Abstract
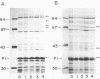
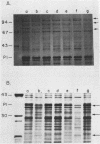
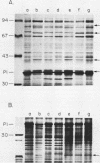
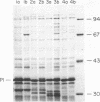
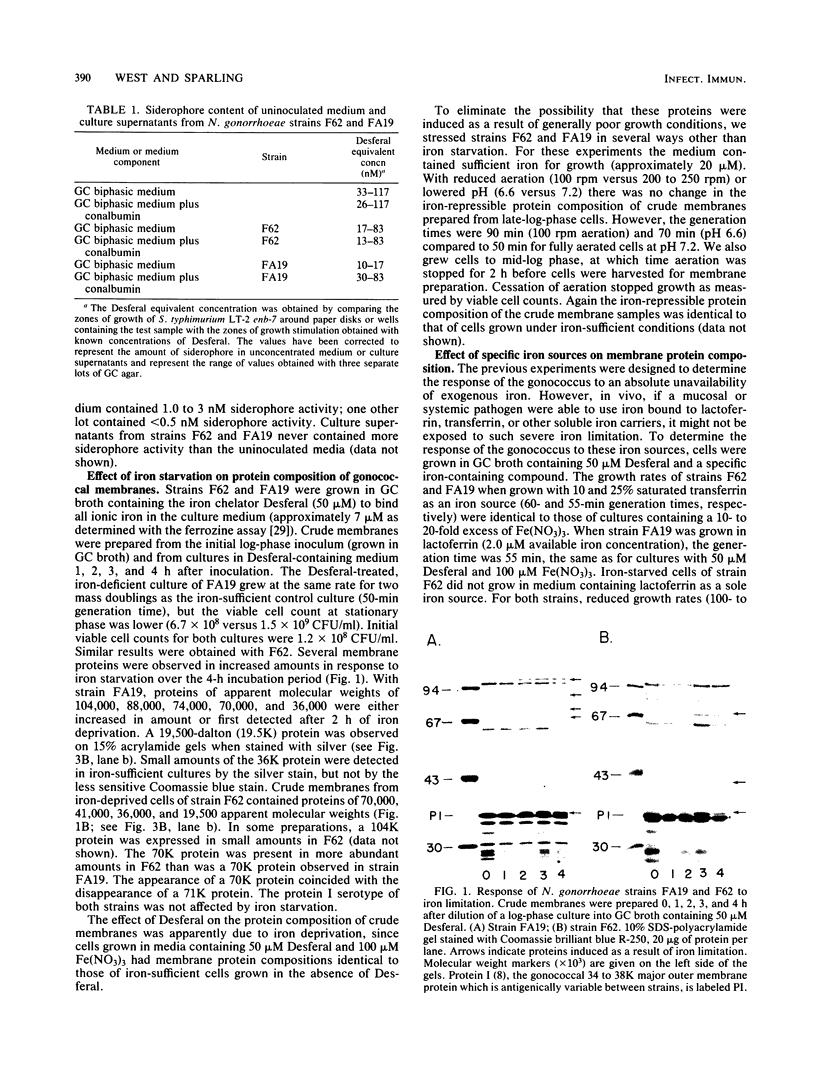
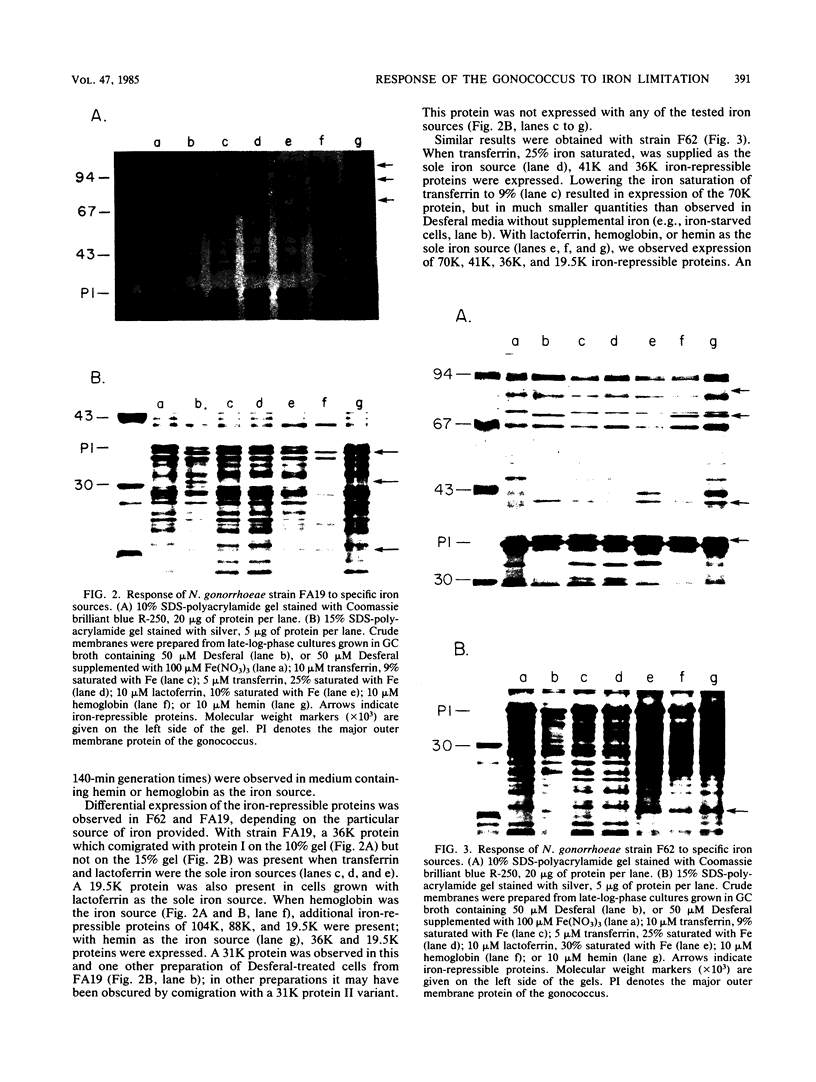
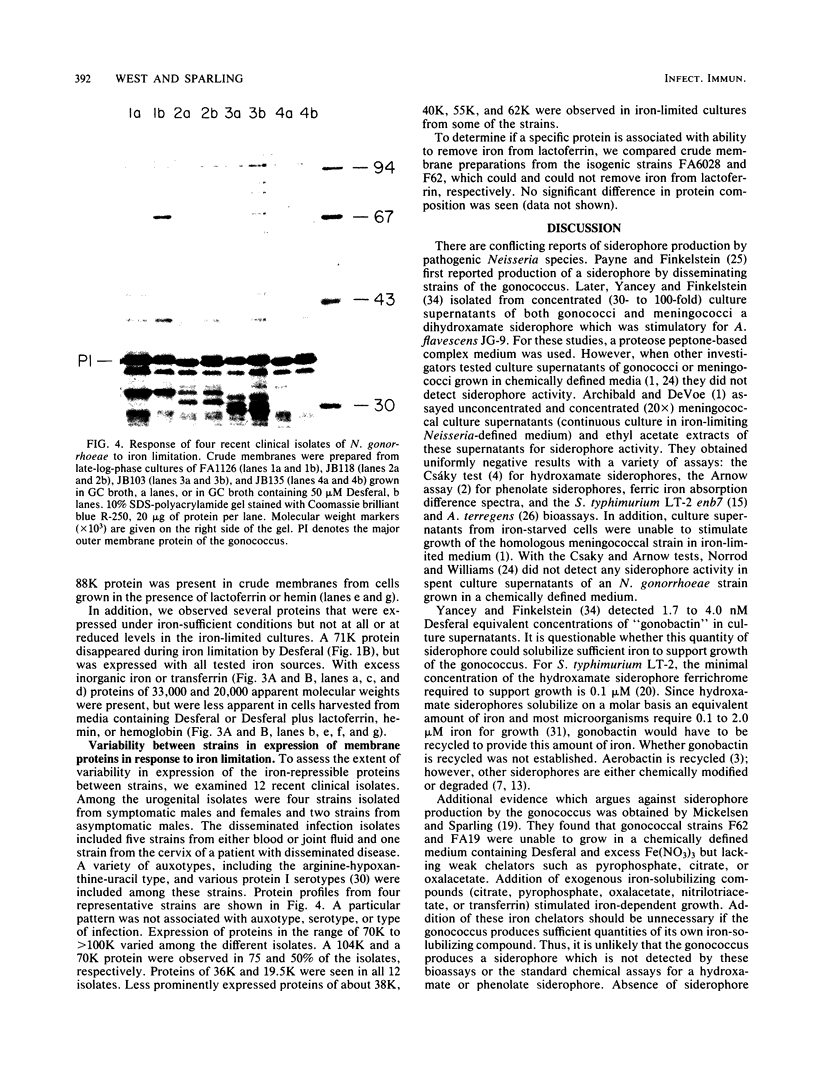
For acquisition of iron, an essential nutrient, most microorganisms produce siderophores (low-molecular-weight iron-chelating compounds) and membrane proteins to serve as receptors for the iron-siderophore complexes. The gonococcus does not appear to produce a siderophore, since the quantity of siderophore detected by bioassays of culture supernatants from strains F62 and FA19 was never greater than the amount present in the uninoculated medium. Iron limitation of the laboratory strains F62 and FA19 and 12 recent clinical isolates resulted in the expression of several iron-repressible membrane proteins. The expression of proteins in the apparent molecular weight range of 70,000 to 100,000 was strain dependent. All strains expressed 36,000-dalton (36K) and 19.5K proteins. FA19 and F62 were also grown in medium containing iron sources commonly encountered in vivo (i.e., transferrin, lactoferrin, hemoglobin, or hemin). Comparison of growth rates indicates that transferrin and lactoferrin were more readily utilized as iron sources than hemin and hemoglobin were. Expression of the iron-repressible proteins varied depending upon the iron source. Fewer iron-repressible proteins were observed when cells were supplied with transferrin or lactoferrin than when the cultures were grown with either hemin or hemoglobin. The 36K protein was expressed with all four iron sources.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., DeVoe I. W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980 Feb;27(2):322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Yancey R. J. Effect of siderophores on virulence of Neisseria gonorrhoeae. Infect Immun. 1981 May;32(2):609–613. doi: 10.1128/iai.32.2.609-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A., Braun V. Iron transport in Escherichia coli: uptake and modification of ferrichrome. J Bacteriol. 1980 Jul;143(1):246–255. doi: 10.1128/jb.143.1.246-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Peterson C. M., Grady R. W., Cerami A. Low molecular weight iron-binding factor from mammalian tissue that potentiates bacterial growth. J Exp Med. 1980 Feb 1;151(2):418–428. doi: 10.1084/jem.151.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P. E., McIntosh M. A., Neilands J. B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982 Mar;149(3):880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Spik G. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim Biophys Acta. 1980 May 7;629(2):399–408. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Luginbuhl G. H., Sandstrom E., Morse S. A. Identification of an iron-regulated 37,000-dalton protein in the cell envelope of Neisseria gonorrhoeae. Infect Immun. 1984 Aug;45(2):410–416. doi: 10.1128/iai.45.2.410-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH C. V., HANKS J. H. USE OF ARTHROBACTER TERREGENS FOR BIOASSAY OF MYCOBACTIN. J Bacteriol. 1964 Jun;87:1317–1320. doi: 10.1128/jb.87.6.1317-1320.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Assmilation of iron by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):592–599. doi: 10.1128/iai.32.2.592-599.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Siderophore production by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):600–608. doi: 10.1128/iai.32.2.600-608.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]