Abstract
Therapeutic vaccines derived from carbohydrate antigen–adjuvant combinations are a promising approach for cancer immunotherapy. One of the critical limitations in this area is access to sufficient quantities of tumour-associated carbohydrate antigens and glycoconjugate adjuvants. At present, availability of the complex oligosaccharide constructs that are needed for the systematic design and evaluation of novel vaccine formulations relies on de novo chemical synthesis. The use of both state-of-the-art and emerging glycosylation technologies has led to significant advances in this field, allowing the clinical exploration of carbohydrate-based antigens in the treatment of cancer.
The identification of distinct glycoprotein and glycolipid constructs that are overexpressed on the cell surfaces of malignant cells1–3 has spurred intense research into exploiting these tumour-associated antigens for the development of anticancer vaccines4–6. Tumour-associated carbohydrate antigens are anchored to the cell surface either by a lipid tail (for example, the glycolipids Globo-H, GM2, GD2 and GD3) or by a protein component (for example, glycoproteins that have an N-acetyl-galactosamine core (GalNAc) oligosaccharide, such as TN, T and sialyl-TN (STN))5. Although glycoprotein and glycolipid carbohydrate epitopes have been used in anticancer vaccine investigations, these molecular subunits typically induce only weak T-cell-independent B-cell (antibody) responses, wherein only a small population of glycolipids might be presented to T cells. However, augmentation of antibody response can be accomplished with three-component vaccine formulations. These constructs involve the covalent conjugation of various carbohydrate antigens to an immunocarrier protein such as keyhole limpet haemocyanin (KLH)7. When this protein is processed and presented by antigen-presenting cells a strong T-cell immune response results, leading to cytokine cascades that increase antibody response, not only to KLH but also to the less immunogenic carbohydrate antigens to which it is attached. Additional immune response potentiation of these antigen conjugates is accomplished through co-administration with an immunological adjuvant such as QS-21 (refs 8–10). Marked eradication of circulating tumour cells and micrometastases in preclinical models, together with prolonged disease-free periods and survival after primary treatment (such as radiation or surgery), highlighted the early potential of carbohydrate vaccines in cancer immunotherapy8,11–13. Several additional three-component anticancer vaccine formulations have since advanced to clinical trials14–20, and large randomized, multicentre trials of polyvalent vaccines such as these will soon address the clinical impact of immunization against carbohydrate antigens.
Low expression levels of tumour-associated carbohydrate antigens in tissue cultures of cancer cells, as well as difficulties associated with isolating homogeneous material from such sources, prompted extensive research into the chemical synthesis of these complex molecules as their primary means of access21–24. Various synthetic strategies have been developed for the preparation not only of these complex oligosaccharide antigens but also of complex carbohydrate immunoadjuvants. The formidable intricacies of regiochemical and stereochemical control in oligosaccharide assembly establish complex carbohydrate construction as one of the most challenging forefronts in chemical synthesis. Here we present an overview of selected glycosylation processes and strategies applied to the synthesis of tumour-associated carbohydrate antigens (TACA) and immunoadjuvants that show promise in anticancer therapeutic vaccines.
Glycosylation strategies
Acetal exchange
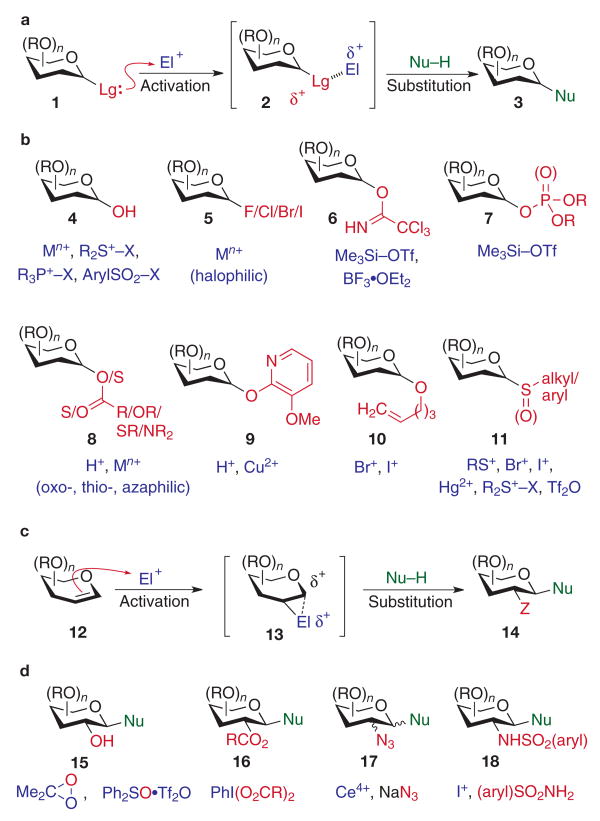
Many of the advances in complex carbohydrate synthesis revolve around methods to form the glycosidic bond, because this is the primary means by which monosaccharide building blocks are assembled into more complex oligosaccharide structures25–32. For more than a century, methods developed for glycosylation have overwhelmingly favoured an approach in which the carbohydrate coupling partner contributing its anomeric carbon in the anomeric linkage (glycosyl donor) serves the role of an electrophile. The corresponding coupling partner (glycosyl acceptor) thus functions as the nucleophilic counterpart. This strategy typically relies on the use of a selectively protected glycosyl donor (1, Fig. 1a), which incorporates at its anomeric centre a latent leaving group (Lg). In the presence of a suitable electrophilic ‘activator’ (El+), the anomeric functionality is rendered highly electron deficient (2), thereby allowing anomeric substitution by the nucleophilic glycosyl acceptor (Nu–H) to form the glycoconjugate 3. This acetal exchange process governs most existing chemical glycosylation processes, and is effective for coupling not only with simple nucleophiles but also complex oligosaccharide, peptide and lipid glycosyl acceptors.
Figure 1. Glycosylation methods.
a, b, Glycosylation of acetal-derived glycosyl donors. Activation of the anomeric leaving group (Lg, red) with an electrophilic promoter (El+, purple) is followed by nucleophilic attack of the acceptor (Nu–H, green) on the resulting electron-deficient anomeric carbon of the carbohydrate donor. c, d, Glycosylation with glycal donors. Activation of glycals with various electrophiles (El+) is followed by coupling with a glycosyl acceptor (Nu–H) at the anomeric carbon. These glycosylations result in functionalization of both the C1 and C2 positions of the donor. M, metal; R, various substituents; Tf, trifluoromethanesulphonyl; X, various leaving groups; Z, various functionalities.
One of the more direct approaches for glycosylation involves the class of C1-hydroxy glycosyl donors (4, Fig. 1b), in which an unprotected anomeric hydroxyl is exchanged under a controlled dehydration process. Various dehydrating reagents have proved effective, including cationic metal Lewis acids, sulphonium and phosphonium salts, and activated sulphonyl halides. Although this approach minimizes the number of distinct anomeric derivatizations in the glycosylation protocol, alternative strategies involving functionalization of the anomeric hydroxyl into an isolable glycosyl donor before anomeric activation and coupling are often desirable. Among the earliest latent leaving groups used in chemical glycosylations are anomeric halides (5), wherein glycosyl fluorides, chlorides, bromides and iodides can be prepared and activated for glycosylation with their respective halophilic reagents. Other anomeric latent leaving groups involving oxygen-derived functionalities include trichloroacetimidates (6), phosphates/phosphites (7), esters/carbonates/thiocarbonates (8) and various aryloxy groups (9), all of which can be efficiently activated by the corresponding oxo-, aza- or thiophilic reagents. Also among the anomeric O-derived glycosyl donors is the 4-pentenyl glycoside 10, in which electrophiles with high affinity for π-electrons (such as electrophilic halogen reagents) have proved to be useful selective activators. Like the 4-pentenyl glycosyl donors, glycosyl sulphides and sulphoxides (11) have been shown to be exceedingly useful not only as latent leaving groups but also as stable anomeric protective groups before the glycosylation event.
Glycal oxidation
In addition to carbohydrate donors with anomeric heteroatom derivatives, the use of glycals (12, Fig. 1c) as glycosyl donors has been explored extensively in complex carbohydrate synthesis. The presence of the 1,2-alkene functionality in this substrate allows the use of various electrophilic oxidants (El+) that are reactive to enol ether nucleophiles (12). The resulting activated glycosyl donor 13 is then poised to receive an appropriate nucleophilic glycosyl acceptor (Nu–H) to form the glycoconjugate 14. These methods allow the introduction of various functionalities ‘Z’ at the C2-position in conjunction with anomeric bond formation. For example, C2-oxygen transfer to glycal donors has proved useful, involving dimethyldioxirane (DMDO)-mediated33 or sulphonium-mediated34 1,2-epoxidation of glycals, followed by anomeric substitution, to generate C2-hydroxy glycosides (15, Fig. 1d). Similarly, I(iii)-containing reagents have recently been applied to the generation of selectively protected C2-acyloxyglycosides (16)35. The synthesis of 2-amino-2-deoxyglycosides has drawn considerable attention owing to the abundance and importance of this class of glycoside in naturally occurring glycoconjugates. The venerable C2-azidonitration reaction of glycals36 to afford C2-azido pyranose derivatives (17) has been, and continues to be, a favoured method by which to introduce the C2-N-functionality. Other reactions have subsequently been developed for C2-nitrogen transfer onto the glycal donor, including stereoselective installation of a sulphonamide group (for instance, 18, by iodosulphonamido glycosylation)33, as well as less-used protocols to install a C2-carbamate functionality37 and the naturally occurring C2-acetamido group38.
This series of acetal exchange couplings and glycal glycosylations (Fig. 1) has been used, as have others, in the chemical synthesis of highly complex oligosaccharide conjugates. However, so far no single coupling method has proved to be broadly effective for all glycosylations, no doubt as a result of the high structural and functional group variability of carbohydrate substrates. This is the case for the exceedingly complex glycoconjugates identified for potential use in cancer immunotherapy, in which chemical glycosylation processes used to prepare these molecules are often highly substrate-specific.
Synthesis of tumour-associated carbohydrate antigens
Globo-H
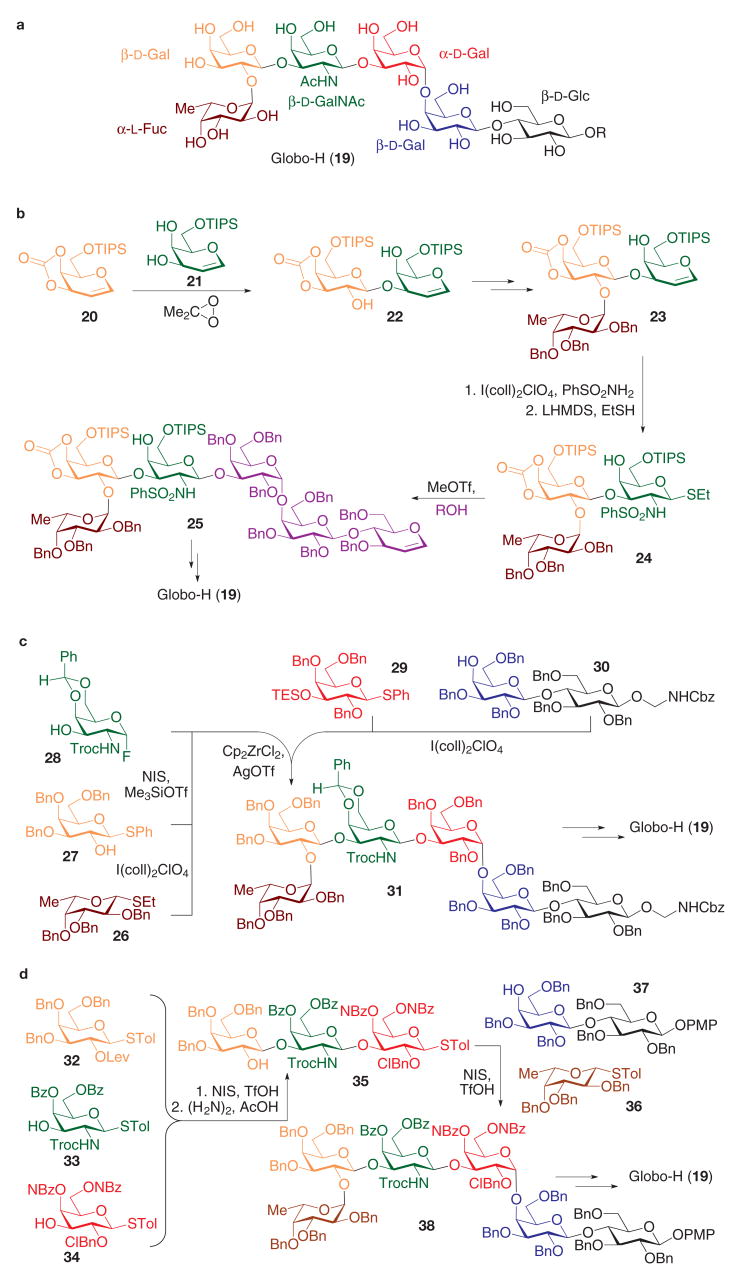
Globo-H (19, Fig. 2a) is a cell-surface glycosphingolipid expressed on a number of epithelial tumours, including those of the breast, prostate and ovary39–41. Its complex hexasaccharide has been a target of many total synthesis approaches. The DMDO-mediated glycal assembly method33 was elegantly applied to the first total synthesis of a Globo-H oligosaccharide42,43 (Fig. 2b). This approach is illustrated by the stereoselective α-epoxidation of galactal 20 followed by regioselective C3-O-glycosylation of galactal diol 21. The resulting glycal disaccharide 22 is then immediately poised for glycosylation with a selectively protected fucose donor to afford the glycal trisaccharide 23. Synthetic incorporation of 2-amino-2-deoxyglycoside derivatives often presents a challenge in complex carbohydrate synthesis, wherein stereoselective nitrogen-transfer to glycal substrates has proved effective. In this late-stage transformation, activation of the glycal enol ether in 23 with iodonium di-sym-collidine perchlorate (I(coll)2ClO4) generates a transient β-iodonium intermediate that rapidly receives a PhSO2NH2 nucleophile at the anomeric position. The resulting glycosyl sulphonamide group is then transferred to the C2-position in the presence of a base (lithium hexamethyldisilazide, or LHMDS) through the generation of a putative aziridine intermediate that is subsequently opened by ethane thiol. The resulting trisaccharide 24, which incorporates the requisite protected C2-aminogalactoside donor, is ready for thioglycoside coupling with an appropriate tri saccharide acceptor (ROH) to afford the hexasaccharide 25, which can be readily elaborated to the Globo-H antigen (19).
Figure 2. Selected syntheses of tumour-associated Globo-H antigen hexasaccharide.
a, The chemical structure of Globo-H. b, Synthesis through glycal assembly using oxidative C2-hydroxyglycosylation and oxidative C2-sulphonamidoglycosylation. c, Synthesis by an orthogonal two-directional glycosylation strategy using thioglycoside and glycosyl fluoride donors. d, Synthesis through a reactivity-based one-pot multiple glycosylation strategy. Bn, benzyl; Bz, benzoyl; Cbz, benzyloxycarbonyl; ClBn, 2-chlorobenzyl; coll, 2,4,6-collidine; Cp, cyclopentadienyl; Fuc, fucose; Lev, laevulinoyl (4-oxopentanoyl); LHMDS, lithium hexamethyldisilazide; NBz, 4-nitrobenzoyl; NIS, N-iodosuccinimide; PMP, 4-methoxyphenyl; TES, triethylsilyl; TIPS, triisopropylsilyl; Tol, toluyl (4-methylphenyl); Troc, trichloroethoxycarbonyl.
Subsequent syntheses of the Globo-H oligosaccharide used the concept of orthogonal glycosylation. By capitalizing on differences in reactivity of various anomeric leaving groups, in conjunction with the control of nucleophilicity of the acceptor, a convergent synthesis of the Globo-H hexasaccharide was accomplished (Fig. 2c). From five distinct carbohydrate building blocks (26–30) with either an anomeric thioalkyl, thioaryl or fluoride functionality, sequential chemoselective anomeric activations were conducted in an orthogonal two-directional glycosylation approach44. For example, the ethylthio fucoside donor 26 could be selectively activated in the presence of the less reactive anomeric phenylthio group of the acceptor 27. After this initial glycosylation of 27 with 26, the remaining anomeric phenylthio group was activated (with N-iodosuccinimide (NIS) in the presence of the galactosyl acceptor 28, whose anomeric fluoride group remained unreactive to the thiophilic NIS reagent. The resulting glycosyl fluoride trisaccharide was then used as the donor for the glycosylation of the remaining Galα1-4Galβ1-4Glc trisaccharide fragment (where Gal denotes galactose and Glc denotes glucose) itself obtained from the glycosylation of the protected lactose acceptor 30 with the thiogalactoside 29. In the late-stage convergent step, fluoride activation (by biscyclopentadienyl zirconium dichloride (Cp2ZrCl2) and silver trifluoro methanesulphonate (AgOTf)) with concomitant triethylsilyl (TES) deprotection allowed stereoselective coupling of both trisaccharide fragments to provide the protected hexasaccharide 31, which served as a suitable advanced substrate for the completion of the synthesis. Notably, no anomeric protecting group interconversions were necessary after any of the glycosylation steps in the synthetic sequence.
The orthogonal reactivity concept was exploited on a different dimension in the reactivity-based one-pot strategy45 for the construction of Globo-H hexasaccharide46 (Fig. 2d). This effort relied on known relative reactivities of various thioglycoside donors, the reactivities of which were finely tuned by the careful choice of proximal protective groups47. The synthesis was initiated through the use of three distinct monosaccharide donors (32–34), all of which incorporated the anomeric thiotoluyl latent leaving group. When this mixture of thioglycosides was treated with electrophilic activators (trifluoromethanesulphonic acid (TfOH) and NIS), the electron-rich thioglycoside donor 32 was activated most rapidly to condense with the most nucleophilic alcohol acceptor 33. Subsequent glycosylation of 34 with the remaining anomeric thiotoluyl group on the resultant disaccharide in the one-pot reaction generated the trisaccharide 35 after selective removal of the laevulinate (Lev) protective group. A similar one-pot multiple glycosylation process was applied to a mixture of the trisaccharide 35, the fucosyl donor 36, and the lactose-derived acceptor 37, securing the hexasaccharide 38 for eventual advancement to the Globo-H hexasaccharide. This one-pot strategy resulted in the assembly of the hexasaccharide core with minimal isolation and purification of oligosaccharide intermediates48. Other notable syntheses of Globo-H have involved exclusive application of glycosyl trichloroacetimidates49 and glycosyl phosphates50, demonstrating the power and versatility of these classes of glycosyl donor.
Sialylated gangliosides
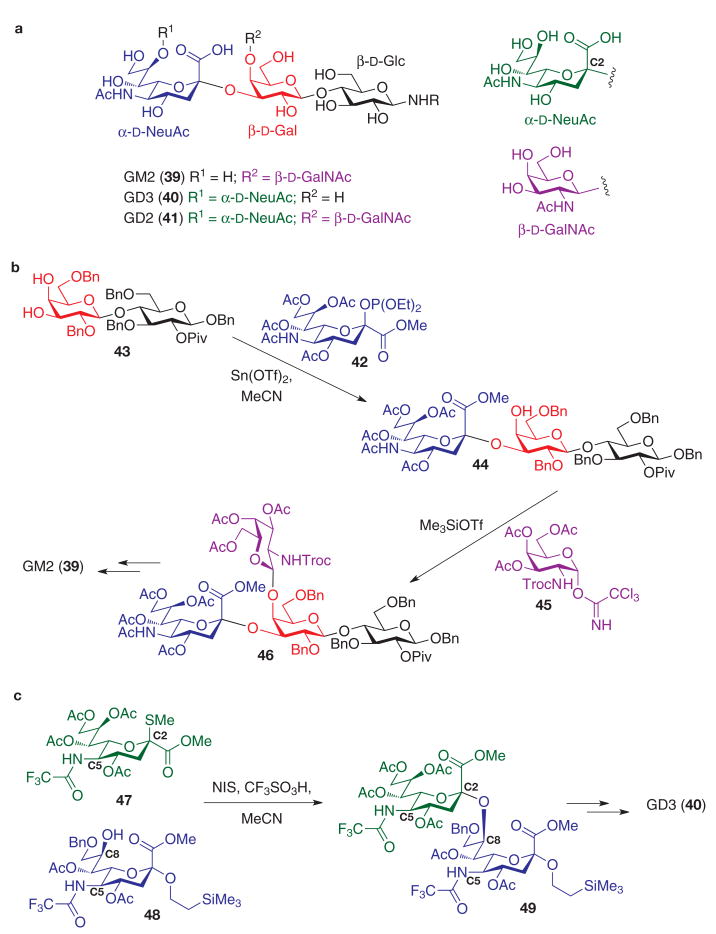
Sialylated gangliosides, exemplified by GM2 (39), GD3 (40), and GD2 (41) (Fig. 3a), are cell-surface glycolipids expressed in a number of neuroectodermal cancers (including melanoma, neuroblastoma, sarcoma and small-cell lung cancer) and also, in the case of GM2, in several epithelial cancers (breast, prostate, ovary and colon)3,51. In addition to the typical challenges associated with the chemical synthesis of glycolipids, an added difficulty is the incorporation of sialic acid residues such as neuraminic acid (NeuAc) into complex oligosaccharides52. Glycosylations with sialic acid donors are often plagued by low yields, because anomeric couplings must occur at the sterically encumbered C2-position through a ketal exchange process. Moreover, naturally occurring sialosides incorporate the α-C2-stereochemical configuration, which is the contra-thermodynamic equatorial isomer, devoid of electronic stabilization through anomeric effects. Anomeric leaving groups in sialylation reactions52 have taken the form of thio-derivatives, halides and phosphites, as well as, to a lesser extent, sialic acid-2,3-glycals and the underivatized hemiketal53. Efforts to control α-anomeric selectivity in these couplings have used removable neighbouring auxiliary groups (either at C3 or at C1), or beneficial solvent participation effects in the anomeric substitution event.
Figure 3. Selected syntheses of tumour-associated sialylated glycosphingolipid oligosaccharides.
a, GM2, GD3 and GD2 have a similar trisaccharide core. b, Synthesis of GM2 tetrasaccharide through α-selective sialyl phosphite donor glycosylation. c, Synthesis of GD3 tetrasaccharide by thiosialoside glycosylation. Incorporation of the C5-trifluoroacetamide group in both donor and acceptor enhances efficiency of the coupling. Piv, pivaloyl (2,2,2-trimethylacetyl).
An early synthesis of the GM2 (39, Fig. 3b) antigen made effective use of the β-sialyl phosphite donor 42 in the regioselective sialylation of the C3-hydroxyl of the lactoside acceptor 43 (ref. 54). By capitalizing on the use of nitrile solvents to effect α-sialylations, presumably through a transient β-nitrilium intermediate55–57, Sn(ii) catalysed glycosylation in acetonitrile provided the α-sialoside 44 in good yield and α-stereoselectivity. Attachment of the remaining protected galactosamine residue 45 was accomplished with the trichloroacetimidate glycosylation28 of the lone hydroxyl group in 44 to provide the tetrasaccharide 46, which was subsequently converted to GM2 (39) by straightforward protective group manipulations.
Further enhancement of reactivity in sialic acid glycosylations is evident in a synthesis of GD3 (40, Fig. 3c), wherein the C5-acetamido functionality in neuraminic acid is replaced with a C5-trifluoroacetamido group in the glycosylation substrates (such as 47)58. With this modification, construction of the NeuAcα2-8NeuAc linkage was possible without the need for installation of neighbouring auxiliary groups. Thus, treatment of the thiosialoside donor 47 with NIS in acetonitrile solvent allowed α-selective C8-O-glycosylation of the sialyl acceptor 48, also derivatized with the C5-trifluoroacetamido substituent. The beneficial effects of these modified amide substrates in both 47 and 48 are thought to arise from the improved reactivity in 47 as a more electron-deficient electrophilic donor and from the attenuation of a putative C5-amido-C8-hydroxyl hydrogen bond in the acceptor 48 that might otherwise compromise its role as a nucleophile. The attainment of 49 allowed elaboration to GD3 (40), after glycosylation of an appropriate lactoside acceptor and facile late-stage exchange of the trifluoroacetamide groups to their native acetamido counterparts.
Mucin-associated glycans
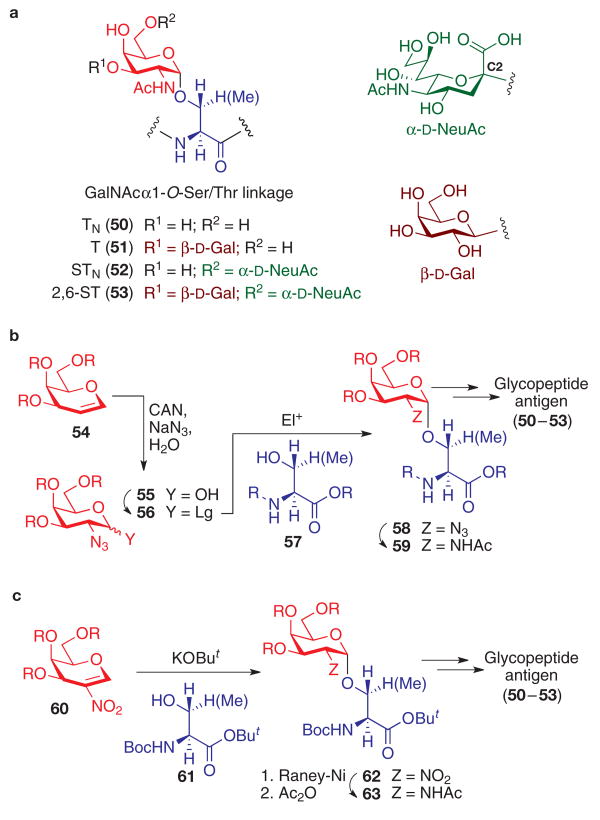
Mucin motifs make up another well-studied class of tumour-associated carbohydrate antigen59. Mucins are high-molecular-weight glycoproteins expressed on the surfaces of many epithelial cells that are characterized by the presence of GalNAc moieties on the hydroxyl side chains of Ser and Thr residues (which are often clustered) in the protein60. Further glycosyl transferase-controlled extension of the carbohydrate moiety results in the formation of oligosaccharides, which, among other functions, provide protection from proteolytic degradation and microbial infection. As a result of altered glycosyltransferase expression in tumour cells, premature termination of oligosaccharide biosynthesis leads to the formation of shortened, often sialylated, saccharide antigens as well as the exposure of peptide epitopes. Examples of tumour-associated carbohydrate antigens (Fig. 4a) include TN (50), T (51), STN (52) and 2,6-ST (53)10,61,62. A number of these tumour-associated structural alterations represent a basis for the design of vaccines for selective eradication of tumours.
Figure 4. Mucin-related tumour-associated carbohydrate antigens.
a, Structures of TN, T, STN and 2,6-ST carbohydrate antigens. O-α-carbohydrate–amino-acid linkages can be synthesized by either (b) glycosylation of suitably protected Ser or Thr derivatives with various galactose-derived C2-azido donors, or (c) conjugate addition of amino-acid alkoxides into nitrogalactals. Boc, tert-butoxycarbonyl; But, tert-butyl; CAN, ceric ammonium nitrate; Y, various carbohydrate C1 groups; Z, various carbohydrate C2 groups.
The chemical synthesis of mucin-derived TACAs involves a glycosylation challenge beyond carbohydrate–carbohydrate coupling: that of the construction of the linkage at the interface between the saccharide and the mucin-derived peptide. Formation of the GalNAcα1-O-Ser/Thr connection (Fig. 4a) highlights one of the key difficulties in achieving C1,C2-cis-glycosylation. Indeed, a C2-acetamido group within a carbohydrate donor is overwhelmingly biased, through oxazoline intermediate formation, to afford the C1,C2-trans glycoside63, a linkage with the anomeric configuration opposite to that of the mucin-related tumour-associated antigen.
To address this challenge, access to the GalNAcα1-O-Ser/Thr substructure is often accomplished through the glycosylation of the side-chain hydroxyl group of protected Ser or Thr derivatives with C2-azido donors (56; Fig. 4b), in which the non-participating nature of the azide substituent allows the stereoselective formation of the desired α-anomer. 2-Deoxy-2-azidogalactopyranose derivatives (55), obtained by azidonitration36 of protected galactal substrates (54), can be converted to various glycosyl donors (56), including anomeric bromides and chlorides64,65, trichloroacetimidates66, thioglycosides67,68 and pentenyl glycosides69, all of which can be used for Ser/Thr glycosylation to generate the carbohydrate–amino-acid conjugate 58. Importantly, these couplings are equally amenable to the construction of oligosaccharide–Ser/Thr conjugates66,70–72. After amino-acid glycosylation, the C2 azido group in 58 is converted, through reductive acetylation, to the naturally occurring acetamide group in 59 to allow subsequent use in iterative peptide synthesis.
A conceptually distinct approach (Fig. 4c) to the construction of mucin-type amino-acid–carbohydrate linkage involves the formation of the critical sugar–amino-acid bond from the potassium tert-butoxide-promoted conjugate addition of protected Ser and Thr nucleophiles (61) to a 2-nitrogalactal donor (60)73. Subsequent C2-nitro reductive acetylation and protective-group modification in 62 results in the preparation of glycosylated building blocks 63, which can be readily elaborated for glycopeptide synthesis.
Once formed, the suitably protected glycosylated amino acids can be incorporated into a growing peptide chain in a modular fashion, allowing the preparation of clustered carbohydrate antigen displays, a motif common to tumour cell surfaces. Various oligopeptide–tumour antigen conjugates have been prepared in this manner, including TN antigen constructs74,75, STN antigen glycopeptides64,76, and 2,3- and 2,6-ST conjugates68,77.
Synthesis of carbohydrate immunoadjuvants
QS-21Aapi
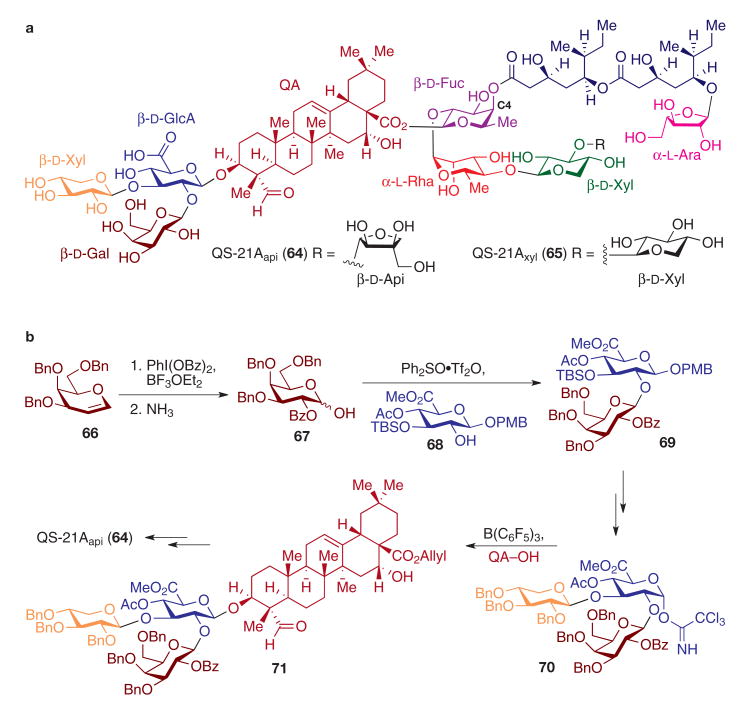
A third component that is required in clinically viable anticancer vaccines is a potent immunological adjuvant, a substance that is itself non-immunogenic but that significantly augments the immune response when administered together with the antigen–carrier conjugate. Among the most potent molecular immunological adjuvants used in antitumour vaccines is QS-21A (ref. 9; Fig. 5), a plant-derived complex saponin from the South American tree Quillaja saponaria Molina. Microgram quantities of this amphiphilic substance in combination with the antigen–carrier conjugate lead to enhancement in both antibody and cell-mediated immune response in a host of promising anticancer and antiviral vaccines78. The structure of QS-21A (Fig. 5a) is that of a complex triterpene–oligosaccharide–normonoterpene conjugate, incorporating quillaic acid as a central lipophilic core flanked by a branched trisaccharide, a linear tetrasaccharide, and an extended glycosylation diester side chain along its periphery. QS-21A has two constitutional isomers, in which the terminal saccharide residue on the linear tetrasaccharide substructure is either d-apiose (QS-21Aapi, 64) or d-xylose (QS-21Axyl, 65)79. Unfortunately, acquiring sufficient quantities of these natural products in pure form is fraught with technical challenges, because they exist in a mixture of more than 100 distinct amphiphilic congeners that are typically only partly purified by repeated high-performance liquid chromatography separation.
Figure 5. Immunological adjuvant QS-21A.
a, QS-21A is a heavily glycosylated saponin isolated from Quillaja saponaria Molina in both apiose (QS21Aapi) and xylose (QS21Axyl) forms. b, Synthesis of QS-21Aapi. Glycosidic linkages were constructed by the use of Ph2SO·Tf2O-promoted dehydrative glycosylation and glycosyl trichloroacetimidate coupling in the construction of the trisaccharide–triterpene substructure. Ara, arabinose; PMB, 4-methoxybenzyl; Rha, rhamnose; TBS, tert-butyldimethylsilyl.
The first report of the chemical synthesis of both oligosaccharide cores of the natural product showcased expert control in stereoselective glycosylation in the synthesis of fully protected versions of both the branched trisaccharide fragment and the linear tetrasaccharide components of QS-21Aapi (64)80. Two glycosylation methods were used: NIS activation of thioglycoside donors and trimethylsilyl triflate activation of trichloroacetimidate donors. Because all of the glycosidic linkages in 64 are composed of the 1,2-trans-linked relative configuration, anomeric stereocontrol was effected through liberal use of C2-ester protective groups that capitalize on neighbouring group participatory effects81.
More recently, a completed synthesis of QS-21Aapi (64) was accomplished82 (Fig. 5b) that relied on minimal use of C2-ester protective groups to avoid potential difficulties in late-stage selective ester deprotection in the presence of the hydrolytically labile C4-O-fucosyl ester side chain78 in 64. In this effort, five of the glycosidic bonds in 64 were constructed with the sulphoxide-mediated dehydrative glycosylation (Ph2SO·Tf2O) using hemiacetal donors83. It is also worth noting that recently developed oxidative glycosylation protocols were applied in the construction of advanced monosaccharide substrates. For example, I(iii)-mediated oxidative bis(C1,C2-acyloxylation)35 of tribenzylgalactal (66) served to install benzoate groups stereoselectively at both the C2 and C1 positions, with the latter being selectively removed by aminolysis to afford the hemiacetal 67. Thus, neighbouring group participatory effects were bestowed on this donor (67) in the subsequent glycosylation, yet the need for excessive ester protective group exchanges in the late stages of the synthesis was minimized. Ph2SO-mediated dehydrative glycosylation of the β-glucuronic acid (β-GlcA) derivative 68 provided disaccharide 69, which was advanced to the branched trisaccharide trichloroacetimidate 70. The stereoselective glycosylation of the quillaic acid triterpene (QA–OH) with trisaccharide 70 proved to be among the most difficult to secure, owing to the significant steric demands at the attachment sites of both coupling partners. Nevertheless, the desired β-glycosidic linkage in 71 was constructed through the use of the less common B(C6F5)3 Lewis acid as the catalyst84 in the trichloroacetimidate glycosylation. The carbohydrate–triterpene conjugate 71 was then advanced to the natural product 64 by way of late-stage conjugation to protected forms of the linear tetrasaccharide fragment and glycosylated acyl side chain, each of which was prepared by de novo multistep synthesis. In vivo immunological evaluation of synthetic QS-21A in vaccine formulations is currently in progress.
Conclusion
Advances in chemical glycosylation have allowed organic synthesis to fulfil its role as a supplier of rare carbohydrate antigens identified as potential targets for cancer immunotherapy6,20. Although several classes of antigen (for example, GM2, fucose-GM1, GD2 and GD3) can be obtained from natural sources in acceptable quantities, the acquisition of sufficient amounts to investigate others (such as Globo-H, STN, TN and T) still relies on chemical synthesis. However, chemical synthesis has evolved far beyond this initial role, as it also makes it possible to design novel versions of otherwise inaccessible antigen and adjuvant constructs, molecules that might hold the key to overcoming critical challenges in the induction of potent yet selective cellular and humoral responses.
Pursuit of non-natural structural modification of carbohydrate antigens that forcibly enhance the non-self identity of specific oligosaccharide conformations has shown promise, although guidelines for such designs are often empirically derived. For example, synthetic TACAs in the form of sialic acid lactone derivatives of GD2 (ref. 15) and GD3 (ref. 14), or N-propionylated derivatives of polysialic acid14,15,85, show potent serological response in immunized patients compared with their ‘natural’ unmodified counterparts. Moreover, as transformed cells have varying degrees of heterogeneity in the type and distribution of antigens on their surfaces86, it is postulated that polyvalent constructs of various antigens could serve as a better mimic of cancer cells19. An approach to incorporate controlled epitope heterogeneity within homogeneous antigen constructs is exemplified by recently prepared pentavalent neoglycopeptides87, whose KLH conjugates have shown encouraging antibody responses in preclinical evaluations in mice.
Further advances on these fronts will probably entail modulation of epitope selection or spacing in clustered constructs, as well as the development of novel molecular platforms for antigen display88–90. These efforts will rely not only on the development and application of new glycosylation technologies but also on innovations in chemoselective ligation reactions for access to increasingly complex and diverse immunogenic molecular arrays. However, chemical synthesis and modification of the immunological adjuvant component is a much less explored area in synthetic vaccine development91,92. Indeed, there have been only limited investigations regarding the chemical modification of QS-21A adjuvant on the basis of natural product degradation and derivatization93,94, providing the first glimpses into its structure–activity relationship profile. The recent synthesis of QS-21Aapi will undoubtedly allow the role of chemical synthesis to extend beyond the arena of antigen construction to that of molecular adjuvant design in the preparation of new conjugate anticancer vaccines.
Acknowledgments
We thank P. O. Livingston for assistance in proof-reading this article. Research on carbohydrate synthesis in the laboratory of D.Y.G. is supported by the National Institutes of Health.
Footnotes
The authors declare no competing financial interests.
References
- 1.Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314:53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 2.Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside–KLH conjugate vaccines. Immunol Rev. 1995;145:147–166. doi: 10.1111/j.1600-065x.1995.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 4.Fung PY, Madej M, Koganty RR, Longenecker BM. Active specific immunotherapy of a murine mammary adenocarcinoma using a synthetic tumor-associated glycoconjugate. Cancer Res. 1990;50:4308–4314. [PubMed] [Google Scholar]
- 5.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 6.Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Synthetic carbohydrate-based antitumor vaccines: challenges and opportunities. Expert Rev Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- 7.Helling F, et al. GD3 vaccines for melanoma: superior immunogenicity of keyhole limpet hemocyanin conjugate vaccines. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 8.Helling F, et al. GM2–KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res. 1995;55:2783–2788. [PubMed] [Google Scholar]
- 9.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 10.Ragupathi G. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother. 1996;43:152–157. doi: 10.1007/s002620050316. [DOI] [PubMed] [Google Scholar]
- 11.Livingston PO, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 12.MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin-associated sialyl-Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. J Immunother Emph Tumor Immunol. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Slovin SF, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragupathi G, et al. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. Int J Cancer. 2000;85:659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Ragupathi G, et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9:5214–5220. [PubMed] [Google Scholar]
- 16.Slovin SF, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 17.Krug LM, et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 18.Slovin SF, et al. Thomsen–Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ragupathi G, Gathuru J, Livingston P. Antibody inducing polyvalent cancer vaccines. Cancer Treat Res. 2005;123:157–180. doi: 10.1007/0-387-27545-2_7. [DOI] [PubMed] [Google Scholar]
- 20.Livingston PO, Ragupathi G. Cancer vaccines targeting carbohydrate antigens. Hum Vaccin. 2006;2:137–143. doi: 10.4161/hv.2941. [DOI] [PubMed] [Google Scholar]
- 21.Toyokuni T, Singhal AK. Synthetic carbohydrate vaccines based on tumour-associated antigens. Chem Soc Rev. 1995:231–242. [Google Scholar]
- 22.Danishefsky SJ, Allen JR. From the laboratory to the clinic: a retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew Chem Int Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Brocke C, Kunz H. Synthesis of tumor-associated glycopeptide antigens. Bioorg Med Chem. 2002;10:3085–3112. doi: 10.1016/s0968-0896(02)00135-9. [DOI] [PubMed] [Google Scholar]
- 24.Buskas T, Ingale S, Boons GJ. Glycopeptides as versatile tools for glycobiology. Glycobiology. 2006;16:113R–136R. doi: 10.1093/glycob/cwj125. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen H. Advances in stereoselective chemical syntheses of complex oligosaccharides. Angew Chem Int Ed Engl. 1982;21:155–224. [Google Scholar]
- 26.Sinay P. Recent advances in glycosylation reactions. Pure Appl Chem. 1991;63:519–528. [Google Scholar]
- 27.Toshima K, Tatsuta K. Recent progress in O-glycosylation methods and its application to natural products synthesis. Chem Rev. 1993;93:1503–1531. [Google Scholar]
- 28.Schmidt RR, Kinzy W. Anomeric-oxygen activation for glycoside synthesis: the trichloroacetimidate method. Adv Carbohydr Chem Biochem. 1994;50:21–123. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 29.Garegg PJ. Thioglycosides as glycosyl donors in oligosaccharide synthesis. Adv Carbohydr Chem Biochem. 1997;52:179–205. doi: 10.1016/s0065-2318(08)60091-8. [DOI] [PubMed] [Google Scholar]
- 30.Davis BG. Recent developments in oligosaccharide synthesis. J Chem Soc Perkin Trans. 2000;1:2137–2160. [Google Scholar]
- 31.Hanessian S, Lou B. Stereocontrolled glycosyl transfer reactions with unprotected glycosyl donors. Chem Rev. 2000;100:4443–4464. doi: 10.1021/cr9903454. [DOI] [PubMed] [Google Scholar]
- 32.Nicolaou KC, Mitchell HJ. Adventures in carbohydrate chemistry: new synthetic technologies, chemical synthesis, molecular design, and chemical biology. Angew Chem Int Ed. 2001;40:1576–1624. [PubMed] [Google Scholar]
- 33.Danishefsky SJ, Bilodeau MT. Glycals in organic synthesis: the evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew Chem Int Ed. 1996;35:1380–1419. [Google Scholar]
- 34.Di Bussolo V, Kim YJ, Gin DY. Direct oxidative glycosylations with glycal donors. J Am Chem Soc. 1998;120:13515–13516. [Google Scholar]
- 35.Shi L, Kim YJ, Gin DY. C2-acyloxyglycosylation with glycal donors. J Am Chem Soc. 2001;123:6939–6940. doi: 10.1021/ja015991a. [DOI] [PubMed] [Google Scholar]
- 36.Lemieux RU, Ratcliffe RM. The azidonitration of tri-O-acetyl-d-galactal. Can J Chem. 1979;57:1244–1251. [Google Scholar]
- 37.Kan C, et al. Photo amidoglycosylation of an allal azidoformate. Synthesis of β-2-amido allopyranosides. Org Lett. 2001;3:381–384. doi: 10.1021/ol0069002. [DOI] [PubMed] [Google Scholar]
- 38.Di Bussolo V, Liu J, Huffman LG, Gin DY. Acetamidoglycosylation with glycal donors: a one-pot glycosidic coupling with direct installation of the natural C(2)-N-acetylamino functionality. Angew Chem Int Ed. 2000;39:204–207. doi: 10.1002/(sici)1521-3773(20000103)39:1<204::aid-anie204>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 39.Kannagi R, et al. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983;258:8934–8942. [PubMed] [Google Scholar]
- 40.Bremer EG, et al. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 41.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 42.Bilodeau MT, et al. Total synthesis of a human breast tumor associated antigen. J Am Chem Soc. 1995;117:7840–7841. [Google Scholar]
- 43.Park TK, et al. Total synthesis and proof of structure of a human breast tumor (globo-H) antigen. J Am Chem Soc. 1996;118:11488–11500. [Google Scholar]
- 44.Zhu T, Boons GJ. A two-directional and highly convergent approach for the synthesis of the tumor-associated antigen globo-H. Angew Chem Int Ed. 1999;38:3495–3497. doi: 10.1002/(sici)1521-3773(19991203)38:23<3495::aid-anie3495>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Douglas NL, Ley SV, Lucking U, Warriner SL. Tuning glycoside reactivity: new tool for efficient oligosaccharide synthesis. J Chem Soc Perkin Trans. 1998;1:51–66. [Google Scholar]
- 46.Burkhart F, Zhang Z, Wacowich-Sgarbi S, Wong CH. Synthesis of the Globo H hexasaccharide using the programmable reactivity-based one-pot strategy. Angew Chem Int Ed. 2001;40:1274–1277. doi: 10.1002/1521-3773(20010401)40:7<1274::aid-anie1274>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, et al. Programmable one-pot oligosaccharide synthesis. J Am Chem Soc. 1999;121:734–753. [Google Scholar]
- 48.Huang CY, et al. Carbohydrate microarray for profiling the antibodies interacting with Globo H tumor antigen. Proc Natl Acad Sci USA. 2006;103:15–20. doi: 10.1073/pnas.0509693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lassaletta J, Schmidt RR. Glycosyl imidates. Part 75. Synthesis of the hexasaccharide moiety of globo H (human breast cancer) antigen. Liebigs Ann. 1996:1417–1423. [Google Scholar]
- 50.Bosse F, Marcaurelle LA, Seeberger PH. Linear synthesis of the tumor-associated carbohydrate antigens Globo-H, SSEA-3, and Gb3. J Org Chem. 2002;67:6659–6670. doi: 10.1021/jo025834+. [DOI] [PubMed] [Google Scholar]
- 51.Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 52.Boons GJ, Demchenko AV. Recent advances in O-sialylation. Chem Rev. 2000;100:4539–4565. doi: 10.1021/cr990313g. [DOI] [PubMed] [Google Scholar]
- 53.Haberman JM, Gin DY. Dehydrative sialylation with C2-hemiketal sialyl donors. Org Lett. 2003;5:2539–2541. doi: 10.1021/ol034815z. [DOI] [PubMed] [Google Scholar]
- 54.Castro-Palomino JC, et al. Efficient synthesis of ganglioside GM2 for use in cancer vaccines. Angew Chem Int Ed. 1997;36:1998–2001. [Google Scholar]
- 55.Gordon JE, Turrell GC. Observations on N-methylacetonitrilium and N-phenylbenzonitrilium hexachloroantimonates. J Org Chem. 1959;24:269–271. [Google Scholar]
- 56.Schmidt RR, Ruecker E. Stereoselective glycosidations of uronic acids. Tetrahedron Lett. 1980;21:1421–1424. [Google Scholar]
- 57.Hasegawa A, et al. Synthetic studies on sialoglycoconjugates 25: reactivity of glycosyl promoters in α-glycosylation of N-acetylneuraminic acid with the primary and secondary hydroxyl groups in the suitably protected galactose and lactose derivatives. J Carbohydr Chem. 1991;10:493–498. [Google Scholar]
- 58.De Meo C, Demchenko AV, Boons GJ. A stereoselective approach for the synthesis of α-sialosides. J Org Chem. 2001;66:5490–5497. doi: 10.1021/jo010345f. [DOI] [PubMed] [Google Scholar]
- 59.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 60.Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17:359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- 61.Müller S, Hanisch FG. Recombinant MUC1 probe authentically reflects cell-specific O-glycosylation profiles of endogenous breast cancer mucin. High density and prevalent core 2-based glycosylation. J Biol Chem. 2002;277:26103–26112. doi: 10.1074/jbc.M202921200. [DOI] [PubMed] [Google Scholar]
- 62.Marcos NT, et al. Polypeptide GalNAc-transferases, ST6GalNAc-transferase I, and ST3Gal-transferase I expression in gastric carcinoma cell lines. J Histochem Cytochem. 2003;51:761–771. doi: 10.1177/002215540305100607. [DOI] [PubMed] [Google Scholar]
- 63.Banoub J, Boullanger P, Lafont D. Synthesis of oligosaccharides of 2-amino-2-deoxy sugars. Chem Rev. 1992;92:1167–1195. [Google Scholar]
- 64.Liebe B, Kunz H. Solid-phase synthesis of a tumor-associated sialyl-TN antigen glycopeptide with a partial sequence of the ‘tandem repeat’ of the MUC-1 mucin. Angew Chem Int Ed. 1997;36:618–621. [Google Scholar]
- 65.Kunz H, Birnbach S. Synthesis of tumor associated TN- and T-antigen type O-glycopeptides and their conjugation to bovine serum albumin. Angew Chem Int Ed. 1986;98:360–362. [Google Scholar]
- 66.Sames D, Chen XT, Danishefsky SJ. Convergent total synthesis of a tumour-associated mucin motif. Nature. 1997;389:587–591. doi: 10.1038/39292. [DOI] [PubMed] [Google Scholar]
- 67.Paulsen H, Rauwald W, Weichert U. Building units of oligosaccharides. LXXXVI. Glycosidation of oligosaccharide thioglycosides to O-glycoprotein segments. Liebigs Ann. 1988:75–86. [Google Scholar]
- 68.George SK, et al. Chemoenzymatic synthesis of sialylated glycopeptides derived from mucins and T-cell stimulating peptides. J Am Chem Soc. 2001;123:11117–11125. doi: 10.1021/ja015570t. [DOI] [PubMed] [Google Scholar]
- 69.Svarovsky SA, Barchi JJ. Highly efficient preparation of tumor antigen-containing glycopeptide building blocks from novel pentenyl glycosides. Carbohydr Res. 2003;338:1925–1935. doi: 10.1016/s0008-6215(03)00323-9. [DOI] [PubMed] [Google Scholar]
- 70.Rademann J, Schmidt RR. Solid-phase synthesis of a glycosylated hexapeptide of human sialophorin, using the trichloroacetimidate method. Carbohydr Res. 1995;269:217–225. doi: 10.1016/0008-6215(94)00364-l. [DOI] [PubMed] [Google Scholar]
- 71.Paulsen H, Peters S, Bielfeldt T, Meldal M, Bock K. Synthesis of the glycosyl amino acids Nα-Fmoc-Ser[Ac4-β-d-Gal p-(1→3)-Ac2-α-d-GalN3p]-OPfp and Nα-Fmoc-Thr[Ac4-β-d-Gal p-(1→3)-Ac2-α-d-GalN3p]-OPfp and the application in the solid-phase peptide synthesis of multiply glycosylated mucin peptides with Tn and T antigenic structures. Carbohydr Res. 1995;268:17–34. doi: 10.1016/0008-6215(94)00292-n. [DOI] [PubMed] [Google Scholar]
- 72.Nakahara Y, Nakahara Y, Ogawa T. Solid-phase synthesis of an O-linked glycopeptide based on a benzyl-protected glycan approach. Carbohydr Res. 1996;292:71–81. doi: 10.1016/s0008-6215(96)91027-7. [DOI] [PubMed] [Google Scholar]
- 73.Winterfeld GA, Khodair AI, Schmidt RR. O-glycosyl amino acids by 2-nitrogalactal concatenation — synthesis of a mucin-type O-glycan. Eur J Org Chem. 2003:1009–1021. [Google Scholar]
- 74.Elofsson M, Salvador LA, Kihlberg J. Preparation of Tn and sialyl Tn building blocks used in Fmoc solid-phase synthesis of glycopeptide fragments from HIV gp120. Tetrahedron. 1997;53:369–390. [Google Scholar]
- 75.Seitz O, Kunz H. A novel allylic anchor for solid-phase synthesis — synthesis of protected and unprotected O-glycosylated mucin-type glycopeptides. Angew Chem Int Ed. 1995;34:803–805. [Google Scholar]
- 76.Dziadek S, Hobel A, Schmitt E, Kunz H. A fully synthetic vaccine consisting of a tumor-associated glycopeptide antigen and a T-cell epitope for the induction of a highly specific humoral immune response. Angew Chem Int Ed. 2005;44:7630–7635. doi: 10.1002/anie.200501594. [DOI] [PubMed] [Google Scholar]
- 77.Dziadek S, Brocke C, Kunz H. Biomimetic synthesis of the tumor-associated (2,3)-sialyl-T antigen and its incorporation into glycopeptide antigens from the mucins MUC1 and MUC4. Chem Eur J. 2004;10:4150–4162. doi: 10.1002/chem.200400228. [DOI] [PubMed] [Google Scholar]
- 78.Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carr Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 79.Jacobsen NE, et al. Structure of the saponin adjuvant QS-21 and its base-catalyzed isomerization product by 1H and natural abundance 13C NMR spectroscopy. Carbohydr Res. 1996;280:1–14. doi: 10.1016/0008-6215(95)00278-2. [DOI] [PubMed] [Google Scholar]
- 80.Zhu X, Yu B, Hui Y, Schmidt RR. Synthesis of the trisaccharide and tetrasaccharide moieties of the potent immunoadjuvant QS-21. Eur J Org Chem. 2004:965–973. [Google Scholar]
- 81.Nukada T, Berces A, Zgierski MZ, Whitfield DM. Exploring the mechanism of neighboring group assisted glycosylation reactions. J Am Chem Soc. 1998;120:13291–13295. [Google Scholar]
- 82.Kim YJ, et al. Synthetic studies of complex immunostimulants from Quillaja saponaria: synthesis of the potent clinical immunoadjuvant QS-21Aapi. J Am Chem Soc. 2006;128:11906–11915. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garcia BA, Gin DY. Dehydrative glycosylation with activated diphenyl sulfonium reagents. Scope, mode of C(1)-hemiacetal activation, and detection of reactive glycosyl intermediates. J Am Chem Soc. 2000;122:4269–4279. [Google Scholar]
- 84.Ishihara K, Yamamoto H. Arylboron compounds as acid catalysts in organic synthetic transformations. Eur J Org Chem. 1999:527–538. [Google Scholar]
- 85.Nores GA, Dohi T, Taniguchi M, Hakomori S. Density-dependent recognition of cell surface GM3 by a certain anti-melanoma antibody, and GM3 lactone as a possible immunogen: requirements for tumor-associated antigen and immunogen. J Immunol. 1987;139:3171–3176. [PubMed] [Google Scholar]
- 86.Zhang S, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int J Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 87.Ragupathi G, et al. Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: a synthetic route to anticancer vaccine candidates. J Am Chem Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 88.Lo-Man R, et al. A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 89.Grigalevicius S, et al. Chemoselective assembly and immunological evaluation of multiepitopic glycoconjugates bearing clustered Tn antigen as synthetic anticancer vaccines. Bioconjug Chem. 2005;16:1149–1159. doi: 10.1021/bc050010v. [DOI] [PubMed] [Google Scholar]
- 90.Svarovsky SA, Szekely Z, Barchi JJ. Synthesis of gold nanoparticles bearing the Thomsen–Friedenreich disaccharide: a new multivalent presentation of an important tumor antigen. Tetrahedron Asymmetry. 2005;16:587–598. [Google Scholar]
- 91.Jiang ZH, Koganty RR. Synthetic vaccines: the role of adjuvants in immune targeting. Curr Med Chem. 2003;10:1423–1439. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 92.Buskas T, Ingale S, Boons GJ. Towards a fully synthetic carbohydrate-based anticancer vaccine: synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated tn antigen. Angew Chem Int Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 93.Marciani DJ, et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–3151. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 94.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003;8:934–943. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]