Abstract
Objective
To modify the skin window technique for extended analysis of acute inflammatory responses in humans, and demonstrate its applicability for investigating disease.
Subjects
15 healthy subjects and 5 Crohn’s patients.
Treatment
Skin windows, created by dermal abrasion, were overlaid for various durations with filter papers saturated in saline, 100 ng/ml muramyl dipeptide (MDP) or 10 μg/ml interleukin-8 (IL-8).
Methods
Exuded leukocytes were analyzed by microscopy, immunoblot, DNA-bound transcription factor arrays and RT-PCR. Inflammatory mediators were quantified by ELISA.
Results
Infiltrating leukocytes were predominantly neutrophils. Numerous secreted mediators were detectable. MDP and IL-8 enhanced responses. Many signalling proteins were phosphorylated with differential patterns in Crohn’s patients, notably PKC α/β hyperphosphorylation (11.3 ± 3.1 vs 1.2 ± 0.9 units, P < 0.02). Activities of 44 transcription factors were detectable, and sufficient RNA isolated for expression analysis of over 400 genes.
Conclusions
The modifications enable broad characterisation of inflammatory responses and administration of exogenous immunomodulators.
Keywords: IBD, Human inflammation models, Skin inflammation and models, Intracellular signalling, Inflammatory mediators
Introduction
Insult or injury to the body elicits an acute inflammatory response, in which extravasation of leukocytes and serum proteins leads to microbial killing, removal of debris and promotion of wound healing. Numerous chemical mediators regulate these events [1, 2], produced first by immune cells resident in the tissues and subsequently by activated leukocytes that have infiltrated the site. Delineating the molecular mechanisms that coordinate this process is crucial for understanding the normal response to tissue damage. Furthermore, abnormalities in this early response have been linked to the pathogenesis of several diseases [3-6], and its modulation by pharmacological agents has important therapeutic implications.
The “skin window” technique permits investigation of the acute inflammatory response in vivo in humans. This typically involves creation of a lesion in the stratum corneum of the skin using a surgical scalpel [7], a high-speed drill [8] or tape stripping [9]. These methods enable quantification of leukocyte emigration, although recovery of fluid exudate for measurement of secreted mediators and application of exogenous chemicals remains practically unreliable. Construction of an overlying chamber to address this drawback [10] can necessitate use of cumbersome apparatus and does not particularly simplify precise administration of immunomodulators. To circumvent these difficulties, others have raised blisters by negative pressure suction [11], from which contents can be aspirated and analyzed. Although effective in principle, these contain only a small volume of fluid with few cells. Larger blisters can be elicited by topical application of cantharidin, a defensive compound derived from Meloidae coleoptera beetles [12]. Unfortunately, this chemical works by inhibiting protein phosphatases-1 and -2α [13] confounding studies of cell function and signalling. A further complication with blister-based methods relates to potential alterations in the nature of the inflammatory infiltrate; these include non-physiological attenuation of monocyte migration and neutrophil degranulation [14].
We report here a refined skin window technique in which the abrasion is overlaid with filter papers onto which cells migrate and soluble mediators are absorbed. These papers can be impregnated with inflammatory modulators to assess their effects on the acute response. A similar previous attempt was only reported for the sole assessment of studying cellular migration [15]. We have successfully employed this method elsewhere to demonstrate major differences in this response in the chronic inflammatory disorder Crohn’s disease [16], and with a collaborating group to demonstrate the extent of leukocyte subpopulation categorization attainable [17]. We provide here full characterisation of the method, and demonstrate its further utility for examining other facets of leukocyte function and the effects of potential topical immunomodulators.
Methods
Subjects
Healthy subjects, defined as individuals with no inflammatory disease and taking no medication, were identified through the Department of Medicine, University College London (UCL). Patients with Crohn’s disease were approached through the gastroenterology outpatient clinic at University College London Hospital (UCLH). The latter had quiescent disease (Harvey-Bradshaw score <3 [18], serum C-reactive protein <5 mg/l, peripheral blood white cell counts within normal range) on no medication, with no signs of malnutrition. They were approximately matched with controls for age, sex and smoking history. Subjects were recruited by DJBM, SM, SB or AWS. The nature and possible consequences of these investigations were fully explained to all volunteers, from whom written informed consent was obtained. These studies were approved by the Joint UCL/UCLH Committee on the Ethics of Human Research.
Skin windows
All equipment used in these experiments was sterile. Forearms were cleaned with 70 % (v/v) ethanol (VWR, Poole, UK) and skin windows created by abrasion of a 30 × 10 mm area on the volar surface using grade C sandpaper (Homebase Ltd, Surrey, UK) until capillaries were visualized but before bleeding commenced. Lesions were always fashioned by the same investigator, and for each subject it was confirmed by a second independent investigator that window sizes, and duration and intensity of abrasion, were uniform.
Abrasions were overlaid with sterile filter papers (Whatman Ltd., Maidstone, UK) saturated in injection-grade normal saline (B. Braun Medical Inc., PA, USA; 0.9 % w/v), either alone or containing muramyl dipeptide (MDP; 100 ng/ml, Sigma-Aldrich) or recombinant human interleukin-8 (IL-8; PeproTech, NJ, USA; 10 μg/ml). These were then covered with a layer of Nescofilm sealing film (Karlan, AZ, USA) and an adhesive dressing. Dressings and filter papers were removed after either 30 min, 6 h, 24 h or 48 h depending on the experiment. Subsequently, windows were washed briefly with water and left open to heal without a dressing.
Cellular analysis
Filter papers were layered for 3 s on a glass slide, which was fixed with methanol and stained by Romanowsky dye. Differential leukocyte counts were determined on the basis of cell morphology under microscopic examination (mean values taken from 5 randomly selected high power fields, more than 150 cells counted per subject).
Filters were next incubated in 400 μl normal saline on a rotating wheel (30 min, 4 °C) and centrifuged (15,000 g, 5 min, 4 °C) to elute secreted proteins. Intracellular contents were then extracted by incubating filter papers and cells in break solution (0.5 M NaCl, 1.5 % Triton X-100) containing Complete Mini protease inhibitor cocktail tablets (Roche, Rotkreuz, Switzerland). These were sonicated (10 × 1 s bursts) and centrifuged (15,000 g, 5 min, 4 °C), then the supernatant measured for myeloperoxidase activity by oxidation of 4-aminoantipyrine (Sigma), using horseradish peroxidase (Sigma) as a standard [19]. Myeloperoxidase is specific for neutrophil granules and its activity is therefore proportional to the number of these cells infiltrating the lesion.
Measurement of secreted mediators
Levels of cytokines and other secreted proteins recovered from filter papers were quantified by ELISA using commercially available kits following the manufacturers’ instructions. These included IL-1β, IL-8, IL-10, IL-12, transforming growth factor-β (TGF-β) and tumour necrosis factor-α (TNF-α) (R&D Systems, Minneapolis, MN), albumin (Alpha Diagnostic International, San Antonio, TX), histamine (IBL, Hamburg, Germany), complement component C3a-desArg (ProGen, Heidelberg, Germany), prostaglandin E2 (PGE2) and leukotriene B4 (LTB4, R&D Systems). To determine recovery efficiency of cytokines, the technique was also performed on filter papers in vitro, which were blocked for 1 h in human serum from healthy volunteers then incubated with recombinant human IL-8 (Peprotech, Rocky Hill, NJ).
Analysis of intracellular protein markers
Leukocyte markers were detected in extracted cellular protein samples by solubilisation in Laemlli sample buffer (containing 0.06 M Tris-HCl pH 6.8, 1 % sodium dodecyl sulphate (SDS), 0.03 M sucrose and 17.9 μM β-mercaptoethanol), electrophoresis through a 10 % SDS-polyacrylamide gel, and transfer to a Hybond-P membrane (Amersham plc, Amersham, UK) using Tris-glycine buffer pH 8.2/20 % methanol in a semi-dry Trans Blot SD blotter (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked for 1 h at room temperature in 5 % non-fat milk then probed for 15 h at 4 °C with antibodies (raised in our laboratory as described previously [20]) against myeloperoxidase or lactoferrin. Membranes were washed three times in phosphate-buffered saline (PBS)/0.05 % Tween-20 and incubated with peroxidase-conjugated donkey anti-rabbit immunoglobulin (Amersham plc) for 1 h at room temperature. After a further three washes, bands were visualized by use of the ECL Plus chemiluminescent system (Amersham plc).
Multi-channel Western blots
In separate experiments, cells from 75 % of the filter paper area were removed using a cell scraper, with simultaneous lysis in 1 ml hypotonic buffer (10 mM HEPES pH 7.9, 10 mM KCl, 10 mM EDTA, 10 mM dithiothreitol (DTT), Protease Inhibitor Cocktail (Sigma), 0.5 % IGEPAL and phosphatase inhibitors: sodium fluoride, sodium orthovanadate and sodium pyrophosphate) [21] under rotation at 4 °C for 10 min. Following centrifugation (15,000 g, 3 min), supernatants were taken as cytosolic fractions, and pellets retained for nuclear proteins.
Cytosolic protein was quantified by Bradford assay. Equal concentrations (250 μg/ml) were run across the top of a 10 % SDS-polyacrylamide gel containing a single long well and transferred to a Hybond-P membrane, and blocked as previously. The membrane was placed on a Mini-protean II multiscreen apparatus (Bio-Rad Laboratories) that allows probing with 16 antibodies on a single blot. Primary antibodies (1:1,300; Cell Signaling Technology, Beverly, MA) were incubated overnight in Tris-buffered saline (TBS)/5 % bovine serum albumin (BSA) at 4 °C. The membrane was then removed from the multiscreen device, washed (5 times, 5 min), and signal detected as before. Developed films were scanned and band density calculated on a densitometer (ChemiGenius 2 Bio Imaging System and Gene Tools software; Syngene, Cambridge, UK). Background activity was automatically deducted from each sample by the software. Densitometry results were normalized to the band obtained with the ERK-Specific Antibody.
DNA-binding proteins
Nuclear pellets prepared as described above were resuspended in 200 μl Complete Lysis Buffer (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 10 % glycerol, Protease Inhibitor Cocktail) and incubated on a shaking platform at 4 °C for 30 min. The extract was centrifuged (15,000 g, 10 min, 4 °C) and the supernatant used to probe TranSignal Protein/DNA Arrays Version I (Panomics, Redwood City, CA) as instructed by the manufacturer. These are spotted with 54 different consensus-binding sequences, each corresponding to a specific transcription factor. In brief, 15 μg of total nuclear proteins were incubated with biotin-labeled DNA-binding probes (TranSignal Probe Mix) to allow the formation of protein-DNA complexes. Free probes were separated by electrophoresis in agarose gels, then complexed probes eluted and hybridized to the TranSignal membrane. Signals were detected by chemiluminescence.
Reverse transcriptase-PCR
In separate experiments, cells attached to one-eighth of each filter paper removed from skin windows were lysed by vortexing in guanidium thiocyanate solution (RLT buffer, Qiagen Inc., Santa Clarita, CA) supplemented with 0.1 M 2-mercaptoethanol. Total RNA was isolated with the RNeasy mini kit (Qiagen), 1 μg of which was then reverse transcribed using dT primers. A cDNA equivalent corresponding to 20 ng of total RNA was amplified in each reaction. Primers used for PCR were: 5′-CACGCCAGAACCTTGTGAG-3′ (human CD14 sense) 5′-CCCAGTCCAGGATTGTCAG-3′ (human CD14 antisense) 5′-GTCACCAGGGCTGCTTTTAAC-3′ (human GAPDH sense) 5′-TGCTTCACCACCTTCTTGATG-3′ (human GAPDH antisense)
As a control of mRNA input, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were determined for each sample in separate reactions. PCR reactions contained dNTPs and buffer as supplied by the manufacturer, 500 pM of each specific primer and 3 U Taq polymerase (RedTaq, Sigma). Transcripts were amplified for 29 cycles with CD14 primers (30 s at 94 °C, 30 s at 56 °C, 30 s at 72 °C) and 20 cycles for GAPDH primers (30 s at 94 °C, 30 s at 58 °C and 45 s at 72 °C), followed by 7 min at 72 °C. PCR products were analyzed by 2.0 % agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV illumination. PCR was performed using different cycle numbers to ensure that amplification was occurring within the linear range.
Statistical analysis
Results are expressed as mean ± standard deviation unless otherwise stated. Statistical tests were performed with GraphPad Prism v4.01 (GraphPad, San Diego, CA). The two-tailed students’ t-test was used for single comparisons, and Kruskal-Wallis Analysis of Variance (ANOVA) with Dunn post-tests or two-way ANOVA with Bonferroni post-tests used for multiple comparisons. Comparison of neutrophil numbers in repeated skin windows was performed using a paired sample t-test.
Results
Acceptability
Dermal abrasion (Fig. 1a) was used to create windows of a standard area (Fig. 1b). This process was described as uncomfortable but not painful until capillary beds were visualized, at which point abrasion was discontinued. There was no bleeding into windows, thrombus formation or evidence of infection in any subject.
Fig. 1.

Creation of skin windows. a. Lesions were fashioned by dermal abrasion of the volar aspect of the forearm. b. Windows had a standard area of 3 cm2, and were then overlaid an individual filter paper. c. After the experiment, healing was complete within 15 days.
Windows were not painful over the course of the experiment, although a small number of subjects described mild tenderness if pressure was applied within the first 24 h. The technique did not interfere with the daily activities of any participant. Scabs developed over windows within 24 h of removing filter papers, and skin re-epithelialized within 15 days (Fig. 1c). A minority of participants reported hypopigmentation at abrasion sites, usually transient lasting no more that 2 months. There were no additional side effects. In terms of acceptability, therefore, this method is comparable to alternative available techniques [7-9, 11].
Cellular infiltrate
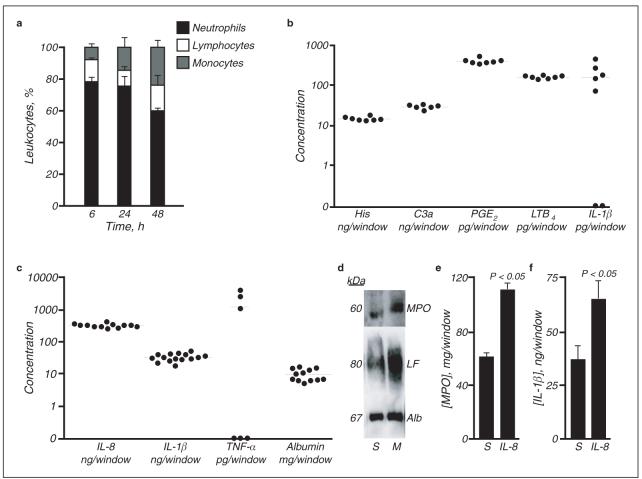
Neutrophils comprised the majority cell population infiltrating skin windows in healthy individuals at each time point (Fig. 2a). Lymphocytes accounted for approximately 15 % of the leukocytes present throughout the experiment time course. Monocyte/macrophage influx increased with time: at 48 h, monocyte/macrophages were more abundant than at 6 or 24 h (P < 0.01 and P < 0.05 respectively), whereas the proportions of neutrophils had decreased (P < 0.001 compared to either time point). Absolute numbers of leukocytes at 24 h were calculated by measurement of neutrophil intracellular myeloperoxidase and from the relative cell numbers previously determined. At this time, 36.2 ± 7.3 × 106 neutrophils, 4.8 ± 2.2 × 106 lymphocytes and 6.7 ± 7.1 × 106 monocytes/macrophages were present. In 4 subjects, a second skin window was created on a subsequent occasion. Neutrophil numbers did not significantly differ between the first and second windows in these subjects (coefficient of variation = 0.62, P = 0.84).
Fig. 2.
Composition of skin window exudates and the effects of immune modulators. a. Differential leukocyte influx over the 48 h following traumatisation. Results are expressed as mean ± SEM, n = 3 for each time point. b, c. Concentration of inflammatory mediators assayed at 30 min (b) and 24 h (c) after creation of lesions. Mean values are shown. d. Representative western blot of cellular exudates showing that application of MDP (M) increases myeloperoxidase (MPO) and lactoferrin (LF) content relative to saline-treated (S) windows. Albumin (Alb) extravasation into exudate fluid was unchanged. e, f. Exogenous IL-8 augments neutrophil recruitment (e) and IL-1β (f) production. Results are expressed as mean ± SEM, n = 3.
Production of inflammatory mediators
The production of inflammatory mediators in healthy subjects was assessed at 30 min (Fig. 2b) and 24 h (Fig. 2c). At 30 min (n = 7), histamine (16.08 ± 1.80 ng/window; mean ± s. d.), activated complement component C3a (27.78 ± 3.25 ng/window; measured as the rapidly generated stable conversion product C3a-desArg, which allows reliable quantitation of C3 activation [22]), and eicosanoids PGE2 (402.65 ± 59.13 pg/window) and LTB4 (193.83 ± 15.98 pg/window) were readily detectable, as well as minimal concentrations of IL-1β (165.18 ± 154.68 pg/window). By 24 h, substantial cytokine production, including IL-8 (329.63 ± 44.50 ng/window, n = 12), IL-1β (36.38 ± 9.68 ng/window, n = 15) and in some subjects TNF-α (1232.90 ± 1620.53 pg/window, n = 6), was observed, as was albumin extravasation (11.30 ± 5.15 mg/window, n = 12). Other cytokines assayed were not detectable at these time points.
The efficiency of cytokine recovery from filter papers was determined by performing the technique in vitro, using filter papers pre-incubated with known concentrations of recombinant human IL-8. Cytokine was then extracted by the same method applied in the in vivo system; recovery efficiency was 76.02 ± 3.80 % (n = 6), and equivalent in the 10−9 and 10−6 g/ml ranges.
Modulation of the acute inflammatory response by exogenous mediators
MDP is a well-established synthetic immune adjuvant, chemically related to a subunit of bacterial peptidoglycan [23]. Its pro-inflammatory action is achieved through induction of cytokines, including IL-8, by mononuclear phagocytes [16, 24]. When applied to skin windows in 3 healthy subjects, myeloperoxidase and lactoferrin content increased but not albumin (Fig. 2d), indicating increased neutrophil emigration consistent with established effects on cytokine production.
One potential confounding issue with this technique was saturation with cells by 24 h such that the entire filter paper surface area was covered. To circumvent this problem, we increased their dimensions to 35 × 15 mm to examine the effects of topical IL-8 administration. IL-8 treatment augmented both myeloperoxidase (Fig. 2e) and IL-1β (Fig. 2f) concentrations in skin windows; this also indicated that these filter papers were not saturated with cells.
Cytosolic signalling pathways
The phosphorylation status of 51 proteins involved in a panel of major cell signal transduction pathways was examined in cytoplasmic extracts of exuded leukocytes. In cells from five healthy individuals, we observed consistent phosphorylation of FKHR at 6 h (not shown); 4E-BP1, p38 MAP kinases, ERK, MEK, Akt, protein kinase C (PKC) and SAPK/JNK at 24 h (Fig. 3a); and Akt and PKC at 48 h (not shown).
Fig. 3.
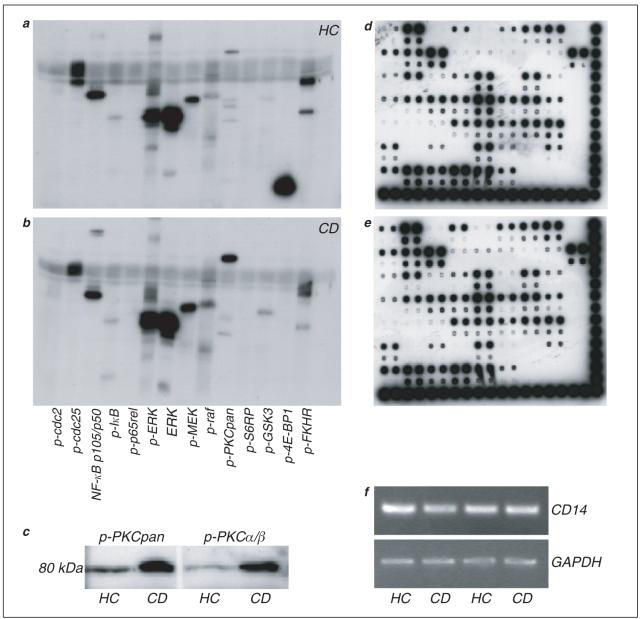
Signal transduction, transcriptional activity and gene expression in exuded leukocytes. a, b. Representative western blots illustrating phosphorylation status of a panel of cytoplasmic proteins in cells recovered 24 h after trauma in a healthy control (a; n = 5) and Crohn’s patient (b; n = 5). c. The most consistent abnormality in Crohn’s patients (CD) was hyperphosphorylation of PKC, and specifically PKC α/β, compared to healthy controls (HC). d, e. DNA-binding activity of various transcription factors from nuclear extracts. Representative membranes (n = 4) are shown from a healthy control (d) and a Crohn’s patient (e), in both of whom the same 44 factors could be detected. (f) Expression of mRNA in exuded cells from 4 subjects. Reverse transcriptase-PCR was performed for CD14 and GADPH, levels of which were similar in all samples. Of the 54 proteins assayed, the activity of 44 could be detected (Fig. 3 d, e). A total of 4 subjects (2 healthy; 2 Crohn’s patients) were compared with minimal variability detected between individuals.
We subsequently searched for abnormalities in five Crohn’s patients. Although the phosphorylation status of the majority of these proteins was unchanged, a few differences were apparent (Fig. 3b). The most striking was supranormal phosphorylation of PKC, specifically the PKC α/β isoform (Fig. 3c), observed in all patients at each time point. Two Crohn’s patients also had diminished phosphorylation of 4E-BP1, whilst that of P70S6 kinase was increased. A third patient had decreased phosphorylation of Akt, and a fourth of p38 MAP kinase. This contrasted with the minimal variability in these proteins observed in healthy subjects.
Levels of phosphorylation of a few proteins were quantified by densitometry. These were normalized against non-phosphorylated ERK determined on the same blot; the amounts of which were similar in all subjects. Levels of phosphorylation of both ERK and NF-kB p105/p50 were not significantly different between healthy controls and Crohn’s patients (phosphorylated ERK: 26.6 ± 6.3 and 28.2 ± 10.2 relative units respectively; phosphorylated NF-kB: 17.5 ± 6.9 and 14.0 ± 4.6 relative units respectively). In contrast, phosphorylation of PKC was significantly greater in Crohn’s patients (1.2 ± 0.9 and 11.3 ± 3.1 relative units respectively, P < 0.02).
Transcription factor activation
We performed DNA/protein arrays (layout of arrays available at http://panomics.multipath.net/pdf/PD_Array_1_with_ap.pdf) to simultaneously screen a large number of transcription factors for DNA binding activity.
Gene expression
Reverse-transcriptase-PCR was utilized to determine the potential for gene expression analysis in exuded cells recovered using this technique. Approximately 1-2 μg of total RNA could be isolated from one-eighth of the total amount of exuded cells. This would be sufficient to analyze expression of over 50 genes, or in excess of 400 genes from the whole sample. CD14 RNA levels were ascertained to evaluate the relative numbers of monocytes within exuded leukocyte populations at 24 h. These were similar in all subjects studied, after normalizing mRNA input with the housekeeping gene GAPDH (Fig. 3f).
Discussion
Although previous skin window techniques have been used to characterize acute inflammatory responses in both health and disease, most possess significant drawbacks in terms of cell or inflammatory mediator recovery [12]. The modifications introduced here facilitate reliable collection of both components. The protocol permits analysis of all facets of the inflammatory response, including cellular influx and phenotype, secretion of inflammatory mediators, and cell function in terms of activation of cytosolic signalling pathways, DNA-binding activity of transcription factors and gene expression. The system also allows investigation of the effects of exogenous mediators, which can be directly applied to abrasions.
Leukocytes migrating into skin windows were predominantly neutrophils, the first cellular line of defence against infection and other insults [25]. Proportionally, these were partially replaced by mononuclear phagocytes over the subsequent 48 h, cells that contribute to removal of neutrophils after the clearance of debris and wound healing [26]. Purification of skin window leukocytes could be achieved using additional steps of either centrifugation through a Percoll gradient [27], or positive or negative selection with antibody-tagged magnetic beads [28]. Such differential separation using our technique has been described elsewhere [17].
In conjunction with cellular extravasation, high concentrations of pro-inflammatory mediators were exuded into the windows. It is probable that a major source of these cytokines were resident and infiltrating leukocytes, although production by epithelial cells is also well-documented [29, 30]. It would be difficult to determine the relative importance of either source without performing skin biopsies in human volunteers (difficult in practice) or use of an animal model. Non-traumatized controls also cannot be obtained, although the non-linear rise in cytokine concentrations provides evidence of stimulated production.
Concentrations of pro-inflammatory mediators were broadly similar amongst healthy individuals studied. This promotes use of our technique as a valuable method for examining the in vivo significance of polymorphisms in genes involved in the inflammatory response, such as cytokines, many of which may predispose to disease [31-34]. The consistency in albumin levels further supports the inter-individual reproducibility of the technique, and provides an appropriate indicator of the extent of trauma for normalization of results when comparing skin windows between individuals or different treatments. This was exemplified when examining the effects of MDP on the inflammatory response. Neutrophil markers were elevated but albumin was unchanged, implying that changes were specific to the immunoadjuvant rather than reflecting differential traumatisation. This could be exploited in the development of topical immunomodulatory drugs.
Analysis of cell signalling events in exuded leukocytes in terms of phosphorylation status of a panel of key proteins revealed highly consistent patterns amongst healthy subjects. Akt could be central within this scheme, documented to induce downstream activation of many of the other molecules found to be phosphorylated [35, 36]. Akt itself plays a critical role in cell survival by inhibiting apoptosis through phosphorylation of FKHR [37] and MEK/ERK [38]. It can also activate other pathways including that utilizing 4E-BP1, which promotes mRNA translation [39]. It is not possible from this model to distinguish definitively pathways that are constitutively active from those stimulated during the inflammatory response, as there can be no non-traumatised control sample. Relevant information could instead be obtained by following the evolution of phosphorylation patterns in serial skin window leukocyte samples, or determining the effects of pharmacological agonists and antagonists of identified pathways in vivo or in vitro.
The functional significance of differences observed in Crohn’s disease remains unclear and merits further investigation, in particular the most consistent finding of hyperphosphorylated PKC α/β. This protein interacts with multiple signal transduction pathways [40]. Altered phosphorylation of molecules involved in protein translation, including P70S6 kinase [41] and 4E-BP1 [39], may contribute to defective synthesis of inflammatory mediators in this condition [16]. Although leukocyte migration into skin windows is diminished in Crohn’s disease [16], the magnitude of the deficiency is insufficient to account for the differences in phosphorylation, especially given the similarities in a large panel of other proteins and transcription factor profiles. Whether these abnormal patterns represent primary pathogenic events will be difficult to prove, although exclusion of patients with active disease on medication removes such potential confounding influences.
The amount of nuclear proteins obtained allowed for maximum sensitivity of DNA/protein arrays. In this model of acute inflammation, activity of 82 % of factors assayed could be detected. Although no differences were observed in Crohn’s patients, further useful information could be provided by time course experiments in this group and healthy subjects. Of the transcription factors that were detected bound to DNA in each subjects, many have been directly associated with the pro-inflammatory response, including NF-kB and STAT-4. These are consistent with an activated state of infiltrating leukocytes. Interestingly, although the transcription factor SMAD has been previously implicated in the pathogenesis of inflammatory bowel disease [42], we observed similar activities in Crohn’s patients and healthy controls. Quantities of mRNA extracted were also sufficient to allow extensive analysis of gene expression by reverse transcriptase-PCR or cDNA microarrays.
In conclusion, the modified skin window technique presented here provides a powerful tool for examining the acute inflammatory response in humans, the effects of immunomodulators, and its dysregulation in disease. As well as delineating molecular mechanisms involved in initiation of inflammation, it could provide insights into processes leading to resolution and healing. These will be of particular relevance in understanding the pathogenesis of both chronic inflammatory diseases and conditions characterised by aberrant healing such as excessive fibrosis.
Acknowledgement
This work was funded by the Wellcome Trust Grant 067287/Z/02/Z.
References
- [1].Ben Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–6. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- [2].Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [3].Segal AW, Loewi G. Neutrophil dysfunction in Crohn’s disease. Lancet. 1976;2:219–21. doi: 10.1016/s0140-6736(76)91024-2. [DOI] [PubMed] [Google Scholar]
- [4].Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182:526–33. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- [5].Gallin JI, Buescher ES. Abnormal regulation of inflammatory skin responses in male patients with chronic granulomatous disease. Inflammation. 1983;7:227–32. doi: 10.1007/BF00917259. [DOI] [PubMed] [Google Scholar]
- [6].Passero FC, Myers AR. Decreased numbers of monocytes in inflammatory exudates in systemic lupus erythematosus. J Rheumatol. 1981;8:62–8. [PubMed] [Google Scholar]
- [7].Rebuck JW, Crowley JH. A method of studying leukocytic functions in vivo. Ann NY Acad Sci. 1955;59:757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- [8].Senn H, Holland JF, Banerjee T. Kinetic and comparative studies on localized leukocyte mobilization in normal man. J Lab Clin Med. 1969;74:742–56. [PubMed] [Google Scholar]
- [9].Mass MF, Dean PB, Weston WL, Humbert JR. Leukocyte migration in vivo: a new method of study. J Lab Clin Med. 1975;86:1040–6. [PubMed] [Google Scholar]
- [10].MacPhee MJ, Rode H, Broadhead M, Christou NV, Meakins JL, Gordon J. Skin window chambers, a novel method for recovering the cells involved in delayed-type hypersensitivity. J Immunol Methods. 1987;103:267–73. doi: 10.1016/0022-1759(87)90299-7. [DOI] [PubMed] [Google Scholar]
- [11].Kiistala U. Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968;50:129–37. doi: 10.1038/jid.1968.15. [DOI] [PubMed] [Google Scholar]
- [12].Day RM, Harbord M, Forbes A, Segal AW. Cantharidin blisters: a technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J Immunol Methods. 2001;257:213–20. doi: 10.1016/s0022-1759(01)00467-7. [DOI] [PubMed] [Google Scholar]
- [13].Honkanen RE. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 1993;330:283–6. doi: 10.1016/0014-5793(93)80889-3. [DOI] [PubMed] [Google Scholar]
- [14].Zimmerli W, Gallin JI. Monocytes accumulate on Rebuck skin window coverslips but not in skin chamber fluid. A comparative evaluation of two in vivo migration models. J Immunol Methods. 1987;96:11–7. doi: 10.1016/0022-1759(87)90361-9. [DOI] [PubMed] [Google Scholar]
- [15].Addison IE, Johnson B, Shaw M. A human skin window technique using micropore membranes. J Immunol Methods. 1982;54:129–39. doi: 10.1016/0022-1759(82)90121-1. [DOI] [PubMed] [Google Scholar]
- [16].Marks DJ, Harbord MW, MacAllister R, Rahman FZ, Young J, Al Lazikani B, et al. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668–78. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- [17].Willment JA, Marshall AS, Reid DM, Williams DL, Wong SY, Gordon S, et al. The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol. 2005;35:1539–47. doi: 10.1002/eji.200425725. [DOI] [PubMed] [Google Scholar]
- [18].Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- [19].Shimizu S, Yasui K, Tani Y, Yamada H. Acyl-CoA oxidase from Candida tropicalis. Biochem Biophys Res Commun. 1979;91:108–13. doi: 10.1016/0006-291x(79)90589-8. [DOI] [PubMed] [Google Scholar]
- [20].Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–7. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- [21].Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–89. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Burger R, Zilow G, Bader A, Friedlein A, Naser W. The C terminus of the anaphylatoxin C3a generated upon complement activation represents a neoantigenic determinant with diagnostic potential. J Immunol. 1988;141:553–8. [PubMed] [Google Scholar]
- [23].Azuma I, Seya T. Development of immunoadjuvants for immuno-therapy of cancer. Int Immunopharmacol. 2001;1:1249–59. doi: 10.1016/s1567-5769(01)00055-8. [DOI] [PubMed] [Google Scholar]
- [24].Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, et al. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–25. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- [25].Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Segal AW, Fortunato A, Herd T. A rapid single centrifugation step method for the separation of erythrocytes, granulocytes and mononuclear cells on continuous density gradients of Percoll. J Immunol Methods. 1980;32:209–14. doi: 10.1016/0022-1759(80)90186-6. [DOI] [PubMed] [Google Scholar]
- [28].Lea T, Vartdal F, Nustad K, Funderud S, Berge A, Ellingsen T, et al. Monosized, magnetic polymer particles: their use in separation of cells and subcellular components, and in the study of lymphocyte function in vitro. J Mol Recognit. 1988;1:9–18. doi: 10.1002/jmr.300010104. [DOI] [PubMed] [Google Scholar]
- [29].Corradi A, Franzi AT, Rubartelli A. Synthesis and secretion of interleukin-1 alpha and interleukin-1 receptor antagonist during differentiation of cultured keratinocytes. Exp Cell Res. 1995;217:355–62. doi: 10.1006/excr.1995.1097. [DOI] [PubMed] [Google Scholar]
- [30].Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–41. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cho J. Update on inflammatory bowel disease genetics. Curr Gastroenterol Rep. 2000;2:434–9. doi: 10.1007/s11894-000-0004-1. [DOI] [PubMed] [Google Scholar]
- [32].Auer J, Weber T, Berent R, Lassnig E, Lamm G, Eber B. Genetic polymorphisms in cytokine and adhesion molecule genes in coronary artery disease. Am J Pharmacogenomics. 2003;3:317–28. doi: 10.2165/00129785-200303050-00003. [DOI] [PubMed] [Google Scholar]
- [33].Imahara SD, O’Keefe GE. Genetic determinants of the inflammatory response. Curr Opin Crit Care. 2004;10:318–24. doi: 10.1097/01.ccx.0000140942.42247.7e. [DOI] [PubMed] [Google Scholar]
- [34].Jin P, Panelli MC, Marincola FM, Wang E. Cytokine polymorphism and its possible impact on cancer. Immunol Res. 2004;30:181–90. doi: 10.1385/IR:30:2:181. [DOI] [PubMed] [Google Scholar]
- [35].Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cantrell D. Protein kinase B (Akt) regulation and function in T lymphocytes. Semin Immunol. 2002;14:19–26. doi: 10.1006/smim.2001.0338. [DOI] [PubMed] [Google Scholar]
- [37].Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Fork-head transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- [38].Fang JY, Richardson BC. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005;6:322–7. doi: 10.1016/S1470-2045(05)70168-6. [DOI] [PubMed] [Google Scholar]
- [39].Sachs AB, Varani G. Eukaryotic translation initiation: there are (at least) two sides to every story. Nat Struct Biol. 2000;7:356–61. doi: 10.1038/75120. [DOI] [PubMed] [Google Scholar]
- [40].Poole AW, Pula G, Hers I, Crosby D, Jones ML. PKC-interacting proteins: from function to pharmacology. Trends Pharmacol Sci. 2004;25:528–35. doi: 10.1016/j.tips.2004.08.006. [DOI] [PubMed] [Google Scholar]
- [41].Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- [42].Fiocchi C. TGF-beta/Smad signaling defects in inflammatory bowel disease: mechanisms and possible novel therapies for chronic inflammation. J Clin Invest. 2001;108:523–6. doi: 10.1172/JCI13863. [DOI] [PMC free article] [PubMed] [Google Scholar]