Abstract
The metabolic syndrome is a common and complex disorder combining obesity, dyslipidemia, hypertension and insulin resistance. It is associated with a high cardiovascular risk that can only partially be explained by its components. There is evidence that low-grade inflammation and high oxidative stress add to this risk. Oxidized LDL, a marker of lipoprotein-associated oxidative stress, is an emerging cardiovascular risk factor. In this review, we demonstrate that the metabolic syndrome exacerbates oxidized LDL in a feedback loop. We introduce molecular mechanisms underlying this loop. Finally, we demonstrate that weight loss and statin treatment lower metabolic syndrome factors associated with a reduction of oxidized LDL. The current data warrant further investigation into the role of lifestyle and therapeutic interventions that inhibit tissue-associated oxidation of LDL in the prevention of the metabolic syndrome.
Keywords: atherosclerosis, inflammation, metabolic syndrome, obesity, oxidative stress, oxidized LDL
The metabolic syndrome is a common and complex disorder combining obesity, dyslipidemia, hypertension and insulin resistance [1–4]. It is a primary risk factor for diabetes and cardiovascular disease [2,5–12]. Typically, Type 2 diabetes begins with insulin resistance; only very few Type 2 diabetics go on to insulin deficiency [13]. Obesity and insulin resistance, and the interaction between these two components, are associated with a high cardiovascular risk [14,15]. Obesity-related Type 2 diabetes is a leading cause of morbidity and mortality in Western societies, and is quickly approaching pandemic proportions [16]. The prevalence of obesity continues to increase, with more than 50% of Europeans currently classified as overweight and up to 30% as clinically obese [17–19]. In the WHO report on integrated management of cardiovascular risk, it was estimated that each year, approximately a quarter of a million deaths in Europe, and more than 2.5 million deaths worldwide, are weightrelated, with cardiovascular disease as the leading cause. The recent rapid increase in childhood overweight and obesity will lead to a further increase in the prevalence of metabolic disease and its associated high cardiovascular risk.
Although insulin resistance and Type 2 diabetes are associated with increased coronary heart disease (CHD) risk, the severity of hyperinsulinemia and hyperglycemia during the diabetic phase can only explain this increased risk to a minor extent. In addition, traditional risk factors do not fully explain this excess risk, and other nontraditional risk factors may be important [20]. Therefore, the European Innovative Medicines Initiative gives priority to the identification of emerging risk factors that are targets for prevention and treatment.
One of the emerging risk factors is subclinical chronic low-grade inflammation [20]. Population studies demonstrated a strong correlation between proinflammatory biomarkers (such as C-reactive protein, IL-6 and TNF-α) and perturbations in glucose homeostasis, obesity and atherosclerosis [21,22]. Adipocytes contribute to this inflammation by producing proinflammatory adipokines. In addition, macrophages that frequently infiltrate the adipose tissue of obese persons produce inflammatory chemokines [23,24].
Another emerging risk factor is oxidized LDL (ox-LDL), which activates circulating monocytes, thereby increasing their ability to infiltrate the vascular wall. This increased infiltration is a primary stage in atherogenesis [25]. Recent data suggest that increased oxidative stress in adipose tissue is an early instigator of the metabolic syndrome and that the redox state in adipose tissue is a potentially useful therapeutic target for the obesity-associated metabolic syndrome [26].
Our aim is to discuss the relation between ox-LDL and the metabolic syndrome. We will outline mechanisms through which the metabolic syndrome can be related to the oxidation of LDL and give examples of interventions that lower metabolic syndrome factors and ox-LDL in experimental models. We will discuss assays for measuring circulating ox-LDL and give an overview of population data highlightling the association between ox-LDL and the metabolic syndrome. Furthermore, we will introduce mechanisms through which ox-LDL could be involved in the pathogenesis of the metabolic syndrome.
Relationship between metabolic syndrome & oxidized LDL: findings in mouse studies
Recently, we obtained a mouse model of the metabolic syndrome that allowed the study of molecular mechanisms, explaining the relationship between the metabolic syndrome components and increased oxidative stress. Indeed, we found that mice with combined leptin (ob/ob) and LDL-receptor deficiency (LDLR-/-; double knockout [DKO] mice) are obese and exhibited severe hypertriglyceridemia, hypertension and insulin resistance and diabetes. This combination of metabolic syndrome components was associated with accelerated atherosclerosis due to an increased accumulation of macrophages in association with endothelial dysfunction evidenced by increased expression of VCAM-1 and ICAM-1 in the aorta of DKO mice [27]. Increased macrophage accumulation was associated with elevated plaque ox-LDL. The latter could be partly attributed to increased myeloperoxidase production by plaque macrophages. In addition, impaired HDL-associated antioxidant activity in the blood [28] was associated with more ox-LDL in the plaques. By means of adenovirus-mediated gene transfer, we demonstrated that over-expression of human paraoxonase (PON)-1 significantly decreased the amount of ox-LDL and the number of macrophages in the plaques, thereby reducing total plaque volume. Interestingly, Hansel and colleagues demonstrated that the metabolic syndrome is associated with dysfunctional dense HDL particles and elevated oxidative stress [29]. The group of Mackness, who collaborated in the PON gene transfer study, later showed a decrease in PON activity that was associated with a defective metabolism of oxidized phospholipids by HDL from patients with Type 2 diabetes [30].
We then further investigated the relationship between metabolic syndrome components and the oxidation of LDL by assessing the effect of weight loss. We selected this intervention because it had been demonstrated that CHD risk factors in obese persons vary as a function of being insulin-resistant or insulin-sensitive; and weight loss is effective in reducing CHD risk in insulin-resistant, obese persons [31]. Figure 1 demonstrates that weight loss in obese mice was associated with a decrease of metabolic syndrome components resulting in reduced inflammation and oxidative stress. Ultimately, these changes led to inhibition of atherosclerosis and an improvement of cardiac function [32].
Figure 1. Effects of weight loss in obese mice.
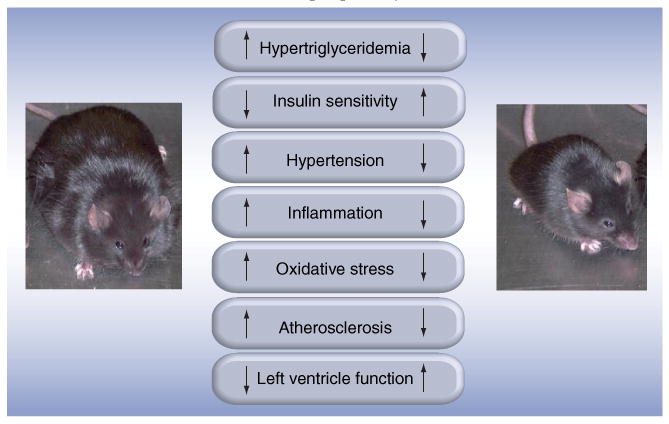
Mice deficient in both the LDL receptor and the leptin gene feature most of the metabolic syndrome components associated with increased oxidative stress and inflammation and, thereby, with accelerated atherogenesis and loss of left ventricle function. Weight loss is associated with an improvement of the metabolic profile associated with inhibition of atherogenesis, increase of plaque stability and improved left ventricle function. Our observations in obese mice are relevant for humans. Indeed, the metabolic syndrome is associated with higher cardiovascular risk, and weight loss decreases this risk.
The inhibition of atherosclerosis was due to a decreased accumulation of macrophages and deposition of ox-LDL. The latter was partly due to improved balance between pro-oxidant and antioxidant enzymes in the adipose tissue. First, weight loss was associated with a reduction of the expression of arachidonate-5-lipoxygenase and of its activating peptide, which catalyzes LDL oxidation. Second, weight loss was associated with increased production of superoxide dismutase (SOD)3, which prevents LDL oxidation [32]. We demonstrated that induction of PPARs in visceral adipose tissue after weight loss correlated positively with SOD3 expression. Decreased ox-LDL in the aorta was also caused by induction of the peroxisome proliferator-activated receptors (PPARs), which correlated with the expression of SOD1 in the aortic arch [32].
We then determined whether those molecular mechanisms were shared with other interventions that were known to decrease insulin sensitivity and the oxidation of LDL. It has previously been demonstrated in man that statins reduce insulin resistance [33–36] and inhibit lipid and lipoprotein oxidation [37–39]. Therefore, we investigated the effect of rosuvastatin on their occurrence in relation to protection against atherosclerosis and sought common mechanisms with weight loss [40]. The selected daily dosage of 10 mg/kg had no effect on weight, cholesterol levels or lipoprotein distribution. However, it reduced triglyceride and free fatty acid levels and decreased glucose and insulin resulting in an increase of insulin sensitivity. Rosuvastatin decreased plaque volume and plaque-ox-LDL. It increased the expression of SOD1, CD36 and LXR-α, ABCA-1 and PPAR-γ, but not of PPAR-α. The expression of PPAR-γ correlated with the expressions of SOD1, LXR-α, ABCA-1 and CD36, which correlated inversely with plaque-ox-LDL. The rosuvastatin-associated increase in SOD1 mRNA expression in the aorta was associated with an increase in SOD1 protein, which was inversely related to the amount of ox-LDL in the plaque. Therefore, we hypothesized that the induction of SOD1, possibly through induction of PPAR-γ, is an important mechanism for preventing oxidation of LDL in the arterial wall. We tested this hypothesis by investigating the effect of rosuvastatin on PPAR-γ and SOD1 expression in endothelial cells in vitro. We demonstrated that rosuvastatin increased PPAR-γ and SOD1 expression in isolated endothelial cells. Both GW9662, a PPAR-γ-specific antagonist, and siRNA raised against PPAR-γ abrogated rosuvastatin's effect [41]. Recently, we demonstrated that rosiglitazone (a PPAR-γ agonist), but not fenofibrate (a PPAR-α agonist), increased SOD1 expression and reduced ox-LDL.
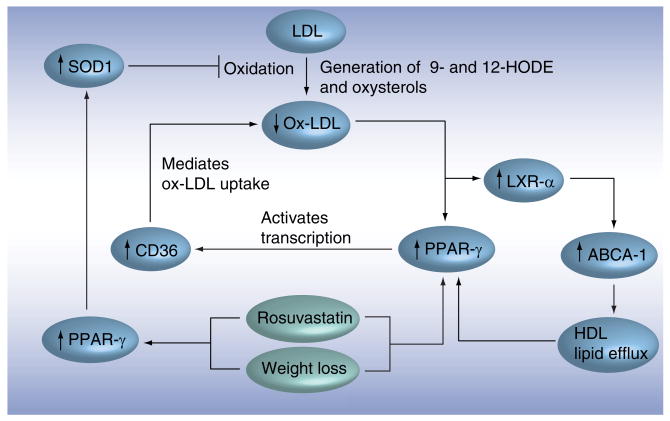
Common mechanisms that explain the similar antiatherogenic effects of weight loss and rosuvastatin treatment in the aorta are presented in Figure 2. We identified SOD1 as a potentially important mediator of the prevention of ox-LDL accumulation within atherosclerotic plaques. The observed induction of PPAR-γ in the aorta and the inverse relation with ox-LDL in the plaque adds to its important role in regulating oxidative stress and inflammation. Our observations in mouse models prompted us to investigate the relationship between the metabolic syndrome and ox-LDL in humans. Those studies required sensitive and specific assays for measuring ox-LDL in the blood.
Figure 2. Common effects of weight loss and rosuvastatin treatment in obese mice.
In the aortic arch, we identified PPAR-γ as a regulator of oxidative stress and inflammation. Induction of PPAR-γ results in an increase of SOD1 that is associated with a reduction of the oxidation of LDL by decreasing reactive oxygen species. Induction of PPAR-γ is also associated with increased expression of CD36, resulting in increased uptake of oxidized LDL. The reduction of plaque-oxidized LDL results in an increased PPAR-γ expression that, through LXR, induces the expression of ABCA-1. This is crucial for the efflux of inflammatory lipids, which tend to reduce PPAR-γ expression, out of the plaque. Arrows indicate activation and flat ends indicate inhibition.
ABCA-1: ATP-binding cassette, subfamily A member 1; LXR: Liver X receptor; PPAR: Peroxisome proliferator-activated receptor; SOD: Superoxide dismutase.
Assays for measuring oxidized LDL
Three groups developed assays for oxidation specific epitopes on plasma LDL. The DLH3 and EO6 assay uses antibodies against oxidized phospholipids [42,43]; our assay uses the monoclonal antibody 4E6 directed against an oxidation-dependent epitope in the ApoB100 moiety of LDL [44,45]. Figure 3 is a schematic representation of a sandwich-type and a competition ELISA using the monoclonal antibody 4E6. Both types of assays are commercially available (Mercodia, Uppsala, Sweden). The tremendous advantage of the competition ELISA is that detection of ox-LDL depends on the highly specific antibody only. We observed good agreement between the original laboratory competition ELISA and Mercodia's competition ELISA [46]. The configuration of the DLH3 assay is similar to that of the 4E6 sandwich-type assay; DLH3 is immobilized. A limitation of the DLH3 assay is that LDL fractions have to be isolated from plasma preventing mass analysis of plasma samples. The E06 assay is also a sandwich-type ELISA. However, the anti-ApoB100 antibody MB47 is the capturing antibody; the monoclonal antibody EO6 is the tagging antibody. This antibody reacts with oxidized phospholipids not only in LDL but also in other lipoproteins, such as Lp(a) [47]. Recent data by Witztum's group point to Lp(a) as a preferential carrier of oxidized phospholipids. This means that for a proper appreciation of the presented clinical data, the levels of Lp(a) have to be taken into account [48].
Figure 3. Configuration of the 4E6 sandwich-type and competition ELISA.
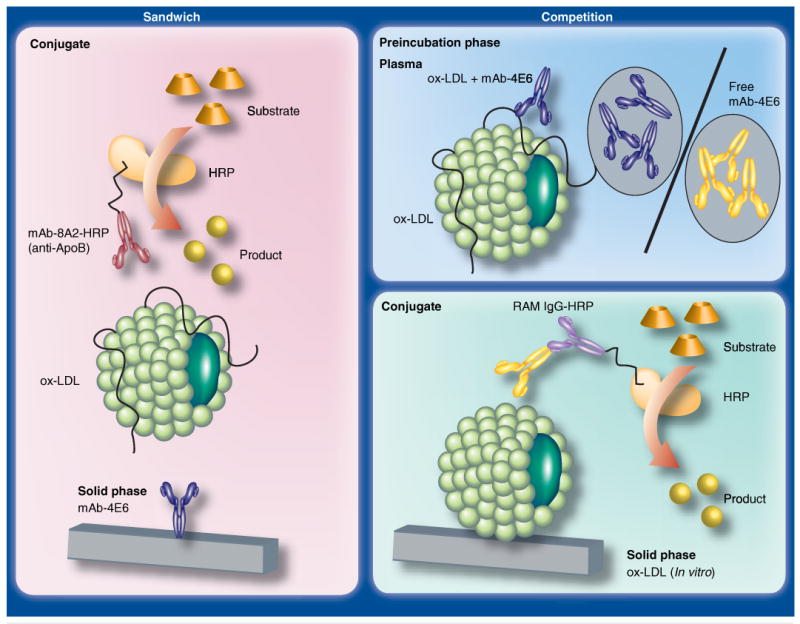
The sandwich-type ELISA uses ox-LDL-specific monoclonal antibody 4E6 as the capturing antibody and the anti-ApoB100-specific antibody 8A2 as the tagging antibody. The latter is conjugated with HRP, which reacts with a specific substrate to yield a yellow-colored reaction product; this is quantified in the spectrophotometer. The competition ELISA requires preincubation of the plasma sample with 4E6. The sample is then applied to a microtiter plate, on which in vitro ox-LDL is immobilized. There, the ox-LDL in the plasma and the in vitro ox-LDL compete for 4E6. After washing, 4E6 bound to the immobilized ox-LDL is detected with HRP conjugated rabbit-anti-mouse antibodies. The reaction is completed as in the sandwich-type ELISA. HRP: Horseradish peroxidase; ox-LDL: Oxidized LDL.
It is generally believed that fully oxidized LDL does not exist in the circulation; blood is rich in antioxidants. In addition, such highly oxidized particles would be rapidly cleared in the liver via scavenger receptors [49]. In contrast, circulating minimally oxidized LDL, in which oxidative modification has not been sufficient to cause changes recognized by scavenger receptors, was demonstrated [50]. Therefore, all assays for ox-LDL presumably detect minimally oxidized LDL. This ox-LDL is only a minor fraction of LDL ranging from 0.001% in healthy controls [51] to approximately 5% in patients with acute coronary events [44]. Since LDL is the substrate for oxidation, concentrations of ox-LDL correlate with LDL concentrations and, in turn, with the cholesterol within LDL. In addition, concentrations of ox-LDL depend on the sensitivity of LDL particles to oxidation; small dense LDLs contain smaller amounts of antioxidants and are, therefore, more prone to oxidation [52]. Figure 4 demonstrates that various mechanisms lead to the oxidation of LDL, indicating that various interventions will be needed to block it efficiently.
Figure 4. Mechanisms of oxidation of LDL.
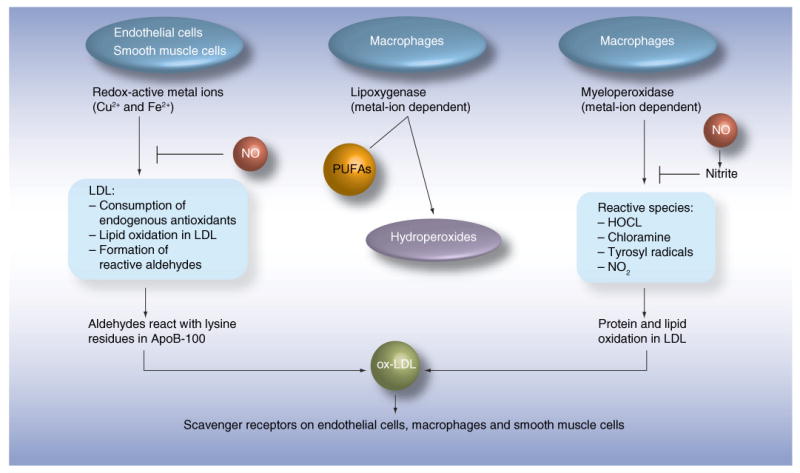
Several cell types and cell-mediated enzymatic reactions can lead to the oxidation of LDL. The common process is the formation of aldehydes, which interact with lysine residues in the ApoB100 protein in LDL. The antibody 4E6 is directed against a conformational epitope generated by this interaction. Interestingly, not only cells in the vessel wall, but also cells in adipose tissues, mediate oxidation reactions. Arrows indicate activation and flat ends indicate inhibition. HOCL: Hypochlorous acid; NO: Nitric oxide; ox-LDL: Oxidized LDL; PUFA: Polyunsaturated fatty acid.
Metabolic syndrome is associated with elevated levels of circulating oxidized LDL & high risk of myocardial infarction
In the Health ABC cohort, comprising 3033 participants aged 70–79 years, we demonstrated that ox-LDL was elevated in persons with high predicted CHD risk (according to adjusted Framingham scoring) before any events [53]. High predicted CHD risk, as defined according to the third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (ATP III), was a 10-year risk for a CHD event above 20% by Framingham scoring, or diabetes or the occurrence of noncoronary forms of cardiovascular disease. The odds ratio (OR) for high predicted CHD risk status for persons in the highest quintile of ox-LDL, compared with those in the lowest quintile and adjusted for age, gender, ethnicity, smoking, LDL-cholesterol (LDL-C)and C-reactive protein, was higher than three. Addition of ox-LDL to the established risk factors may improve cardiovascular risk prediction [45]. We then demonstrated that the metabolic syndrome in this cohort was associated with a higher cardiovascular risk [54]. Therefore, our objective was to establish the association between the metabolic syndrome and ox-LDL and to determine the risk for CHD in relation to the metabolic syndrome and ox-LDL.
Metabolic syndrome components were defined according to ATP III [1]:
Waist circumference greater than or equal to 102 cm in men and greater than or equal to 88 cm in women;
Fasting triglycerides greater than or equal to 150 mg/dl (1.70 mmol/l);
HDL-cholesterol (HDL-C) less than 40 mg/dl (1.03 mmol/l) in men and less than 50 mg/dl (1.29 mmol/l) in women;
Blood pressure greater than or equal to 130/85 mmHg or on antihypertensive medication;
Fasting-glucose greater than or equal to 100 mg/dl (5.55 mmol/l) or on antidiabetic medication.
Persons with at least three of these components were defined as having metabolic syndrome [1–4].
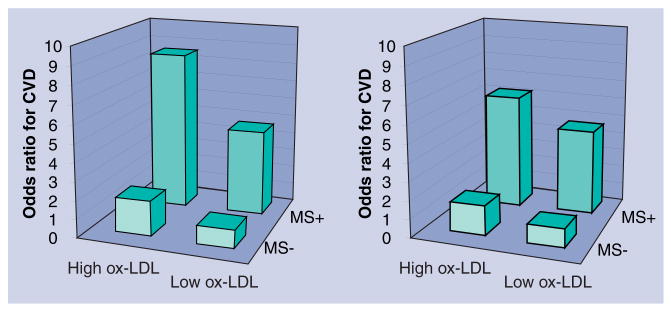
Compared with participants without the metabolic syndrome, the OR for high ox-LDL (>1.90 mg/dl) in participants with the metabolic syndrome was 1.82, after adjusting for age, sex, ethnicity and smoking status. No interaction with sex and ethnicity was observed. After further adjustment for LDL-C, the OR was 2.01. When ox-LDL was expressed as the percentage of LDL, the adjusted OR for high ox-LDL (>1.58%) was 2.56. Higher waist circumference and levels of triglycerides, insulin, glucose and HbA1c (adjusted for glucose and insulin) and lower levels of HDL-C were associated with higher adjusted prevalence of high ox-LDL. No significant association between blood pressure and ox-LDL was observed [55]. The prevalence of cardiovascular disease was higher in individuals with the metabolic syndrome and with higher levels of ox-LDL. It was the highest in persons with the metabolic syndrome combined with elevated ox-LDL (Figure 5).
Figure 5. Prevalence of cardiovascular disease according to the presence of the metabolic syndrome and high oxidized LDL.
As expected, the metabolic syndrome was associated with a higher odds ratio for cardiovascular disease. In agreement with our earlier studies, high ox-LDL was associated with a higher prevalence. The highest prevalence was observed in persons with the metabolic syndrome and high ox-LDL. In the left panel, absolute levels of ox-LDL (expressed in mg/dl) were used. In the right panel, the ox-LDL-to-LDL-cholesterol ratios were used. Data are from the Health ABC study [56].
CVD: Cardiovascular disease; MS: Metabolic syndrome; OR: Odds ratio; ox-LDL: Oxidized LDL.
We determined the relative risk of myocardial infarction (MI) in relation to the metabolic syndrome and ox-LDL, adjusted for age, sex, ethnicity and smoking status. Those with the metabolic syndrome had a twofold higher risk. We also divided the cohort into five groups by levels of ox-LDL. The risk ratio for persons in the highest quintile was 2.25 (95% CI: 1.22–24.15). After adjusting for the metabolic syndrome, the risk ratio for persons in the highest quintile of ox-LDL was 1.9 (95% CI: 1.1–3.5).
In summary, we demonstrated in a population cohort that the metabolic syndrome is associated with a higher fraction of ox-LDL and, thus, with higher levels of circulating ox-LDL. As expected, dyslipidemia (low HDL-C and high triglycerides) was associated with high levels of ox-LDL. It had been demonstrated in healthy, nondiabetic volunteers that plasma glucose and insulin levels correlate with a higher susceptibility of ex vivo oxidation of LDL. Here, we demonstrated that hyperinsulinemia and impaired glycemic control, independent of lipid levels, are associated with increased in vivo LDL oxidation, as reflected by the higher prevalence of high ox-LDL. This association was consistent across sex and ethnicity. Our data further support the importance of identifying individuals with the metabolic syndrome as a high-risk group for developing CHD. Finally, our study identified the oxidation of LDL as a potential mechanism explaining the increased risk for MI among those with the metabolic syndrome.
Interestingly, we confirmed the relationship between the metabolic syndrome components and the increase in circulating ox-LDL in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, and demonstrated that ox-LDL is associated with subclinical cardiovascular disease by its relationship with many cardiovascular risk factors [56]. The association between the metabolic syndrome and elevated levels of ox-LDL has been confirmed in European and Japanese cohorts [57–59]. In addition, two recent studies indicated that levels of circulating ox-LDL predict future cardiovascular events, even after adjustment for traditional cardiovascular risk factors and C-reactive protein [60,61].
Circulating oxidized LDL is associated with high risk of the metabolic syndrome
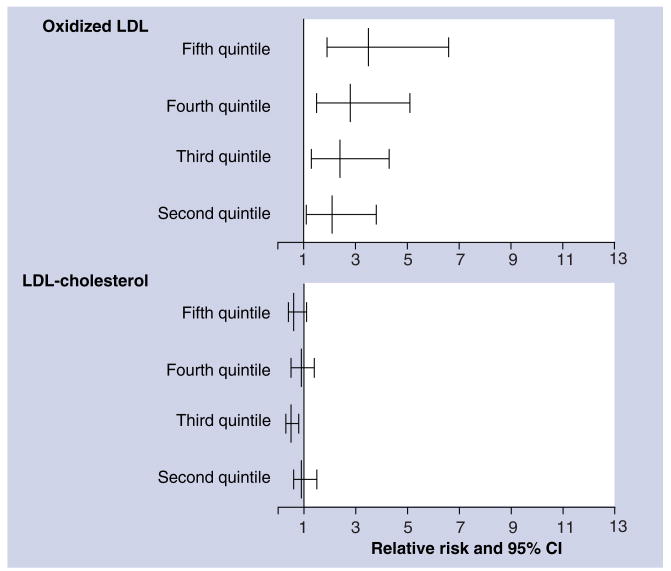
We determined the longitudinal association of ox-LDL and incident metabolic syndrome in 1889 participants of the Cardiovascular Risk Development in Young Adults (CARDIA) study [62,63]. Participants were recruited between 1985 and 1986 at four clinical sites in the USA: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. The studied CARDIA sample was balanced by age (45% aged 33–39 years, 55% aged 40–45 years), race (52% African–American, 48% white), gender (46% men, 54% women), and education (40% having completed ≤12 years of education, 60% having completed >12 years). Figure 6 shows that elevated ox-LDL, but not elevated LDL-C, was associated with a higher risk of future metabolic syndrome. The adjusted ORs for incidence of dichotomous components of the metabolic syndrome in the highest quantile of ox-LDL compared with the lowest were 2.1 (95% CI: 1.2–3.6) for abdominal obesity, 2.1 (95% CI: 1.1–4.0) for high fasting glucose and 2.4 (95% CI: 1.5–3.8) for high triglycerides [64].
Figure 6. Elevated oxidized LDL is associated with a high risk of the metabolic syndrome.
The data are odds ratios (and 95% CI intervals) for incident metabolic syndrome after 5 years' follow-up in the second to fifth quintiles compared with the lowest quintile of oxidized LDL or LDL-cholesterol. These ratios were adjusted for age, gender, race, study center, cigarette smoking, BMI, physical activity and LDL-cholesterol or oxidized LDL. Data are from the Cardiovascular Risk Development in Young Adults (CARDIA) study [65].
Mechanisms underlying the relationship between oxidized LDL & the metabolic syndrome
A possible explanation for the relationship between ox-LDL and obesity is that ox-LDL may be associated with the increase of adipose tissue. This is in agreement with experimental findings that ox-LDL induces adipocyte proliferation either directly [65] or indirectly by increasing the infiltration of inflammatory monocytes/macrophages that increase adipogenesis [66]. The increase in adipose tissue mass may also be explained by a cellular hypertrophy due to an increased lipid accumulation in the pre-existing adipocytes rather than an increase in cell number or differentiation. Indeed, ox-LDL increases triglyceride production by inducing the expression of lipoprotein lipase [67] and by inducing the accumulation of fatty acids in adipocytes [68]. Interestingly, fatty acids stimulate the accumulation of ceramide, which contributes to inflammation that, as discussed above, is associated with adipose hyperplasia. Ox-LDL was also found to decrease the production of adiponectin that, in contrast with other adipokines, is reduced in obese persons and suppresses excess reactive oxygen species production under high-glucose conditions – an effect that has implications for vascular protection in diabetes [69]. The observed relationship between obesity and ox-LDL and between ox-LDL and the metabolic syndrome, are important to understand the association between obesity and the metabolic syndrome [70].
The association between ox-LDL and hyperglycemia could be due to ox-LDL reducing insulin signaling [71] and glucose uptake [72]. Moreover, ox-LDL causes death of islet β-cells in the pancreas [73]. Hyperglycemia is associated with increased LDL oxidation as glucose impairs the antioxidant properties of serum albumin [74].
Finally, the association between ox-LDL and hypertriglyceridemia could be due to impairment of triglyceride storage and secretion by ox-LDL [75]. Hypertriglyceridemia is associated with higher levels of small dense LDL [76], which is particularly prone to oxidation and has been proven to be more atherogenic than larger LDL particles [77–80].
Lifestyle & therapeutic interventions that lower oxidized LDL
Several studies demonstrated that the relationship between obesity and ox-LDL and weight loss was associated with a decrease of circulating ox-LDL [81–86]. Information concerning the effect of aerobic exercise and dietary supplements is too sparse to draw firm conclusions. Small studies suggest that a gluten-free diet [87], dietary supplementation with grape juice and vitamin E [88] and a cocktail of antioxidants containing a thiol-containing antioxidant (N-acetylcysteine 600 g/d), a bound antioxidant (vitamin E 300 g/d) and an aqueous phase antioxidant (vitamin C 250 mg/d) [89], reduce concentrations of ox-LDL. However, the relationship with the progression of metabolic syndrome remains to be determined.
Some studies demonstrated that statin treatment reduced concentrations of circulating ox-LDL [90–94]. As yet, it is unclear whether this reduction is independent of cholesterol lowering or reduction of metabolic syndrome components as insulin resistance and whether this antioxidant effect is different for different statins. To our knowledge, there are no large studies of the effect of the PPAR agonists on the oxidation of LDL in relation to their capacity to decrease the severity of metabolic syndrome components.
Although numerous in vitro and animal studies have supported the role of ox-LDL in atherosclerosis, and epidemiologic cohort studies with large numbers of men, women and diverse populations have been largely supportive of this hypothesis, interventional trials of isolated antioxidant compounds have been controversial, with some positive findings, many null findings and some suggestion of harm in certain high-risk populations [95]. Owing to the mismatch between the epidemiologic studies and the interventional trials, some researchers have advocated ending antioxidant work. However, we have to bear in mind that most antioxidant therapies that have been tested were not chosen because they were proved to be the best antioxidants, but rather owing to their easy availability. An excellent example is vitamin E. Although easily available, it has many limitations as an anti-oxidant. In fact, in some studies, vitamin E has been shown to have some pro-oxidant effects. Furthermore, the antioxidant defense system is complex and the naturally occurring mix of antioxidants obtained from food may be much more effective in reducing oxidative stress from a multiplicity of oxidative stressors than is any single isolated antioxidant compound. However, it is difficult to carry out clinical trials of food. Another possible explanation for the lack of benefit in clinical trials is that the trials have not lasted long enough. It may be impossible to show the benefits of antioxidant therapy over several years if the therapy is trying to reverse the results of several decades of oxidative stress [96]. In addition, it is critical to select the ideal study patients, in other words, patients in whom high oxidative stress is proven, for example, patients with the metabolic syndrome. Finally, one has to make sure that the selected antioxidant accumulates and exerts its effect in tissues, such as heart and adipose tissues, where the oxidation of LDL preferentially occurs. One possible approach is to target the antioxidant by gene therapy. However, this area is in need of development, including the improvement of gene transfer vectors and transfer protocols to more efficiently transduce different cell types of the cardiovascular system, development of regulatable vectors and diagnostic means for better identification of patients most likely to benefit from gene therapy interventions [97].
Conclusion
We demonstrated that metabolic syndrome components in obese mice are associated with increased oxidative stress and impaired HDL-associated antioxidant defense, which correlated with accelerated atherosclerosis due to increased macrophage infiltration and accumulation of ox-LDL in the aorta. The impaired HDL-associated antioxidant defense was largely due to a decrease in PON1. Our mouse data are relevant for humans as lower PON1 activity was found to be associated with higher concentrations of oxidized phospholipids in patients with Type 2 diabetes.
We established an important relationship between PPAR-γ and SOD1 for the prevention of the oxidation of LDL in the arterial wall. The observed induction of PPAR-γ in the aorta and the inverse relationship with ox-LDL in the plaque adds to its important role in regulating oxidative stress, as well as inflammation.
We demonstrated that the metabolic syndrome is associated with a higher fraction of ox-LDL and, thus, with higher levels of circulating ox-LDL in humans. Hyperinsulinemia and impaired glycemic control, independent of lipid levels, were associated with increased in vivo LDL oxidation, as reflected by the higher prevalence of high ox-LDL. High concentrations of ox-LDL were associated with increased risk of future myocardial infarction, even after adjustment for LDL-C and other established cardiovascular risk factors.
We demonstrated, in a longitudinal study, that higher concentrations of ox-LDL were associated with higher risk of future metabolic syndrome. In particular, we found an association with obesity, hyperglycemia and hypertriglyceridemia. These associations in population cohorts are supported by mechanistic studies in experimental models.
Overall, the reviewed data support the hypothesis that the metabolic syndrome exacerbates ox-LDL in a feedback loop.
Future perspective
Although an association between ox-LDL and the metabolic syndrome has been demonstrated, important mechanistic and clinical questions remain to be addressed. Further studies in cellular and animal models are needed to determine:
Precise molecular mechanisms by which ox-LDL contributes to inflammation in adipose tissue, and how chronic inflammation in adipose tissue contributes to insulin resistance;
Whether ox-LDL contributes to adipose hypertrophy by inducing adipocyte proliferation and/or lipid accumulation;
The effect of ox-LDL on insulin receptor substrate proteins that regulate the metabolic capacity of the liver;
Common mechanisms of LDL oxidation in adipose tissues and in the vessel wall;
How adipokines induce oxidation of LDL or protect ox-LDL from oxidation in the vessel wall.
In addition, large prospective studies are needed to further establish the relationship of ox-LDL levels to lifestyle and therapeutic interventions. Little is known about:
The effect of low-carbohydrate and Mediterranean diets, which seem to improve lipid profiles of ox-LDL and decrease weight more than low-fat diets;
The nature of antioxidant foods or supplements, which effectively prevent LDL oxidation in adipose and cardiac tissues;
The relationship between ox-LDL and the prevalence and incidence of overweight and obesity in the nutritional transition countries where the health burden of obesity-related complications is growing;
Effect of antiobesity medication and bariatric surgery on concentrations of ox-LDL;
Effect of some newer antioxidants (e.g., oxygenated carotenoids [xanthophylls]) [98] in prevention and treatment of cardiovascular disease.
We must be aware that owing to differences in specificity and sensitivity, studies using different assays may yield different outcomes. Therefore, comparative studies of the various assays are needed; various assays should be compared using the same sample sets. In addition, there is a need for an international reference standard and for automated tests.
Executive summary.
Metabolic syndrome
Disorder combining obesity, dyslipidemia, hypertension and insulin resistance.
Its prevalence increases with that of obesity in Western and in nutritional transition countries.
Associated with high cardiovascular risk.
Oxidized LDL
Oxidation of LDL results in the production of oxidized phospholipids and aldehyde-conjugated lysine residues in its ApoB100 moiety, which are targets for antibodies.
Plasma concentration of oxidized LDL depends on the concentration and the size of LDL particles (small, dense LDL are more prone to oxidation) and the imbalance between pro-oxidant and antioxidant molecules in blood and tissues.
Elevated plasma concentrations are associated with a higher incidence of the metabolic syndrome and with higher cardiovascular risk.
Leads to tissue infiltration of macrophages and inflammation.
Pro-oxidant & antioxidant enzymes related to the oxidation of LDL
Myeloperoxidase and lipoxygenases are oxidant enzymes associated with macrophages that oxidize LDL in the arterial wall.
Paraoxonase is an antioxidant enzyme associated with HDL. Its plasma activity is reduced in persons with the metabolic syndrome with elevated plasma oxidized LDL.
Superoxide dismutases are antioxidant enzymes associated with macrophages. They may provide cardiac protection by inhibiting insulin resistance-associated oxidation of LDL.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Paul Holvoet, Atherosclerosis & Metabolism Unit, Department of Cardiovascular Diseases, Katholieke Universiteit Leuven, Herestraat 49, B-3000 Leuven, Belgium, Tel.: +32 16 347 149, Fax: + 32 16 347 114, paul.holvoet@med.kuleuven.be.
Dieuwke De Keyzer, Atherosclerosis & Metabolism Unit, Department of Cardiovascular Diseases, Katholieke Universiteit Leuven, Herestraat 49, B-3000 Leuven, Belgium, Tel.: +32 16 346 228, Fax: +32 16 347 114, Dieuwke.DeKeyzer@med.kuleuven.be.
David R Jacobs, Jr, Division of Epidemiology & Community Health, University of Minnesota, 1300 South 2nd Street, Ste 300, Minneapolis, MN 55454, USA, Tel.: +1 612 624 4196, Fax: +1 612 624 0315, jacob004@umn.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Adult Treatment Panel III: Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173(2):309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 5.Girman CJ, Rhodes T, Mercuri M, et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 2004;93(2):136–141. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110(10):1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 7.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with Type II diabetes. Diabetologia. 2001;44(9):1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 8.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100(2):123–128. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 11.Onat A, Ceyhan K, Basar O, Erer B, Toprak S, Sansoy V. Metabolic syndrome: major impact on coronary risk in a population with low cholesterol levels – a prospective and cross-sectional evaluation. Atherosclerosis. 2002;165(2):285–292. doi: 10.1016/s0021-9150(02)00236-8. [DOI] [PubMed] [Google Scholar]
- 12.Stern MP, Williams K, Hunt KJL. Impact of diabetes/metabolic syndrome in patients with established cardiovascular disease. Atheroscler Suppl. 2005;6(2):3–6. doi: 10.1016/j.atherosclerosissup.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Goetz FC, Roel J, Jacobs DR, Jr, et al. Declining β-cell function in Type 2 diabetes: 5-year follow-up and immunologic studies of the population of Wadena, MN. Metabolism. 2002;51(2):144–148. doi: 10.1053/meta.2002.29974. [DOI] [PubMed] [Google Scholar]
- 14.Fagerberg B, Bondjers L, Nilsson P. Low birth weight in combination with catch-up growth predicts the occurrence of the metabolic syndrome in men at late middle age: the Atherosclerosis and Insulin Resistance study. J Intern Med. 2004;256(3):254–259. doi: 10.1111/j.1365-2796.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 15.Sinaiko AR, Steinberger J, Moran A, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors and oxidative stress during adolescence. Circulation. 2005;111(15):1985–1991. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 16.Sjoholm A, Nystrom T. Inflammation and the etiology of Type 2 diabetes. Diabetes Metab Res Rev. 2006;22(1):4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 17.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9 4:228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 18.James PT, Rigby N, Leach R. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Prev Rehabil. 2004;11(1):3–8. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 19.York DA, Rossner S, Caterson I, et al. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group I: worldwide demographics of obesity. Circulation. 2004;110(18):E463–E470. doi: 10.1161/01.CIR.0000140125.26161.49. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca V, Desouza C, Asnani S, Jialal I. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev. 2004;25(1):153–175. doi: 10.1210/er.2002-0034. [DOI] [PubMed] [Google Scholar]
- 21.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of Type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing Type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 23.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14(12):1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 24.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 25.Cipolletta C, Ryan KE, Hanna EV, Trimble ER. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes. 2005;54(9):2779–2786. doi: 10.2337/diabetes.54.9.2779. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertens A, Verhamme P, Bielicki JK, et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107(12):1640–1646. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- 28.Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J. 2001;15(12):2073–2084. doi: 10.1096/fj.01-0273rev. [DOI] [PubMed] [Google Scholar]
- 29.Hansel B, Giral P, Nobecourt E, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89(10):4963–4971. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 30.Mastorikou M, Mackness M, Mackness B. Defective metabolism of oxidized phospholipid by HDL from people with Type 2 diabetes. Diabetes. 2006;55(11):3099–3103. doi: 10.2337/db06-0723. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin T, Abbasi F, Kim HS, Lamendola C, Schaaf P, Reaven G. Relationship between insulin resistance, weight loss and coronary heart disease risk in healthy, obese women. Metabolism. 2001;50(7):795–800. doi: 10.1053/meta.2001.24210. [DOI] [PubMed] [Google Scholar]
- 32.Verreth W, De Keyzer D, Pelat M, et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-γ correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110(20):3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]; ▪▪ Reveals the associations between weight loss-induced improvement of insulin sensitivity in adipose tissue, the induction of PPARs and the reduction of oxidative stress in the vascular cells. This finding is important in view of the earlier report [31] demonstrating that weight loss reduces cardiovascular risk more in insulin-resistant than in insulin-sensitive obese women.
- 33.Costa A, Casamitjana R, Casals E, et al. Effects of atorvastatin on glucose homeostasis, post- prandial triglyceride response and C-reactive protein in subjects with impaired fasting glucose. Diabet Med. 2003;20(9):743–745. doi: 10.1046/j.1464-5491.2003.00993.x. [DOI] [PubMed] [Google Scholar]
- 34.Guclu F, Ozmen B, Hekimsoy Z, Kirmaz C. Effects of a statin group drug, pravastatin, on the insulin resistance in patients with metabolic syndrome. Biomed Pharmacother. 2004;58(10):614–618. doi: 10.1016/j.biopha.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Paniagua JA, Lopez-Miranda J, Escribano A, et al. Cerivastatin improves insulin sensitivity and insulin secretion in early-state obese Type 2 diabetes. Diabetes. 2002;51(8):2596–2603. doi: 10.2337/diabetes.51.8.2596. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates that a statin increases insulin sensitivity associated with decreased lipid oxidation.
- 36.Sonmez A, Baykal Y, Kilic M, et al. Fluvastatin improves insulin resistance in nondiabetic dyslipidemic patients. Endocrine. 2003;22(2):151–154. doi: 10.1385/endo:22:2:151. [DOI] [PubMed] [Google Scholar]
- 37.Parker RA, Huang Q, Tesfamariam B. Influence of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors on endothelial nitric oxide synthase and the formation of oxidants in the vasculature. Atherosclerosis. 2003;169(1):19–29. doi: 10.1016/s0021-9150(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 38.Stoll LL, McCormick ML, Denning GM, Weintraub NL. Antioxidant effects of statins. Drugs Today (Barc) 2004;40(12):975–990. doi: 10.1358/dot.2004.40.12.872573. [DOI] [PubMed] [Google Scholar]
- 39.Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20(1):61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Verreth W, De Keyzer D, Davey PC, et al. Rosuvastatin restores superoxide dismutase expression and inhibits accumulation of oxidized LDL in the aortic arch of obese dyslipidemic mice. Br J Pharmacol. 2007;151(3):347–355. doi: 10.1038/sj.bjp.0707231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desjardins F, Sekkali B, Verreth W, et al. Rosuvastatin increases vascular endothelial PPARγ expression and corrects blood pressure variability in obese dyslipidaemic mice. Eur Heart J. 2008;29(1):128–137. doi: 10.1093/eurheartj/ehm540. [DOI] [PubMed] [Google Scholar]
- 42.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103(15):1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 43.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41(3):360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 44.Holvoet P, Vanhaecke J, Janssens S, Van deWerf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98(15):1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 45.Holvoet P, Mertens A, Verhamme P, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21(5):844–848. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 46.Holvoet P, Macy E, Landeloos M, et al. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin Chem. 2006;52(4):760–764. doi: 10.1373/clinchem.2005.064337. [DOI] [PubMed] [Google Scholar]
- 47.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 48.Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49(10):2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266(4):2282–2289. [PubMed] [Google Scholar]
- 50.Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. 1988;8(1):79–87. [PubMed] [Google Scholar]
- 51.Shoji T, Nishizawa Y, Fukumoto M, et al. Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis. 2000;148(1):171–177. doi: 10.1016/s0021-9150(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 52.Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron Artery Dis. 1998;9(8):473–481. doi: 10.1097/00019501-199809080-00002. [DOI] [PubMed] [Google Scholar]
- 53.Holvoet P, Harris TB, Tracy RP, et al. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly: findings from the Health, Aging and Body Composition study. Arterioscler Thromb Vasc Biol. 2003;23(8):1444–1448. doi: 10.1161/01.ATV.0000080379.05071.22. [DOI] [PubMed] [Google Scholar]
- 54.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47(8):1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 55.Holvoet P, Kritchevsky SB, Tracy RP, et al. The metabolic syndrome, circulating oxidized LDL and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53(4):1068–1073. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]; ▪ First report supporting the relationship between metabolic syndrome and elevated plasma oxidized LDL and the high risk of acute cardiovascular events.
- 56.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;194(1):245–252. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Lapointe A, Couillard C, Piche ME, et al. Circulating oxidized LDL is associated with parameters of the metabolic syndrome in postmenopausal women. Atherosclerosis. 2007;191(2):362–368. doi: 10.1016/j.atherosclerosis.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 58.Weinbrenner T, Schroder H, Escurriol V, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006;83(1):30–35. doi: 10.1093/ajcn/83.1.30. [DOI] [PubMed] [Google Scholar]
- 59.Yamagishi SI, Matsuoka H, Kitano S, et al. Elevated circulating oxidized LDL levels in Japanese subjects with the metabolic syndrome. Int J Cardiol. 2007;118(2):270–272. doi: 10.1016/j.ijcard.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 60.Johnston N, Jernberg T, Lagerqvist B, Siegbahn A, Wallentin L. Improved identification of patients with coronary artery disease by the use of new lipid and lipoprotein biomarkers. Am J Cardiol. 2006;97(5):640–645. doi: 10.1016/j.amjcard.2005.09.123. [DOI] [PubMed] [Google Scholar]
- 61.Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112(5):651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]; ▪▪ Demonstrates that plasma oxidized LDL is the strongest predictor of acute cardiovascular events compared with a conventional lipoprotein profile and other traditional cardiovascular risk factors.
- 62.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12 1:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 63.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 64.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299(19):2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Identifies plasma oxidized LDL, independent of LDL cholesterol, as a risk factor for metabolic syndrome and its components obesity, hyperglycemia and hypertriglyceridemia.
- 65.Masella R, Vari R, D'Archivio M, et al. Oxidised LDL modulate adipogenesis in 3T3-L1 preadipocytes by affecting the balance between cell proliferation and differentiation. FEBS Lett. 2006;580(10):2421–2429. doi: 10.1016/j.febslet.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura S, Manabe I, Nagasaki M, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56(6):1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 67.Stengel D, Antonucci M, Gaoua W, et al. Inhibition of LPL expression in human monocyte-derived macrophages is dependent on LDL oxidation state: a key role for lysophosphatidylcholine. Arterioscler Thromb Vasc Biol. 1998;18(7):1172–1180. doi: 10.1161/01.atv.18.7.1172. [DOI] [PubMed] [Google Scholar]
- 68.Merkel M, Heeren J, Dudeck W, et al. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J Biol Chem. 2002;277(9):7405–7411. doi: 10.1074/jbc.M107914200. [DOI] [PubMed] [Google Scholar]
- 69.Ouedraogo R, Wu X, Xu SQ, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55(6):1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]; ▪ Demonstrates that adiponectin reduces oxidative stress associated with high-glucose in vascular cells.
- 70.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]; ▪▪ Demonstrates that visceral obesity, associated with elevated oxidized LDL, is related to high risk of the metabolic syndrome.
- 71.Maziere C, Morliere P, Santus R, et al. Inhibition of insulin signaling by oxidized low density lipoprotein. Protective effect of the antioxidant vitamin E. Atherosclerosis. 2004;175(1):23–30. doi: 10.1016/j.atherosclerosis.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of α-lipoic acid. Diabetes. 2001;50(2):404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 73.Cnop M, Hannaert JC, Grupping AY, Pipeleers DG. Low density lipoprotein can cause death of islet β-cells by its cellular uptake and oxidative modification. Endocrinology. 2002;143(9):3449–3453. doi: 10.1210/en.2002-220273. [DOI] [PubMed] [Google Scholar]
- 74.Bourdon E, Loreau N, Blache D. Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J. 1999;13(2):233–244. doi: 10.1096/fasebj.13.2.233. [DOI] [PubMed] [Google Scholar]
- 75.Koonen DP, Jacobs RL, Febbraio M, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 76.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23(1):97–104. [PubMed] [Google Scholar]
- 77.Chancharme L, Therond P, Nigon F, Lepage S, Couturier M, Chapman MJ. Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler Thromb Vasc Biol. 1999;19(3):810–820. doi: 10.1161/01.atv.19.3.810. [DOI] [PubMed] [Google Scholar]
- 78.Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95(1):69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 79.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 80.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 81.Knopp RH, Paramsothy P. Oxidized LDL and abdominal obesity: a key to understanding the metabolic syndrome. Am J Clin Nutr. 2006;83(1):1–2. doi: 10.1093/ajcn/83.1.1. [DOI] [PubMed] [Google Scholar]
- 82.Linna MS, Borg P, Kukkonen-Harjula K, et al. Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int J Obes (Lond) 2007;31(2):245–253. doi: 10.1038/sj.ijo.0803413. [DOI] [PubMed] [Google Scholar]
- 83.Porreca E, Di FC, Moretta V, et al. Circulating leptin is associated with oxidized LDL in postmenopausal women. Atherosclerosis. 2004;175(1):139–143. doi: 10.1016/j.atherosclerosis.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Rector RS, Warner SO, Liu Y, et al. Exercise and diet induced weight loss improves measures of oxidative stress and insulin sensitivity in adults with characteristics of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293(2):500–506. doi: 10.1152/ajpendo.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes Lond. 2006;30(10):1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 86.Vasankari T, Fogelholm M, Kukkonen-Harjula K, et al. Reduced oxidized low-density lipoprotein after weight reduction in obese premenopausal women. Int J Obes Relat Metab Disord. 2001;25(2):205–211. doi: 10.1038/sj.ijo.0801533. [DOI] [PubMed] [Google Scholar]
- 87.Elkan AC, Sjoberg B, Kolsrud B, Ringertz B, Hafstrom I, Frostegard J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther. 2008;10(2):34. doi: 10.1186/ar2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castilla P, Davalos A, Teruel JL, et al. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am J Clin Nutr. 2008;87(4):1053–1061. doi: 10.1093/ajcn/87.4.1053. [DOI] [PubMed] [Google Scholar]
- 89.Neri S, Signorelli SS, Torrisi B, et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: a single-blind, 15-day clinical trial in patients with untreated Type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin Ther. 2005;27(11):1764–1773. doi: 10.1016/j.clinthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Choi SH, Chae A, Miller E, et al. Relationship between biomarkers of oxidized low-density lipoprotein, statin therapy, quantitative coronary angiography, and atheroma: volume observations from the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study. J Am Coll Cardiol. 2008;52(1):24–32. doi: 10.1016/j.jacc.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 91.Ky B, Burke A, Tsimikas S, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51(17):1653–1662. doi: 10.1016/j.jacc.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 92.Paniagua JA, Lopez-Miranda J, Perez-Martinez P, et al. Oxidized-LDL levels are changed during short-term serum glucose variations and lowered with statin treatment in early Type 2 diabetes: a study of endothelial function and microalbuminuria. Diabet Med. 2005;22(12):1647–1656. doi: 10.1111/j.1464-5491.2005.01703.x. [DOI] [PubMed] [Google Scholar]
- 93.Shin MJ, Chung N, Lee JH, et al. Effects of simvastatin on plasma antioxidant status and vitamins in hypercholesterolemic patients. Int J Cardiol. 2007;118(2):173–177. doi: 10.1016/j.ijcard.2006.03.089. [DOI] [PubMed] [Google Scholar]
- 94.Tavridou A, Efthimiadis A, Efthimiadis I, Paschalidou H. Antioxidant effects of simvastatin in primary and secondary prevention of coronary heart disease. Eur J Clin Pharmacol. 2006;62(6):485–489. doi: 10.1007/s00228-006-0097-z. [DOI] [PubMed] [Google Scholar]
- 95.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am J Cardiol. 2008;101(10A):75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101(10A):14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]; ▪▪ Although numerous in vitro and animal studies have supported the role of oxidized LDL in atherosclerosis, and epidemiologic cohort studies with large numbers of men, women and diverse populations have been largely supportive of this hypothesis, interventional trials have been controversial. Together with [95] this paper discusses reasons for this mismatch.
- 97.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117(16):2142–2150. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 98.Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol. 2008;101(10A):58D–68D. doi: 10.1016/j.amjcard.2008.02.010. [DOI] [PubMed] [Google Scholar]