Abstract
Background/Aims
To examine the incremental effect of patients’ dependence on others, on cost of medical and nonmedical care, and on informal caregiving hours over time.
Methods
Data are obtained from 172 patients from the Predictors Study, a large, multicenter cohort of patients with probable Alzheimer disease (AD) followed annually for 4 years in 3 University-based AD centers in the USA. Enrollment required a modified Mini-Mental State Examination score ≥30. We examined the effects of patient dependence (measured by the Dependence Scale, DS) and function (measured by the Blessed Dementia Rating Scale, BDRS) on medical care cost, nonmedical care cost, and informal caregiving time using random effects regression models.
Results
A one-point increase in DS score was associated with a 5.7% increase in medical cost, a 10.5% increase in nonmedical cost, and a 4.1% increase in caregiving time. A one-point increase in BDRS score was associated with a 7.6% increase in medical cost, a 3.9% increase in nonmedical cost and an 8.7% increase in caregiving time.
Conclusions
Both functional impairment and patient dependence were associated with higher costs of care and caregiving time. Measures of functional impairment and patient dependence provide unique and incremental information on the overall impact of AD on patients and their caregivers.
Key Words: Alzheimer's disease; Patient dependence; Costs of care, longitudinal changes
Introduction
The high cost of caring for patients with Alzheimer disease (AD) is widely known, with annual costs exceeding USD 80–100 billion in the USA [1]. As the disease progresses, the patients’ function worsens and they become increasingly dependent on others to supervise and perform cognitively demanding and even basic physical tasks. The relationship between patients’ functional deficits and higher cost of care has been clearly demonstrated in cross-sectional settings [2,3,4,5]. In the Predictors Study, we examined longitudinal relationships between patients’ functional deficits and several cost outcomes, including medical (e.g. hospitalizations) and informal caregiving costs (e.g. cost of caregivers’ time) [4, 5]. We found the relationship between increasing functional deficits and higher costs consistent over time.
However, functional deficits do not fully describe patients’ dependence on other individuals and may only provide a partial explanation of variation in AD-related costs [6,7,8,9,10]. To address this issue, the Dependence Scale (DS) was developed to directly measure the amount of assistance AD patients require [7]. Earlier studies demonstrated that, aside from patients’ cognition and function, the DS measures related but distinct aspects of disability in AD [8,9,10]. Two recent studies began to examine the effect of dependence on costs of care [11, 12]. Murman et al. [11] conducted a pathway analysis to examine the effect of the DS on cost by payer (e.g. Medicare). The results showed that the DS was partially explained by a combination of clinical characteristics (e.g. cognitive impairment), and that the DS, in turn, partially explained variations in total societal costs. While this provides additional support for the DS as a predictor of cost, the omission of a functional measure limits any conclusions on the utility of the DS as a predictor separate from functional deficits. To address this issue, we conducted an analysis using baseline data of the Predictors Study and examined the incremental effect of the DS on cost [12]. We showed that, after controlling for functional deficits, the DS was significantly associated with total cost of care, and that functional deficits and dependence related differently to different cost components. However, since all patients were at early disease stages at baseline, few used any nonmedical care (e.g. home health aides), precluding a detailed analysis on the relationship between the DS and this important cost component. Also, since both studies were cross-sectional, whether this relationship is consistent over time has yet to be determined.
In this study, we aim to extend previous work in two directions: (1) to investigate whether the relationships between increasing dependence and costs of care observed in cross-sectional settings are consistent over time, and (2) to examine the nonmedical care cost trajectory and estimate its relationship with patient dependence. By estimating these relationships, we hope to provide useful data for future evaluations of disease cost trajectories, provide insights into future areas for research, clinical practice and disease management, and influence health care policy and financing.
Methods
Sample
The sample was drawn from the Predictors 2 cohort, and consisted of 204 patients with probable AD recruited between 1998 and 2004 from the Columbia University Medical Center, Johns Hopkins School of Medicine, and the Massachusetts General Hospital [13, 14]. The inclusion and exclusion criteria have been fully described elsewhere [13, 14]. Briefly, subjects met DSM-III-R criteria for primary degenerative dementia of the Alzheimer type and NINDS-ADRDA criteria for probable AD. Enrollment required a modified Mini-Mental State Examination (mMMS) score ≥30 [15]. The mMMS (range 0–57) is an expanded measure of global cognitive status based on the original Folstein Mini-Mental State Exam (MMSE) [16], and includes the Wechsler Adult Intelligence Scale Digit Span subtest, as well as additional attention/calculation, general knowledge, language, and construction items. A conversion equation, mMMS = 1.73 MMSE + 2.81, can be used to relate the mMMS to the original MMSE. An mMMS score ≥30 therefore is equivalent to a score of approximately ≥16 on the original MMSE. Because patients were followed at academic AD centers, they were well characterized, with high degrees of certainty in their AD diagnosis. To date, postmortem diagnoses have been completed for 96 patients, 92 (96%) of whom had AD-type pathological changes based on CERAD and NIA-Reagan criteria [17, 18].
Recruitment of patients began in 1998 and is ongoing. After the baseline visit, all patients were re-evaluated semiannually, with annual assessments of health care utilization and costs. At the time of these analyses, baseline data were collected for 13.3% of patients in 1998, 8.3% in 1999, 24.3% in 2000, 26.0% in 2001, 15.5% in 2002, 11.1% in 2003, and 1.1% in 2004. At present, 82.4% have had at least one follow-up assessment and about a third had 3 assessments. Median follow-up for the cohort at this point was 2.5 years; maximum was 7. Patients who did not respond at a particular visit could respond at a subsequent visit. Differences in the number of observations during follow-up reflect both continuous accrual of patients and patient deaths (7%). Missed visits during follow-up were rare: 15.6% missed 1, 2.5% missed 2, and 1% missed 3 visits.
The construction of our analysis sample has been described in detail in earlier reports [4, 5]. Briefly, because patterns of health care utilization differ substantially for community and institutionalized patients [19, 20], we excluded visits during which patients lived in an institutional setting. We further excluded visits for which there were missing cost and DS data. In addition, we excluded from the analysis 3 different visits (from 2 patients) with reports of zero use of any resource items. Because the number of observations with zero use was so small, excluding them did not change the distribution of the dependent variables in the sample. The analysis sample consisted of 428 observations from 172 patients who lived at home throughout the study period.
Measures
Patient characteristics and cost outcomes used in this study are briefly described below. Details of the measures and the costing methods used were reported in earlier studies [4, 5].
Dependent Variables
Informants reported patients’ utilization of medical, nonmedical, and informal care. Domains of medical care included hospitalization, outpatient treatment and procedures, assistive devices, and medications. Domains of nonmedical care included overnight respite care, adult daycare, and home health aides. Quantities of care used were annualized and converted into costs using prices obtained from public databases. All costs values were adjusted to constant 2005 dollars. Informal caregiving time for basic and instrumental activities of daily living and for supervision was obtained from up to 3 caregivers for each patient. Hours of informal care provided per day for each caregiving task were asked in the following categories: 0, ≤3, 3–6, 6–9, 9–12, and >12 h. We transformed these categories into continuous values using the mean of each category and top-coded the last category to 12 h. We summed the hours for each patient to obtain an estimate of total caregiving hours.
Independent Variables
Our main independent variables are the DS and the Blessed Dementia Rating Scale (BDRS).
Dependence Scale
The DS consists of 13 items, representing a wide range of care required by a patient, from items related to early disease stages, such as needing reminders, to those related to more advanced disease stages, such as needing to be fed [7]. Table 1 presents the entire DS questionnaire. All items deal with patients’ needs, rather than what they actually received. The instrument is designed to be administered to a reliable informant who lives with the patient or one who is well informed about the patient's daily activities and needs. With the exception of the first 2 items (needs reminders to manage chores, needs help to remember important things such as appointments) which are coded as 0 (no), 1 (occasionally, at least once a month), and 2 (frequently, at least once a week), responses to the rest of the items are coded dichotomously and indicate whether the patient requires assistance with a particular item (0 = no, 1 = yes). The total DS score is the sum of scores on all 13 items (range = 0–15), and provides a continuous index of progressively greater dependence, with higher scores indicating increasing dependence. Reliability and validity of the scale have been established, with reliability coefficients ranging from 0.66 to 0.93 [7].
Table 1.
The DS Questionnaire
| A | Does the patient need reminders or advice to manage chores, do shopping, cooking, play games, or handle money? |
| B | Does the patient need help to remember important things such as appointments, recent events, or names of family or friends? |
| C | Does the patient need frequent (at least once a month) help finding misplaced objects, keeping appointments, or main- taining health or safety (locking doors, taking medication)? |
| D | Does the patient need household chores done for him/her? |
| E | Does the patient need to be watched or kept company when awake? |
| F | Does the patient need to be escorted when outside? |
| G | Does the patient need to be accompanied when bathing or eating? |
| H | Does the patient have to be dressed, washed, and groomed? |
| I | Does the patient have to be taken to the toilet regularly to avoid incontinence? |
| J | Does the patient have to be fed? |
| K | Does the patient need to be turned, moved, or transferred? |
| L | Does the patient wear a diaper or a catheter? |
| M | Does the patient need to be tube fed? |
Items A and B are coded as follows: no = 0; occasionally (i.e., at least once a month) = 1; frequently (i.e., at least once a week) = 2. The other items are coded as follows: no = 0; yes = 1. The total needs, rather than DS score is the sum of scores on all 13 items (range = 0–15).
Blessed Dementia Rating Scale
Functional capacity was measured by the BDRS parts I (instrumental activities of daily living) and II (basic activities of daily living) [6]. Response options for the 7 instrumental activities of daily living items (e.g. difficulty in doing chores around the house) were none (0), some difficulty (0.5), and a lot of difficulty (1). Response options for the 3 basic activities of daily living items (e.g. eating) ranged from 0 to 3, with higher scores indicating more difficulty. For example, for the item on eating, the response options were eat cleanly (0), messily or only with a spoon (1), only able to eat simple solids such as pudding (2), and need to be fed (3). The total BDRS score is the sum of scores on all 10 items (range = 0–17), with higher scores indicating worse function. Reliability and validity of the scale have been established, with reliability coefficients ranging from 0.60 to 0.80 [6].
Other Independent Variables
Patients’ medical histories at baseline were used to construct a modified version of the Charlson Comorbidity Index [21, 22]. Patients’ age, ethnicity, sex, and education were recorded at baseline and marital status was recorded at each visit.
Analysis
We used random effects models to estimate the effects of patient characteristics on medical cost, nonmedical cost, and informal caregiving time [23]. With this framework, we hypothesize that costs are predicted by a combination of fixed effects that are common to all individuals in the population or common to groups of individuals, and random effects that indicate individual specific variations. The fixed effects parameters have the usual interpretation as the average effect of each explanatory variable on costs. The random effects are interpreted as deviations from the mean for each individual, and therefore model the magnitude of unobserved heterogeneity. Specifically, to deal with clustering of observations within the individual over time, we included a random intercept to estimate the between-individual variations in cost at baseline, and a random slope to estimate between-individual variations in changes in cost over time.
Because the distributions of the dependent variables were highly positively skewed, we examined log-transformed dependent variables. Therefore, the coefficient estimates are semi-elasticities, for which the interpretation requires some care. For continuous explanatory variables, the coefficient beta estimates proportional change in the cost outcome for a unit change in the explanatory variable. That is, for a unit increase in the explanatory variable, cost increases by 100 beta %. For dichotomous explanatory variables, the corresponding proportional change in cost of the explanatory variable from the reference group is estimated by Kennedy [24]. We interpreted these proportional changes as marginal effects of the explanatory variables, and estimated the positive portion of each dependent variable. All analyses were performed using Stata 9.0 [25].
As in our baseline analysis, we were concerned about the relationship between our main independent variables and other clinical characteristics (e.g. MMSE). We followed our baseline analysis and examined two sets of models, a trimmed model that controlled for demographic variables only, and a full model that also controlled for other clinical variables [12]. We present data for the trimmed models only because results suggest that they performed just as well as the full models.
Results
Sociodemographic and Clinical Characteristics
Descriptive statistics of patients’ sociodemographic characteristics at baseline and at each follow-up year are provided in table 2 to give a sense of the characteristics of the cohort over time. At baseline, the average patient was 75.1 years old. Slightly over half were women (55.8%). Most patients were non-Hispanic white (95.3%), well-educated (average 14.5 years of schooling), and married (67.4%).
Table 2.
Descriptive statistics of sociodemographic characteristics
| All samples (n = 428) | Baseline (n = 172) | Year 1 (n = 103) | Year 2 (n = 71) | Year 3 (n = 43) | Year 4 (n = 32) | |
|---|---|---|---|---|---|---|
| Age at baseline (mean ± SD), years | 74.9±7.6 | 75.1±7.5 | 75.1±8.0 | 75.7±8.1 | 74.0±6.1 | 73.9±7.8 |
| Female, % | 50.0 | 55.8 | 46.6 | 50.7 | 30.2 | 53.1 |
| Race, % | ||||||
| White | 95.8 | 95.3 | 97.1 | 94.4 | 95.3 | 96.9 |
| Black | 4.2 | 4.7 | 2.9 | 5.6 | 4.7 | 3.1 |
| Years of schooling (mean ± SD) | 14.7±3.3 | 14.5±3.3 | 14.9±3.1 | 14.8±3.3 | 15.2±3.5 | 14.1±3.7 |
| Marital status, % | ||||||
| Married | 71.0 | 67.4 | 70.9 | 71.8 | 86.0 | 71.9 |
| Widowed | 23.1 | 25.0 | 22.3 | 22.5 | 14.0 | 25.0 |
| Other | 5.8 | 7.6 | 6.8 | 5.6 | 0.0 | 3.1 |
| Site, % | ||||||
| Columbia | 53.5 | 52.3 | 43.7 | 57.7 | 60.5 | 65.6 |
| Johns Hopkins | 20.8 | 22.1 | 22.3 | 14.1 | 18.6 | 28.1 |
| Massachusetts General | 25.7 | 25.6 | 34.0 | 28.2 | 20.9 | 6.3 |
At baseline, data were collected from 172 patients. At year 1 and each year thereafter through year 4, data were collected from 103, 71, 43, and 32 patients for a total number of 428 observations. Differences in the number of follow-up visits mainly reflected the staggered nature of sample recruitment and to a lesser extent patient deaths (7%).
Table 3 presents patients’ clinical characteristics at baseline for the entire sample and for the subset of patients with complete 4-year follow-up data. Because of study inclusion criteria, all patients were at the early stages of AD at baseline, with a mean MMSE score of 22.1 (SD = 3.8), mean BDRS score of 2.8 (SD = 1.4), and mean DS score of 5.1 (SD = 2.3). Behavioral problems (42.4%) and psychotic symptoms (31.0%) were common. About 20% had depressive symptoms; 14.5% exhibited extrapyramidal symptoms (EPS). At baseline, the subset of patients with complete 4-year follow-up data was similar to other patients regarding DS, BDRS, and MMSE scores. However, the prevalence of behavioral problems, psychotic symptoms, depressive symptoms, and EPS was lower. As expected, patients’ cognition and function declined and dependence increased over time. The presence of EPS and psychotic symptoms seemed to have increased over time, while the presence of behavioral problems and depressive symptoms fluctuated. The number of comorbidities remained relatively stable over time (mean = 0.7, SD = 0.9).
Table 3.
Patient clinical characteristics
| Subsample with complete 4-year follow-up data (n = 32) |
||||||
|---|---|---|---|---|---|---|
| All samples at baseline (n = 172) | baseline | year 1 | year 2 | year 3 | year 4 | |
| DS total score (mean ± SD) | 5.1±2.3 | 5.2±2.0 | 5.9±2.1 | 6.9±2.9 | 8.3±3.4 | 8.9±2.9 |
| BDRS total score (mean ± SD) | 2.8±1.4 | 2.9±1.3 | 3.3±1.6 | 4.0±1.8 | 4.6±1.9 | 5.2±1.4 |
| MMSE score (mean ± SD) | 22.1±3.8 | 23.0±3.5 | 21.4±5.8 | 19.6±6.9 | 18.2±7.6 | 17.28 |
| Modified comorbidity index | 0.7 (0.9) | 0.7 (0.8) | 0.6 (0.7) | 0.7 (0.8) | 0.7 (0.8) | 0.8 (0.9) |
| Behavioral problems, % | 42.4 | 28.9 | 55.9 | 57.6 | 54.8 | 60.0 |
| EPS, % | 14.5 | 10.8 | 16.7 | 21.4 | 22.7 | 37.5 |
| Depressive symptoms, % | 19.6 | 15.8 | 23.5 | 15.2 | 16.1 | 26.7 |
| Psychotic symptoms, % | 31.0 | 21.1 | 20.6 | 30.3 | 40.0 | 46.7 |
DS score: range = 0–15; BDRS score: range = 0–17; MMSE score: range = 0–30. EPS = Extrapyramidal signs.
Unadjusted Medical and Nonmedical Costs and Weekly Caregiving Hours
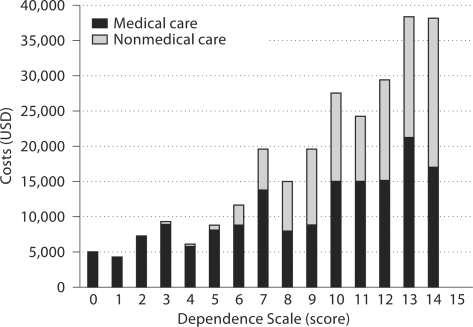
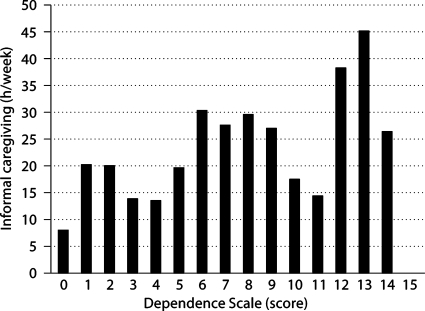
At baseline, informants reported an average annual cost of USD 8,600 for medical care and USD 1,500 for nonmedical care. Figure 1 presents descriptive data on medical and nonmedical cost by DS scores for all observations, regardless of visit. Medical care cost increased from USD 4,831 when DS score = 0 to USD 16,937 when DS score ≥14. Nonmedical cost followed a somewhat different trend. Patients had little nonmedical cost until reaching mild levels of dependence before cost started to increase, from USD 2,828 when DS score = 6 to USD 21,072 when DS score ≥14. These results were combined to show that total direct cost increased from USD 4,831 when DS score = 0 to over USD 38,009 when DS score ≥14. At baseline, informants reported that patients received an average of 21.2 h of informal care per week. Figure 2 presents descriptive data on informal caregiving hours per week by DS scores for all observations, regardless of visit. Informal caregiving time increased from 7.9 h per week when DS score = 0 to 26.5 h per week when DS score ≥14.
Fig. 1.
Medical and nonmedical costs by DS score.
Fig. 2.
Informal caregiving hours by DS score.
Adjusted Medical and Nonmedical Costs and Weekly Caregiving Hours
Table 4 presents our multivariate results on the effects of patient characteristics on medical cost, nonmedical cost, and informal caregiving time. Results showed that, after controlling for other covariates, both DS and BDRS scores were significantly associated with all three dependent variables. Specifically, each additional point in DS score was associated with a 4.1% increase in informal caregivers’ time (p = 0.05), a 5.7% increase in medical cost (p = 0.031), and, although the effect was only marginally significant, a 10.5% increase in nonmedical cost (p = 0.106). Each additional point in BDRS score was associated with an 8.7% increase in informal caregivers’ time (p = 0.032), a 7.6% increase in medical cost (p = 0.109), and a 3.9% increase in nonmedical cost (p = 0.019). The results also showed that one more comorbidity was associated with a 19.2% increase in medical cost (p = 0.002).
Table 4.
Random effects models of medical cost, nonmedical cost, and informal caregiving hours over time
| Medical costs |
Nonmedical costs |
Informal caregiving hours |
||||
|---|---|---|---|---|---|---|
| coefficient | marginal effect | coefficient | marginal effect | coefficient | marginal effect | |
| Year | –0.038 (0.036) | –0.038 | 0.171∗ (0.099) | 0.180 | 0.031 (0.029) | 0.032 |
| DS score | 0.055∗∗ (0.026) | 0.057 | 0.109∗ (0.067) | 0.105 | 0.040∗∗ (0.022) | 0.041 |
| BDRS score | 0.074∗ (0.046) | 0.076 | 0.034∗∗ (0.144) | 0.038 | 0.084∗∗ (0.039) | 0.087 |
| Charlson's comorbidity score | 0.177∗∗∗ (0.056) | 0.192 | 0.086 (0.152) | 0.077 | –0.016 (0.043) | –0.017 |
| Women (1 = yes, 0 = no) | –0.319∗∗∗ (0.102) | –31.942 | 0.110∗∗∗ (0.030) | 11.030 | –0.042∗ (0.079) | –4.151 |
| Younger than 65 (1 = yes, 0 = no) | –0.106 (0.162) | –10.573 | 0.457 (0.506) | 45.724 | –0.012 (0.123) | –1.227 |
| Site (reference = Columbia) | ||||||
| Johns Hopkins | –0.151 (0.137) | –15.089 | –0.604∗ (0.371) | –60.363 | –0.215∗∗ (0.103) | –21.481 |
| Massachusetts General | –0.237 (0.123) | –23.726 | –0.354 (0.387) | –35.448 | –0.487∗∗∗ (0.097) | –48.732 |
DS score: range = 0–15; BDRS score: range = 0–17. Figures in parentheses indicate standard errors.
p < 0.10,
p < 0.05,
p < 0.01.
Secondary Analysis Estimating Informal Caregiving Costs
There is controversy in the approaches to value cost of caregivers’ time [26]. The replacement wage approach is often used in the literature for its simplicity with the assumption that wage rates used approximate prices paid for purchasing similar caregiving activities in the market. The alternative, opportunity cost approach, predicts the caregivers’ wage rate from individuals with similar characteristics (e.g. age, gender, education, work experience). Although the opportunity cost approach is more consistent with economic theory, lack of data on caregivers’ labor force participation, as in this study, often precludes a detailed estimation of caregivers’ wage rates. To gauge the sensitivity of using different wage rates to value informal caregiving time, we used three replacement wage rates. The national average hourly earning for all private industries [27] was used as a high estimate of caregivers’ time, the average wage rate for home health aides as a middle estimate, and the federal minimum wage as a low estimate. In each valuation, informal caregiving cost was estimated by multiplying the hourly wage rate with the weekly hours of caregiving reported, and converting weekly cost to annual cost by multiplying by 52 (weeks per year). Using these wage rates, we estimated the low (high) cost of informal caregiving time at USD 2,391 (USD 18,560) at baseline, increasing to USD 3,923 (USD 32,862) in year 4. As the DS score increased, we estimated the cost of informal caregivers’ time to increase from USD 2,919 to USD 7,998 (low estimate) or from USD 7,065 to USD 31,863 (high estimate).
Discussion
AD has been characterized as impairments involving three main areas: cognition, function and behavior. Each of these characteristics has individually been analyzed with respect to their effect on patients’ health care cost. The DS was developed as a brief measure of AD patients’ needs, and provides a useful method for measuring patients’ dependence on others and tracking changes in dependence over time [7]. It has been shown to change over time after accounting for changes in cognition or function, supporting its utility as a construct separate from functional disability [8,9,10]. Until recently, however, there has been limited information on the ability of the DS to discern differences in cost of care [4, 5, 11, 28]. The current analysis builds on recent literature by establishing the relationship between DS and cost over time. This question was explored for medical cost, nonmedical cost, and informal caregiving time. Our results show after controlling for functional deficits, patients’ dependence was significantly associated with medical cost and informal caregiving time, and marginally significantly associated with nonmedical cost.
This study has several limitations. First, patients in our sample, selected from academic AD centers and predominantly white and highly educated, may represent a nonrandom sample of AD patients in the community. Caution is needed in generalizing the results of this study. Second, data on patients’ health care costs were reported by informants, most of whom were patients’ primary caregivers. Although there is no reason to believe that caregivers’ reports of patients’ health care utilization are inaccurate [29, 30], it is possible that there are additional costs beyond those collected in the study. Third, it should be noted that we estimated costs associated with caring for patients with AD which are not incremental costs due to AD.
The large variations in informal caregiving cost (and hence total cost) estimates using different wage rates should be noted. This wide variation in cost of care for AD patients is well known in the literature [31]. Two of the main reasons for variation are types of resources (e.g. informal care, medical care services used) included in the studies and the methods of valuing these resources. Depending on the wage rates assigned to caregivers’ time, the magnitude of imputed informal cost and its proportion in total societal cost would vary. To address these issues and to increase transparency we have provided caregiving hours prior to applying any cost estimates on caregiving time. However, valuation of informal caregiving time is important in order to obtain a sense of its magnitude as compared to direct cost of care. We estimated that total cost of caring for AD patients ranged from USD 13,178 at baseline to USD 22,385 at year 4 (low estimate using minimum wage) to USD 30,544 at baseline to USD 50,174 at year 4 (high estimate using national average wage rate). These estimates are within the ranges reported in the literature and indicate the validity of the data collection process used in the Predictors Study.
Confidence in our findings is strengthened by several factors. A major contribution of the present analysis lies in the careful diagnosis and clinical follow-up that patients received. Patients were followed prospectively, which eliminates the potential biases inherent in deriving information from retrospective chart reviews. Clinical signs of interest were ascertained and coded in a standardized fashion at each visit. Evaluations were performed annually, which provides multiple assessments of cost and therefore permits more accurate coefficient estimates. Finally, patients were recruited at early disease stages and followed for long periods of time with high follow-up rates. Analysis is not compressed in time and the cohort describes the full range of progression over time. At the time of these analyses, 5.5% of the cohort had missing follow-up information before the most updated data entry.
The results of this analysis suggest that patients’ dependence provides a significant contribution in explaining variations in health care cost in AD and that small changes in dependence are associated with large changes in costs of care. Thus, interventions that enhance patient independence (or delay patients’ dependence) may be associated with cost savings. The traditional approach to managing AD focusing on identifying and handling patient symptoms may have been missing an important component in how we evaluate patients’ needs or dependence on others. Focus on this area is of particular importance for clinicians and policymakers in providing support to patients, planning patients’ future health care needs, and approaching management of AD patients.
Acknowledgements
The Predictors Study is supported by Federal grants R01 AG07370, M01 RR000645, and U01AG010483. Funding for this analysis was also partially provided by Elan Pharmaceuticals, Inc., and Wyeth Pharmaceuticals. Drs. Zhu and Sano are supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1.Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion: Unrealized Prevention Opportunities: Reducing the Health and Economic Burden of Chronic Illness. November 2000.
- 2.Taylor DH, Jr, Schenkman M, Zhou J, Sloan FA. The relative effect of Alzheimer's disease and related dementias, disability, and comorbidities on cost of care for elderly persons. J Gerontol B Psychol Sci Soc Sci. 2001;56:S285–S293. doi: 10.1093/geronb/56.5.s285. [DOI] [PubMed] [Google Scholar]
- 3.Small GW, McDonnell DD, Brooks RL, Papadopoulos G. The impact of symptom severity on the cost of Alzheimer's disease. J Am Geriatr Soc. 2002;50:321–327. doi: 10.1046/j.1532-5415.2002.50065.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu CW, Scarmeas N, Torgan R, et al. Clinical characteristics and longitudinal changes of informal cost of Alzheimer's disease in the community. J Am Geriatr Soc. 2006;54:1596–1602. doi: 10.1111/j.1532-5415.2006.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu CW, Scarmeas N, Torgan R, et al. Longitudinal study of effects of patient characteristics on direct costs in Alzheimer disease. Neurology. 2006;67:998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]
- 6.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 7.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer's disease. J Gerontol. 1994;49:M216–M222. doi: 10.1093/geronj/49.5.m216. [DOI] [PubMed] [Google Scholar]
- 8.Brickman AM, Riba A, Bell K, et al. Longitudinal assessment of patient dependence in Alzheimer disease. Arch Neurol. 2002;59:1304–1308. doi: 10.1001/archneur.59.8.1304. [DOI] [PubMed] [Google Scholar]
- 9.Holtzer R, Tang MX, Devanand DP, et al. Psychopathological features in Alzheimer's disease: course and relationship with cognitive status. J Am Geriatr Soc. 2003;51:953–960. doi: 10.1046/j.1365-2389.2003.51308.x. [DOI] [PubMed] [Google Scholar]
- 10.Sarazin M, Stern Y, Berr C, et al. Neuropsychological predictors of dependency in patients with Alzheimer disease. Neurology. 2005;64:1027–1031. doi: 10.1212/01.WNL.0000154529.53488.30. [DOI] [PubMed] [Google Scholar]
- 11.Murman DL, Von Eye A, Sherwood PR, Liang J, Colenda CC. Evaluated need, costs of care, and payer perspective in degenerative dementia patients cared for in the United States. Alzheimer Dis Assoc Disord. 2007;21:39–48. doi: 10.1097/WAD.0b013e31802f2426. [DOI] [PubMed] [Google Scholar]
- 12.Zhu CW, Leibman C, McLaughlin T, et al: The effects of patient function and dependence on costs of care in Alzheimer's disease. J Am Geriatr Soc, in press. [DOI] [PMC free article] [PubMed]
- 13.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the ‘predictors study’). 1. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7:3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Richards M, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the ‘predictors study’). II. Neurological, psychiatric, and demographic influences on baseline measures of disease severity. Alzheimer Dis Assoc Disord. 1993;7:22–32. doi: 10.1097/00002093-199307010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- 17.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 18.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease: Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 19.Leon J, Cheng CK, Neumann PJ. Alzheimer's disease care: costs and potential savings. Health Aff (Millwood) 1998;17:206–216. doi: 10.1377/hlthaff.17.6.206. [DOI] [PubMed] [Google Scholar]
- 20.Menzin J, Lang K, Friedman M, Neumann P, Cummings JL. The economic cost of Alzheimer's disease and related dementias to the California Medicaid program (‘Medi-Cal’) in 1995. Am J Geriatr Psychiatry. 1999;7:300–308. [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station: Stata Press; 2005. [Google Scholar]
- 24.Kennedy P. Estimation with correctly interpreted dummy variables in semilogarithmic equations. Am Econ Rev. 1981;71:801. [Google Scholar]
- 25.Stata Statistical Software: Release 9. College Station, StataCorp, 2005.
- 26.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. Oxford: Oxford University Press; 1996. [Google Scholar]
- 27.Transmitted to the Congress, February 2006. Washington: United States Government Printing Office; 2006. Economic Report of the President. [Google Scholar]
- 28.Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59:1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- 29.Corder LS, Woodbury MA, Manton KG. Proxy response patterns among the aged: effects on estimates of health status and medical care utilization from the 1982–1984 long-term care surveys. J Clin Epidemiol. 1996;49:173–182. doi: 10.1016/0895-4356(95)00507-2. [DOI] [PubMed] [Google Scholar]
- 30.Neumann PJ, Araki SS, Gutterman EM. The use of proxy respondents in studies of older adults: lessons, challenges, and opportunities. J Am Geriatr Soc. 2000;48:1646–1654. doi: 10.1111/j.1532-5415.2000.tb03877.x. [DOI] [PubMed] [Google Scholar]
- 31.Bloom BS, de Pouvourville N, Straus WL. Cost of illness of Alzheimer's disease: how useful are current estimates? Gerontologist. 2003;43:158–164. doi: 10.1093/geront/43.2.158. [DOI] [PubMed] [Google Scholar]