Abstract
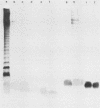
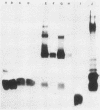
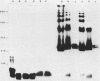
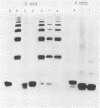
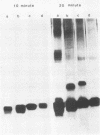
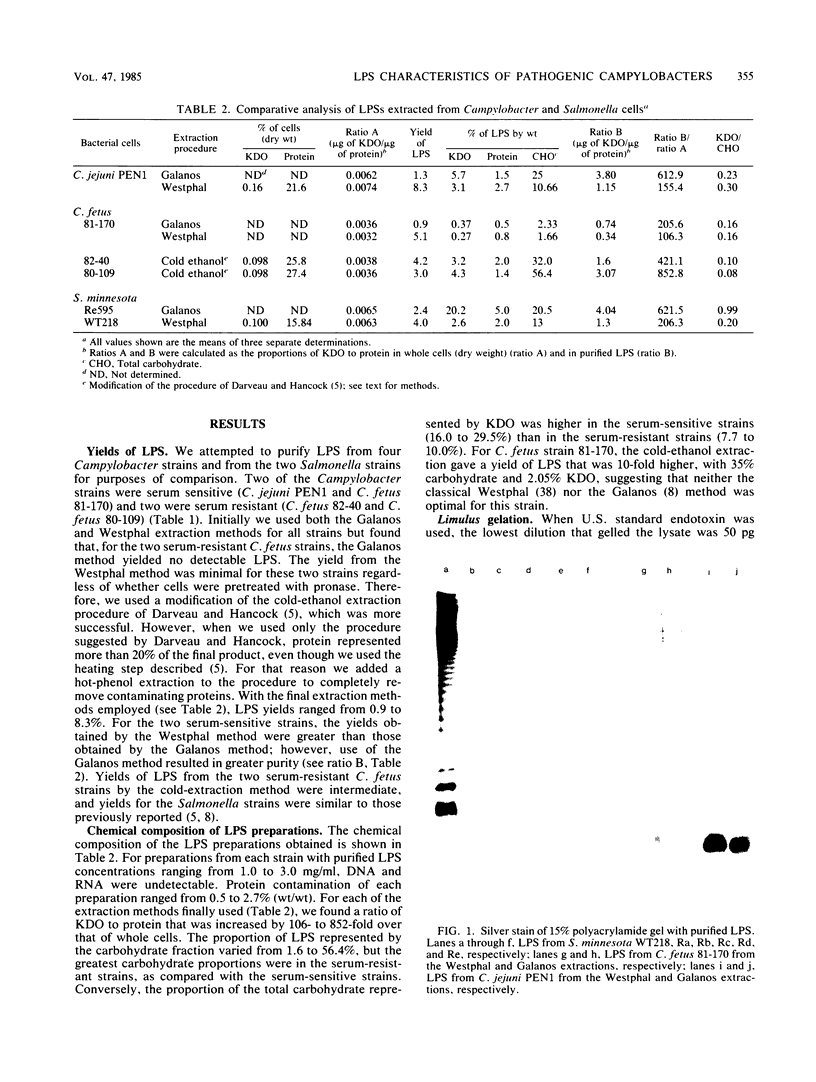
Most Campylobacter jejuni strains are sensitive and most Campylobacter fetus strains are resistant to the bactericidal activity in normal human serum. We purified lipopolysaccharides from Campylobacter strains to determine whether their composition and structure relate to serum susceptibility. The lipopolysaccharide of two serum-sensitive strains was best isolated by the Galanos procedure, but for two serum-resistant strains a cold-ethanol extraction was optimal. For each lipopolysaccharide preparation, the ratio of 2-keto-3-deoxyoctonate to protein was increased by 100 to 1,000-fold over that of whole cells. For serum-resistant strains, total carbohydrates was a high proportion of lipopolysaccharide weight; for serum-sensitive strains, 2-keto-3-deoxyoctanate was a high proportion of total carbohydrates. By polyacrylamide gel electrophoresis, the lipopolysaccharide of serum-sensitive strains appeared rough, but for serum-resistant strains a smooth-type ladder was seen, with a minimal core region and several high-molecular-weight complexes. Proteinase K-treated whole-cell lysates showed polyacrylamide gel electrophoresis profiles similar to that of pure lipopolysaccharide. Proteinase K-treated whole-cell lysates from seven serum-sensitive C. jejuni strains all had rough profiles, and five serum-resistant C. fetus strains all had smooth profiles. These studies indicate that lipopolysaccharide composition may be an important determinant of serum susceptibility among Campylobacter species and that serum resistance is usually associated with a smooth-type lipopolysaccharide.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- DENNIS S. M. Isolation of a lipopolysaccharide from Vibrio fetus. Nature. 1959 Jan 17;183(4655):186–186. doi: 10.1038/183186a0. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Aberrant migration of lipopolysaccharide in sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Eur J Biochem. 1983 Jul 1;133(3):685–688. doi: 10.1111/j.1432-1033.1983.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A., Hollis D. G., Weaver R. E., Lambert M. A., Blaser M. J., Moss C. W. 30 years of campylobacters: biochemical characteristics and a biotyping proposal for Campylobacter jejuni. J Clin Microbiol. 1982 Jun;15(6):1065–1073. doi: 10.1128/jcm.15.6.1065-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McKevitt A. I., Woods D. E. Characterization of Pseudomonas cepacia isolates from patients with cystic fibrosis. J Clin Microbiol. 1984 Feb;19(2):291–293. doi: 10.1128/jcm.19.2.291-293.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz C. S., Apicella M. A., Morse S. A. Electrophoretic and serological characterization of the lipopolysaccharide produced by Neisseria gonorrhoeae. J Infect Dis. 1984 Apr;149(4):544–552. doi: 10.1093/infdis/149.4.544. [DOI] [PubMed] [Google Scholar]
- Munford R. S. Quantitative Limulus lysate assay for endotoxin activity: aggregation of radioiodinated coagulogen monomers. Anal Biochem. 1978 Dec;91(2):509–515. doi: 10.1016/0003-2697(78)90537-7. [DOI] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Isolation and chemical composition of lipopolysaccharide from Campylobacter jejuni. Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):135–139. doi: 10.1111/j.1699-0463.1982.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Niwa M., Milner K. C., Ribi E., Rudbach J. A. Alteration of physical, chemical, and biological properties of endotoxin by treatment with mild alkali. J Bacteriol. 1969 Mar;97(3):1069–1077. doi: 10.1128/jb.97.3.1069-1077.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Lipopolysaccharide banding patterns of Neisseria meningitidis and Neisseria gonorrhoeae. J Clin Microbiol. 1984 Apr;19(4):558–560. doi: 10.1128/jcm.19.4.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progulske A., Holt S. C. Isolation and characterization of the outer membrane and lipopolysaccharide from Eikenella corrodens. Infect Immun. 1984 Jan;43(1):166–177. doi: 10.1128/iai.43.1.166-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig P. J. Campylobacter infections in human beings. J Pediatr. 1979 Jun;94(6):855–864. doi: 10.1016/s0022-3476(79)80202-4. [DOI] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Rowley D. Sensitization of complement resistant bacterial strains. Nature. 1969 Mar 29;221(5187):1259–1261. doi: 10.1038/2211259a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Torres J., Torres N. I., Escamilla E., Ruiz-Palacios B. R., Tamayo J. Cholera-like enterotoxin produced by Campylobacter jejuni. Characterisation and clinical significance. Lancet. 1983 Jul 30;2(8344):250–253. doi: 10.1016/s0140-6736(83)90234-9. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Chun P. W. The dispersion of gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J Biol Chem. 1980 Feb 10;255(3):1221–1226. [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Sensitivity of some smooth strains of Escherichia coli to the bactericidal action of normal human serum. J Clin Pathol. 1974 Aug;27(8):626–629. doi: 10.1136/jcp.27.8.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Winter A. J. An antigenic analysis of Vibrio fetus. 3. Chemical, biologic, and antigenic properties of the endotoxin. Am J Vet Res. 1966 May;27(118):653–658. [PubMed] [Google Scholar]
- Winter A. J., McCoy E. C., Fullmer C. S., Burda K., Bier P. J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978 Dec;22(3):963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]