with water and extracted with dichloromethane. The solution was extracted with 3 M HCl and the organic layer was discarded. The aqueous layer was made basic with sodium hydroxide and extracted with dichloromethane. The organic layer was dried over sodium sulfate, filtered and concentrated on a rotary evaporator to give 1.2 g (59 % yield) of 1c as a colorless liquid, which was used in the preparation of 2c without further purification: 1H NMR (CDCl3) δ 1.26 (t, 3H, J = 6.4 Hz), 1.28 (d, 6H, J = 6.8 Hz), 2.83 (broad, 4H), 4.05–4.09 (m, 2H), 4.16 (q, 2H, J = 7.1 Hz).
Sodium 1-[(4-Ethoxycarbonyl)-3-methylpiperazin-1-yl]diazen-1-ium-1,2-diolate (2a)
UV (0.1 M NaOH) λmax (ε) 251 nm (7.3 mM−1cm−1); 1H NMR (D2O, NaOD) δ 1.27 (t, 3H, J = 7.1 Hz), 1.35 (d, 3H, J = 6.9 Hz), 3.04–3.08 (m, 2H), 3.14–3.21 (m, 2H), 3.35–3.43 (m, 1H), 4.07–4.10 (m, 1H), 4.17 (q, 2H, J = 7.1 Hz), 4.51–4.54 (m, 1H); 13C NMR (D2O, NaOD) δ δ16.6, 17.9, 41.1, 50.1, 54.4, 58.6, 65.6, 159.7.
Sodium 1-[(4-Ethoxycarbonyl)-2-methylpiperazin-1-yl]diazen-1-ium-1,2-diolate (2b)
UV (0.01 M NaOH) λmax (ε) 250 nm (9.0 mM−1cm−1); 1H NMR (D2O, NaOD) δ 0.95 (d, 3H, J = 6.1 Hz), 1.27 (t, 3H, J = 7.1 Hz), 2.79–2.86 (m, 1H), 3.08–3.22 (m, 4H), 4.14–4.19 (m, 4H); 13C NMR (D2O, NaOD) δ 16.6, 17.4, 45.5, 51.4, 54.3, 57.8, 65.6, 159.7.
Sodium 1-[(4-Ethoxycarbonyl)-3,5-dimethylpiperazin-1-yl]diazen-1-ium-1,2-diolate (2c)
UV (0.01 M NaOH) λmax (ε) 252 nm (7.6 mM−1cm−1); 1H NMR (D2O, NaOD), δ 1.28 (t, 3H, J = 7.1 Hz), 1.42 (d, 6H, J = 6.9 Hz), 3.07–3.18 (m, 4H), 4.15–4.20 (q, 2H, J = 7.1 Hz), 4.39–4.46 (m, 2H); 13C NMR (D2O, NaOD) δ 16.7, 22.6, 50.4, 58.8, 65.4, 159.9.
Sodium 1-[(4-Ethoxycarbonyl)-2,5-dimethylpiperazin-1-yl]diazen-1-ium-1,2-diolate (2d)
UV (0.01 M NaOH) λmax (ε) 251 nm (9.5 mM−1cm−1); 1H NMR (D2O, NaOD), δ 0.99 (t, 3H, J = 7.1 Hz), 1.42 (d, 6H, J = 6.9 Hz), 3.07–3.18 (m, 4H), 4.15–4.20 (q, 2H, J = 7.1 Hz), 4.39–4.46 (m, 2H); 13C NMR (D2O, NaOD) δ 14.6, 16.65, 18.45, 46.8, 51.3, 53.3, 56.8, 65.4, 160.5.
O2-(2,4-Dinitrophenyl) 1-[(4-Ethoxycarbonyl)-3-methylpiperazin-1-yl]diazen-1-ium-1,2-diolate (3a)
mp 110–111 °C; UV (ethanol) λmax (ε) 298 nm (17.1 mM−1cm−1); 1H NMR (CDCl3), δ 1.29 (t, 3H, J = 7.1 Hz), 1.36 (d, 3H, J = 6.8 Hz), 3.01–3.09 (m, 1H), 3.17 (dd, 3H, J = 4.1, 11.4 Hz), 3.33–3.40 (m, 1H), 3.49–3.51 (m, 1H), 3.67–3.75 (m, 4H), 4.16–4.28 (m, 2H), 4.61–4.62 (m, 2H), 7.71 (d, 1H, J = 9.3 Hz), 8.47 (dd, 1H, J = 2.7, 9.3 Hz), 8.88 (d, 1H, J = 2.7 Hz); 13C NMR (CDCl3) δ 14.6, 15.8, 37.7, 46.8, 50.8, 54.9, 62.0, 117.7, 122.1, 129.1, 137.6, 142.4, 153.7, 154.8. Anal. Calcd for C14H18N6O8: C, 42.21, H, 4.51, N, 21.10. Found: C, 42.06, H, 4.49, N, 20.97.
O2-(2,4-Dinitrophenyl) 1-[(4-Ethoxycarbonyl)-2-methylpiperazin-1-yl]diazen-1-ium-1,2-diolate (3b)
mp 105–106 °C; UV (ethanol) λmax (ε) 297 nm (16.5 mM−1cm−1); 1H NMR (CDCl3), δ 1.15 (d, 3H, J = 6.3 Hz), 1.29 (t, 2H, J = 7.1 Hz), 3.35–3.37 (m, 1H), 3.49–3.51 (m, 1H), 3.67-3.75 (m, 4H), 4.16–4.28 (m, 2H), 4.61–4.62 (m, 2H), 7.71 (d, 1H, J = 9.3 Hz), 8.47 (dd, 1H, J = 2.7, 9.3 Hz), 8.89 (d, 1H, J = 2.7 Hz); 13C NMR (CDCl3) δ 10.5, 13.0, 14.6, 42.3, 48.0, 48.8, 54.7, 62.0, 118.1, 122.15, 129.1, 137.6, 142.7, 153.5, 155.2. Anal. Calcd for C14H18N6O8: C, 42.21, H, 4.55, N, 21.10. Found: C, 42.16, H, 4.51, N, 21.12.
O2-(2,4-Dinitrophenyl) 1-[(4-Ethoxycarbonyl)-3,5-dimethylpiperazin-1-yl]diazen-1-ium-1,2-diolate (3c)
mp 133–134 °C; UV (ethanol) λmax (ε) 297 nm (11.2 mM−1cm−1); 1H NMR (CDCl3) δ 1.30 (t, 3H, J = 7.1 Hz), 1.43 (d, 6H, J = 6.9 Hz), 3.12–3.16 (dd, 2H, J = 4.5, 11.3 Hz, axial) 4.06–4.09 (m, 2H, equatorial), 4.17–4.23 (q, 2H, J = 7.1 Hz), 4.51–4.58 (m, 2H), 7.69 (d, 1H, J = 9.3 Hz), 8.46–8.49 (dd, 1H, J = 2.7, 9.3 Hz), 8.88 (d, 1H, J = 7.6 Hz); 13C NMR (CDCl3) δ 14.6, 20.6, 47.1, 55.3, 61.8, 117.8, 122.2, 129.1, 137.0, 142.5, 153.65, 154.9. Anal. Calcd for C15H20N6O8: C, 43.69, H, 4.89, N, 20.38. Found: C, 43.46, H, 4.78, N, 19.98.
O2-(2,4-Dinitrophenyl) 1-[(4-Ethoxycarbonyl)-2,5-dimethylpiperazin-1-yl]diazen-1-ium-1,2-diolate (3d)
mp 133–134 °C; UV (ethanol) λmax (ε) 301 nm (14.8 mM−1cm−1); 1H NMR (CDCl3), δ 1.81 (d, 3H, J = 6.5 Hz), 1.29 (t, 3H, J = 7.1 Hz), 1.34 (d, 3H, J = 6.8 Hz), 3.46–3.54 (m, 2H), 3.76–3.81 (m, 1H), 3.98–4.02 (m, 1H), 4.15–4.24 (m, 2H), 4.61–4.62 (m, 2H), 7.63 (d, 1H, J = 9.3 Hz), 8.47 (dd, 1H, J = 2.7, 9.3 Hz), 8.88 (d, 1H, J = 2.7 Hz); 13C NMR (CDCl3) δ 10.5, 14.6, 16.1, 54.5, 46.2, 47.9, 53.0, 61.9, 117.6, 122.2, 129.0, 137.4, 142.3, 153.9, 155.5. Anal. Calcd for C15H20N6O8: C, 43.69, H, 4.89, N, 20.38. Found: C, 43.64, H, 4.89, N, 20.39.
O2-(2-Nitrophenyl) 1-[(4-Ethoxycarbonyl)piperazin-1-yl)]diazen-1-ium-1,2-diolate (5a)
The general procedure for arylation was used except that the desired product was isolated after flash chromatography as a yellow oil: UV (ethanol) λmax (ε) 251 nm (10.4 mM−1cm−1); 1H NMR (CDCl3) δ 1.28 (t, J = 7.2 Hz, 3H), 3.54–3.57 (m, 4H), 3.69–3.71 (m, 4H), 4.17 (q, J = 7.2 Hz, 2H), 7.25–7.30 (m, 1H), 7.46 (dd, J = 1.2 Hz, 8.4 Hz, 1H), 7.58–7.62 (m, 1H), 7.96 (dd, J = 1.6 Hz, 8.0 Hz, 1H); 13C NMR δ 14.6, 42.3, 50.8, 62.0, 119.0, 124.6, 125.9, 134.3, 139.3, 149.3, 155.0. Anal. Calcd for C13H17N6O8: C, 46.02, H, 5.05, N, 20.64. Found: C, 46.09, H, 5.06, N, 20.45.
O2-(4-Nitrophenyl) 1-[(4-Ethoxycarbonyl)piperazin-1-yl)]diazen-1-ium-1,2-diolate (5b)
mp 156–157 °C; UV (ethanol) λmax (ε) 305 nm (13.5 mM−1cm−1); 1H NMR (CDCl3) δ 1.29 (t, J = 7.2 Hz, 3H), 3.57–3.59 (m, 4H), 3.69–3.72 (m, 4H), 4.16 (q, J = 7.2 Hz, 2H), 7.28–7.32 (m, 2H), 8.22–8.25 (m, 2H); 13C NMR (CDCl3) δ 14.6, 42.3, 50.8, 62.0, 115.1, 125.8, 143.8, 155.0, 160.7. Anal. Calcd for C13H17N5O6·0.25 H2O: C, 45.22, H, 5.15, N, 20.28. Found: C, 45.37, H, 5.16, N, 20.28.
4.3 Kinetics of decomposition in the presence of GSH
In a quartz cuvette, 3 mL of 1 mM glutathione in pH 7.4 phosphate buffered saline and the O2-(2,4-dinitrophenyl) diazeniumdiolate (10 µL, 10 mM) were placed. The changes in absorbance maxima (roughly 300 nm) and 340 nm (corresponding to DNP-SG) were monitored over 2 h. Half-lives were calculated from the rate constant, which was obtained by fitting the decomposition curve to a first order equation.
4.4 Nitric oxide release in the presence of GSH
Calibration of the Nitric Oxide Analyzer was performed by injection of various volumes of known concentrations of NO/He (50 ppm, 500 ppm and 5 %) certified standards into the reaction chamber and recording the peaks. Samples and reaction chambers were incubated at 37 °C and the contents were sparged with argon and swept into the chemiluminescence detector. Data were recorded using Agilent Chemstation software and processed using Microsoft Excel. Approximately 3 mL of pH 7.4 buffer containing 1 mM GSH was placed into the reaction chamber and then sparged for several minutes with argon. A solution of the analyte in 50 µM diethylenetriaminepentaacetic acid (DTPA) prepared in pH 7.4 buffer was injected into the reaction chamber and nitric oxide release was recorded. Total amount of NO released was determined by measuring the area under the curve.
4.5 Specific activity of human GSTs (A1, M1 and P1) towards JS-K and its analogues
Purified preparations of recombinant hGSTA1-1 and hGSTM1-1 were obtained from Invitrogen (Carlsbad, CA). hGSTP1-1 was purified using a reported method.45,46 The activity of human GST toward CDNB was determined as previously described,45,46 to ensure that the enzyme preparations were catalytically active. For activity measurements toward the compounds, the reaction mixture in a final volume of 1 mL contained 0.1 M phosphate buffer (pH 7.4), 1 mM GSH, 50 µM of compound, and a catalytic amount of hGSTA1-1, hGSTM1-1 or hGSTP1-1. The reaction was started by addition of compound (5 µL, 10 mM), and the rate of reaction was monitored by measuring decrease in absorbance, for example of JS-K at 298 nm. The specific activity toward each of these compounds was calculated using the change in extinction coefficient at their absorbance maxima. Earlier, we reported the specific activity of JS-K under different reaction conditions (pH 6.5, 25 °C).32 The relative values of specific activities of JS-K in this study are comparable to those in the previous study.
Determination of kcat/Km
Enzyme kinetics experiments were performed at varying concentrations of compounds (1–20 µM) and the other conditions were same as mentioned above. The parameters were calculated by using Michaelis-Menten equation using SigmaPlot.
4.6 Cell culture and cytotoxicity assays
Cell lines HL-60, U937, H441 and A549 were obtained from American Type Culture Collection (ATCC, Manassas, VA) and CaCo-2, H1395, H838, HCT-116, HCT-15, HT- 29, OVCAR-3, OVCAR-5, and PC-3 were from NCI-Frederick DCTD Tumor/Cell Line Repository (Frederick, MD). Cell lines TK-10, 786-O, UO-3O, HREpC, and BJ-5ta were obtained from other laboratories at the National Cancer Insitute at Frederick. All cells except BJ-5ta were maintained in RPMI 1640 medium (Gibco, Invitrogen, Carlsbad, CA) supplemented with 10 % fetal calf serum (Gemini Bio-Products, Sacramento, CA), 100 U/mL penicillin and 2 mM glutamine, at 37 °C and 5 % CO2. BJ-5ta cells were maintained in DMEM(Gibco, Invitrogen, Carlsbad, CA):199 (Sigma) medium (4:1) supplemented with 10 % fetal calf serum (Gemini Bio-Products, Sacramento, CA), 100 U/mL penicillin and 2 mM glutamine.
The CellTiter 96 non-radioactive cell proliferation assay (MTT assay, Promega, Madison, WI), performed according to the manufacturer's protocol, was used to measure cell growth. Cells were seeded in 96-well plates at the density 104 per well and allowed to grow for 24 h before addition of the drugs. Diazeniumdiolate prodrugs were prepared as 10 mM stock solution in DMSO (Sigma, St. Louis, MO). Increasing drugs concentrations in 10 µL of PBS were added to 100 µL of the culture medium for 72 h. Each compound concentration was represented in six repeats, and the screening was performed as at least two independent experiments.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, as well as by National Cancer Institute contract N01-CO-12400 to SAIC-Frederick. We are grateful to Dr. James Phang, Metabolism and Cancer Susceptibility Section, NCI and Ms. Susan Kenney, NCI-Frederick Screening Technology Branch for providing us cell lines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furchgott RF. Angew. Chem. Int. Ed. Engl. 1999;38:1870–1880. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1870::AID-ANIE1870>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ. Angew. Chem. Int. Ed. Engl. 1999;38:1882–1892. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1882::AID-ANIE1882>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Murad F. Angew. Chem. Int. Ed. Engl. 1999;38:1856–1868. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1856::AID-ANIE1856>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Wink DA, Mitchell JB. Free Rad. Biol. Med. 2003;34:951–954. doi: 10.1016/s0891-5849(02)01362-x. [DOI] [PubMed] [Google Scholar]
- 5.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 6.Wink DA, Mitchell JB. Free Rad. Biol. Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 7.Magrinat G, Mason SN, Shami PJ, Weinberg JB. Blood. 1992;80:1880–1884. [PubMed] [Google Scholar]
- 8.Shami PJ, Moore JO, Gockerman JP, Hathorn JW, Misukonis MA, Weinberg JB. Leukemia Res. 1995;19:527–533. doi: 10.1016/0145-2126(95)00013-e. [DOI] [PubMed] [Google Scholar]
- 9.Shami PJ, Sauls DL, Weinberg JB. Leukemia. 1998;12:1461–1466. doi: 10.1038/sj.leu.2401131. [DOI] [PubMed] [Google Scholar]
- 10.Bonavida B, Khineche S, Huerta-Yepez S, Garban H. Drug Res. Updates. 2006;9:157–173. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ. Chem. Rev. 2002;102:1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 12.King SB. Free Rad. Biol. Med. 2004;37:735–736. doi: 10.1016/j.freeradbiomed.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Thatcher GR. J. Curr. Top. Med. Chem. 2005;5:597–601. doi: 10.2174/1568026054679281. [DOI] [PubMed] [Google Scholar]
- 14.Megson IL. Drugs Fut. 2000;25:701–715. [Google Scholar]
- 15.Scatena R, Bottoni P, Martorana GE, Giardina B. Expert Opin. Investig. Drugs. 2005;14:835–846. doi: 10.1517/13543784.14.7.835. [DOI] [PubMed] [Google Scholar]
- 16.Keefer LK, Nims RW, Davies KM, Wink DA. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 17.Hrabie JA, Keefer LK. Chem. Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 18.Keefer LK. Annu. Rev. Pharmacol. Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 19.Keefer LK. Curr. Top. Med. Chem. 2005;5:625–636. doi: 10.2174/1568026054679380. [DOI] [PubMed] [Google Scholar]
- 20.Saavedra JE, Dunams TM, Flippen-Anderson JL, Keefer LK. J. Org. Chem. 1992;57:6134–6138. [Google Scholar]
- 21.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. J. Med. Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 22.Saavedra JE, Shami PJ, Wang LY, Davies KM, Booth MN, Citro ML, Keefer LK. J. Med. Chem. 2000;43:261–269. doi: 10.1021/jm9903850. [DOI] [PubMed] [Google Scholar]
- 23.Saavedra JE, Srinivasan A, Bonifant CL, Chu J, Shanklin AP, Flippen-Anderson JL, Rice WG, Turpin JA, Davies KM, Keefer LK. J. Org. Chem. 2001;66:3090–3098. doi: 10.1021/jo0016529. [DOI] [PubMed] [Google Scholar]
- 24.Cai TB, Lu D, Landerholm M, Wang PG. Org. Lett. 2004;6:4203–4206. doi: 10.1021/ol048397p. [DOI] [PubMed] [Google Scholar]
- 25.Showalter BM, Reynolds MM, Valdez CA, Saavedra JE, Davies KM, Klose JR, Chmurny GN, Citro ML, Barchi JJ, Jr, Merz SI, Meyerhoff ME, Keefer LK. J. Am. Chem. Soc. 2005;127:14188–14189. doi: 10.1021/ja054510a. [DOI] [PubMed] [Google Scholar]
- 26.Chakrapani H, Showalter BM, Kong L, Keefer LK, Saavedra JE. Org. Lett. 2007;9:3409–3412. doi: 10.1021/ol701419a. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong RN. Chem. Res. Toxicol. 1991;4:131–140. doi: 10.1021/tx00020a001. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong RN. Adv. Enzymol. 1994;69:1–44. doi: 10.1002/9780470123157.ch1. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong RN. Chem. Res. Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 30.Townsend DM, Tew KD. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keen JH, Habig WH, Jakoby WB. J. Biol. Chem. 1976;251:6183–6188. [PubMed] [Google Scholar]
- 32.Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV, Gu Y, Fox SD, Buzard GS, Citro ML, Waterhouse DJ, Davies KM, Ji X, Keefer LK. Mol. Cancer Ther. 2003;2:409–417. [PubMed] [Google Scholar]
- 33.Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI. J. Cell. Physiol. 2003;197:426–434. doi: 10.1002/jcp.10380. [DOI] [PubMed] [Google Scholar]
- 34.Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, Ji X, Tew KD. Mol. Pharmacol. 2004;65:1070–1079. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Li C, Qu W, Leslie E, Bonifant CL, Buzard GS, Saavedra JE, Keefer LK, Waalkes MP. Mol. Cancer Ther. 2004;3:709–714. [PubMed] [Google Scholar]
- 36.Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. J. Med. Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 37.Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K, Wilde TC, Citro ML, Cuellar M, Deschamps JR, Parrish D, Shami PJ, Findlay VJ, Townsend DM, Tew KD, Singh S, Jia L, Ji X, Keefer LK. J. Med. Chem. 2006;49:1157–1164. doi: 10.1021/jm050700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udupi V, Yu M, Malaviya S, Saavedra JE, Shami PJ. Leukemia Res. 2006;30:1279–1283. doi: 10.1016/j.leukres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li C-Q, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakrapani H, Goodblatt MM, Udupi V, Malaviya S, Shami PJ, Keefer LK, Saavedra JE. Bioorg. Med. Chem. Lett. 2008;18:950–953. doi: 10.1016/j.bmcl.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakrapani H, Wilde TC, Citro ML, Goodblatt MM, Keefer LK, Saavedra JE. Bioorg. Med. Chem. 2008;16:2657–2664. doi: 10.1016/j.bmc.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kushner S, Brancone LM, Hewitt RI, McEwen WL, Subbarow Y, Stewart HW, Turner RJ, Denton JJ. J. Org. Chem. 1948;13:144–153. doi: 10.1021/jo01159a019. [DOI] [PubMed] [Google Scholar]
- 43.Charles ES, Sharma S. Indian J. Chem., Sec. B. 1987;26B:752–756. [Google Scholar]
- 44.Bishop MJ, Garrido DM, Boswell GE, Collins MA, Harris PA, McNutt RW, O'Neill SJ, Wei K, Chang K-J. J. Med. Chem. 2003;46:623–633. doi: 10.1021/jm020395s. [DOI] [PubMed] [Google Scholar]
- 45.Simons PC, Vander Jagt DL. Anal. Biochem. 1977;82:334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- 46.Habig WH, Pabst MJ, Jakoby WB. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]